See article vol. 28: 604-610
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is a common inherited and X-chromosome linked enzyme defect, and affects more than 400 million people worldwide 1) . While patients with G6PD deficiency often remain asymptomatic throughout their life, some patients exhibit severe clinical manifestation associated with oxidative stress such as hemolytic anemia and bilirubin-induced neurological damage, particularly in newborns. Hemolytic anemia associated with G6PD is usually triggered by exogenic agents such as fava bean 2) and primaquines (an anti-malaria drug) and rapid and severe mode of the onset of this manifestation is associated with the toxic or allergic effects of the exposures and degree of G6PD deficiency in the patents.
In this issue of Journal of Atherosclerosis and Thrombosis, another precipitating factor for G6PD deficiency pathogenies is proposed, that is, aging and cardiovascular disease. Dore et al. conducted a retrospective cross-sectional study investigating association between G6PD deficiency and prevalence of cardiovascular disease among 9,604 patients aged between 18 and 95 years, who had undergone gastroendoscopy for the screening of H. Pylori 3) . The overall prevalence of G6PD deficiency in their participants was unsurprisingly high (11.3%) because their study was conducted in Sardinia, Italy, which is a common area of G6PD deficiency in Mediterranean lesion. In the multivariate models, the adjusted odds ratio (OR) of G6PD deficiency for cardiovascular disease (CVD) was 3.24, which is the second highest OR following age (OR=3.80). More importantly, age-stratified analysis revealed that G6PD deficiency is significantly associated with increased OR of CVD only among those aged 60 years and older. They confirmed the age-related increase in OR for CVD both male and female participants.
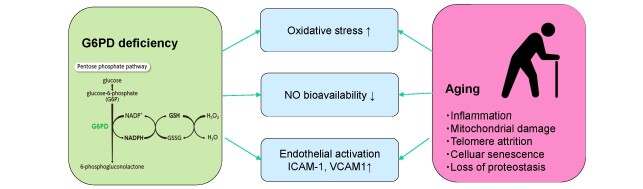
So far, results from observational studies on the association between G6PD deficiency and CVD are controversial 4 , 5) , possibly due to its pro- and antioxidant effects in the organisms and the differences in the cohort characteristics. In a study by the US military center with 17, 338 individuals with mean age of 37 years, G6PD deficiency is associated with 39.6% increase in OR of developing CVD 5) . Although the differences in potential causative mutations and degree of enzymatic deficiency need to be taken account, the finding by Dore et al. that G6PD deficiency is associated with highly elevated OR for CVD in the affected individuals aged 60 years or older may be worth discussing. There are some biological mechanisms underlying enhanced association between G6PD deficiency and CVD in the elderly. First, G6PD catalyzes the rate-limiting step in the pentose phosphate pathway, providing reduced form of nicotinamide adenine dinucreotide phosphate, which contribute to recycling in the reduced form of glutathione, a strong antioxidant in the body. Therefore, defects in G6PD may accumulate oxidative stress and subsequent pathogenesis of atherosclerosis. Second, G6PD deficiency in endothelial cells contributes to decrease in nitric oxide (NO) production along with glutathione depletion 6) . Endothelial NO synthesis is critical for maintaining vascular relaxation; hence, depletion of NO leads to hypertension, atherosclerosis, and CVD. Additionally, experimental models show G6PD deficiency is associated with increased expression of cell adhesion molecules such as ICAM-1 and VCAM-1 7) . Intriguingly, these molecular and cellular mechanisms associated with G6PD deficiency overlap on the effects of aging in the vasculature, thus the older adults with these genetic mutations may have accelerated atherogenic process, which underlie high prevalence of CVD ( Fig.1 ) .
Fig. 1.
Potential molecular effects of G6PD deficiency and aging on cardiovascular disease
The paper by Dore et al. presents an interesting point of contention, but the many challenges are ahead. First, their findings should be replicated in a large-scale prospective cohort study of general population. Such studies should include biomarker measurement of oxidative stress and NO signaling. It is also of interest to compare areas with high and low prevalence of G6PD deficiency. As the global population aging and threat of cardiovascular disease increases, elucidation of G6PD deficiency in CVD may be important not only for clinical management of patients with the mutations but also for further understanding of intricate associations between aging, oxidative stress, and atherosclerosis.
Conflicts of Interest
Yasumichi Arai received a research grant from Daiichi Sankyo Co, Ltd.
References
- 1).Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet, 2008; 371: 64-74 [DOI] [PubMed] [Google Scholar]
- 2).Luzzatto L, Arese P. Favism and Glucose-6-Phosphate Dehydrogenase Deficiency. N Engl J Med, 2018; 378: 60-71 [DOI] [PubMed] [Google Scholar]
- 3).Dore MP, Portoghese M, Pes GM. The Elderly with Glucose-6-Phosphate Dehydrogenase Deficiency are More Susceptible to Cardiovascular Disease. J Atheroscler Thromb, 2021; 28: 604-610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Cocco P, Todde P, Fornera S, Manca MB, Manca P, Sias AR. Mortality in a cohort of men expressing the glucose-6-phosphate dehydrogenase deficiency. Blood, 1998; 91: 706-709 [PubMed] [Google Scholar]
- 5).Thomas JE, Kang S, Wyatt CJ, Kim FS, Mangelsdorff AD, Weigel FK. Glucose-6-Phosphate Dehydrogenase Deficiency is Associated with Cardiovascular Disease in U.S. Military Centers. Tex Heart Inst J, 2018; 45: 144-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Parsanathan R, Jain SK. Glucose-6-phosphate dehydrogenase deficiency increases cell adhesion molecules and activates human monocyte-endothelial cell adhesion: Protective role of l-cysteine. Arch Biochem Biophys, 2019; 663: 11-21 [DOI] [PubMed] [Google Scholar]
- 7).Parsanathan R, Jain SK. L-Cysteine in vitro can restore cellular glutathione and inhibits the expression of cell adhesion molecules in G6PD-deficient monocytes. Amino Acids, 2018; 50: 909-921 [DOI] [PubMed] [Google Scholar]