Abstract
Background
Intraventricular hemorrhage (IVH) is a common complication in preterm infants that has poor outcomes, especially in severe cases, and there are currently no widely accepted effective treatments. Erythropoietin has been shown to be neuroprotective in neonatal brain injury.
Objective
The objective of this study was to evaluate the protective effect of repeated low-dose recombinant human erythropoietin (rhEPO) in preterm infants with IVH.
Methods
This was a single-blinded prospective randomized controlled trial. Preterm infants ≤ 32 weeks gestational age who were diagnosed with IVH within 72 h after birth were randomized to receive rhEPO 500 IU/kg or placebo (equivalent volume of saline) every other day for 2 weeks. The primary outcome was death or neurological disability assessed at 18 months of corrected age.
Results
A total of 316 eligible infants were included in the study, with 157 in the rhEPO group and 159 in the placebo group. Although no significant differences in mortality (p = 0.176) or incidence of neurological disability (p = 0.055) separately at 18 months of corrected age were seen between the rhEPO and placebo groups, significantly fewer infants had poor outcomes (death and neurological disability) in the rhEPO group: 14.9 vs. 26.4%; odds ratio (OR) 0.398; 95% confidence interval (CI) 0.199–0.796; p = 0.009. In addition, the incidence of Mental Development Index scores of < 70 was lower in the rhEPO group than in the placebo group: 7.2 vs. 15.3%; OR 0.326; 95% CI 0.122–0.875; p = 0.026.
Conclusions
Treatment with repeated low-dose rhEPO improved outcomes in preterm infants with IVH.
Trial Registration
The study was retrospectively registered on ClinicalTrials.gov on 16 April 2019 (NCT03914690).
Key Points
| Erythropoietin is a promising neuroprotective drug for preterm infants, and treatment with repeated low-dose recombinant human erythropoietin (rhEPO) is well-tolerated. |
| Treatment with repeated low-dose rhEPO improved poor outcomes in preterm infants with intraventricular hemorrhage. |
Introduction
Intraventricular hemorrhage (IVH) is one of the most common complications in preterm infants [1] and is associated with low birth weight and low gestational age [2–4]. IVH-induced brain injuries are caused not only by hematoma but also by neurotoxic compounds released from the hematoma [5]. Nearly 60% of very preterm infants with grade III–IV IVH develop severe neurodevelopmental outcomes [6, 7]. There are currently no specific treatments to prevent preterm infants with IVH from developing serious neurological disabilities [8]. However, a promising candidate is erythropoietin, which is commonly used for treating anemia in preterm infants [9].
Erythropoietin and its receptor are expressed in brain cells and play critical roles in promoting brain maturation, and recent studies showed that erythropoietin is a promising neuroprotective drug [10–15]. Animal experiments have shown that erythropoietin exerts its neuroprotective effects by reducing inflammation, oxidative stress, and apoptosis and by promoting the regeneration of neuronal stem cells and blood vessels [16–21]. Erythropoietin treatment had positive effects on short- and long-term neurological outcomes in a preclinical model of IVH [22], and some observational clinical studies also showed that erythropoietin treatment may improve neurodevelopmental outcomes in preterm infants with IVH [10, 23]. However, the use of erythropoietin remains controversial with regard to poor outcomes [9], and both the dose and the course of erythropoietin in preterm infants is uncertain [24, 25]. Many clinical follow-up studies on preterm infants primarily treated with low-dose recombinant human erythropoietin (rhEPO) for anemia of prematurity showed improved neurodevelopmental outcomes [10, 26–28]. Our previous study showed that prophylactic use of low-dose rhEPO 500 IU/kg decreased the incidence of IVH in preterm infants [24]. In this study, we aimed to assess whether treatment with repeated low-dose rhEPO 500 IU/kg improved poor outcomes, including mortality and neurological disability, in preterm infants with IVH.
Methods
Study Design and Participants
Between July 2014 and December 2017, preterm infants with a gestational age of ≤ 32 weeks who were diagnosed with IVH by cerebral ultrasound within 72 h after birth in the neonatal intensive care unit (NICU) of the Third Affiliated Hospital and Children’s Hospital of Zhengzhou University were eligible for enrollment. IVH was classified as grade I–IV according to Papile et al. [29] as follows: grade I—blood in the periventricular germinal matrix regions or germinal matrix hemorrhage; grade II—blood within the lateral ventricular system without ventricular dilatation; grade III—blood acutely distending into the lateral ventricles; grade IV—blood within the ventricular system and parenchyma. We excluded preterm infants with congenital cerebral malformation, chromosome disease, genetic metabolic disease, large venous thrombus, hypertension, or polycythemia and infants whose parents declined to participate or withdrew treatment. The study protocol was approved by the Ethics Committee of Zhengzhou University and Henan Medical Academy, and written informed consent was obtained from all parents. The study was retrospectively registered on ClinicalTrials.gov on 16 April 2019 (NCT03914690).
Sample Size
The sample size was calculated assuming an incidence of long-term neurological disability of 47% in very preterm infants [30] and that rhEPO treatment can reduce the incidence by 40%. Thus, 140 premature infants were needed in each group for a significance of 5% with 90% power, and a total of 308 infants were needed for a 10% loss to follow-up.
Randomization and Blinding
Each patient was randomly assigned to the rhEPO group or placebo group in a 1:1 ratio using a computer-based random number generator, and the group assignment for each eligible patient was concealed in a sealed envelope before the patients were included. The parents, neurologists, and examiners who performed the neurodevelopmental screening were blinded to the infants’ group allocation. The neonatologists and nurses responsible for treatment in the NICU were not blinded.
Intervention
Infants in the rhEPO group received intravenous rhEPO 500 IU/kg immediately after diagnosis with IVH and then every other day for 2 weeks, whereas infants in the placebo group received an equivalent volume of saline. Infants in the two groups received the same nursing and treatment strategy except for rhEPO.
Data Collection
Cerebral ultrasound was performed through the anterior fontanelle within 72 h after birth and then performed once a week until the bleeding was stable (the absorption period). The day of IVH onset, degree, unilateral or bilateral IVH, and whether combined with periventricular leukomalacia (PVL) or lateral ventricular dilatation were recorded.
Baseline information were collected, including gestational age, birth weight, sex, delivery mode, Apgar score, mechanical ventilation, and maternal factors during the perinatal period. Complications during hospitalization such as retinopathy of prematurity (ROP) [31], necrotizing enterocolitis (NEC) [32], and bronchopulmonary dysplasia (BPD) [33] were recorded. Infants who developed rhEPO-related side effects, including large venous thrombus, polycythemia, or hypertension, were immediately taken off the rhEPO treatment and given emergency therapy.
Follow-up and Outcomes
All subjects were followed-up every 3 months until they were aged 18 months of corrected age to undergo measurement of body weight and length and a standardized physical examination of the nervous system. Two experienced examiners evaluated the Mental Development Index (MDI) score at 18 months of corrected age using the Bayley Infant Development Scale (2nd edition). Deafness was defined as hearing loss that needed external hearing aids [34]. Blindness was defined as a best corrected visual acuity of < 0.05 or a sight radius < 10° [34]. Neurological disability was defined as surviving with one or more of the following complications: cerebral palsy, MDI score < 70, blindness, or deafness [24]. The primary outcome was death or neurological disability at 18 months of corrected age.
Statistical Analysis
The data were analyzed using SPSS v23.0. We used Mann–Whitney U test to compare the quantitative data including gestational age, birth weight, Apgar score, and onset day of IVH between the two groups. We used the chi-squared test to compare the count data including sex, delivery mode, maternal and neonatal complications, and mortality and long-term neurological disability between the two groups. Multivariate logistic regression was performed to adjust for the effect of sociodemographic and perinatal risk factors on poor outcomes. Fisher’s exact test and the Mantel–Haenszel test were used for subgroup analysis and interaction analysis.
Results
Characteristics of Preterm Infants
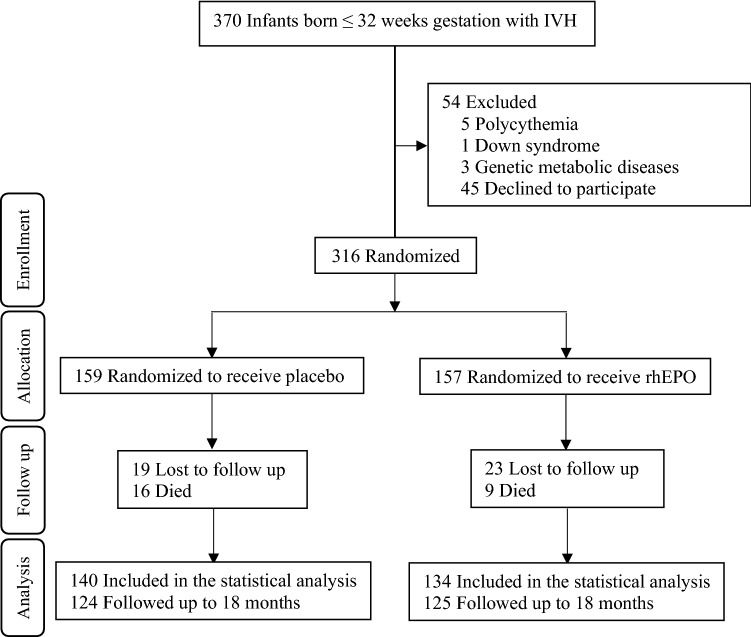
During the study period, a total of 939 preterm infants ≤ 32 weeks gestational age were admitted to the NICUs, and 370 were diagnosed with IVH within 72 h after birth. In total, 54 infants were excluded (five had polycythemia, one had Down syndrome, three had genetic metabolic diseases, and 45 declined to participate). A total of 316 eligible preterm infants were randomly divided into the placebo group (159 infants) or the rhEPO group (157 infants) (Fig. 1). The gestational age range was 25+4–32 weeks in the rhEPO group, and 25+5–32 weeks in the placebo group. The median age of IVH onset was 2 days (quartile range 2–3). In the total group of 316 infants, the number of those with grade I, II, III, and IV IVH was 133 (42.1%), 162 (51.3%), 10 (3.2%), and 11 (3.2%), respectively, with 89 (28.2%) having unilateral IVH and 227 (71.8%) having bilateral IVH. There was no significant difference between the two groups at the beginning of the study (p > 0.05) (Table 1). In total, 42 infants were lost to follow-up, and 274 (86.7%) infants attended the assessment at 18 months of corrected age (Fig. 1).
Fig. 1.
Study flow. Schematic flowchart showing the number of participants and the procedure of assigning patients to recombinant human erythropoietin or placebo and follow-up to 18 months of corrected age. Preterm infants with grade I–IV intraventricular hemorrhage were enrolled in the study. IVH intraventricular hemorrhage, rhEPO recombinant human erythropoietin
Table 1.
Baseline information of preterm infants with intraventricular hemorrhage in the placebo and recombinant human erythropoietin groups
| Characteristic | Placebo (n = 159) | rhEPO (n = 157) | p value |
|---|---|---|---|
| Gestational age, weeks | 29.6 (28.6–30.6) | 29.4 (28.4–30.3) | 0.997 |
| Birth weight, g | 1250 (1050–1400) | 1200 (1030–1350) | 0.948 |
| Male | 96 (60.4) | 88 (56.1) | 0.436 |
| SGA | 30 (18.9) | 37 (23.6) | 0.307 |
| Premature rupture of membrane | 59 (37.1) | 62 (39.5) | 0.663 |
| Maternal hypertension | 32 (20.1) | 25 (15.9) | 0.331 |
| Placental abruption | 6 (3.8) | 11(7.0) | 0.203 |
| Caesarean section | 80 (50.3) | 91 (58.0) | 0.173 |
| 5-Minute Apgar | 8 (7–9) | 8 (8–9) | 0.688 |
| IVH onset, days | 2 (2–3) | 2 (1–3) | 0.951 |
| Bilateral IVH | 111 (69.8) | 116 (73.9) | 0.421 |
| IVH (grade III–IV) | 14 (8.8) | 7 (4.5) | 0.121 |
| Post-hemorrhagic ventricular dilation | 7 (4.4) | 5 (3.2) | 0.571 |
| Early onset sepsis | 18 (11.3) | 16 (10.2) | 0.856 |
| Chorioamnionitis | 26 (16.4) | 29 (18.5) | 0.658 |
| Number of transfusions | 2 (1–4) | 2 (1–4) | 0.249 |
Data are presented as n (%) or median (quartile range) unless otherwise indicated
The p value was calculated using Mann–Whitney U test or the chi-squared test to compare the placebo and rhEPO groups. p < 0.05 was considered significantly different
IVH intraventricular hemorrhage, rhEPO recombinant human erythropoietin, SGA small for gestational age
Mortality and Neurological Outcomes at 18 Months of Corrected Age
A total of 249 survivors (124 in the placebo group and 125 in the rhEPO group) with IVH were followed up to 18 months of corrected age. During the study period, 16 infants in the placebo group died (11 died during hospitalization and five died during follow-up; six died of BPD and severe pneumonia, four died of severe pneumonia, three died of NEC, two died of sepsis, and one died of respiratory distress syndrome [RDS]) and nine in the rhEPO group died (four during hospitalization and five during follow up; three died of BPD and severe pneumonia, three died of severe pneumonia, two died of RDS, and one died of multiple organ failure). The median (quartile range) age of death was 57.5 (38.3–71.5) days in the placebo group and 40.0 (22.5–48.0) days in the rhEPO group, with no significant difference between the two groups (p > 0.05). Among the 16 infants who died in the placebo group, the incidence of IVH grade I, II, III, and IV was 5 (31.3%), 9 (56.3%), 1 (6.3%), and 1 (6.3%), respectively. Among the nine patients who died in the rhEPO group, the incidence of IVH grade I, II, III, and IV was 4 (44.4%), 4 (44.4%), 0, and 1 (11.1%), respectively.
The mortality and the incidence of neurological disability at 18 months of corrected age were not significantly different between the two groups (p > 0.05). However, significantly fewer infants had poor outcomes (death and neurological disability) in the rhEPO group (20/134 [14.9%]) than in the placebo group (37/140 [26.4%]) (relative risk [RR] 0.488; 95% confidence interval [CI] 0.267–0.895; p = 0.019). The incidence of MDI score < 70 was also significantly lower in the rhEPO group than in the placebo group (7.2 vs. 15.3%; RR 0.429; 95% CI 0.186–0.989; p = 0.043). There was no significant difference in the incidence of cerebral palsy (p = 0.231), deafness (p = 0.566), or blindness (p = 0.154) between the two groups (Table 2).
Table 2.
Poor outcomes with intraventricular hemorrhage at 18 months of corrected age
| Outcome | Placebo | rhEPO | RR (95% CI) | p value |
|---|---|---|---|---|
| Primary outcome | ||||
| Death | 16/140 (11.4) | 9/134 (6.7) | 0.558 (0.238–1.310) | 0.176 |
| Disability | 21/124 (16.9) | 11/125 (8.8) | 0.473 (0.218–1.029) | 0.055 |
| Death + disability | 37/140 (26.4) | 20/134 (14.9) | 0.488 (0.267–0.895) | 0.019 |
| Secondary outcomea | ||||
| Cerebral palsy | 8/124 (6.5) | 4/125 (3.2) | 0.479 (0.141–1.635) | 0.231 |
| MDI < 70 | 19/124 (15.3) | 9/125 (7.2) | 0.429 (0.186–0.989) | 0.043 |
| Deafness | 1/124 (0.8) | 2/125 (1.6) | 2.000 (0.179–22.344) | 0.566 |
| Blindness | 2/124 (1.6) | 0/125 (0) | 0.984 (0.962–1.006) | 0.154 |
Data are presented as n/N (%) unless otherwise indicated. Disability is defined as surviving infants with one or more of the following complications: cerebral palsy, MDI < 70, blindness, or deafness. p < 0.05 was considered statistically significant
CI confidence interval, MDI Mental Developmental Index, rhEPO recombinant human erythropoietin, RR relative risk
aOne infant with more than one disability could be counted repeatedly
The sociodemographic and perinatal risk factors of preterm infants may affect their outcomes, and multivariate logistic regression was performed to adjust for these factors. Maternal education, family economic status, gestational age, birth weight, sex, premature rupture of membrane, mechanical ventilation > 7 days, maternal hypertension, placental abruption, caesarean section, severe asphyxia, grade III–IV IVH, PVL, post-hemorrhagic ventricular dilation, sepsis, respiratory distress, NEC, and BPD were included as covariates. For death or neurological disability, after adjusting for confounding factors (mechanical ventilation > 7 days, grade III–IV IVH, PVL, and BPD), infants in the rhEPO group had lower odds ratios (ORs) than those in the placebo group: 14.9 versus 26.4%; OR 0.398; 95% CI 0.199–0.796; p = 0.009. For MDI score < 70, after adjusting for confounding factors (grade III–IV IVH and PVL), infants in the rhEPO group also had lower ORs than those in the placebo group: 7.2 versus 15.3%; OR 0.326; 95% CI 0.122–0.875; p = 0.026) (Table 3).
Table 3.
Multivariate analysis for poor outcomes at 18 months of corrected age
| Outcome | Placebo | rhEPO | p value | Odds ratio (95% CI) |
|---|---|---|---|---|
| Death + disability | 37/140 (26.4) | 20/134 (14.9) | 0.009 | 0.398 (0.199–0.796) |
| MDI < 70 | 19/124 (15.3) | 9/125 (7.2) | 0.026 | 0.326 (0.122–0.875) |
Data are presented as n/N (%) unless otherwise indicated. Poor outcomes are defined as infants with death or disability
CI confidence interval, MDI Mental Developmental Index, rhEPO recombinant human erythropoietin
Subgroup Analysis
Subgroup analysis was performed using gestational age (< 28, 28–296/7, and 30–32 weeks), birth weight (< 1000, 1000–1499, and ≥ 1500), sex (males and females), and the grade of IVH (I–II and III–IV). Treatment with rhEPO reduced the incidence of death or neurological disability in infants born at 30–32 weeks of gestational age (p < 0.05), in infants with a birth weight of 1000–1499 g (p < 0.05), in girls (p < 0.05), and in infants with grade I–II IVH (p < 0.05) (Table 4), and treatment with rhEPO reduced the incidence of MDI score <70 in infants born at 28–296/7 weeks of gestational age (p < 0.05) and infants with a birth weight of 1000–1499 g (p < 0.05) (Table 5). However, interaction analysis showed that gestational age, birth weight, sex, and degree of IVH had no significant interaction effect on rhEPO treatments in terms of improving neurological outcomes and mortality (Table 4) and MDI score (Table 5) in preterm infants.
Table 4.
Subgroup interaction analysis for recombinant humanerythropoietin on neurological disability or death
| Subgroups | Placebo | rhEPO | RR (95% CI) | Interaction p value |
|---|---|---|---|---|
| Sex | ||||
| Male | 23/84 (27.4) | 14/78 (17.9) | 0.580 (0.274–1.230) | |
| Female | 14/56 (25.0) | 6/56 (10.7)* | 0.360 (0.127–1.019) | |
| Total | 0.488 (0.267–0.895) | 0.465 | ||
| Gestational age | ||||
| < 28 weeks | 6/19 (31.6) | 5/21 (23.8) | 0.677 (0.168–2.730) | |
| 28–296/7 weeks | 18/72 (25.0) | 10/66 (15.2) | 0.536 (0.227–1.264) | |
| 30–32 weeks | 13/49 (26.5) | 5/47 (10.6)* | 0.330 (0.107–1.014) | |
| Total | 0.488 (0.267–0.895) | 0.694 | ||
| Birth weight, g | ||||
| < 1000 | 7/18 (38.9) | 6/19 (31.6) | 0.725 (0.187–2.809) | |
| 1000–1499 | 24/102 (23.5) | 12/96 (12.5)* | 0.464 (0.217–0.991) | |
| ≥ 1500 | 6/20 (30.0) | 2/19 (10.5) | 0.275 (0.048–1.579) | |
| Total | 0.488 (0.267–0.895) | 0.683 | ||
| Degree of IVH | ||||
| Grade I–II | 30/126 (23.8) | 17/128 (13.3)* | 0.490 (0.255–0.943) | |
| Grade III–IV | 7/14 (50.0) | 3/6 (50.0) | 1.000 (0.148–6.772) | |
| Total | 0.488 (0.267–0.895) | 0.487 |
Data are presented as n/N (%) unless otherwise indicated. The p value is for the interaction analysis in subgroups using the Mantel–Haenszel test. p < 0.05 was considered statistically significant
CI confidence interval, IVH intraventricular hemorrhage, rhEPO recombinant human erythropoietin, RR relative risk
*p < 0.05 using Fisher’s exact test to compare the placebo group and the rhEPO group
Table 5.
Subgroup interaction analyses for recombinant human erythropoietin on Mental Development Index < 70
| Subgroups | Placebo | rhEPO | RR (95% CI) | Interaction p value |
|---|---|---|---|---|
| Sex | ||||
| Male | 12/75 (16.0) | 7/72 (9.7) | 0.565 (0.209–1.528) | |
| Female | 7/49 (14.3) | 2/53 (3.8) | 0.235 (0.046–1.193) | |
| Total | 0.429 (0.186–0.989) | 0.361 | ||
| Gestational age | ||||
| < 28 weeks | 2/15 (13.3) | 3/19 (15.8) | 1.219 (0.176–8.423) | |
| 28–296/7 weeks | 9/64 (14.1) | 2/60 (3.3)* | 0.211 (0.044–1.019) | |
| 30–32 weeks | 8/45 (17.8) | 4/46 (8.7) | 0.440 (0.123–1.583) | |
| Total | 0.429 (0.186–0.989) | 0.366 | ||
| Birth weight, g | ||||
| < 1000 | 1/12 (8.3) | 2/15 (13.3) | 1.692 (0.135–21.270) | |
| 1000–1499 | 14/93 (15.1) | 5/91 (5.5)* | 0.328 (0.113–0.952) | |
| ≥ 1500 | 4/19 (21.1) | 2/19 (10.5) | 0.441 (0.070–2.761) | |
| Total | 0.429 (0.186–0.989) | 0.470 | ||
| Degree of IVH | ||||
| Grade I–II | 14/112 (12.5) | 7/120 (5.8) | 0.434 (0.168–1.118) | |
| Grade III–IV | 5/12 (41.7) | 2/5 (40.0) | 0.933 (0.111–7.820) | |
| Total | 0.429 (0.186–0.989) | 0.516 |
Data are presented as n/N (%) unless otherwise indicated. The p value is for the interaction analysis in subgroups using the Mantel–Haenszel test. p < 0.05 was considered statistically significant
CI confidence interval, IVH intraventricular hemorrhage, rhEPO recombinant human erythropoietin, RR relative risk
*p < 0.05 using Fisher’s exact test to compare the placebo and rhEPO groups
Safety
Compared with infants in the placebo group, those in the rhEPO group had a significantly lower incidence of BPD (85/159 [53.5%] vs. 60/157 [38.2%]; RR 0.539; 95% CI 0.344–0.843; p = 0.007). The incidence of ROP (13/159 [8.2%] vs. 9/157 [5.7%]; RR 0.683; 95% CI 0.283–1.647; p = 0.393) and NEC (11/159 [6.9%] vs. 9/157 [5.7%]; RR 0.818; 95% CI 0.329–2.032; p = 0.665) between the control group and the rhEPO group were not significantly different. No adverse events were observed during the study period.
Discussion
Preterm infants with IVH are susceptible to poor neurodevelopmental outcomes, including cerebral palsy, intellectual deficits, deafness, and blindness [35]. Although administration of antenatal corticosteroids and postnatal prophylactic indomethacin is associated with a reduction in preterm infants with IVH [36, 37], no specific therapies or drugs are available for improving long-term neurological outcomes in preterm infants with IVH. In the current study, we showed that treatment with repeated low-dose rhEPO 500 IU/kg improved the incidence of poor outcomes in preterm infants with IVH.
Death and neurological disorders are poor outcomes for preterm infants [38], so we analyzed both in our study when evaluating whether rhEPO could improve poor outcomes in preterm infants with IVH. Multivariate analysis showed that treatment with rhEPO could reduce the incidence of death or neurological disability in preterm infants with IVH, which was consistent with findings from previous observational studies [10, 23]. Our previous study indicated that repeated low-dose rhEPO decreased the incidence of grade III–IV IVH in preterm infants [24], and this study added evidence that treatment with low-dose rhEPO could improve rates of death or the neurological outcomes of IVH. The incidence of death declined after treatment with rhEPO (11.4 vs. 6.7%) but without significant difference. One possible reason might be the small sample size (16 vs. 9 infant deaths). Another reason might be that some of the infants died from other diseases, such as NEC or sepsis, rather than IVH-induced brain injury itself. In addition to the anti-inflammatory, anti-oxidative, and anti-apoptotic effects of rhEPO and its promotion of nerve and vascular regeneration [39], rhEPO might also play a role in alleviating IVH-induced secondary injury due to the release of free iron and cell-free hemoglobin [40–42]. An experimental study showed that iron supplementation aggravated white-matter injury in newborn mice [43], and a clinical study found that unbound iron was elevated in the cerebral spinal fluid of preterm infants with white-matter damage [44]. These studies indicated that free iron plays an important role in premature brain injury [45, 46]. Erythropoietin increases iron utilization and reduces free iron oxidative toxicity [47], which might play a key role in preventing free-iron-induced brain injury. Experimental studies have shown that cell-free hemoglobin is distributed in areas of periventricular white matter [41], which induces inflammation and cell death in the choroid plexus [47]. Treatment with rhEPO promotes erythrocytopoiesis and reduces free hemoglobin levels, which can reduce the toxicity of free hemoglobin.
In the subgroup analysis, interaction analysis showed that gestational age, birth weight, sex, and degree of IVH had no significant interaction effect on the ability of rhEPO treatments to improve poor outcomes. This means that rhEPO treatment could improve poor outcomes in all preterm infants with gestational age ≤ 32 weeks. However, the preterm infants in our study had a large range of gestational ages, from 25+4 to 32 weeks, and fewer infants aged < 28 weeks (40/274 [14.6%] at < 28 weeks, 138/274 [50.4%] at 28–29+6 weeks, and 96/274 [35.0%] at 30–32 weeks), which might complicate the interpretation of the neuroprotective role of rhEPO in different subgroups, especially in extremely preterm infants [13, 48]. Therefore, we conducted a further study focusing on preterm infants born at < 28 weeks of gestational age (NCT02745990) to further explore the protective effect of rhEPO.
Sex differences have been noticed not only in adults but also in children, both in terms of the incidence of disease and therapeutic effects [49–51], and our previous animal studies showed sex differences in the immature brain after insult and treatment [52, 53]. These studies demonstrated that sex plays an important role in neuronal cell death and brain injury that is not related to sex hormone. Other studies further demonstrated that microglia activation and inflammatory responses are sex related [54–56]. Some studies showed that the effect of erythropoietin is even sex specific with regard to stimulating respiration in neonates [57] and regulating metabolism and hypothalamic inflammation [58]. In the current study, we found that treatment with rhEPO reduced death and neurological disability more significantly in females than in males. However, interaction analysis showed the neuroprotective effect of rhEPO was not influenced by sex. This needs to be confirmed further with a greater number of infants.
Preterm infants of < 32 weeks gestational age are at high risk of ROP, and whether rhEPO, which has properties similar to those of vascular endothelial growth factor, is a risk factor for the development of ROP remains a concern [9, 59], even though some clinical studies showed the high-dose rhEPO or longer treatment durations did not increase the risk of ROP in extremely preterm infants [38, 60]. Our study showed that treatment with rhEPO did not increase the risk of ROP, which supports other studies that also reported that different dosages of rhEPO did not increase the incidence of ROP [59, 61, 62] in very low birth weight infants. Thus, again, rhEPO appears to be an effective and safe drug that does not increase ROP in preterm infants.
There are some limitations to this study. First, this trial was a follow-up study of the protective effect of rhEPO in terms of long-term outcomes in preterm infants with IVH born in 2014, which was retrospectively registered in 2019. Although this study was retrospectively registered, our group has been engaged in studies of this area since 2009 (NCT02036073, NCT03919500), and we found that repeated low-dose rhEPO was neuroprotective and safe for preterm infants. Second, the sample sizes of preterm infants with severe IVH (20/274 [7.3%]) and < 28 weeks of gestational age (40/274 [14.6%]) were small. Considering that preterm infants with severe IVH and < 28 weeks of gestational age are more likely to develop poor outcomes, further study focusing on extremely preterm infants with low-dose rhEPO for a long treatment duration is needed, even though recent studies with extremely preterm infants did not show that rhEPO is neuroprotective [60]. Third, the follow-up period to 18 months of corrected age was not long enough. Even though there was nothing abnormal in the imaging examination after the absorption of the hematoma, these patients probably experienced some neurological or psychological complications [63]. Such complications might not appear until school age or even later [64, 65], so longer follow-up to allow comprehensive assessments of neurodevelopment would be valuable [66].
Conclusions
This study showed that treatment with repeated low-dose rhEPO improved poor outcomes (mortality or neurodevelopmental outcomes) in preterm infants with IVH. Therefore, rhEPO might be a useful therapeutic option for improving long-term outcomes in preterm infants with IVH.
Acknowledgements
The authors thank all the infants and their parents who participated in this study and the neurologists who performed the neurodevelopmental screening and diagnosis.
Declarations
Funding
Open access funding provided by University of Gothenburg. This study was supported by the National Key Research and Development Program of China (2018YFC1004604), the National Nature Science Foundation of China (U1704281, 31761133015, 81771418), the Department of Science and Technology of Henan Province of China (134200510023, 171100310200), the Swedish Research Council (2018-02267), and Swedish governmental grants to scientists working in healthcare of Gothenburg, Sweden (ALFGBG-717791).
Conflicts of interest
Juan Song, Yong Wang, Falin Xu, Huiqing Sun, Xiaoli Zhang, Lei Xia, Shan Zhang, Kenan Li, Xirui Peng, Bingbing Li, Yaodong Zhang, Wenqing Kang, Xiaoyang Wang, and Changlian Zhu have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
The study protocol was approved by the Ethics Committee of Zhengzhou University and Henan Medical Academy and was carried out in accordance with the Helsinki declaration.
Informed consent
Written informed consent was obtained from all parents included in the study prior to any study-related activities.
Consent for publication
All authors consent to the publication of the manuscript and accept the terms of related agreements.
Code availability
Not applicable.
Data availability statement
The datasets generated and/or analyzed during the current trial are available from the corresponding author on reasonable request.
Author contributions
Study concept and design: CZ. Data acquisition: JS, YW, FX, HS, XZ, LX, SZ, KL, XP, BL, and YZ. Data analysis: JS and YW. Drafting the manuscript: JS and YW. Reviewing the manuscript: WK, XW, and CZ. All authors approved the final version of the manuscript and agreed to be accountable for the work described in the manuscript.
Footnotes
Juan Song and Yong Wang contributed equally to the manuscript.
References
- 1.Bolisetty S, Dhawan A, Abdel-Latif M, Bajuk B, Stack J, Lui K. Intraventricular hemorrhage and neurodevelopmental outcomes in extreme preterm infants. Pediatrics. 2014;133(1):55–62. doi: 10.1542/peds.2013-0372. [DOI] [PubMed] [Google Scholar]
- 2.Szpecht D, Szymankiewicz M, Nowak I, Gadzinowski J. Intraventricular hemorrhage in neonates born before 32 weeks of gestation-retrospective analysis of risk factors. Childs Nerv Syst. 2016;32(8):1399–1404. doi: 10.1007/s00381-016-3127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang S, Yan W, Li S, Zhang L, Zhang Y, Shah PS, et al. Mortality and morbidity in infants < 34 weeks’ gestation in 25 NICUs in China: A prospective cohort study. Front Pediatr. 2020;8:33. doi: 10.3389/fped.2020.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He L, Zhou W, Zhao X, Liu X, Rong X, Song Y. Development and validation of a novel scoring system to predict severe intraventricular hemorrhage in very low birth weight infants. Brain Dev. 2019;41(8):671–677. doi: 10.1016/j.braindev.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Garton T, Hua Y, Xiang J, Xi G, Keep RF. Challenges for intraventricular hemorrhage research and emerging therapeutic targets. Expert Opin Ther Targets. 2017;21(12):1111–1122. doi: 10.1080/14728222.2017.1397628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cizmeci MN, de Vries LS, Ly LG, van Haastert IC, Groenendaal F, Kelly EN, et al. Periventricular hemorrhagic infarction in very preterm infants: characteristic sonographic findings and association with neurodevelopmental outcome at age 2 years. J Pediatr. 2020;217(79–85):e1. doi: 10.1016/j.jpeds.2019.09.081. [DOI] [PubMed] [Google Scholar]
- 7.Romantsik O, Calevo MG, Bruschettini M. Head midline position for preventing the occurrence or extension of germinal matrix-intraventricular hemorrhage in preterm infants. Cochrane Database Syst Rev. 2017;7:Cd012362. doi: 10.1002/14651858.CD012362.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitelaw A. Periventricular hemorrhage: a problem still today. Early Hum Dev. 2012;88(12):965–969. doi: 10.1016/j.earlhumdev.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Ohlsson A, Aher SM. Early erythropoiesis-stimulating agents in preterm or low birth weight infants. Cochrane Database Syst Rev. 2020;2(2):Cd004863. doi: 10.1002/14651858.CD004863.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neubauer AP, Voss W, Wachtendorf M, Jungmann T. Erythropoietin improves neurodevelopmental outcome of extremely preterm infants. Ann Neurol. 2010;67(5):657–666. doi: 10.1002/ana.21977. [DOI] [PubMed] [Google Scholar]
- 11.Fauchere JC, Dame C, Vonthein R, Koller B, Arri S, Wolf M, et al. An approach to using recombinant erythropoietin for neuroprotection in very preterm infants. Pediatrics. 2008;122(2):375–382. doi: 10.1542/peds.2007-2591. [DOI] [PubMed] [Google Scholar]
- 12.O'Gorman RL, Bucher HU, Held U, Koller BM, Huppi PS, Hagmann CF. Tract-based spatial statistics to assess the neuroprotective effect of early erythropoietin on white matter development in preterm infants. Brain. 2015;138(Pt 2):388–397. doi: 10.1093/brain/awu363. [DOI] [PubMed] [Google Scholar]
- 13.Ohls RK, Ehrenkranz RA, Das A, Dusick AM, Yolton K, Romano E, et al. Neurodevelopmental outcome and growth at 18 to 22 months' corrected age in extremely low birth weight infants treated with early erythropoietin and iron. Pediatrics. 2004;114(5):1287–1291. doi: 10.1542/peds.2003-1129-L. [DOI] [PubMed] [Google Scholar]
- 14.Zhu C, Kang W, Xu F, Cheng X, Zhang Z, Jia L, et al. Erythropoietin improved neurologic outcomes in newborns with hypoxic–ischemic encephalopathy. Pediatrics. 2009;124(2):e218–e226. doi: 10.1542/peds.2008-3553. [DOI] [PubMed] [Google Scholar]
- 15.Robinson S, Conteh FS, Oppong AY, Yellowhair TR, Newville JC, Demerdash NE, et al. Extended combined neonatal treatment with erythropoietin plus melatonin prevents posthemorrhagic hydrocephalus of prematurity in rats. Front Cell Neurosci. 2018;12:322. doi: 10.3389/fncel.2018.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juul SE, Beyer RP, Bammler TK, McPherson RJ, Wilkerson J, Farin FM. Microarray analysis of high-dose recombinant erythropoietin treatment of unilateral brain injury in neonatal mouse hippocampus. Pediatr Res. 2009;65(5):485–492. doi: 10.1203/PDR.0b013e31819d90c8. [DOI] [PubMed] [Google Scholar]
- 17.Juul SE, McPherson RJ, Bammler TK, Wilkerson J, Beyer RP, Farin FM. Recombinant erythropoietin is neuroprotective in a novel mouse oxidative injury model. Dev Neurosci. 2008;30(4):231–242. doi: 10.1159/000110348. [DOI] [PubMed] [Google Scholar]
- 18.Xiong Y, Mahmood A, Zhang Y, Meng Y, Zhang ZG, Qu C, et al. Effects of posttraumatic carbamylated erythropoietin therapy on reducing lesion volume and hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome in rats following traumatic brain injury. J Neurosurg. 2011;114(2):549–559. doi: 10.3171/2010.10.JNS10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reitmeir R, Kilic E, Kilic U, Bacigaluppi M, ElAli A, Salani G, et al. Post-acute delivery of erythropoietin induces stroke recovery by promoting perilesional tissue remodelling and contralesional pyramidal tract plasticity. Brain. 2011;134(Pt 1):84–99. doi: 10.1093/brain/awq344. [DOI] [PubMed] [Google Scholar]
- 20.Lee HS, Song J, Min K, Choi YS, Kim SM, Cho SR, et al. Short-term effects of erythropoietin on neurodevelopment in infants with cerebral palsy: a pilot study. Brain Dev. 2014;36(9):764–769. doi: 10.1016/j.braindev.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Zhu C, Wang X, Gerwien JG, Schrattenholz A, Sandberg M, et al. The nonerythropoietic asialoerythropoietin protects against neonatal hypoxia–ischemia as potently as erythropoietin. J Neurochem. 2004;91(4):900–910. doi: 10.1111/j.1471-4159.2004.02769.x. [DOI] [PubMed] [Google Scholar]
- 22.Hierro-Bujalance C, Infante-Garcia C, Sanchez-Sotano D, Del Marco A, Casado-Revuelta A, Mengual-Gonzalez CM, et al. Erythropoietin improves atrophy, bleeding and cognition in the newborn intraventricular hemorrhage. Front Cell Dev Biol. 2020;8:571258. doi: 10.3389/fcell.2020.571258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rüegger CM, Hagmann CF, Bührer C, Held L, Bucher HU, Wellmann S. Erythropoietin for the repair of cerebral injury in very preterm infants (EpoRepair) Neonatology. 2015;108(3):198–204. doi: 10.1159/000437248. [DOI] [PubMed] [Google Scholar]
- 24.Song J, Sun H, Xu F, Kang W, Gao L, Guo J, et al. Recombinant human erythropoietin improves neurological outcomes in very preterm infants. Ann Neurol. 2016;80(1):24–34. doi: 10.1002/ana.24677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Natalucci G, Latal B, Koller B, Ruegger C, Sick B, Held L, et al. Effect of early prophylactic high-dose recombinant human erythropoietin in very preterm infants on neurodevelopmental outcome at 2 years: a randomized clinical trial. JAMA. 2016;315(19):2079–2085. doi: 10.1001/jama.2016.5504. [DOI] [PubMed] [Google Scholar]
- 26.Bierer R, Peceny MC, Hartenberger CH, Ohls RK. Erythropoietin concentrations and neurodevelopmental outcome in preterm infants. Pediatrics. 2006;118(3):e635–e640. doi: 10.1542/peds.2005-3186. [DOI] [PubMed] [Google Scholar]
- 27.Ohls RK, Kamath-Rayne BD, Christensen RD, Wiedmeier SE, Rosenberg A, Fuller J, et al. Cognitive outcomes of preterm infants randomized to darbepoetin, erythropoietin, or placebo. Pediatrics. 2014;133(6):1023–1030. doi: 10.1542/peds.2013-4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohls RK, Cannon DC, Phillips J, Caprihan A, Patel S, Winter S, et al. Preschool assessment of preterm infants treated with darbepoetin and erythropoietin. Pediatrics. 2016;137(3):e20153859. doi: 10.1542/peds.2015-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–534. doi: 10.1016/S0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 30.Patra K, Wilson-Costello D, Taylor HG, Mercuri-Minich N, Hack M. Grades I–II intraventricular hemorrhage in extremely low birth weight infants: effects on neurodevelopment. J Pediatr. 2006;149(2):169–173. doi: 10.1016/j.jpeds.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Sun H, Song J, Kang W, Wang Y, Sun X, Zhou C, et al. Effect of early prophylactic low-dose recombinant human erythropoietin on retinopathy of prematurity in very preterm infants. J Transl Med. 2020;18(1):397. doi: 10.1186/s12967-020-02562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Song J, Sun H, Xu F, Li K, Nie C, et al. Erythropoietin prevents necrotizing enterocolitis in very preterm infants: a randomized controlled trial. J Transl Med. 2020;18(1):308. doi: 10.1186/s12967-020-02459-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibrahim J, Bhandari V. The definition of bronchopulmonary dysplasia: an evolving dilemma. Pediatr Res. 2018;84(5):586–588. doi: 10.1038/s41390-018-0167-9. [DOI] [PubMed] [Google Scholar]
- 34.McAdams RM, McPherson RJ, Mayock DE, Juul SE. Outcomes of extremely low birth weight infants given early high-dose erythropoietin. J Perinatol. 2013;33(3):226–230. doi: 10.1038/jp.2012.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis AS, Hintz SR, Goldstein RF, Ambalavanan N, Bann CM, Stoll BJ, et al. Outcomes of extremely preterm infants following severe intracranial hemorrhage. J Perinatol. 2014;34(3):203–208. doi: 10.1038/jp.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelin TD, Pena E, Giacomazzi T, Lee S, Logan JW, Moallem M, et al. Outcomes following indomethacin prophylaxis in extremely preterm infants in an all-referral NICU. J Perinatol. 2017;37(8):932–937. doi: 10.1038/jp.2017.71. [DOI] [PubMed] [Google Scholar]
- 37.Norman M, Piedvache A, Børch K, Huusom LD, Bonamy AE, Howell EA, et al. Association of short antenatal corticosteroid administration-to-birth intervals with survival and morbidity among very preterm infants: results from the EPICE cohort. JAMA Pediatr. 2017;171(7):678–686. doi: 10.1001/jamapediatrics.2017.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Juul SE, Comstock BA, Wadhawan R, Mayock DE, Courtney SE, Robinson T, et al. A randomized trial of erythropoietin for neuroprotection in preterm infants. N Engl J Med. 2020;382(3):233–243. doi: 10.1056/NEJMoa1907423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Juul SE, Pet GC. Erythropoietin and neonatal neuroprotection. Clin Perinatol. 2015;42(3):469–481. doi: 10.1016/j.clp.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ley D, Romantsik O, Vallius S, Sveinsdottir K, Sveinsdottir S, Agyemang AA, et al. High presence of extracellular hemoglobin in the periventricular white matter following preterm intraventricular hemorrhage. Front Physiol. 2016;7:330. doi: 10.3389/fphys.2016.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Wu Y, Li T, Wang X, Zhu C. Iron metabolism and brain development in premature infants. Front Physiol. 2019;10:463. doi: 10.3389/fphys.2019.00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dommergues MA, Gallego J, Evrard P, Gressens P. Iron supplementation aggravates periventricular cystic white matter lesions in newborn mice. Eur J Paediatr Neurol. 1998;2(6):313–318. doi: 10.1016/S1090-3798(98)80006-8. [DOI] [PubMed] [Google Scholar]
- 44.Imamura T, Ariga H, Kaneko M, Watanabe M, Shibukawa Y, Fukuda Y, et al. Neurodevelopmental outcomes of children with periventricular leukomalacia. Pediatr Neonatol. 2013;54(6):367–372. doi: 10.1016/j.pedneo.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 45.Wu Y, Song J, Wang Y, Wang X, Culmsee C, Zhu C. The potential role of ferroptosis in neonatal brain injury. Front Neurosci. 2019;13:115. doi: 10.3389/fnins.2019.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gram M, Sveinsdottir S, Cinthio M, Sveinsdottir K, Hansson SR, Mörgelin M, et al. Extracellular hemoglobin—mediator of inflammation and cell death in the choroid plexus following preterm intraventricular hemorrhage. J Neuroinflamm. 2014;11:200. doi: 10.1186/s12974-014-0200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishimura K, Tokida M, Katsuyama H, Nakagawa H, Matsuo S. The effect of hemin-induced oxidative stress on erythropoietin production in HepG2 cells. Cell Biol Int. 2014;38(11):1321–1329. doi: 10.1002/cbin.10329. [DOI] [PubMed] [Google Scholar]
- 48.Juul SE, Mayock DE, Comstock BA, Heagerty PJ. Neuroprotective potential of erythropoietin in neonates; design of a randomized trial. Matern Health Neonatol Perinatol. 2015;1:27. doi: 10.1186/s40748-015-0028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bi D, Chen M, Zhang X, Wang H, Xia L, Shang Q, et al. The association between sex-related interleukin-6 gene polymorphisms and the risk for cerebral palsy. J Neuroinflamm. 2014;11:100. doi: 10.1186/1742-2094-11-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Charriaut-Marlangue C, Besson VC, Baud O. Sexually dimorphic outcomes after neonatal stroke and hypoxia–ischemia. Int J Mol Sci. 2017;19(1):61. [DOI] [PMC free article] [PubMed]
- 51.Battarbee AN, Glover AV, Vladutiu CJ, Gyamfi-Bannerman C, Aliaga S, Manuck TA, et al. Sex-specific differences in late preterm neonatal outcome. Am J Perinatol. 2019;36(12):1223–1228. doi: 10.1055/s-0039-1683886. [DOI] [PubMed] [Google Scholar]
- 52.Li K, Li T, Wang Y, Xu Y, Zhang S, Culmsee C, et al. Sex differences in neonatal mouse brain injury after hypoxia–ischemia and adaptaquin treatment. J Neurochem. 2019;150(6):759–775. doi: 10.1111/jnc.14790. [DOI] [PubMed] [Google Scholar]
- 53.Zhu C, Sun Y, Gao J, Wang X, Plesnila N, Blomgren K. Inhaled nitric oxide protects males but not females from neonatal mouse hypoxia-ischemia brain injury. Transl Stroke Res. 2013;4(2):201–207. doi: 10.1007/s12975-012-0217-2. [DOI] [PubMed] [Google Scholar]
- 54.Al Mamun A, Yu H, Romana S, Liu F. Inflammatory responses are sex specific in chronic hypoxic–ischemic encephalopathy. Cell Transplant. 2018;27(9):1328–1339. doi: 10.1177/0963689718766362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Charriaut-Marlangue C, Leconte C, Csaba Z, Chafa L, Pansiot J, Talatizi M, et al. Sex differences in the effects of PARP inhibition on microglial phenotypes following neonatal stroke. Brain Behav Immun. 2018;73:375–389. doi: 10.1016/j.bbi.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 56.Nelson LH, Peketi P, Lenz KM. Microglia regulate cell genesis in a sex-dependent manner in the neonatal hippocampus. Neuroscience. 2021;453:237–255. doi: 10.1016/j.neuroscience.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 57.Iturri P, Bairam A, Soliz J. Efficient breathing at neonatal ages: a sex and epo-dependent issue. Respir Physiol Neurobiol. 2017;245:89–97. doi: 10.1016/j.resp.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 58.Dey S, Cui Z, Gavrilova O, Zhang X, Gassmann M, Noguchi CT. Sex-specific brain erythropoietin regulation of mouse metabolism and hypothalamic inflammation. JCI Insight. 2020;5(5):e134061. doi: 10.1172/jci.insight.134061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohlsson A, Aher SM. Early erythropoiesis-stimulating agents in preterm or low birth weight infants. Cochrane Database Syst Rev. 2017;11:Cd004863. [DOI] [PMC free article] [PubMed]
- 60.Natalucci G, Latal B, Koller B, Rüegger C, Sick B, Held L, et al. Effect of early prophylactic high-dose recombinant human erythropoietin in very preterm infants on neurodevelopmental outcome at 2 years: a randomized clinical trial. JAMA. 2016;315(19):2079–2085. doi: 10.1001/jama.2016.5504. [DOI] [PubMed] [Google Scholar]
- 61.Schneider JK, Gardner DK, Cordero L. Use of recombinant human erythropoietin and risk of severe retinopathy in extremely low-birth-weight infants. Pharmacotherapy. 2008;28(11):1335–1340. doi: 10.1592/phco.28.11.1335. [DOI] [PubMed] [Google Scholar]
- 62.Juul SE, McPherson RJ, Bauer LA, Ledbetter KJ, Gleason CA, Mayock DE. A phase I/II trial of high-dose erythropoietin in extremely low birth weight infants: pharmacokinetics and safety. Pediatrics. 2008;122(2):383–391. doi: 10.1542/peds.2007-2711. [DOI] [PubMed] [Google Scholar]
- 63.Roland EH, Hill A. Germinal matrix-intraventricular hemorrhage in the premature newborn: management and outcome. Neurol Clin. 2003;21(4):833–851. doi: 10.1016/S0733-8619(03)00067-7. [DOI] [PubMed] [Google Scholar]
- 64.Hack M, Wilson-Costello D, Friedman H, Taylor GH, Schluchter M, Fanaroff AA. Neurodevelopment and predictors of outcomes of children with birth weights of less than 1000 g: 1992–1995. Arch Pediatr Adolesc Med. 2000;154(7):725–731. doi: 10.1001/archpedi.154.7.725. [DOI] [PubMed] [Google Scholar]
- 65.Marlow N, Morris T, Brocklehurst P, Carr R, Cowan F, Patel N, et al. A randomised trial of granulocyte-macrophage colony-stimulating factor for neonatal sepsis: childhood outcomes at 5 years. Arch Dis Child Fetal Neonatal Ed. 2015;100(4):F320–F326. doi: 10.1136/archdischild-2014-307410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Volpe JJ. Commentary—do the negative results of the PENUT trial close the book on erythropoietin for premature infant brain? J Neonatal Perinatal Med. 2020;13(2):149–152. doi: 10.3233/NPM-200444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current trial are available from the corresponding author on reasonable request.