Abstract
In the last two decades, we have witnessed three major epidemics of the coronavirus human disease namely, severe acute respiratory syndrome (SARS), Middle Eastern respiratory syndrome, and more recently an ongoing global pandemic of coronavirus disease 2019 (COVID-19). Iran, a country of nearly 84 million, in the Middle East, severely involved with the COVID-19 disease. A documented multidimensional approach to COVID-19 disease is therefore mandatory to provide a well-balanced platform for the concerned medical community in our county and beyond. In this review, we highlight the disease status in Iran and attempt to provide a multilateral view of the fundamental and clinical aspects of the disease including the clinical features of the confirmed cases, virology, pathogenesis, epidemiology, and laboratory methods needed for diagnosis.
Keywords: Coronaviruses, Coronavirus disease 2019, COVID-19, SARS-CoV-2
Introduction
In less than two decades humanity have encountered three outbreaks of the coronavirus, including SARS coronavirus (SARS-CoV in 2003), MERS coronavirus (MERS-CoV in 2012) and COVID-19 disease (SARS-CoV- 2) in 2019. COVID-19 is a respiratory infectious disease that caused by a pathogen closely linked to the SARS coronavirus. The causative virus of COVID-19 disease recently named SARS Coronavirus 2 (SARS-CoV-2) (1). Although the clinical manifestations of the disease are broad, the most cases show mild symptoms similar to common colds with the development of pneumonia and multi-organ failure in the minority of patients (2). People with minimal symptoms constitute asymptomatic carriers who play an important role in hindering effective containment of COVID-19.
COVID-19 disease soon attracted global attention, especially because of its potentials for rapid progression to severe respiratory infections, particularly in susceptible hosts with high morbidity and mortality, leading the WHO to declare it as a public health emergency of international concern (PHEIC) on 30 Jan 2020. Although the first two corona outbreaks resulted in major epidemics, no case of SARS and only one cluster of five cases of MERS-CoV were reported in Iran (3). Nevertheless, the Iran’s situation with COVID-19 pandemic is quite different compared to the past two outbreaks. Although Iran is regarded as the second COVID-19 center in Asia after China, the situation may be temporary and some changes may occur in the near future.
To curtail the spread of COVID-19 as a ‘newly emerging’ infectious disease, such as previous epidemics including Zika, SARS and MERS with the potency to cause serious public health problems (4), we need a better understanding of this virus and its impact on people’s health particularly those who are most vulnerable.
Virology of Coronavirus
The novel coronavirus (2019-nCoV) that renamed SARS-CoV-2 by the international committee on taxonomy of viruses is a member of the Coronaviridae family and Nidovirales order. All the coronaviruses enveloped and possess a linear single-stranded positive RNA genome, with an overall size of 26-32 kb. Coronaviruses virions possess four main structural proteins; the spike (S), membrane (M), envelope (E), and nucleocapsid (N) proteins. The first three (M, E and S) are envelope proteins. The Coronaviruses family consist of two subfamilies, Torovirinae and Coronavirinae (5). The latter subfamily subdivided into four genera; alpha, beta, gamma, and delta coronavirus. Among them, numerous efforts have been performed to identify the virus, including the whole genome of the SARS-CoV-2 sequence. The whole genome of the SARS-CoV-2 sequence is under investigation, but the sequence of the dead gene sequenced in the first 15 patients (6).
Epidemiology
Global
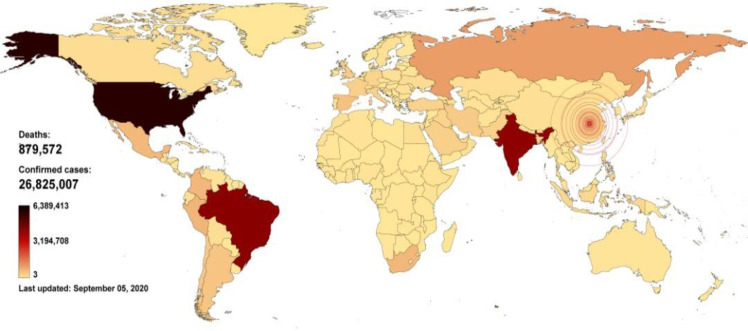
Although the first confirmed report of COVID-19 from Wuhan to the WHO country office in China was on 31.Dec. 2019, it can be traced to the beginning of Dec 2019 according to media reports on unpublished Chinese government data. As of 20 Oct 2020, it is affecting over 217 countries and 40.755.458 people. According to the rate of increase (over 50% per month), the number of infected patients will reach between 95 to 100 million by the end of 2020. All identified cases are across the world and are consistent with laboratory results as well as clinical diagnosis (Fig. 1). Growing numbers of cases have also reported in other countries across all continents, and the rate of recent cases outside of China has outpaced the rate in China. These cases initially occurred mainly among travelers from China and those who have had contact with travelers from China (7–9). However, ongoing local transmission has driven outbreaks in some locations outside of China, including South Korea, Italy, Iran, Japan, Spain, Germany, France, USA and Brazil and infections elsewhere (10). Therefore, according to the rapid change of the epidemic, the usual method for classifying countries is not correct, in which we have used only the announced statistics of incidence or mortality. It is, therefore recommended that another item be replaced, such as the percentage of incidence or mortality rate to the entire population. With such information, we can have a more accurate view of the level of personal hygiene, the prevalence of epidemic, and other cases in that country.
Fig. 1:
Global COVID-19 outbreak spread through 05, SEP, 2020. For more up-to-date information on reported cases, visit https://experience.arcgis.com/experience and https://www.worldometers.info/coronavirus
Iran
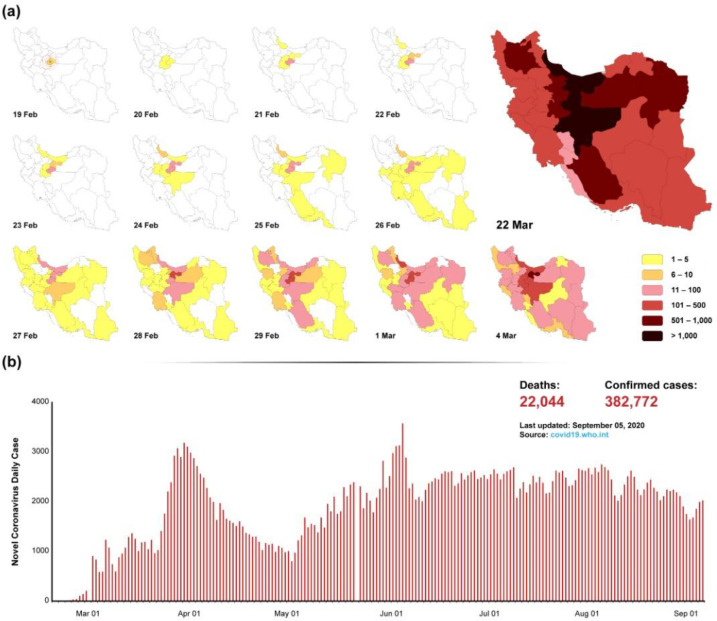
On 19 Feb 2020, the first confirmed four cases of SARS-CoV-2 infections sensed in the National Influenza Center (NIC) and reported from Qom Province, Iran (Fig. 2A). Shortly after the initial outbreak, several clusters of COVID-19 with the local transmission were identified throughout Iran (11). Most reports have been from Tehran and surrounding provinces, but it has also reported many cases in other provinces throughout Iran. As claimed by the Iranian health authorities, there had been 22044 COVID-19 deaths with around 382.772 confirmed infections as of 5 Sep 2020. According to the mentioned statistics, Iran is among the top 10 countries in terms of COVID-19 mortality new cases and recovery (12). Iran is a country with different religious customs and also under the conditions of economic sanctions, all of which challenge the ways to deal with this virus. Therefore, after removing some restrictions, we are witnessing the second wave of the virus outbreak, which could be other temples for other countries that pass the first peak of its outbreak (Fig. 2B). From this perspective, we can point to the possibility of reopening schools as well as gathering people for religious mourning, which raises many concerns about the occurrence of the third peak of the disease. One differences between Iran and other countries is the incidence of people living in nursing homes, which is a few of positive cases reported in the elderly because of molecular testing on nurses at the time of departure and also before entering the centers. Therefore, by identifying positive cases among staff and isolating them, the incidence of the disease in the elderly group is low.
Fig. 2:
Distribution of infection by city and daily statistics. 2A) The distribution of infection is schematically illustrated by the province. The prevalence of the disease from the center to the outside can be considered. 2B) The daily incidence rate indicates the occurrence of the second wave, which occurred after the removal of the restrictions. Therefore, the third wave can be predicted according to the upward trend and the possibility of reopening schools.
Immunopathogenesis
Although the exact pathogenesis of COVID-19 has not been fully elucidated, knowledge derived from related SARS-CoV and MERS-CoV infections, as well as the limited studies on COVID-19 cases have provided some insight regarding the pathogenesis of SARS-CoV-2. Similar to SARS-CoV, SARS-CoV2 binds to target cells through interactions of viral spike glycoprotein with angiotensin-converting enzyme 2 (ACE2) (13) a dipeptidyl peptidase 4 (DPP4) expressed at the surface of endothelial cells (14). This is followed by the entry of viral RNA into the cytoplasm, expression of structural and non-structural genes and formation of new virions, released from the host cells (13, 15, 16). Released virions infect various cell types including T cells, cells of the lower respiratory tract and cells of the liver and kidney (13, 17, 18). Tissue injury most likely results from both direct (i.e. virus-induced) and indirect (i.e. immunological) mechanisms. Immunological alterations are a prominent aspect of COVID-19 disease. Like most other systemic viral diseases, COVID-19 is associated with a type 1 interferon response in the initial stages of infection. This is followed by a dramatic increase in the levels of innate immune cytokines in the blood, e.g. TNF, IL1 and IL6. This so-called ‘cytokine storm’ likely underlies severe systemic clinical manifestations of the disease (19, 20). Indeed, the reported efficacy of treatment with tocilizumab, a humanized anti-IL6 receptor monoclonal antibody in COVID-19 patients, supports this view (21, 22).
Lymphopenia is a frequently reported finding in patients who suffer from SARS-Cov-2 infection (23–28). Different mechanisms have been suggested for virus-induced lymphopenia, including decreased lymphopoiesis (29), redistribution of lymphocytes (30) and enhanced lymphocyte apoptosis/death (31). Interferon response can initially lead to lymphocyte redistribution (i.e. tissue infiltration), but a protracted interferon response might also diminish lymphopoiesis. Severe systemic diseases are also associated with the activation of the hypothalamic pituitary adrenal (HPA) axis, which in turn diminishes lymphopoiesis and enhances lymphocyte apoptosis. Perhaps the most prominent pathological feature of COVID-19 is the substantial infiltration of lymphocytes into the lungs and the associated lung inflammation (32). The severity of the disease is correlated with both T lymphopenia and increased lymphocyte infiltration in the lungs (11). Lymphocytic lung infiltration can lead to tissue injury, a phenomenon that results from an inflammatory microenvironment. Whether inflammation-induced lung injury in COVID-19 is entirely due to injurious effects of leukocyte-derived cytokines/mediators or that a specific autoreactive component might also exist remains to be explored. This latter phenomenon might be worth investigating, considering the fact that widespread lymphocyte activation might be associated with lymphocyte auto-reactivity, itself a consequence of perturbed regulatory mechanisms.
COVID-19 has been found to be more severe in the elderly and in peoples with cardiovascular disorders (33). Older people with cardiovascular problems are generally more susceptible to systemic viral infections. In the case of COVID-19, a weakened blood-tissue barrier and endothelial dysfunction might also contribute to disease, i.e. exacerbating the infiltration of cells from blood to the lungs (34–37). Inhibition of lymphocyte migration from blood to the lungs might have therapeutic potential in this disease. In the most severe cases, lymphocytic lung inflammation is followed by the acute respiratory distress syndrome (ARDS) pulmonary edema, bilateral diffuse alveolar damage, desquamation of pneumocytes, and significant hyaline membrane formation. Histological examination and biopsy samples of liver from COVID-19 patients have shown that inflammation in COVID-19 is not limited to the lungs, but also exists in other organs including the liver. Liver injury can be due to direct viral invasion of hepatocytes or a consequence of the systemic inflammation. Drug–induced hepatotoxicity could also contribute to liver injury in the COVID-19 disease. Pathological examination of liver tissue in a patient with COVID-19 has shown mild lobular and portal infiltration and, microvesicular steatosis which could either be because of viral infection, or drug-induced liver injury (32).
Diagnosis
Developing an appropriate, specific and sensitive laboratory approach is a crucial concern for screening patients, confirming suspects, and monitoring disease status because the clinical symptoms of COVID-19 (SARS-CoV-2) infection is highly nonspecific. However, we present recent laboratory, serological and molecular findings that play an important role in identifying COVID-19 patients among the large suspected population.
Laboratory findings
Although there is controversy in the white blood cell count (WBCs) which mostly appears to be normal, lymphocytopenia and eosinophilia appear to be the predominant finding in patients, consistent with some previous findings (28, 38, 39). However, these findings, along with other laboratory parameters such as C-reactive protein (CRP), liver enzymes and inflammatory cytokines are being carefully studied in detail.
Serological
Although the virus-specific IgM is detectable in the serum approximately 3–5 days after the onset, and serum IgG levels rise at least 4-fold in serum samples in acute and convalescent phase (40) but our findings were inconsistent with this report. Therefore, according to our finding, the using serological methods is not recommended, especially in acute cases of the disease (early stages) because some patients exhibit a negative result in serological tests up to 4 wk after the detection of the virus by molecular method. The understanding of the cross-reactivity of human serological responses to other coronaviruses is important for serological diagnosis of COVID disease.
Since half of the patients develop clinical symptoms by the 5th-day post-infection, qualitative antibody tests are not reliable tools in the diagnosis of infected people especially in early phases of infection.
Molecular Assays
Because of the limitations of serological methods, it can be said that molecular methods like as “reverse-transcriptase-qPCR (RT-qPCR)” are at the forefront in identifying positive cases. However, despite the specificity and high sensitivity of RT-qPCR method, false positives and false negatives are common, which is depend highly to the sampling and the type of genes used. In this sense, one week after the identification of the virus genome in China, a team of German scientists introduced the first RT-PCR-based diagnostic protocol using specimens swab of the upper respiratory tract (URT; naso- and oropharyngeal) (41), subsequently selected and approved by the WHO as the standard global method. In this protocol, the assays were performed with three virus-specific targets, namely RNA-dependent RNA polymerase (RdRp), envelope (E), and nucleocapsid (N) genes. Corman et al. used in-vitro transcribed RNA for checking the sensitivity of their primers and recommend the E gene assay as the first-line screening tool, followed by confirmatory testing with the RdRp gene assay (41). In Iran, sampling is done first from the throat and then from the nasal and nasopharynx and the virus is sent to the laboratory in the viral transport media. For molecular testing, two genes, N and RdRp are firstly used. However, it is noteworthy to mention, we found that the RdRp is less sensitive in clinical samples because the copy number of E and N genes is much greater than RdRp in virus replication (42). According to The WHO recommendation, the respiratory sample should be obtained from upper respiratory (nasopharyngeal and oropharyngeal swab or wash in ambulatory patients) and/or lower respiratory (sputum and/or endotracheal aspirate or bronchoalveolar lavage. The additional specimens may be used such as blood and stool (43, 44).
Ultimately, the rate of viral load in patients’ respiratory tract specimens was associated with the severity of lung injury (45). The current challenge is the development of rapid antigen detection and nucleic acid detection via PCR- and microarray-based assays to distinguish novel coronavirus pneumonia (NCP) from the other pneumonia infections agents including viral, mycoplasma and even common respiratory pathogens (46).
Radiology
Understanding of the radiological features of COVID-19 is evolving very rapidly. In the initial phase of this pandemic in Wuhan, the disease epicenter, chest imaging soon became the forefront of investigation for patients with suspected or confirmed COVID-19 infection (47). Computed tomography (CT) of the chest, more than plain chest radiography, has been utilized extensively in different stages of COVID-19 disease. Over the past two months, there has been a plethora of reports and publications regarding the role of high-resolution CT findings in the early diagnosis, timing features, association with symptoms, laboratory data, disease progression and outcome (47).
Plain chest radiography can be normal in the early stages of this illness, whereas high resolution, non-contrast chest CT scans performed in the supine position at 1 mm collimations, shows a variety of findings, in different stages of COVID-19 disease, from asymptomatic patients, those with minimal symptoms to severe respiratory infection (SARI) and acute respiratory distress syndrome (ARDS). The CT findings, depending on the stage and severity of the disease, have been round or linear ground-glass attenuation in peripheral lung regions, interlobular and intralobular septal thickening (crazy-paving pattern), lobar consolidations, air-bronchogram, cystic changes and the ominous signs of diffuse bilateral ground glass opacities with air-bronchogram (white lungs) (47).
Ying-Hui Jin et al. have recently stratified the CT findings in progressive stages of ultra-early (asymptomatic), early [(1–3 d after fever, cough, dry cough, etc.), rapid progression (3–7 d with increased respiratory symptoms), consolidation), (7–14 d, respiratory failure) and dissipation phase), (2–3 wk with progressive patchy consolidations, grid-like and strip-like twist of bronchial walls)] (48).
There was a slight predilection for the right lower lobe, although all segments of the lungs could be involved. In one study 225 (27%) of 849 involved segments were in the right lower lobe region and more segments and lobes were involved as patients progressed in to the second and third weeks of the illness. There are some CT features that are atypical in this disease such as, pleural effusion, pericardial effusion, solid nodules and cavity formation (48).
Although CT scan is more sensitive, it is more useful when the lung parenchyma involved. However, the indiscriminate use of this method due to the high level of radiation raises the concern that we will see an increase in the incidence of breast cancer in women and even men in the future.
Medical ethics concerns in COVID-19
Many countries have been affected by the socio-economic aspects of the COVID-19 outbreak and many ethical concerns have been raised. Although ethical concerns are almost identical in different countries, the priorities may vary in health emergencies regarding the characteristics of the local context. Therefore, the concerns and solutions presented in Iran can be summarized in four sub-headings.
○ Obtain reliable national evidence: Issuance of the official authorization for fast track scientific and ethical evaluation of research proposals by medical universities has the first response to this epidemic in order to non-repetition of lessons learned from previous outbreaks, such as delays in community response (49). The general justification behind this policy is increasing the probability of generating useful data for developing more reliable evidence, for better management of the disease across the country.
○ Application of Unapproved Medical Interventions: Concerns related to the usage of unproven medical interventions have been raised from the beginning of the outbreak because, there is no proven treatment strategy and it may take a long time to develop the vaccine (50). Therefore, a national guideline was immediately published for the COVID-19 diagnosis and treatment based on the Chinese researcher’s findings which authorize the application of some other interventions such as ECMO, blood perfusion, and interferon or heparin for severe cases under paragraph 37 of the Helsinki Declaration 2013. (51).
○ Researchers’ rights with social media: Another problem is the rights and responsibilities of researchers in their relations with media, as the media hype emerged during the COVID-19 epidemics (52). Because reports of interviews about the ongoing trials had noticeable news feed, again ministry of health and medical education (MOHME) asked researchers to announce any achievements which may help to improve the condition of patients only after receiving approval from the allowed organizations including MOHME and Iran food and drug administration (IFDA).
○ Administration of medical donations: Although all of these may be known as research medications, some of them are routinely used in clinical practice. Therefore, the management of donated medications is under the supervision of a clinical research team that can perform various aspects of a clinical trial according to the international standards with a fair selection of participants.
Environmental transmission and controls in COVID-19
SARS-CoV-2 has several unique features that make it difficult to diagnose and control the disease, including its long-term stability and survival in the environment, insidious onset of symptoms, prolonged virus shedding and the unclear border between incubation period and symptomatic phase. The latter leads to an increased possibility of false-negative results based on a high similarity between COVID-19 and other mild respiratory infections. According to the mentioned characteristics as well as the limitations of health systems, three important strategies can be considered to respond to the tsunami-like increase in demand for care in mild and severe cases. First, is a three-layer triage plan from home to hospital to reduce the burden of mild cases? Second, the development of strict infection prevention and control programs in the community and health care centers. Third, parallel diagnostic measures to find more severe cases that might require hospital care such as radiological and health measures. We use evidence-based user-friendly applications on smartphones to handle triage cases and take advantage of social potentials to counter this “invisible enemy”. Although some studies have shown that the virus can be isolated from pets and sewage (53, 54), however, no positive cases of pets (dogs and cats) and hints of the SARS-CoV-2 in sewage (treated/raw) have been reported so far. However, the possibility of transmission of the SARS-CoV-2 through water or agricultural products should not be overlooked as one way of transmitting COVID may be irrigation of agricultural products with treated effluents or spreading biosolids on farm lands (55). Despite the possibility of reducing the prevalence of influenza because of personal hygiene and social distance, we will encounter the severe peak of COVID-19 infection due to the spread of other respiratory viral infections, also known as common cold, in fall and winter.
Extracorporeal Membrane Oxygenation (ECMO)
ECMO has been widely used during recent years for cardiopulmonary support (56). This device has greatly helped patients with severe ARDS in viral epidemics like influenza H1N1 with a success rate of up to 60% (56). Considering lung involvement and the high incidence of ARDS in COVID-19 disease, the use of this tool has been considered in China, but the results have not been very encouraging so far (57). This tool has also been used in Iran for seven COVID-19 patients with severe ARDS (PaO2/FIO2 < 100 mmHg) who required mechanical ventilation despite conventional management and recently one patient has been successfully weaned.
Conclusion
The viruses are continuously changing as a result of genetic selection and their ability to alter genetically, by recombination, gene exchange, deletion or addition of a gene fragment. These transformations can result in the generation of novel viruses, carrying unique abilities to cause human disease with serious public health risks of global proportions. As the management strategies of these emerging disease lag behind by many months or years, we apply strict infection prevention and control through rapid resource allocation, mass education, social distancing, case finding via rapid testing, contact tracing, appropriate use of personal protective equipment, protocolized patient care/isolation in the outpatient and hospital settings, emergency clinical research during the pandemics and global co-operation.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses (2020). The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol, 5: 536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet, 395 (10223): 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yavarian J, Rezaei F, Shadab A, et al. (2015). Cluster of Middle East respiratory syndrome coronavirus infections in Iran, 2014. Emerg Infect Dis, 21(2): 362–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noorbakhsh F, Abdolmohammadi K, Fatahi Y, et al. (2019). Zika Virus Infection, Basic and Clinical Aspects: A Review Article. Iran J Public Health, 48 (1): 20–31. [PMC free article] [PubMed] [Google Scholar]
- 5.Smits SL, Lavazza A, Matiz K, et al. (2003). Phylogenetic and evolutionary relationships among torovirus field variants: evidence for multiple intertypic recombination events. Journal of Virology, 77 (17): 9567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jila Yavarian N-ZS-J, et al. (2020). First Cases of SARS-CoV-2 in Iran, 2020:Case Series Report. Iran J Public Health, 49 (8): 1564–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haynes B, Messonnier NE, Cetron MS. (2020). First travel-related case of 2019 novel coronavirus detected in United States. https://www.cdc.gov/media/releases/2020/p0121-novel-coronavirus-travel-case.html
- 8.Haynes B, Messonnier NE, Cetron MS. (2020). Second travel-related case of 2019 novel coronavirus detected in United States : press release, Friday, January 24, 2020. https://stacks.cdc.gov/view/cdc/84536
- 9.Wang C, Horby PW, Hayden FG, et al. (2020). A novel coronavirus outbreak of global health concern. Lancet, 395(10223):470–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO (2020). Coronavirus disease 2019 (COVID-19) Situation Report – 36. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200225-sitrep-36-covid-19.pdf?sfvrsn=2791b4e0_2.
- 11.Tuite AR, Bogoch I, Sherbo R, et al. (2020). Estimation of COVID-2019 burden and potential for international dissemination of infection from Iran. Ann Intern Med, 172: 699–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ECDC (2020). COVID-19 situation update worldwide, as of week 10, updated 18 March 2021. https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases
- 13.Lambeir A-M, Durinx C, Scharpé S, et al. (2003). Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci, 40(3): 209–94. [DOI] [PubMed] [Google Scholar]
- 14.Raj VS, Mou H, Smits SL, et al. (2013). Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature, 495 (7440): 251–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luk HK, Li X, Fung J, et al. (2019). Molecular epidemiology, evolution and phylogeny of SARS coronavirus. Infect Genet Evol, 71:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahin AR, Erdogan A, Agaoglu PM, et al. (2020). 2019 Novel Coronavirus (COVID-19) Outbreak: A Review of the Current Literature. EJMO, 4 (1): 1–7. [Google Scholar]
- 17.Lai C-C, Shih T-P, Ko W-C, et al. (2020). Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents, 55(3):105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu H, Zhou J, Wong BH-Y, et al. (2014). Productive replication of Middle East respiratory syndrome coronavirus in monocyte-derived dendritic cells modulates innate immune response. Virology, 454-455:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song P, Li W, Xie J, et al. (2020). Cytokine storm induced by SARS-CoV-2. Clin Chim Acta, 509:280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coperchini F, Chiovato L, Croce L, et al. (2020). The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev, 53:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. (2020). Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol, 2(8):e474–e484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biran N, Ip A, Ahn J, et al. (2020). Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study. Lancet Rheumatol, 2(10):e603–e612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin C, Zhou L, Hu Z, et al. (2020). Dysregulation of Immune Response in Patients with COVID-19 in Wuhan, China. Clin Infect Dis, 71 (15): 762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bermejo-Martin JF, Almansa R, Menéndez R, et al. (2020). Lymphopenic community acquired pneumonia as signature of severe COVID-19 infection: Lymphopenia in severe COVID-19 infection. J Infect, 80(5):e23–e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diao B, Wang C, Tan Y, et al. (2020). Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (COVID-19). Front Immunol, 11: 827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu F, Zhao S, Yu B, et al. (2020). A new coronavirus associated with human respiratory disease in China. Nature, 579(7798):265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan L, Wang Q, Zhang D, et al. (2020). Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther, 5(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou P, Yang X-L, Wang X-G, et al. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 588(7836):E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fathi N, Rezaei N. (2020). Lymphopenia in COVID-19: Therapeutic opportunities. Cell Biol Int, 44: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng Z, Zhang M, Zhu T, et al. (2020). Dynamic changes in peripheral blood lymphocyte subsets in adult patients with COVID-19. Int J Infect Dis, 98:353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li S, Zhang Y, Guan Z, et al. (2020). SARS-CoV-2 triggers inflammatory responses and cell death through caspase-8 activation. Signal Transduction and Targeted Therapy, 5 (1): 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Z, Shi L, Wang Y, et al. (2020). Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med, 8(4):420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang D, Hu B, Hu C, et al. (2020). Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA, 323 (11): 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bermejo-Martin JF, Martín-Fernandez M, López-Mestanza C, et al. (2018). Shared features of endothelial dysfunction between sepsis and its preceding risk factors (aging and chronic disease). J Clin Med, 7 (11): 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herrera MD, Mingorance C, Rodríguez-Rodríguez R, de Sotomayor MA. (2010). Endothelial dysfunction and aging: an update. Ageing Res Rev, 9 (2): 142–52. [DOI] [PubMed] [Google Scholar]
- 36.Vischer U. (2006). von Willebrand factor, endothelial dysfunction, and cardiovascular disease. J Thromb Haemost, 4 (6): 1186–93. [DOI] [PubMed] [Google Scholar]
- 37.Zhang C. (2008). The role of inflammatory cytokines in endothelial dysfunction. Basic Res Cardiol, 103 (5): 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang Y, Zhou H, Yang R, Xu Y, Feng X, Gong P. (2020). Clinical characteristics of 36 non-survivors with COVID-19 in Wuhan, China.medRxiv, 10.1101/2020.02.27.20029009 [DOI]
- 39.Guan W-j, Ni Z-y, Hu Y, et al. (2020). Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med, 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WHO (2020). Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases: interim guidance. https://apps.who.int/iris/handle/10665/331501
- 41.Corman VM, Landt O, Kaiser M, et al. (2020). Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill, 25 (3): 2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shirvani A, Azimi L, Mansour Ghanaie R, et al. (2020). Utility of Available Methods for Diagnosing SARS-CoV-2 in Clinical Samples. Archives of Pediatric Infectious Diseases, 8 (3): e103677. [Google Scholar]
- 43.Zhang Y, Chen C, Song Y, et al. (2020). Excretion of SARS-CoV-2 through faecal specimens. Emerg Microbes Infect, 9(1):2501–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang W, Du R-H, Li B, et al. (2020). Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect, 9 (1): 386–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y, Yang Y, Zhang C, et al. (2020). Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci, 63 (3): 364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan JF, Yip CC, To KK, et al. (2020). Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-polymerase chain reaction assay validated in vitro and with clinical specimens. J Clin Microbiol, 58 (5): e00310–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi H, Han X, Jiang N, et al. (2020). Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis, 20(4):425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin YH, Cai L, Cheng ZS, et al. (2020). A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res, 7: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bain LE, Ngwain CG, Nwobegahay J, et al. (2018). Research Ethics Committees (RECs) and epidemic response in low and middle income countries. Pan Afr Med J, 31: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu S. (2020). Timely development of vaccines against SARS-CoV-2. Emerg Microbes Infect, 9 (1): 542–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shrestha B, Dunn L. (2020). The Declaration of Helsinki on Medical Research involving Human Subjects: A Review of Seventh Revision. J Nepal Health Res Counc, 17(4):548–552. [DOI] [PubMed] [Google Scholar]
- 52.Ippolito G, Hui DS, Ntoumi F, et al. (2020). Toning down the 2019-nCoV media hype-and restoring hope. Lancet Respir Med, 8 (3): 230–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhowmick GD, Dhar D, Nath D, et al. (2020). Coronavirus disease 2019 (COVID-19) outbreak: some serious consequences with urban and rural water cycle. npj Clean Water, 3 (1): 32. [Google Scholar]
- 54.Sit THC, Brackman CJ, Ip SM, et al. (2020). Infection of dogs with SARS-CoV-2. Nature, 586(7831):776–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sabzali A. (2020). The Coronavirus Transmission by Wastewater and Biosolids Reuse for Agricultural Usages: A Literature Review. Am J Biomed Sci, 8 (5): 365–8. [Google Scholar]
- 56.Jahangirifard A, Ahmadi ZH, Daneshvar Kakhaki A, et al. (2018). ECMO-assisted resection of huge thoracic mass. J Cardiovasc Thorac Res, 10 (3): 174–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.MacLaren G, Fisher D, Brodie D. (2020). Preparing for the Most Critically Ill Patients With COVID-19: The Potential Role of Extracorporeal Membrane Oxygenation. JAMA, 323 (13): 1245–1246. [DOI] [PubMed] [Google Scholar]