Abstract
Obsessive-compulsive disorder (OCD), a leading cause of disability, affects ~1–2% of the population, and can be distressing and disabling. About 1/3 of individuals demonstrate poor responsiveness to conventional treatments. A small proportion of these individuals may be deep brain stimulation (DBS) candidates. Candidacy is assessed through a multidisciplinary process including assessment of illness severity, chronicity, and functional impact. Optimization failure, despite multiple treatments, is critical during screening. Few patients nationwide are eligible for OCD DBS and thus a multi-center approach was necessary to obtain adequate sample size. The study was conducted over a six-year period and was a NIH-funded, eight-center sham-controlled trial of DBS targeting the ventral capsule/ventral striatum (VC/VS) region. There were 269 individuals who initially contacted the sites, in order to achieve 27 participants enrolled. Study enrollment required extensive review for eligibility, which was overseen by an independent advisory board. Disabling OCD had to be persistent for ≥5 years despite exhaustive medication and behavioral treatment. The final cohort was derived from a detailed consent process that included consent monitoring. Mean illness duration was 27.2 years. OCD symptom subtypes and psychiatric comorbidities varied, but all had severe disability with impaired quality of life and functioning. Participants were randomized to receive sham or active DBS for three months. Following this period, all participants received active DBS. Treatment assignment was masked to participants and raters and assessments were blinded. The final sample was consistent in demographic characteristics and clinical features when compared to other contemporary published prospective studies of OCD DBS. We report the clinical trial design, methods, and general demographics of this OCD DBS sample.
Keywords: Obsessive-compulsive disorder, Deep brain stimulation, Neurosurgery, Psychiatry
1. Introduction
Obsessive-compulsive disorder (OCD) is a leading cause of disability in developed countries, with a one-year prevalence of about 1% [1]. It is characterized by distressing and highly disabling intrusive, anxiety-provoking obsessions and compulsive rituals. About a third of affected individuals are poorly responsive to medication or behavioral therapies [[2], [3], [4], [5]]. A much smaller proportion, including those with the greatest illness severity, chronicity, and functional impact despite optimized behavioral and medication treatments, might be candidates for either ablation or deep brain stimulation (DBS; [[6], [7], [8], [66]].
Stereotactic surgeries for OCD developed empirically in the mid-20th century and targeted nodes within fronto-basal networks. Subsequent functional neuroimaging studies in patients with OCD illustrated the importance of these networks [9]; thereby providing support for the surgical targets. DBS for OCD was introduced by Nuttin and colleagues [10] in Europe. Nuttin's DBS target was initially based on anterior capsulotomy, arguably the most effective ablation.
Deep brain stimulation for OCD was first used in the United States several years later [11,12]. As with lesion procedures, selection criteria are strict (see Methods): candidates must have severe, chronic, and otherwise intractable illness. Sustained and optimized medication and behavioral treatments must have failed to provide adequate relief.
There are few randomized controlled trials of DBS for OCD, though two prior studies (anterior limb of the internal capsule (ALIC)/bed nucleus of the stria terminalis (BST) and nucleus accumbens) using crossover designs indicated significant decreases in obsessive-compulsive, depressive, and anxiety symptoms with active stimulation (Damiaan [13,14]. Prospective, open-label DBS studies using the ventral anterior limb of the internal capsule/ventral striatum (VC/VS) target in a combined Belgian-U.S. sample (N = 26) were reported in 2010 [15]. Most patients had sustained benefit, with full responses (a 35%+ reduction in Yale-Brown Obsessive-Compulsive Scale [YBOCS] severity) in 48% and 61% of the sample at one and three years, respectively. YBOCS improvement reached stability on a group basis at three months. Other open-label studies had similar responses, with 50–67% judged to be responders [11]; Damiaan [13,16,17]. A meta-analysis of 16 studies [18] found a 60% response rate for OCD overall, with most DBS targets overlapping in the VC/VS region. However, given these data, collaborative groups of investigators have indicated additional trials of DBS for OCD are needed [19].
Controlled data on the efficacy of the procedure remain limited. Here, we describe the design and study sample of our collaborative, sham-controlled trial of VC/VS DBS. We used a delayed-start design, across eight U.S. centers, and enrolled 27 patients.
2. Materials and methods
2.1. Participating centers and recruitment
This was a collaborative multi-center study. We started with three clinical sites (Butler Hospital [BH], the Cleveland Clinic [CC], and the University of Florida [UF]); all had experience with open-label DBS for OCD research. Due to recruiting difficulties given the stringent entry criteria, we expanded progressively, adding Massachusetts General Hospital (MGH), George Washington University (GW), Wake Forest, Mount Sinai, Kaiser Permanente, University of Chicago, and Mayo Clinic. All sites either had or developed teams with appropriate expertise in DBS for OCD. Two sites, University of Chicago and Wake Forest, ultimately did not implant patients. Several factors slowed the pace of patient recruitment but the primary factor was the relative rarity of the target population (estimated at under 1% of treatment-seeking OCD patients [8]). A second limiting factor was ensuring that prospective surgical candidates had appropriate U.S. insurance coverage that made ongoing, and in fact indefinite, access to this intensive treatment after the trial highly likely in case it proved effective for them. In many cases this required a formal petition to a regional insurance carrier which varied by site. The majority of the participants had US Medicare by virtue of disability due to OCD. Relevant approvals including an Investigator-Sponsored Investigational Device Exemption (IDE G070235/R006) from the U.S. FDA and IRB approvals centrally (at Butler Hospital) and at each local site (NCT00640133) required significant time and effort to move through the appropriate processes. In addition, Kaiser Permanente had to seek state approval through the county mental health department, and were the first in their state to have formal approval for psychiatric neurosurgery since a ban in the 1970s.
2.2. General entry criteria
Patient selection criteria were informed by previous experience (e.g. Refs. [15,20]). Study procedures emphasized cautious, comprehensive assessment, treatment, and follow-up of patients. Patients approved met our criteria for intractable illness and had had extensive prior treatment.
2.3. Inclusion criteria
-
(a)
OCD, diagnosed by Structured Clinical Interview for DSM-IV (SCID-IV), of disabling severity with Yale-Brown Obsessive Compulsive Scale (YBOCS) severity of at least 28 (“severe”). This meant that the majority of an individual's waking life was consumed by obsessive thoughts, compulsive urges/behaviors, and avoidance of environments evoking OCD symptoms. Serious functional impairment indicated by a Global Assessment of Functioning (GAF) score of 45 (serious impairment in social, occupational, or school functioning) or less.
-
(b)
Highly treatment-refractory illness, documented after review of medical records as well as discussions with treating clinicians, both psychiatrists and psychologists. Persistence of severe symptoms and impairment for five or more years despite at least three first-line and 2 s-line treatments: i) at least three adequate trials of, or documented intolerance to, different serotonin reuptake inhibitors (SRIs) for ≥ three months at the maximum tolerated dose, including an adequate course of clomipramine, either alone or in combination with a more selective serotonin reuptake inhibitor; ii) Augmentation of one of the selective SRIs with a neuroleptic and with clonazepam (each for at least two weeks); and iii) adequate behavior therapy: ≥20 sessions of exposure and response prevention (ERP) by a therapist with substantial expertise in OCD treatment as determined by the investigators. In practice, several trials of ERP were usually attempted and proved ineffective or intolerable (just as the number and types of medication trials exceeded the required minimum in practice). At least one trial of exposure-based therapy must have been in combination with pharmacotherapy.
-
(c)
Age 18–75 years.
-
(d)
Able to understand and comply with instructions.
-
(e)
Able to give fully informed, written consent in the judgment of the site Consent Monitor.
-
(f)
Either medication free or on a stable regimen for at least six weeks.
-
(g)
Good general health, including a platelet count >125,000/mm3 and normal coagulation indices.
-
(h)
The local referring psychiatrist indicated in writing their commitment to provide ongoing conventional care during and after the trial. The local psychiatrist had to agree that the study psychiatrist would prescribe medications during the three-month masked phase.
It was also very helpful, though not an absolute requirement, for a family member/significant other in close touch with the patient to communicate with the study team to provide collateral information as needed and if necessary accompany the patient to study visits.
2.4. Exclusion criteria
-
(a)
Current or past psychotic disorder, thought to worsen responses to psychiatric neurosurgery.
-
(b)
Full-scale IQ below 75 on the Wechsler Abbreviated Scale of Intelligence (WASI), or cognitive impairment that would affect a participant's ability to give informed consent or provide interview or self-report data reliably, as determined by the Consent Monitor (consent) or site psychiatrist (providing data).
-
(c)
A clinical history of bipolar I mood disorder, as DBS may induce mania or hypomania.
-
(d)
Current clinically significant neurological disorder or medical illness affecting brain function, beyond a tic disorder.
-
(e)
Clinically significant abnormality on preoperative magnetic resonance imaging (MRI).
-
(f)
Any labeled DBS contraindication, and/or inability to undergo presurgical MRI (cardiac pacemaker, pregnancy, metal in body, severe claustrophobia), infection, coagulopathy, inability to undergo an awake operation, significant cardiac or other medical risk factors for surgery.
-
(g)
Current or unstably remitted substance abuse, dependence, or a positive urine toxicology screen. Stable remission had to last 1+ years.
-
(h)
Pregnancy and women of childbearing age not using effective contraception.
-
(i)
Unable to adhere to operational and administrative study requirements, in the investigators' judgment.
-
(j)
Clinical history of severe personality disorder that would interfere with participation.
-
(k)
An inability to control suicide attempts, imminent risk of suicide in the investigator's judgment, or a history of serious suicidal behavior. This was defined, using the Columbia-Suicide Severity Rating Scale (C-SSRS), as: either i) one or more actual suicide attempts in the preceding 3 years the lethality of which was rated at 3 or higher (i.e. defined as moderately severe physical damage with medical hospitalization and likely intensive care required) or ii) one or more interrupted suicide attempts with a potential lethality judged to result in serious injury or death.
-
(l)
Current comorbid diagnosis of body dysmorphic disorder, an OCD-spectrum illness where responses to neurosurgery are largely unknown.
Screening and determination of candidacy. After consent, patients underwent an initial phone screening and extensive medical record review. Those passing initial screening then underwent comprehensive evaluation at each site. Of note, during evaluation, candidates' expectations of improvement after surgery were explored, as unrealistic expectations of dramatic or rapid improvement can be problematic in postoperative management. After evaluation, candidates underwent review through an external Independent Review Group (IRG), including experts in OCD diagnosis and treatment (a psychiatrist and psychologist), in DBS (a neurologist), and a clinical ethicist. Kaiser Permanente required review by a Northern California DBS Movement Disorders group, in addition to review by two outside (non- Kaiser Permanente) psychiatrists for San Mateo County medical supervisor approval as added measures to protect patients prior to moving forward with surgery. It is notable that Redwood City Kaiser resides fairly close to Herrick Memorial Hospital in San Jose, where Walter Freeman had performed lobotomies including his last, and was the first hospital to gain formal county approval after various state statutes were instituted in the late 1960's [21].
After review and approval, participants were scheduled for DBS implantation. Written informed consent was obtained from each participant. At each site, a Consent Monitor, available family members/significant others and an investigator, were present for the full protocol consent session. The monitor's role was to ensure the participant understood the study, its potential risks and benefits, study logistics, and that all participant's questions were satisfactorily answered. The monitor administered a modified Informed Consent Evaluation Feedback Tool [22] as a consent process aid. This helped assure that all participants fully understood the salient aspects of the study. The majority of the patients scored perfectly on the measure without additional discussion. If an individual participant did not understand a feature of the study, the consent monitor would provide further teaching until they demonstrated adequate understanding to the monitors' satisfaction.
2.5. DBS procedures
Participants were randomized to masked sham or active DBS for three months, beginning after the usual postoperative recovery interval of 3–4 weeks after bilateral DBS system implantation. Thus, other than test stimulation during surgery (see below), patients did not receive stimulation until the optimization period. Leads were implanted into the ventral capsule/ventral striatum (VC/VS) as in previous work [15]. One DBS lead (Model 3387, Medtronic Inc.) was implanted on each side, connected to one implantable neurostimulator (INS) on each side usually in the chest. We chose the 3387 to give us better control of stimulation within the ventral part of the capsule, determined to be the most important region for a therapeutic effect based both on experience with the larger 3391 lead and anatomical studies indicating connections within putative OCD circuitry are greatest in this region [23,24]. Intraoperative test stimulation, standard in DBS implantations for different diagnoses, was conducted to optimize lead placement, particularly to avoid negative effects such as panic or fear as previously observed with the VC/VS target [25] Contacts 0, 1, 2 and 3 were individually stimulated for approximately 1 min each at the following settings: Frequency = 135 Hz Pulse Width = 90 μSec & 150 μSec Voltage = 2V, 4V, 6V (only if no effect at 2V or 4V). If no adverse effects were observed, the lead position was unchanged. If adverse effects (e.g., panic) were observed at contact 0, the lead was withdrawn 1.5 mm. If adverse effects were observed at both contacts 0 and 1, the lead was withdrawn 3 mm.
The INS used during the three-month masked phase was the non-rechargeable Activa PC (Medtronic, Inc), since recharging would unblind participants and raters. Active or sham DBS began after a several day period of DBS optimization similar to that used in DBS clinical applications. Optimization involved systematic surveys of acute behavioral effects (on mood, affect, and anxiety) of DBS at individual unilateral electrode contacts in monopolar mode [26]. The programming device was shielded from the view of all participants. Optimization procedures for those randomized to sham stimulation were identical in length, DBS settings were changed with amplitude remaining at 0 V. For active stimulation monopolar surveys used a frequency of 135 Hz, pulse widths of 90 and 150 μs at 0, 2, 4, 6 or 8V (the higher two amplitudes used if no responses observed at 2 or 4V). After the three-month masked phase, all participants received open-label DBS after a repeated optimization session.
The protocol specified that no medication or behavior therapy changes be allowed during the masked phase. However, emergency medication adjustments were allowed when necessary. The Steering Committee (PIs at all sites) decided in cases that did not follow those guidelines whether the deviation from protocol was sufficient to warrant removing that patient from the masked phase data analysis. Otherwise, medication and therapy changes were not controlled during the 2 year follow-up, in part due to prior evidence indicating that post-DBS responders may be able to reduce number of prescribed medications. Thus, medication and behavioral treatments were recorded throughout.
2.6. Clinical assessments
Baseline clinical assessment was completed just prior to implantation, with follow-up ratings at Week 2 and then Months 1, 2, 3, 6, 9, and 12, then every 6 months thereafter for at least two years. See Table 1 for rating schedule. The three co-primary endpoints were the Yale-Brown Obsessive-Compulsive Scale (YBOCS), Global Assessment of Functioning (GAF), and Social and Occupational Functioning Scale (SOFAS). These scales were intended to capture symptom burden but equally importantly global functioning, since patients were selected for surgery only if they had both chronically severe and otherwise intractable OCD symptoms and markedly impaired functioning. Secondary outcomes were selected to add additional information about mood, generalized anxiety, quality of life, functioning, and constructs thought related to clinical response (e.g. behavioral activation). Raters were trained on measures through video and observational training, as well as co-rating. Ratings were audiotaped and reviewed at the main site for fidelity. Baseline neuropsychological and neuroimaging data will be presented elsewhere.
Table 1.
Schedule of assessments.
| Masked Phase |
Open Phase |
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial | Baseline | Pre-Implant | Implant | Pre-Optimization | Optimization | Week 2 | Month 1 | Month 2 | Month 3 | Optimization | Month 6 | Month 9 | Month 12 | Month 18 | Month 24 | Month 30 | Month 36 | Month 42 | Month 48 | ||||||||||||||||||
| Clinical Measures | ICEFT-R | X | |||||||||||||||||||||||||||||||||||
| SCID | X | ||||||||||||||||||||||||||||||||||||
| MMSE | X | only if cognitive impairment is present | |||||||||||||||||||||||||||||||||||
| CBTH | X | ||||||||||||||||||||||||||||||||||||
| YBOCS-SC | X | ||||||||||||||||||||||||||||||||||||
| YBOCS | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||||||||||
| GAF & SOFAS | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||||||||||||
| MADRS | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||||||||||
| HDRS | X | X | |||||||||||||||||||||||||||||||||||
| HARS | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||||||||||
| CGI | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||||||||||||
| GIT | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||||||||||||
| PGI | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||||||||||||
| mania screen | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||||||||||||
| YMRS | X | if mania screen is positive | X | if screen positive | X | if mania screen is positive | |||||||||||||||||||||||||||||||
| C-SSRS | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||||||||||||
| LIFE-RIFT | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||||||||||||||||
| Clinical Summary | X | X | X | X | X | ||||||||||||||||||||||||||||||||
| Neuropsych Battery | X | X | |||||||||||||||||||||||||||||||||||
| Q-LES-Q | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||||||||||||||
| CBAS | X | X | X | X | X | X | X | X | X | ||||||||||||||||||||||||||||
| BADS | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||||||||||||
| Case Report Forms | Phone Screen | X | |||||||||||||||||||||||||||||||||||
| Baseline | X | ||||||||||||||||||||||||||||||||||||
| Pre-op Physical | X | ||||||||||||||||||||||||||||||||||||
| Pre-op Neurological | X | ||||||||||||||||||||||||||||||||||||
| Pre-Op Labs | X | ||||||||||||||||||||||||||||||||||||
| Initial Implant | X | ||||||||||||||||||||||||||||||||||||
| Intra-op DBS Testing | X | ||||||||||||||||||||||||||||||||||||
| DBS Setting Optimization | X | X | |||||||||||||||||||||||||||||||||||
| Masked Phase First Post-Op Visit | X | ||||||||||||||||||||||||||||||||||||
| DBS Setting Record | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||||||||||||
| Masked Phase Follow-up Visit | X | X | X | X | |||||||||||||||||||||||||||||||||
| Open Phase Follow-up Visit | X | X | X | X | X | X | X | X | X | X | |||||||||||||||||||||||||||
| Medication Changes | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||||||||||
| Adverse Event | as adverse events occur | ||||||||||||||||||||||||||||||||||||
| Phone Visit | as phone visits occur | ||||||||||||||||||||||||||||||||||||
| System Modification | only if modification occurs | ||||||||||||||||||||||||||||||||||||
| Study Termination | only at point of termination | ||||||||||||||||||||||||||||||||||||
2.7. Descriptive measures
Informed Consent Evaluation Feedback Tool (Revised) (ICEFT-R) [22]: The ICEFT-R was developed at Dartmouth Medical School, modified to suit the particulars of this study. It was administered by the Consent Monitor at the time of consent to improve research participants’ understanding of study.
Structured Clinical Interview for the DSM IV (SCID) [27]: The SCID-I/P (Patient Edition) was used to determine comorbid diagnoses at baseline.
Mini Mental State Examination (MMSE [28]). The MMSE is a 30-point clinician-administered screen of cognitive functioning. This form was administered at baseline, and then only if there was concern about cognitive impairment at future visits.
Cognitive-Behavioral Treatment History Form (CBTH) [29]: This form assessed adequacy of previous behavior therapy (≥20 sessions at minimum). This form was administered only at baseline.
YBOCS Symptom Checklist (YBOCS-SC [30]): The YBOCS-SC has 58 self-administered items comprising 16 subgroups of OCD symptoms. It is administered before the YBOCS and facilitates OCD severity ratings. It was completed once only at baseline. Assessment of subtypes was carried out in order to complete secondary analyses for assessment of whether certain subtypes predict outcome. Symptoms were characterized using the five factors in Pinto's item-level analysis [31].
2.8. Primary outcome measures
YBOCS [32]. The YBOCS captures the severity of OCD symptoms over the preceding 2 weeks. Obsessions and compulsions are evaluated separately and the final overall score, which ranges from 0 to 40, reflects overall severity. A YBOCS severity score of 28 (severe illness) was required for study entry. YBOCS severity was rated at all time points.
Global Assessment of Functioning (GAF [33]). The GAF is a numeric scale (0–100) used to rate social, occupational and psychological functioning of adults during the week of poorest functioning in the past month. Used as a primary measure of global functioning. GAF was rated at all time points with the exception of during the optimization period.
Social and Occupational Functioning Assessment Scale (SOFAS [33]). The SOFAS assesses an individual's level of social and occupational functioning during the week of poorest functioning in the past month. It is the primary measure of social, occupational, and interpersonal functioning. SOFAS was rated at all time points with the exception of during the optimization period.
2.9. Secondary outcome measures
Montgomery Asberg Depression Rating Scale (MADRS [34]). The MADRS is a widely-used 10-item interviewer-administered measure of depression severity often used in clinical trials, rating apparent sadness, reported sadness, inner tension, sleep, appetite, concentration, lassitude, inability to feel, pessimistic and suicidal thoughts. MADRS was administered at all timepoints to assess participant report of depressive symptoms and rating of apparent sadness, as it was designed to be sensitive to change in clinical trials. This was used to assess the timeline of change in depressive symptoms after treatment, the relationship between these depression and changes in OCD and non-OCD related anxiety, and functioning.
Hamilton Depression Rating Scale (HDRS-17 [35]). Through the HDRS-17 an interviewer rates 17 items, including questions regarding depressed mood, guilt, suicide, insomnia, work/activities, retardation, agitation, psychic and somatic anxiety, genital symptoms, hypochondriasis, and insight during the past week. HDRS was administered at baseline and the end of the masked period.
Hamilton Anxiety Rating Scale (HARS [36]). The HARS measures severity of psychic anxiety (mental agitation and psychological distress), and somatic anxiety (anxiety-related physical complaints). The HARS was administered at all time points in order to assess changes in non-OCD anxiety symptoms.
Clinical Global Impressions Scale (CGI [37]). The CGI is a clinician assessment of the patient's global functioning, as well as change in global functioning post-DBS. This was administered at baseline and all other time points.
Patient Global Impressions Scale (PGI [38]). The PGI is a patient's impression of their own global functioning, as well as change in global functioning post-DBS. This was administered at baseline and all other time points.
Clinical Global Impression- Behavior Therapy (GIT). The GIT was created for this study, and assesses a therapist's impression of how well a patient is able to engage in exposures to OCD triggers, as well as impression of change post-DBS of how well they can engage in exposures.
2.10. Safety measures
Mania Screen & Young Mania Rating Scale (YMRS [39]). The 16-item mania screen is adapted from the NIH mania questions. The mania screen was administered at all time points. If the patient scored 3 or greater on any item in the screen, the full YMRS was administered.
Columbia-Suicide Severity Rating Scale (C-SSRS [40]). The C-SSRS addresses suicidal ideation and behavior. Subscales include ideation severity and intensity, behavior (actual, aborted, and interrupted suicide attempts, preparatory behavior, non-suicidal self-injurious behavior) and lethality. Given the high comorbidity of depression with risk of suicidal ideation, the C-SSRS was administered at all time points with the exception of optimization.
2.11. Functioning measures
The Range of Impaired Functioning Tool (LIFE-RIFT [41]). This rater-administered tool measures functioning over four domains (work, interpersonal relationships, global satisfaction and recreation). The LIFE-RIFT was measured at baseline and the end of the masked phase, and then at all visits thereafter. This measure was administered in order to determine the extent of functional impairment secondary to OCD.
Quality of Life Enjoyment and Satisfaction Questionnaire-Short Form (Q-LES-Q SF [42]). The self-administered 16-item Q-LES-Q SF assesses enjoyment and satisfaction experienced in various areas of daily functioning. The Q-LES-Q was administered at baseline, pre-optimization, week 2, month 3, and then all visits thereafter.
Cognitive-Behavioral Avoidance Scale (CBAS [43]). Developed initially for depression, this 32-item scale measures both cognitive and behavioral avoidance, which are often associated symptoms in OCD. The CBAS was administered at baseline, at month 6, and all visits thereafter.
Behavioral Activation for Depression Scale (BADS; (J. R [44]). This questionnaire is designed to measure changes in avoidance and activation over the course of treatment. Subscales include Activation, Avoidance/Rumination, Work/School Impairment, and Social Impairment. The BADS was administered at all timepoints with the exception of optimization.
2.12. Planned statistical analyses
We plan to carry out a mixed effect regression model in which we will regress follow-up observation of baseline value of the outcome and treatment groups. The estimate of the treatment effect will be derived from the regression coefficient for treatment assignment, and statistical significance by the ratio of parameter estimate to its standard error. Control variables will also include dummy variables for site. Consistent with the intent-to-treat principle, all randomized persons are included in the analysis using maximum likelihood estimation procedures. We will report time-point specific and omnibus (over all time points) DBS treatment effect differences over the first 12 weeks of follow-up for the primary outcomes. We will carry out the same approach for all secondary outcomes. For analyses related to crossover to the active phase, DBS treatment effects will be examined taking advantage of the delayed start of the initially randomized to sham patients. Treatment effects will be analyzed for each group and capturing regression to the mean (initial resolution of symptoms) and time from initiation of active DBS.
3. Results
3.1. Demographics
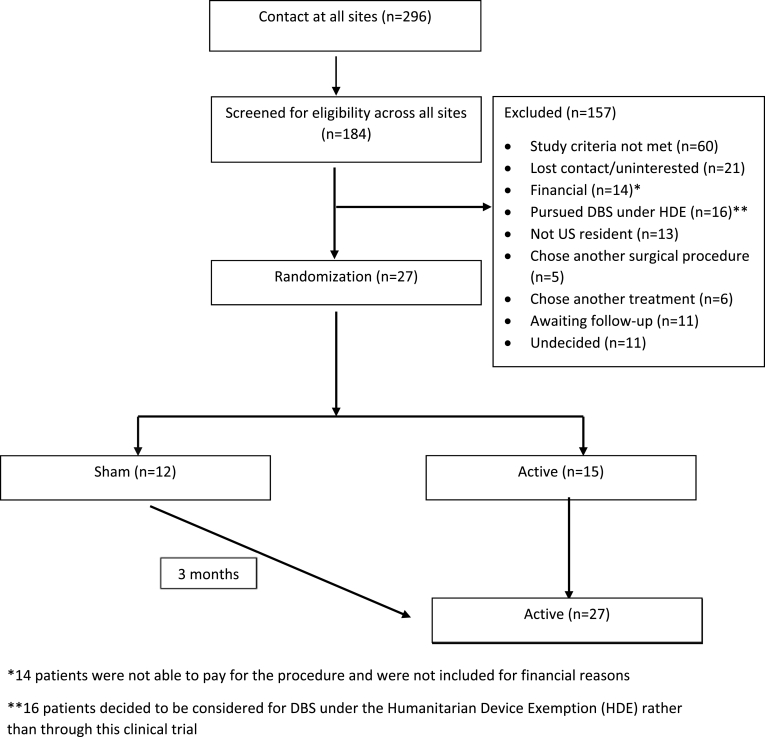
Two hundred and ninety-six individuals underwent initial screening across eight clinical sites. After extensive in-person evaluations and discussions, as noted above, the majority of the patients scored perfectly on an informed consent tool measure without additional discussion. Twenty-seven individuals were enrolled in this study over 6 years. See Fig. 1 for CONSORT diagram. As seen in the diagram, 296 individuals contacted all sites, and 184 of these participants advanced to screening. Sixty out of 296 screened participants (20%) did not meet study criteria, 14 of 296 (5%) were unable to pay for the procedure, and 16 of 296 (5%) chose open-label treatment under the Humanitarian Device Exemption.
Fig. 1.
CONSORT flow chart.
Mean age at pre-surgical baseline was 40 years (SD = 12, range 21–64 years). The majority (59%) were men. Mean age at onset of obsessive-compulsive symptoms was 12.4 years (SD = 5.4, range 4–23 years), with mean illness duration of 27 (SD = 11) years. Most identified as white, not Hispanic (89%), with one person reporting as Hispanic, one as Asian, and one as American Indian/Alaska Native. Please see Table 2 for overall clinical characteristics and Table 3 for individual participant characteristics. Breakdown of enrollment across sites included Massachusetts General Hospital (n = 7), Butler Hospital (n = 4), University of Florida (n = 4), Cleveland Clinic (n = 3), Mayo Clinic (n = 3), Mount Sinai (n = 2), George Washington University (n = 2), and Kaiser Permanente (n = 2).
Table 2.
Clinical features.
| Mean (SD) |
Observed |
||
|---|---|---|---|
| Characteristic | or n (%) | range | |
| Total [n (%)] | 27 | (100) | |
| Age at baseline years [M (SD)] | 39.6 | (12.4) | [21.0–64.0] |
| Age of symptom onset years [M (SD)] | 12.4 | (5.4) | [4.0–23.0] |
| Duration of OCD years [M (SD)] | 27.2 | (11.4) | [10.0–51.0] |
| Gender (male vs. female) [n (%)] Female | 11 | (40.7) | |
| Male | 16 | (59.3) | |
| Race ethnicity [n (%)] White | 24 | (88.9) | |
| Hispanic | 1 | (3.7) | |
| All other race ethnicity groups | 2 | (7.4) | |
| YBOCS [M (SD)] | 33.4 | (2.3) | [29.0–39.0] |
| GAF [M (SD)] | 39.0 | (5.8) | [25.0–45.0] |
| SOFAS [M (SD)] | 39.9 | (6.6) | [25.0–50.0] |
| Q-LES-Q-SF [M (SD)] | 37.5 | (8.8) | [23.0–58.0] |
| MADRS [M (SD)] | 22.7 | (11.0) | [2.0–41.0] |
| HDRS (17) [M (SD)] | 16.3 | (7.2) | [6.0–31.0] |
| HDRS (25) [M (SD)] | 26.1 | (10.3) | [8.0–46.0] |
| HARS [M (SD)] | 17.6 | (8.9) | [3.0–39.0] |
| BADS (sum of BADS) [M (SD)] | 69.7 | (21.8) | [24.0–117.0] |
| LIFE-RIFT [M (SD)] | 15.6 | (2.5) | [11.0–20.0] |
| CBAS [M (SD)] | 82.1 | (20.0) | [36.0–125.0] |
| Characteristic |
or n (%) |
range |
|
| YMRS [M (SD)]a | 3.8 | (2.7) | [0.0–9.0] |
| C-SSRS Suicidal Ideation Score [M (SD)] | 2.0 | (1.8) | [0.0–5.0] |
YMRS mean and standard deviation based on 8 participants with positive mania screen.
Table 3.
Individual patient data (n = 27).
| ID | Age | Gender | Age of Onset (Years) | Duration (Years) | Subtype | Incompletenessa |
|---|---|---|---|---|---|---|
| B1 | 48 | Female | 5 | 43 | Symmetry, ordering | Partial |
| B2 | 59 | Male | 8 | 51 | Doubt, checking | Primary |
| B3 | 47 | Male | 11 | 36 | Doubt, checking | Partial |
| B4 | 34 | Male | 5 | 29 | Contamination, cleaning | None |
| C1 | 39 | Male | 12 | 27 | Taboo thoughts | Partial |
| C2 | 36 | Male | 14 | 22 | Contamination, cleaning | Primary |
| C3 | 29 | Female | 7 | 22 | Symmetry, ordering | Partial |
| F1 | 49 | Male | 16 | 33 | Taboo thoughts | None |
| F2 | 41 | Male | 18 | 23 | Symmetry, ordering | Primary |
| F3 | 59 | Female | 12 | 47 | Symmetry, ordering | Partial |
| F4 | 56 | Female | 19 | 37 | Doubt, checking | Primary |
| G1 | 29 | Male | 4 | 25 | Doubt, checking | Partial |
| G2 | 33 | Male | 20 | 13 | Contamination, cleaning | None |
| K1 | 31 | Male | 7 | 24 | Taboo thoughts | None |
| K2 | 48 | Male | 16 | 32 | Contamination | None |
| M1 | 58 | Male | 17 | 41 | Doubt, checking | Primary |
| M2 | 25 | Female | 12 | 13 | Doubt, checking | None |
| M3 | 64 | Female | 20 | 44 | Contamination, cleaning | None |
| M4 | 21 | Male | 11 | 10 | Doubt, checking | Primary |
| M5 | 26 | Female | 15 | 11 | Doubt, checking | Partial |
| M6 | 42 | Female | 15 | 27 | Contamination, cleaning | None |
| M7 | 36 | Male | 6 | 30 | Contamination, cleaning | None |
| S1 | 27 | Female | 5 | 22 | Symmetry, ordering | Primary |
| S2 | 31 | Female | 10 | 21 | Doubt, checking | Partial |
| Y1 | 31 | Male | 17 | 14 | Doubt, checking | Primary |
| Y2 | 47 | Male | 23 | 24 | Doubt, checking | Primary |
| Y3 | 24 | Female | 10 | 14 | Contamination, cleaning | None |
Participants were rated on the “core feature” of incompleteness, or ‘just right’ symptoms, using the baseline clinical summary and discussion with site clinicians. Individuals were rated as having primary (majority of symptoms were incompleteness), partial (some incompleteness and some harm avoidant), or none (no significant incompleteness).
3.2. Comorbid diagnoses
The most common current psychiatric comorbidity was major depression in 19 of the 27 patients (70%). Other comorbidities included Dysthymia in 4/27 (15%), Generalized Anxiety Disorder 4/27 (15%), Alcohol Abuse (2/27,7%; not current or unstably remitted at time of surgery), Panic Disorder (1/27, 4%), Specific Phobia (1/27, 4%), Anorexia Nervosa (1/27, 4%), and Binge Eating Disorder (1/27, 4%). See Table 4 for details.
Table 4.
Symptom subtypes and comorbidities.
| Mean (SD) | Characteristic | or n (%) |
|---|---|---|
| Total [n (%)] | 27 | (100) |
| Symptom subtype [n (%)] Symmetry ordering | 5 | (19.2) |
| Taboo thoughts | 3 | (11.5) |
| Doubt checking | 11 | (42.3) |
| Contamination cleaning | 7 | (26.9) |
| Major Depression [n (%)] Not present | 8 | (29.6) |
| Present | 19 | (70.4) |
| Dysthymia [n (%)] | ||
| Not present | 23 | (85.2) |
| Present | 4 | (14.8) |
| GAD [n (%)] Not present |
23 | (85.2) |
| Present | 4 | (14.8) |
| Panic Disorder [n (%)] | ||
| Not present | 26 | (96.3) |
| Present | 1 | (3.7) |
| Specific Phobia [n (%)] | ||
| Not present | 26 | (96.3) |
| Present | 1 | (3.7) |
| Anorexia [n (%)] Not present | 26 | (96.3) |
| Present | 1 | (3.7) |
| Binge Eating Disorder [n (%)] | ||
| Not present | 26 | (96.3) |
| Present | 1 | (3.7) |
| Substance Abuse [n (%)] | ||
| Not present | 25 | (92.6) |
| Present | 2 | (7.4) |
3.3. Functioning
Baseline and “best ever” functioning were rated based on clinical summaries, in conjunction with treating clinicians and patient reports. See Table 5. At baseline, 5 were unable to live independently, 11 required some support, typically from family, and 11 were living independently.
Table 5.
Ratings of baseline and ‘best ever’ functioning.
| Best Ever Functioning |
Baseline Functioning |
||||
|---|---|---|---|---|---|
| Working/School | Social Engagement | Working/School | Social Engagement | Family Support | |
| Minimal/None | 2/27 (7%) | 4/27 (15%) | 24/27 (89%) | 16/27 (59%) | 2/27 (7%) |
| Limited/Some | 11/27 (41%) | 10/27 (37%) | 3/27 (11%) | 10/27 (37%) | 25/27 (93%) |
| Good | 14/27 (52%) | 13/27 (48%) | 0/27 (0%) | 1/27 (4%) | 0/27 (0%) |
Baseline and “best ever” functioning were rated based on clinical summaries, in conjunction with treating clinicians and patient reports. Numbers in cells represent number of participants in each category.
3.4. OCD Symptom subtypes
We assessed primary symptoms at baseline using the five factors in Pinto's item-level analysis [31]. The most common subtype (see Table 4) was doubt/checking (42.3%), followed by contamination (30%), symmetry/ordering (19%), and taboo thoughts (12%). There were no cases with primary hoarding (excluded by design). Participants were also rated on the “core feature” of incompleteness, or ‘just right’ symptoms, using the baseline clinical summary and discussion with site clinicians. As seen in Table 4, individuals were rated as having primary (majority of symptoms were incompleteness), partial (some incompleteness and some harm avoidant), or none (no significant incompleteness). One third (33%), were primary, 29% partial incompleteness, and 37% no significant incompleteness.
3.5. Treatment
Twenty-two participants had full trials of exposure and response prevention (ERP). The remaining five were deemed unable to undergo ERP. Mean number of trials of ERP, defined as a continuous period of treatment, followed by a break, and a re-initiation of treatment, was 2.4. Of the 27 participants, 16 had prior inpatient hospitalization, 15 had residential OCD treatment, and 4 had day hospital treatment.
Participants had a lifetime mean of 5.04 SRI trials (independent of a retrial of the same medication), 2.9 trials of a SRI with neuroleptic, 1.6 trials of a SRI with benzodiazepine, and 6.5 trials of another type of psychotropic medication (e.g., stimulant, mood stabilizer, atypical antidepressant). At baseline, participants were taking a mean of 4.0 medications (range 0–9). Most were taking at least one SRI at baseline (mean 0.9; range 0–2).
3.6. Clinical assessments
3.6.1. Primary outcome measures
OCD Symptoms. Mean YBOCS severity score was 33.4 (SD = 2.3), range 29–39 (severe OCD).
General Functioning. General functioning, as assessed by the GAF, was a mean of 38.8 (SD = 5.8), consistent with major impairment in several areas of functioning. Social and occupational functioning, as assessed by the SOFAS, was in the same range, with a mean of 39.7 (SD = 6.6).
3.6.2. Secondary outcomes
Depression. Scores on the MADRS (M = 23; SD = 11) and the HDRS (M = 16.3; SD = 7.2) indicated moderate depression.
Anxiety. The mean baseline HARS score of 17.6 (SD = 8.9) indicated mild to moderate nonspecific anxiety.
Safety. Eight of the 27 participants had a positive mania screen at baseline (with a severity score of 3 or greater on any item in the screen), and were administered the full YMRS. Score on the YMRS for those participants who received the entire measure was a mean of 4.9 (SD = 2.4), below the recognized threshold for mania on this measure [39]. Mean C-SSRS score of 2.0 (SD = 1.8), indicated no imminently serious levels of suicidal ideation.
Functioning. Mean total score on the LIFE-RIFT was 15.6 (SD = 2.5), indicating significant impairment, more than has been previously reported in a general group of treatment-seeking OCD patients: 2.4 (SD = 3.4) [45]. The mean baseline BADS score was 70 (SD = 22), reflecting low behavioral activation. This BADS score was the same or lower than in studies of depression [46]. Mean CBAS rating was 82 (SD = 20), reflecting high levels of behavioral avoidance, similar to studies in depressed adults [47], and higher than seen in remitted major depression or healthy individuals [47,48].
Quality of Life. Mean Q-LES-Q total score was 37.5 (SD = 8.8), representing poor quality of life, lower than has been reported in a healthy group, a general OCD population [49], subthreshold OCD [50,51], remitted bipolar disorder, or remitted schizophrenia [51].
4. Discussion
We describe the design of the first collaborative multi-center NIH-funded randomized controlled trial for intractable OCD (clinicaltrials.gov NCT00640133), and the clinical sample obtained. Recruitment, screening, and baseline evaluations were exhaustive and time-consuming, typically taking months from initial contact to final approval, to assure candidates met surgical criteria. Multidisciplinary expertise was essential in case evaluation, as well as for subsequent study procedures and long-term follow-up, at all sites undertaking DBS for OCD. All available psychiatric and behavioral therapy records were reviewed. These were typically numerous and extensive, from outpatient psychiatric care and behavioral therapy to multiple inpatient and/or residential treatment episodes. Despite such intensive treatments, candidates remained markedly impaired in social and occupational functioning. Many patients were impaired in activities of daily living such that it was impossible for them to live independently. Our sample was similar to other neurosurgical samples (e.g. Ref. [12]) and individuals with severe OCD generally [52,53]. Given our concern that those who benefitted from DBS in this study should have ongoing access to this costly treatment, not guaranteed in the US despite FDA humanitarian approval, only those with insurance plans that would pay for the procedure and ongoing treatment were considered for enrollment. In practice, this meant the majority had US Medicare for which they qualified by virtue of chronic disability due to OCD.
Based on an analysis of naturalistic data after the study began [8], only a small subset (<1%) of treatment-seeking affected individuals would be appropriate candidates. Recruitment of this sample thus took more time, and required eight centers instead of the three initially proposed. In addition to IRB approval, as well as local CMS approval for Medicare coverage, Kaiser Permanente in California was required to obtain state approval through the county mental health department, and were the first in their state to have formal approval for psychiatric neurosurgery since a ban in the 1970s. Though small in number, average implantations per year across all sites were comparable to studies in other countries. For example, Denys et al. [13] enrolled 70 patients over the course of 12.5 years (5.6 patients/year). After an initial delay starting enrollment due to the need to submit an IDE application to the FDA as well as establish regional contracts with Medicare, we enrolled 27 patients over the course of 5 years (5.4 patients/year). As noted above, there are concerns regarding obtaining insurance coverage for DBS for OCD, which likely accounted for the seemingly larger effort in recruitment in this study. Even within U.S. Medicare, a Federal U.S. insurance program that includes insurance for those disabled by conditions such as intractable OCD, and which includes costs for approved clinical trials, there is major regional variability across the country - in some U.S. regions DBS for OCD reimbursement is denied even when patients ostensibly have the “same” insurance. As noted in the manuscript, we chose to only enroll and implant individuals who would have access to continuing insurance coverage in order for DBS to be available essentially indefinitely. With continued randomized controlled trials such as this, we may be able to address some of these barriers to parity coverage for mental health, resulting in increased access to treatments such as this.
Our final sample was similar to those of other studies of psychiatric neurosurgery for intractable OCD and characteristic of the general OCD population. Mean onset of major OC symptoms was early adolescence, and all participants had substantial illness by their early 20s, as in prior studies of general OCD populations [20,54]. The average duration of illness was 27 years which is expected since illness onset was typically early in life and participants presented for surgery at a mean age of 39.6 years (comparable to DBS or lesion studies) [12,[55], [56], [57], [58], [59]]. Major depression was the most common lifetime comorbidity (at 70%), consistent with other neurosurgical studies [20,60]. Other psychiatric comorbidities, in order of decreasing prevalence, included dysthymia, generalized anxiety disorder, history of substance abuse (not active or unstably remitted at baseline), panic disorder, specific phobia, anorexia nervosa, and binge eating disorder. While baseline depression severity was generally moderate, participants showed low levels of behavioral activation and high levels of behavioral avoidance, features associated with depression [61] as well as OCD and frequently comorbid illnesses. Nonspecific anxiety symptoms were relatively mild on average, consistent with other studies [12,20,55,59,60].
Since OCD symptoms are heterogeneous, we also categorized the sample using symptom subtypes established in general OCD populations (e.g, Ref. [62]). The most common symptom subtype in our sample was doubt/checking as also seen in a study of ventral capsulotomy [20]. Other OCD subtypes, in order of decreasing frequency, were contamination, symmetry/ordering, and taboo-related symptoms. Another important dimensional distinction in OCD is that symptoms can be motivated by harm avoidance or incompleteness [63,64]. In our sample, incompleteness dominated the clinical picture in a third, a third had both incompleteness and harm avoidance, and the remaining third only had harm avoidance.
Our study team chose a randomized, sham-controlled design given concerns about safety and rigor resulting from prior crossover RCT designs in this population. When stimulation is stopped in severely ill patients, there is often rapid return of symptoms, which is a safety concern in this population. These concerns were evidenced in the Luyten et al. trail [14] in which 14/17 (82%) of participants used the escape procedure during the OFF phase due to worsening of symptoms (Luyten et al.). In the Denys et al. trial [13]; the sham portion of the crossover phase was reduced from 3 months to 2 weeks due to “concerns about patient cooperation.” Thus, we decided to avoid a crossover component, and all patients received active stimulation after the first 3 months of the trial. Another important concern is that blinding of patients once exposed to a period of active stimulation is extremely difficult.
In summary, we describe a multicenter RCT of DBS for intractable OCD, in which all patients underwent device implantation followed by randomization to either active or sham DBS (i.e., a delayed start design). Primary outcomes are OCD symptom severity (YBOCS) and general functioning (GAF, SOFAS). The surgical target has been referred to as the ventral capsule/ventral striatum, or VC/VS [15], representing white matter in the anterior limb of the internal capsule itself as well as fibers in the cortico-basal-thalamic circuitry extending below the capsule proper in ventral striatum [65]. Non-rechargeable DBS devices (Medtronic, Inc.) were initially implanted to maintain the blind, attached to the quadripolar Medtronic 3387 brain lead in the VC/VS. The masked study phase lasted three months after a postsurgical recovery period of approximately one month.
Our selection process was generally consistent with that of other ablative or DBS studies of neurosurgery for OCD. Consequently, it is unsurprising that the resulting sample was also broadly similar to that reported previously. We characterized the resulting sample using categorical diagnoses of OCD and comorbid conditions as well as along clinically important dimensions including OCD symptom subtypes and the broader dimension of whether symptoms were motivated by harm avoidance, incompleteness, or both in our study sample. In addition, we assessed functional impairment and quality of life using several metrics. This broad approach to baseline symptoms, behaviorally-relevant dimensions, functioning and quality of life is important to judge effects of DBS on a range of critical outcomes. In addition, these phenotypic clinical features might prove useful in tailoring treatment (i.e., stimulation or site) and predicting responses to an invasive treatment that requires specialized resources and clinicians over the long term or even lifetime for some patients.
Funding
This work was supported by NIH: U01MH076179, P20GM130452 (NCM, RNJ, BDG), K23MH100607 (NCM), P50MH106435 (NCM, BDG, SAR), UH3NS100549, P41EB015896, T32GM007739 (DLR), 1R01 MH118514 (RMB). This work was also supported by the Picower Family Foundation (ASW), the Brain & Behavior Research Foundation (ASW; KL), Michael J Fox Foundation (MSO), Tourette Association of America (MSO), NCATS (3UL1TR00142; RMB), VA RR&D (B9256; RMB), and VAORD N9228TC (BDG).
Acknowledgements
The authors wish to acknowledge David Shire (consent monitor), Brittney Blanchette, Roberta McMahon, Rouba Youssef, Ihtsham Haq, MD, James Kimball, MD, Jennifer Bernier Coleman, PhD, Lynn Hana, and Kenneth Rickler, MD.
References
- 1.Baxter A.J., Vos T., Scott K.M., Ferrari A.J., Whiteford H.A. The global burden of anxiety disorders in 2010. Psychol. Med. 2014;44(11):2363–2374. doi: 10.1017/S0033291713003243. [DOI] [PubMed] [Google Scholar]
- 2.Fava M., Davidson K.G. Definition and epidemiology of treatment-resistant depression. Psychiatr. Clin. 1996;19(2):179–200. doi: 10.1016/s0193-953x(05)70283-5. [DOI] [PubMed] [Google Scholar]
- 3.Husted D.S., Shapira N.A. A review of the treatment for refractory obsessive-compulsive disorder: from medicine to deep brain stimulation. CNS Spectr. 2004;9(11):833–847. doi: 10.1017/s109285290000225x. [DOI] [PubMed] [Google Scholar]
- 4.Jenike M.A., Rauch S.L., Baer L., Rasmussen S.A. Neurosurgical treatment of obsessive-compulsive disorder. In: Jenike M.A., Baer L., Minichiello W.E., editors. Obsessive-compulsive Disorders: Practical Management. Mosby; St. Louis, MO: 1998. [Google Scholar]
- 5.Kennedy S.H., Giacobbe P., Rizvi S.J., Placenza F.M., Nishikawa Y., Mayberg H.S., Lozano A.M. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am. J. Psychiatr. 2011;168(5):502–510. doi: 10.1176/appi.ajp.2010.10081187. [DOI] [PubMed] [Google Scholar]
- 6.Brennan B.P., Wang D., Li M., Perriello C., Ren J., Elias J.A.…Liu H. Use of an individual-level approach to identify cortical connectivity biomarkers in obsessive-compulsive disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4(1):27–38. doi: 10.1016/j.bpsc.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dougherty D.D., Brennan B.P., Stewart S.E., Wilhelm S., Widge A.S., Rauch S.L. Neuroscientifically informed formulation and treatment planning for patients with obsessive-compulsive disorder: a review. JAMA Psychiatry. 2018;75(10):1081–1087. doi: 10.1001/jamapsychiatry.2018.0930. [DOI] [PubMed] [Google Scholar]
- 8.Garnaat S.L., Greenberg B.D., Sibrava N., Goodman W., Mancebo M., Eisen J., Rasmussen S. Who qualifies for deep brain stimulation for OCD? Data from a naturalistic clinical sample. J. Neuropsychiatry Clin. Neurosci. 2013 doi: 10.1176/appi.neuropsych.12090226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bandelow B., Baldwin D., Abelli M., Altamura C., Dell'Osso B., Domschke K.…Riederer P. Biological markers for anxiety disorders, OCD and PTSD - a consensus statement. Part I: neuroimaging and genetics. World J. Biol. Psychiatr. 2016;17(5):321–365. doi: 10.1080/15622975.2016.1181783. [DOI] [PubMed] [Google Scholar]
- 10.Nuttin B., Cosyns P., Demeulemeester H., Gybels J., Meyerson B. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet. 1999;354(9189):1526. doi: 10.1016/S0140-6736(99)02376-4. [DOI] [PubMed] [Google Scholar]
- 11.Abelson J.L., Curtis G.C., Sagher O., Albucher R.C., Harrigan M., Taylor S.F.…Giordani B. Deep brain stimulation for refractory obsessive-compulsive disorder. Biol. Psychiatr. 2005;57(5):510–516. doi: 10.1016/j.biopsych.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg B.D., Malone D.A., Friehs G.M., Rezai A.R., Kubu C.S., Malloy P.F.…Rasmussen S.A. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31(11):2384–2393. doi: 10.1038/sj.npp.1301165. [DOI] [PubMed] [Google Scholar]
- 13.Denys D., Graat I., Mocking R., de Koning P., Vulink N., Figee M.…Schuurman R. Efficacy of deep brain stimulation of the ventral anterior limb of the internal capsule for refractory obsessive-compulsive disorder: a clinical cohort of 70 patients. Am. J. Psychiatr. 2020;177(3):265–271. doi: 10.1176/appi.ajp.2019.19060656. [DOI] [PubMed] [Google Scholar]
- 14.Luyten L., Hendrickx S., Raymaekers S., Gabriëls L., Nuttin B. Electrical stimulation in the bed nucleus of the stria terminalis alleviates severe obsessive-compulsive disorder. Mol. Psychiatr. 2016;21(9):1272–1280. doi: 10.1038/mp.2015.124. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg B.D., Gabriels L.A., Malone D.A., Jr, Rezai A.R., Friehs G.M., Okun M.S.…Nuttin B.J. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol. Psychiatr. 2010;15(1):64–79. doi: 10.1038/mp.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodman W.K., Foote K.D., Greenberg B.D., Ricciuti N., Bauer R., Ward H.…Okun M.S. Deep brain stimulation for intractable obsessive compulsive disorder: pilot study using a blinded, staggered-onset design. Biol. Psychiatr. 2010;67(6):535–542. doi: 10.1016/j.biopsych.2009.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nuttin B., Gybels J., Cosyns P., Gabriels L., Meyerson B., Andreewitch S.…Fins J.J. Deep brain stimulation for psychiatric disorders. Neurosurg. Clin. 2003;14(2):xv–xvi. doi: 10.1016/s1042-3680(03)00007-x. [DOI] [PubMed] [Google Scholar]
- 18.Alonso P., Cuadras D., Gabriels L., Denys D., Goodman W., Greenberg B.D.…Menchon J.M. Deep brain stimulation for obsessive-compulsive disorder: a meta-analysis of treatment outcome and predictors of response. PloS One. 2015;10(7) doi: 10.1371/journal.pone.0133591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu H., Hariz M., Visser-Vandewalle V., Zrinzo L., Coenen V.A., Sheth S.A.…Coyne T. Deep brain stimulation for refractory obsessive-compulsive disorder (OCD): emerging or established therapy? Mol. Psychiatr. 2021;26(1):60–65. doi: 10.1038/s41380-020-00933-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rasmussen S.A., Noren G., Greenberg B.D., Marsland R., McLaughlin N.C., Malloy P.J.…Lindquist C. Gamma ventral capsulotomy in intractable obsessive-compulsive disorder. Biol. Psychiatr. 2018;84(5):355–364. doi: 10.1016/j.biopsych.2017.11.034. [DOI] [PubMed] [Google Scholar]
- 21.Nadler R., Chandler J.A. Legal regulation of psychosurgery: a fifty-state survey. J. Leg. Med. 2019;39(4):335–399. doi: 10.1080/01947648.2019.1688208. [DOI] [PubMed] [Google Scholar]
- 22.Bankert E.A., Amdur R.J. Informed Consent Evaluation Feedback Tool Institutional Review Board: Management and Function. 2 ed. Jones and Bartlett Publishers; Sudbury, Massachussets: 2006. pp. 246–248. [Google Scholar]
- 23.Lehman J.F., Greenberg B.D., McIntyre C.C., Rasmussen S.A., Haber S.N. Rules ventral prefrontal cortical axons use to reach their targets: implications for diffusion tensor imaging tractography and deep brain stimulation for psychiatric illness. J. Neurosci. 2011;31(28):10392–10402. doi: 10.1523/JNEUROSCI.0595-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makris N., Rathi Y., Mouradian P., Bonmassar G., Papadimitriou G., Ing W.I., Dougherty D.D. Variability and anatomical specificity of the orbitofrontothalamic fibers of passage in the ventral capsule/ventral striatum (VC/VS): precision care for patient-specific tractography-guided targeting of deep brain stimulation (DBS) in obsessive compulsive disorder (OCD) Brain Imaging Behav. 2015 doi: 10.1007/s11682-015-9462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shapira N., Okun M., Wint D., Foote K., Byars J., Bowers D.…Haber S. Panic and fear induced by deep brain stimulation. J. Neurol. Neurosurg. Psychiatr. 2006;77(3):410–412. doi: 10.1136/jnnp.2005.069906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Widge A.S., Dougherty D.D. Cambridge University Press; 2015. Managing Patients with Psychiatric Disorders with Deep Brain Stimulation. [Google Scholar]
- 27.First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. SCID-I/P. Patient Edition. Biometrics Research, New York State Psychiatric Institute; New York: 2002. Structured clinical interview for DSM-IV-TR Axis I disorders, research version. [Google Scholar]
- 28.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Abramowitz J.S. Mahwah; NJ, London: 2006. Understanding and Treating Obsessive-Compulsive Disorder a Cognitive-Behavioral Approach. [Google Scholar]
- 30.Feinstein S.B., Fallon B.A., Petkova E., Liebowitz M.R. Item-by-item factor analysis of the Yale-Brown obsessive compulsive scale symptom checklist. J. Neuropsychiatry Clin. Neurosci. 2003;15(2):187–193. doi: 10.1176/jnp.15.2.187. [DOI] [PubMed] [Google Scholar]
- 31.Pinto A., Greenberg B.D., Murphy D.L., Nestadt G., Rasmussen S.A. Using individual items to clarify OCD symptom structure: the case for five factors. Am. J. Psychiatr. 2009;166(6):728–729. doi: 10.1176/appi.ajp.2009.09020287. [DOI] [PubMed] [Google Scholar]
- 32.Goodman W.K., Price L.H., Rasmussen S.A., Mazure C., Delgado P., Heninger G.R., Charney D.S. The Yale-Brown obsessive compulsive scale. II. Validity. Arch. Gen. Psychiatr. 1989;46(11):1012–1016. doi: 10.1001/archpsyc.1989.01810110054008. [DOI] [PubMed] [Google Scholar]
- 33.Hilsenroth M.J., Ackerman S.J., Blagys M.D., Baumann B.D., Baity M.R., Smith S.R.…Holdwick D.J., Jr. Reliability and validity of DSM-IV axis V. Am. J. Psychiatr. 2000;157(11):1858–1863. doi: 10.1176/appi.ajp.157.11.1858. [DOI] [PubMed] [Google Scholar]
- 34.Montgomery S.A., Asberg M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamilton M. A rating scale for anxiety. Br. J. Med. Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 37.Busner J., Targum S.D. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont) 2007;4(7):28. [PMC free article] [PubMed] [Google Scholar]
- 38.Hossack T., Woo H. Validation of a patient reported outcome questionnaire for assessing success of endoscopic prostatectomy. Prostate international. 2014;2(4):182–187. doi: 10.12954/PI.14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young R.C., Biggs J.T., Ziegler V.E., Meyer D.A. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 40.Posner K., Brown G.K., Stanley B., Brent D.A., Yershova K.V., Oquendo M.A.…Mann J.J. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am. J. Psychiatr. 2011;168(12):1266–1277. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leon A.C., Solomon D.A., Mueller T.I., Turvey C.L., Endicott J., Keller M.B. The Range of Impaired Functioning Tool (LIFE-RIFT): a brief measure of functional impairment. Psychol. Med. 1999;29(4):869–878. doi: 10.1017/s0033291799008570. [DOI] [PubMed] [Google Scholar]
- 42.Endicott J., Nee J., Harrison W., Blumenthal R. Quality of life enjoyment and satisfaction questionnaire: a new measure. Psychopharmacol. Bull. 1993;29(2):321–326. [PubMed] [Google Scholar]
- 43.Ottenbreit N.D., Dobson K.S. Avoidance and depression: the construction of the cognitive-behavioral avoidance scale. Behav. Res. Ther. 2004;42(3):293–313. doi: 10.1016/S0005-7967(03)00140-2. [DOI] [PubMed] [Google Scholar]
- 44.Kanter J.R., Lc, Busch A.M., Sedivy S.K. Validation of the behavioral activation for depression scale(BADS) in a community sample with elevated DepressiveSymptoms. J. Psychopathol. Behav. Assess. 2009;31(1):36–42. [Google Scholar]
- 45.Eisen J.L., Mancebo M.A., Pinto A., Coles M.E., Pagano M.E., Stout R., Rasmussen S.A. Impact of obsessive-compulsive disorder on quality of life. Compr. Psychiatr. 2006;47(4):270–275. doi: 10.1016/j.comppsych.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alexopoulos G.S., Raue P.J., Gunning F., Kiosses D.N., Kanellopoulos D., Pollari C.…Arean P.A. Engage" therapy: behavioral activation and improvement of late-life major depression. Am. J. Geriatr. Psychiatr. 2016;24(4):320–326. doi: 10.1016/j.jagp.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quigley L., Wen A., Dobson K.S. Avoidance and depression vulnerability: an examination of avoidance in remitted and currently depressed individuals. Behav. Res. Ther. 2017;97:183–188. doi: 10.1016/j.brat.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 48.Moulds M.L., Kandris E., Starr S., Wong A.C. The relationship between rumination, avoidance and depression in a non-clinical sample. Behav. Res. Ther. 2007;45(2):251–261. doi: 10.1016/j.brat.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 49.Jacoby R.J., Leonard R.C., Riemann B.C., Abramowitz J.S. Predictors of quality of life and functional impairment in obsessive-compulsive disorder. Compr. Psychiatr. 2014;55(5):1195–1202. doi: 10.1016/j.comppsych.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 50.Goracci A., Martinucci M., Kaperoni A., Fagiolini A., Sbaragli C., Corsi E., Castrogiovanni P. Quality of life and subthreshold obsessive-compulsive disorder. Acta Neuropsychiatr. 2007;19(6):357–361. doi: 10.1111/j.1601-5215.2007.00257.x. [DOI] [PubMed] [Google Scholar]
- 51.Latalova K., Prasko J., Diveky T., Kamaradova D., Velartova H. Quality of life in patients with bipolar disorder--a comparison with schizophrenic patients and healthy controls. Psychiatr. Danub. 2011;23(1):21–26. [PubMed] [Google Scholar]
- 52.Bystritsky A., Liberman R.P., Hwang S., Wallace C.J., Vapnik T., Maindment K., Saxena S. Social functioning and quality of life comparisons between obsessive-compulsive and schizophrenic disorders. Depress. Anxiety. 2001;14(4):214–218. doi: 10.1002/da.1069. [DOI] [PubMed] [Google Scholar]
- 53.Calvocoressi L., Libman D., Vegso S.J., McDougle C.J., Price L.H. Global functioning of inpatients with obsessive-compulsive disorder, schizophrenia, and major depression. Psychiatr. Serv. 1998;49(3):379–381. doi: 10.1176/ps.49.3.379. [DOI] [PubMed] [Google Scholar]
- 54.Brakoulias V., Starcevic V., Belloch A., Brown C., Ferrao Y.A., Fontenelle L.F.…Viswasam K. Comorbidity, age of onset and suicidality in obsessive-compulsive disorder (OCD): an international collaboration. Compr. Psychiatr. 2017;76:79–86. doi: 10.1016/j.comppsych.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 55.Jung H.H., Kim C.H., Chang J.H., Park Y.G., Chung S.S., Chang J.W. Bilateral anterior cingulotomy for refractory obsessive-compulsive disorder: long-term follow-up results. Stereotact. Funct. Neurosurg. 2006;84(4):184–189. doi: 10.1159/000095031. [DOI] [PubMed] [Google Scholar]
- 56.Lopes A.C., Greenberg B.D., Canteras M.M., Batistuzzo M.C., Hoexter M.Q., Gentil A.F., Pereira C.A.B., Joaquim M.A., de Mathis M.E., D'Alcante C.C., Taub A., de Castro D.G., Tokeshi L., Sampaio L.A.N.P.C., Leite C.C., Shavitt R.G., Diniz J.B., Busatto G., Norén G., Rasmussen S.A., Miguel E.C. Gamma ventral capsulotomy for obsessive-compulsive disorder A randomized clinical trial. JAMA Psychiatry. 2014;71(9):1066–1076. doi: 10.1001/jamapsychiatry.2014.1193. [DOI] [PubMed] [Google Scholar]
- 57.Ruck C., Karlsson A., Steele J.D., Edman G., Meyerson B.A., Ericson K.…Svanborg P. Capsulotomy for obsessive-compulsive disorder: long-term follow-up of 25 patients. Arch. Gen. Psychiatr. 2008;65(8):914–921. doi: 10.1001/archpsyc.65.8.914. [DOI] [PubMed] [Google Scholar]
- 58.Sheth S.A., Neal J., Tangherlini F., Mian M.K., Gentil A., Cosgrove G.R.…Dougherty D.D. Limbic system surgery for treatment-refractory obsessive-compulsive disorder: a prospective long-term follow-up of 64 patients. J. Neurosurg. 2013;118(3):491–497. doi: 10.3171/2012.11.JNS12389. [DOI] [PubMed] [Google Scholar]
- 59.Zhan S., Liu W., Li D., Pan S., Pan Y., Li Y.…Sun B. Long-term follow-up of bilateral anterior capsulotomy in patients with refractory obsessive-compulsive disorder. Clin. Neurol. Neurosurg. 2014;119:91–95. doi: 10.1016/j.clineuro.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 60.Denys D., Mantione M., Figee M., van den Munckhof P., Koerselman F., Westenberg H.…Schuurman R. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch. Gen. Psychiatr. 2010;67(10):1061–1068. doi: 10.1001/archgenpsychiatry.2010.122. [DOI] [PubMed] [Google Scholar]
- 61.Kanter J.W., Mulick P., Busch A.M., Berlin K.S., Martell C.R. The behavioral activation for depression scale (BADS): psychometric properties and factor structure. J. Psychopathol. Behav. Assess. 2007;29:191–202. [Google Scholar]
- 62.Pinto A., Greenberg B.D., Murphy D.L., Nestadt G., Rasmussen S.A. Using individual items to clarify OCD symptom structure: the case for five factors. Am. J. Psychiatr. 2009;166(6):728–729. doi: 10.1176/appi.ajp.2009.09020287. author reply 729-731. [DOI] [PubMed] [Google Scholar]
- 63.Sibrava N.J., Boisseau C.L., Eisen J.L., Mancebo M.C., Rasmussen S.A. An empirical investigation of incompleteness in a large clinical sample of obsessive compulsive disorder. J. Anxiety Disord. 2016;42:45–51. doi: 10.1016/j.janxdis.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Summerfeldt Lj K.P., Antony M.M., Swinson R.P. Examining an obsessive-compulsive core dimensions model: structural validity of harm avoidance and incompleteness. Journal of Obsessive-Compulsive and Related Disorders. 2014;3(2):83–94. doi: 10.1016/j.jocrd.2014.01.003. [DOI] [Google Scholar]
- 65.Lehman J.F., Greenberg B.D., McIntyre C.C., Rasmussen S.A., Haber S.N. Rules ventral prefrontal cortical axons use to reach their targets: implications for diffusion tensor imaging tractography and deep brain stimulation for psychiatric illness. J. Neurosci. 2011;31(28):10392–10402. doi: 10.1523/JNEUROSCI.0595-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koran L, Hanna G, Hollander E, Nestadt G, Simpson HB. Practice Guidelines for the Treatment of Patients with Obsessive-Compulsive Disorder. American Psychiatric Association. 2010 [PubMed] [Google Scholar]