Abstract
Coronavirus is a novel human pathogen causing fulminant respiratory syndrome (COVID-19). Although COVID-19 is primarily a disease of the lungs with florid respiratory manifestations, there are increasing reports of cardiovascular, musculoskeletal, gastrointestinal, and thromboembolic complications. Developing an effective and reliable vaccine was emergently pursued to control the catastrophic spread of the global pandemic. We report a fatal case of vaccine-induced immune thrombotic thrombocytopenia (VITT) after receiving the first dose of the ChAdOx1 nCoV-19 vaccine. We attribute this fatal thrombotic condition to the vaccine due to the remarkable temporal relationship. The proposed mechanism of VITT is production of rogue antibodies against platelet factor-4 resulting in massive platelet aggregation. Healthcare providers should be aware of the possibility of such fatal complication, and the vaccine recipients should be warned about the symptoms of VITT.
Key Words: COVID-19, Vaccine, Vaccine-induced Immune Thrombotic Thrombocytopenia, Cerebral Venous Sinus Thrombosis, Disseminated Intravascular Coagulation, Death
Introduction
Coronavirus is a novel human pathogen causing fulminant respiratory syndrome (COVID-19) that was first identified in December 2019 as a cluster of cases with fatal pneumonia in Wuhan, China.1 In March 2020, the World Health Organization declared a worldwide pandemic and designated the disease taxonomy as COVID-19.2 Although COVID-19 is primarily a disease of the lungs with florid respiratory manifestations, there are increasing reports of cardiovascular, musculoskeletal, gastrointestinal, and thromboembolic complications.3 Developing an effective and reliable vaccine was emergently pursued to control the catastrophic spread of the global pandemic. Traditionally, vaccine development progresses through several pre-clinical and clinical stages occurring sequentially, and each may take a considerable time for completion. This was not the case with the COVID-19 vaccine as these stages were accelerated to an unprecedented pace with a seamless transition from one stage to the other over a few months. Inactivated or live-attenuated viruses as well as recombinant proteins and vectors technologies have been deployed to develop the COVID-19 vaccine. In addition, new platforms such as RNA and DNA vaccines are also used for the first time in a licensed vaccine.4 We report a fatal case of vaccine-induced immune thrombotic thrombocytopenia (VITT) after receiving the first dose of the ChAdOx1 nCoV-19 vaccine with emphasis on the possible pathophysiology behind this complication.
Case report
A 36-year-old female, known case of diabetes mellitus on oral therapy, presented to the emergency room with sudden onset of focal left-sided convulsions for 5 minutes followed by weakness in the left arm. She had a fever of 38.2 C° at home with vomiting and severe headache that started a few hours prior to presentation. Two weeks prior to the presentation, she received the first dose of the ChAdOx1 nCoV-19 vaccine. She had no history of any thromboembolic or connective tissue disorders. She was not on oral contraceptive pills or herbal remedies. On examination, the patient was conscious and oriented with tachycardia at 117 bpm. Her Glasgow coma scale (GCS) was 15/15, and cranial nerves examination was unremarkable. Motor examination showed left upper limb weakness at 3/5 on the Medical Research Council's scale with normal power of the right upper and bilateral lower limbs. Deep tendon reflexes were brisk on the left with Babinski sign. Sensory and coordination examinations were normal. Initial complete blood count upon admission showed elevated white blood cell count at 18.7×10"9/L (mainly neutrophils), low hemoglobin at 10.4 g/dL, and clumped platelets. Her liver enzymes (AST, ALT, lactate dehydrogenase, and GGT) were slightly elevated. Her coagulation profile showed prolonged PT (45 s), PTT (98 s), and INR (4.1) with D-dimer more than 35 mg/L. Blood smear showed leukocytosis of neutrophils with mild left shift, polychromasia, anisocytosis, Burr cells, and moderate thrombocytopenia with a few large platelets. A septic screen of blood, urine, and respiratory cultures was negative. COVID-19 PCR was negative. Brain computed tomography (CT) scan showed superior sagittal thrombosis with thickened cortical veins and bilateral hypodensites in the parietal lobes (Fig. 1 ). She was started on enoxaparin 80 mg, antibiotics, and antivirals. Two hours later, the patient became hypotensive and tachycardic with a drop in GCS from 15 to 8 necessitating transfer to the Intensive Care Unit (ICU) where she was intubated, mechanically ventilated, and started on ionotropic support. One day later, the patient deteriorated as she developed florid DIC with a drop in hemoglobin to 4 g/dL requiring massive blood transfusion. A repeat blood work showed a highly elevated white blood cell count at 48.4×10"9/L (mainly neutrophils), low platelets at 94×10"9/L, low fibrinogen at 0.6 g/L, and high creatinine at 254 umol/L. She developed acute kidney injury and severe acidosis requiring emergent hemodialysis. Enoxaparin was stopped due to DIC. A repeat brain CT scan showed multiple new bifrontal and biparietal hypodensites. The CT-venogram demonstrated extensive dural venous sinus thrombosis of the superior sagittal sinus and its cortical tributaries as well as the proximal left transverse sinus (Fig. 2 ). CT abdomen and pelvis showed extensive portal vein thrombosis with superior mesenteric vein thrombosis and potential splenic and hepatic infarction. The patient had a complicated course in the ICU given her multiple thromboses, DIC, difficulty to use anticoagulants, lactic acidosis, acute kidney injury, and multi-organ failure. She developed cardiac arrest with pulseless electrical activity and died four days after admission.
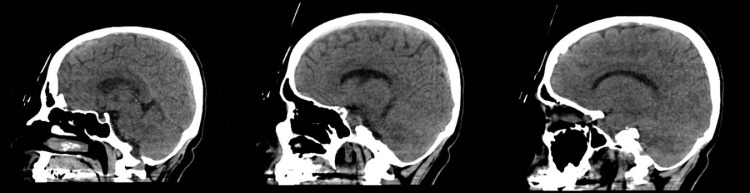
Fig. 1.
Brain computed tomography scan showing superior sagittal thrombosis with thickened cortical veins and bilateral hypodensites in the parietal lobes.
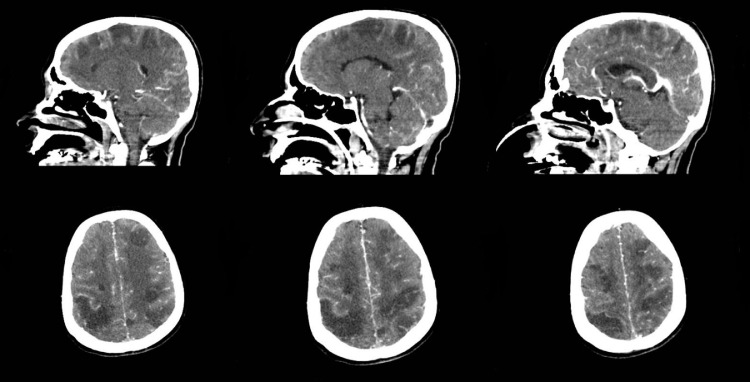
Fig. 2.
Brain computed tomography scan showing bilateral variable-sized hypodensities in the cerebral hemispheres involving the frontal and parietal lobes bilaterally with loss of gray-white matter differentiation. The CT-venogram is demonstrating extensive dural venous sinus thrombosis of the superior sagittal sinus and its cortical tributaries as well as the proximal left transverse sinus.
Discussion
The outbreak of COVID-19 infection as a serious emerging disease was accompanied by a host of neurological complications of variable degrees of severity in up to 30% of hospitalized patients. The spectrum of neurological complications includes meningitis, encephalitis, stroke, cerebral venous sinus thrombosis, encephalopathy, and Guillain-Barre syndrome.5 Several cases of cerebral venous sinus thrombosis complicating COVID-19 infection have been reported with a relevant temporal relationship since the infection preceded the thrombosis by up to two weeks.6 , 7 COVID-19 has been associated with a spectrum of hypercoagulable states including elevation of D-dimer, fibrinogen, and fibrin degradation products. Moreover, a direct endothelial injury may result from the interaction of the virus with the angiotensin-converting enzyme receptor that promotes a hypercoagulable state.8 Venous thromboembolism with COVID-19 infection is most prominent in the second week after symptom onset, which suggests a relationship to the peak of the cytokine storm9.
The ChAdOx1 nCoV-19 vaccine is a viral vector vaccine that uses modified chimpanzee adenovirus ChAdOx1 as a vector. The safety profile of the vaccine is acceptable with commonly reported mild side effects such as injection-site pain, nausea, and headache that resolve within a few days.10 The vaccine was linked to severe thrombotic events with the majority of the cases occurring in women under the age of 60 within 2 weeks of receiving the first dose. In addition, cerebral venous sinus thrombosis was found to usually occur with low levels of platelets (thrombocytopenia).11 The mechanism of thrombosis is that the viral proteins and free DNA in the vaccine bind to platelet factor 4 to generate a neoantigen that subsequently leads to the development of antibodies against platelet factor 4 which activate platelets and promote clotting.12 The phenomenon is similar to autoimmune heparin-induced thrombocytopenia (aHIT).13 Our case reflects this mechanism with fulminant consumptive coagulopathy and thrombocytopenia leading to extensive thrombosis co-occurring with bleeding diathesis.
The antibodies associated with the ChAdOx1 nCoV-19 vaccine can be detected by platelet factor 4/heparin enzyme-immunoassays, which are commercially available to diagnose HIT.14 However, this test was not performed for our patient. Recent articles reported successful treatment of VITT using intravenous immunoglobulin (IVIG), which prevents platelet activation by anti-platelet factor 4 antibodies.15 VITT is a rapidly fatal disorder if not recognized and treated early. A recent post-mortem study of VITT reported extensive involvement of large venous vessels with thrombotic occlusions in the microcirculation of multiple organs as well as increased inflammatory infiltrates. These findings indicated the progression of an inflammatory process that culminates in microvascular injury of multiple organs by iatrogenic activation of the innate immune system along with the complement pathway.16
Conclusion
The report presents a rare occurance of a florid thrombotic thrombocytopenia after the first dose of the ChAdOx1 nCoV-19 vaccine. We attribute this fatal thrombotic condition to the vaccine due to the remarkable temporal relationship. The proposed mechanism of VITT is production of rogue antibodies against platelet factor-4 resulting in massive platelet aggregation. The case should alert healthcare providers to the possibility of such precipitously fatal complication while vaccine recipients should be warned about the symptoms of VITT.
Conflict of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y-D, Chi W-Y, Su J-H, Ferrall L, Hung C-F, Wu T-C. Coronavirus vaccine development: from SARS and MERS to COVID-19. J Biomed Sci. 2020;27(1):104. doi: 10.1186/s12929-020-00695-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koralnik IJ, Tyler KL. Covid-19: a global threat to the nervous system. Ann Neurol. 2020;88(1):1–11. doi: 10.1002/ana.25807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dakay K, Cooper J, Bloomfield J, et al. Cerebral venous sinus thrombosis in covid-19 infection: a case series and review of the literature. J Stroke Cerebrovasc Dis. 2021;30(1) doi: 10.1016/j.jstrokecerebrovasdis.2020.105434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes C, Nichols T, Pike M, Subbe C, Elghenzai S. Cerebral venous sinus thrombosis as a presentation of covid-19. Eur J Case Rep Intern Med. 2020;7(5) doi: 10.12890/2020_001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N Engl J Med. Published online April 9, 2021. [DOI] [PMC free article] [PubMed]
- 12.Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after chadox1 ncov-19 vaccination. N Engl J Med. Published online April 16, 2021. [DOI] [PMC free article] [PubMed]
- 13.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S.Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N Engl J Med. Published online April 9, 2021. [DOI] [PMC free article] [PubMed]
- 14.Handtke S, Wolff M, Zaninetti C, et al. A Flow cytometric assay to detect platelet activating antibodies in VITT after ChAdOx1 nCov-19 vaccination. Blood. Published online May 4, 2021. [DOI] [PMC free article] [PubMed]
- 15.Thaler J, Ay C, Gleixner KV, et al. Successful treatment of vaccine-induced prothrombotic immune thrombocytopenia (Vipit). J Thromb Haemost. Published online April 20, 2021. [DOI] [PMC free article] [PubMed]
- 16.Pomara C, Sessa F, Ciaccio M, et al. Post-mortem findings in vaccine-induced thrombotic thombocytopenia. Haematologica. Published online May 20, 2021. [DOI] [PMC free article] [PubMed]