Abstract
Background
Assessing biomarker profiles in various body fluids is of large value to discern between the sole use of nicotine products. In particular, the assessment of the product compliance is required for long-term clinical studies. The objective of this study was the identification of biomarkers and biomarker patterns in body fluids, to distinguish between combustibles, heated tobacco products, electronic cigarettes, oral tobacco and oral/dermal nicotine products used for nicotine replacement therapy (NRT), as well as a control group of non-users.
Methods
A controlled, single-center study was conducted with 60 healthy subjects, divided into 6 groups (5 nicotine product user groups and one non-user group) based on their sole use of the products of choice. The subjects were confined for 76 h, during which, free and uncontrolled use of the products was provided. Sample collections were performed according to the study time schedule provided in Table 2. The primary outcome will be validated through analysis of the collected biospecimens (urine, blood, saliva, exhaled breath and exhaled breath condensate) by means of untargeted omics approaches (i.e. exposomics, breathomics and adductomics). Secondary outcome will include established biomarker quantification methods to allow for the identification of typical biomarker patterns. Statistical analysis tools will be used to specifically discriminate different product use categories.
Results/Conclusions
The clinical trial was successfully completed in May 2020, resulting in sample management and preparations for the quantitative and qualitative analyses. This work will serve as a solid basis to discern between biomarker profiles of different nicotine product user groups. The knowledge collected during this research will be required to develop prototype diagnostic tools that can reliably assess the differences and evaluate possible health risks of various nicotine products.
Keywords: Biomarkers of exposure, Biomarkers of potential harm, Exposomics, Breathomics, Adductomics, Nicotine delivery products
1. Introduction
Smoking is the single greatest preventable cause of death and disability in the world today [[1], [2], [3]]. Moreover, due to the introduction of strict tobacco control measures and constantly increasing worldwide awareness to the negative effects of conventional cigarette smoking, users are turning to ever-evolving alternative nicotine-delivery products, which are often advertised as potentially reduced risk products [[4], [5], [6], [7], [8], [9], [10], [11]].
Electronic cigarettes (EC) are electronic devices, turning liquid by heating into an aerosol for inhalation. Due to the possibility to be vaped with or without nicotine and additional flavors, they have been used by smokers to reduce the risks of smoking [6] and aid cessation [6,7]. Cessation studies have collected substantial data indicating EC efficacy and safety [4,5,7,[11], [12], [13]]. Generally, EC generate lower up to comparable levels of nicotine and produce lower concentrations of biomarkers of exposure (BoE), i.e., reduce exposure to harmful chemicals in comparison to conventional cigarettes (CC) [[14], [15], [16], [17]].
Heated tobacco products (HTP) contain a tobacco substrate, designed to be heated and not combusted, to produce a nicotine-containing aerosol [18]. This new approach has been tested in studies organized by tobacco industry and independent governmental institutions, in first line indicating a reduced exposure to the harmful and potentially harmful chemicals [[19], [20], [21], [22], [23], [24]].
Oral tobacco (OT) products, especially the Swedish snus, have been widely distributed and used across the Nordic countries [25]. Smoking cessation due to transition to OT, as well as dual use have been reported [26,27]. Due to the large variety of smokeless tobacco products on the market, their constituents may vary widely, as reported [[28], [29], [30], [31]].
Nicotine replacement therapy (NRT) includes products e.g. nicotine chewing gum, sublingual tablets/lozenge, transdermal patch, intranasal spray and inhaled oral spray, which all aim to help quit smoking by replacing the nicotine from cigarettes and thus reducing craving and withdrawal symptoms [32,33]. Aiming the complete abstinence from nicotine dependence, the NRTs are generally well tolerated and have minimal adverse effects [34]. BoE (except those derived from nicotine) from NRT are expected to be at non-user levels [35,36].
The systematic and objective assessment of biomarkers, i.e. biomarkers of exposure (BoE) and biomarkers of potential harm (BoPH), in various body fluids (e.g., urine, blood, plasma, saliva) is routinely performed as part of the risk assessment and pharmacokinetics of potentially reduced risk products like EC or HTP [15].
However, the categorization of a user to one of those product categories becomes rather difficult. While smokers are readily distinguished from non-smokers by measuring nicotine metabolites, the development and use of potentially reduced risk products implies a more diverse assessment of biomarkers to discriminate different product use groups. Especially with respect to long-term studies in a free setting, robust biomarkers to assess compliance are needed to monitor the participants’ sole product use. Philip Morris International (PMI) recently filed its HTP IQOS as a modified risk tobacco product application (MRTPA) to the US FDA which included a 6 month clinical study where participants visited a clinical site on a regular basis and inter alia biological samples were collected for biomarker analysis. Subjects allocated to the HTP arm were instructed to use solely the provided HTP over a period of 6 months [21]. The regulators criticized the lack of robust compliance measures besides self-report in this ambulatory study [37].
However, setting up robust biomarkers of compliance is challenging. In addition to metabolic differences between subjects, biomarker levels may vary due to differing consumption patterns and other exposure sources. Thus, it is likely that a combination of different biomarkers and specimens will be required to unequivocally determine the volunteers’ compliance in a free setting. Biomarkers in alternative matrices like exhaled breath (EB) and exhaled breath condensate (EBC) can also add powerful data for the intended purpose.
Exhaled breath (EB) is a very interesting matrix for analysis, mainly due to its easy, non-invasive collection [38]. Until now, it has been mainly used for monitoring inflammation and oxidative stress in the airways [[39], [40], [41]]. It is typically targeting VOCs by means of GC-MS analysis, and was previously used for discrimination between smokers and non-smokers based on characteristic biomarker profiles [[42], [43], [44], [45], [46]].
Exhaled breath condensate (EBC) presents a matrix which includes the non-volatile compounds which are not present in EB [47,48]. Therefore, the EBC composition should mainly resemble the content of the respiratory tract lining fluid [49], which can be analyzed by LC-MS/MS [50,51].
This manuscript will focus mainly on the design of a clinical study, which was organized and performed in order to identify biomarkers and/or biomarker patterns to distinguish between combustibles (CC), heated tobacco products (HTP), electronic cigarettes (EC), oral tobacco (OT) and oral/dermal nicotine delivery products (used for nicotine replacement therapy, NRT), and compare them with a control group of non-users (NU).
2. Methods
2.1. Trial design
This is a controlled, single-center, open label trial comparing 5 nicotine product user groups, namely smokers of CC, EC vapers, HTP, OT, NRT users, and one group of never users of any product category in terms of their body fluids' biomarker profile after consumption of their respective nicotine-delivery products. In order to ensure compliance within each of the groups, i.e. to provide clear discrimination between the specific biomarkers, a strict separated confinement of subjects was ensured. Each group was confined separately (up to 10 subjects) at the clinical site of Clinical Trial Center (CTC) North (Hamburg, Germany) for four consecutive days. Special care was devoted keeping apart the groups that could cause cross-contamination during consumption, due to product emissions and exhaled breath, especially CC, HTP and EC users.
The enrolment period lasted about 3 months prior to the study start and was prolonged until all the remaining subjects of the last groups were included. Screening numbers of three digits, starting with 001, were assigned sequentially to all subjects as they consented to take part in the clinical trial. All candidates participated in a preliminary examination, ensuring that none of the exclusion and all of the inclusion criteria were fulfilled. Before the baseline visit, upon the arrival to the study site, an additional pre-eligibility check was conducted by a physician. Once confirmed suitable, the patients within the group were assigned randomized study numbers of three digits starting at 201, after verbal and written information about the study has been received and their signed consent to participate in the study has been collected.
The total duration of the study was 7 months, with all participants divided into study groups (Table 1). The study group 1 (non-smokers) visit was performed from 1. – 4. November 2019. From 7. – 10. November 2019, the study was carried out with study group 2 – smokers of conventional cigarettes. Study group 3 (users of EC) was examined from 15. – 18. November 2019. In order to accelerate the study conduct and facilitate subject recruitment, from December 2019, the remaining groups were enrolled with a mix of subjects across the different nicotine product users.
Table 1.
Distribution of subjects by product use into 7 study groups.
| User Groups | Study Group |
||||||
|---|---|---|---|---|---|---|---|
| Study Group 1 (Nov 1–4 2019) | Study Group 2 (Nov 7–10 2019) | Study Group 3 (Nov 15–18 2019) | Study Group 4 (Dec 13–16 2019) | Study Group 5 (Jan 13–16 2020) | Study Group 6 (Feb 21–24 2020) | Study Group 7 (May 26–29 2020) | |
| Non-Users | 10 NU | ||||||
| Smokers of Cigarettes | 10 CC | ||||||
| Users of E-Cigarettes | 10 EC | ||||||
| Users of Heated Tobacco Products | 4 HTP | 6 HTP | |||||
| Users of Nicotine Replacement Therapy | 3 NRT | 3 NRT | 1 NRT | 3 NRT | |||
| Users of Oral Tobacco | 2OT | 3 OT | 4 OT | 1 OT | |||
Study group 4, which consisted of four HTP-users, three NRT-users and two OT-users, was confined at the study site from 13. – 16. December 2019. Study group 5 took place from 13. – 16. January 2020, comprising six HTP users, three NRT users and three OT users. From 21. – 24. February 2020, the study was carried out with study group 6, consisting of one NRT and four OT users. The HTP users were allowed to use their products only in a room strictly separated from the common areas, to avoid cross-contamination between subjects. The final study group 7, which consisted of three NRT users and one OT user, was confined at the study site from 26.–29. May 2020. For the last group, special measures (i.e. social distancing, hygiene plan) have been taken due to the COVID-19 crisis.
During the confinement, for 76 h from Day −1 04.00 p.m. until Day 3 08.00 p.m., subjects used their own brand, which they were provided based on the regular daily consumption. The free, uncontrolled use was expected to conform the usual consumption habits of each study subject.
This cohort study was designed and performed according to the rules of chapter §15 (Research) of the Berufsordnung der Hamburger Ärzte und Ärztinnen, based on the categorized nicotine consumption. All protocols were prepared in accordance with the quality standards of Good Clinical Practice (GCP) and the Helsinki declaration of the World Medical Association (WMA) and approved by the responsible ethics committee before study start. The 60 healthy adult subjects were confined at the study site, each 10 of them as part of one of the following user groups: non-smokers, smokers, e-cigarette users, heated tobacco product users, oral tobacco users and nicotine replacement therapy users (Fig. 1).
Fig. 1.
Graphical illustration of the trial design.
2.2. Sample size
The main rationale for the sample size calculation in this study was based on two observations. Firstly, a significant reduction in toxicant concentrations was reported for the majority of the potentially reduced risk products. Depending on the product category, reduced exposure of up to 99% compared to CC was observed in product characterization studies by the manufacturers and reproduced by independent (governmental) organizations [[19], [20], [21], [22],24,52]. The decrease in toxicant exposure should be resembled in significantly reduced levels of the corresponding biomarkers of exposure compared to smokers of CCs [53]. Secondly, the differing product characteristics shall imply a unique biomarker pattern in the analysis of the different specimens for each product [[54], [55], [56], [57], [58]].
Considering this, with differences being expected between groups – at least for a few biomarkers in one or several biological matrices, a small sample size is considered adequate in this strictly controlled study. The strict prerequisites in the trial design, i.e. recruiting only experienced, exclusive users of one product, the diet-control, and the clinical setting, create distinct, highly homogenous groups, which lower the standard deviations expected within the groups, confirming the indicated smaller sample size being in line with the study design.
However, a larger sample size would lead to more accurate parameter estimates, giving a greater ability to detect differences between the groups. Therefore, taking the desired power (80%) and significance level (α ≤ 0.05) into consideration, a size that would be sufficient to show difference in the biomarker profile was calculated.
Using G*Power 3.1.9.7 software (gpower.hhu.de) for size estimation between independent groups, a sample size of 60 subjects – 10 subjects per each of the 5 cohort groups, plus 10 subjects in the control group was calculated. Based on the previous experience with the similar controlled use trials organized by ABF [17], the sample size was considered sufficient to meet the set investigation objectives. In order to prevent a possible case of subject drop out, additional subjects were recruited to ensure study completion with 60 subjects.
Moreover, it has to be emphasized that this trial was planned as a proof-of-concept study to narrow down suitable biological matrices and user groups for future, larger cohort studies.
2.3. Screening
Healthy subjects were recruited with the support from the clinical study site subject pool and public advertisement. The preliminary examination was performed in order to determine their eligibility to participate in the study. The screening process comprised collection of the following information:
-
•
Medical history and demographics (including product use status);
-
•
Physical examination (including body weight and height; BMI);
-
•
Vital signs;
-
•
Alcohol breath test;
-
•
Cotinine test;
-
•
Carbon monoxide (CO)-breath test;
-
•
Urine pregnancy test for women in child-bearing age;
-
•
Questioning for drug abuse;
-
•
Questionnaire regarding the typical nicotine consumption.
In total, 93 potential subjects were screened, out of which 66 subjects (11 per each group) were recruited as non-users, or the exclusive experienced users of one specific product category, as defined in the study plan and based on the inclusion list. In case that all the 11 recruited subjects fulfilled the set criteria, one of them (which obtained the highest randomized number within the group) was automatically designated as a replacement subject. After confirming through the cotinine and CO-breath test that no other nicotine or tobacco product was used in parallel, the enrolled subjects were entitled to the sole use of one type of product during the controlled clinical confinement.
2.4. Inclusion criteria
Only the subjects meeting all of the inclusion criteria (both general and additional) were included in the clinical trial. The general inclusion criteria included males and females between 19 and 65 years of age, physically and mentally healthy, without a legal guardian.
For each use group, additional inclusion criteria were defined. Cigarette smokers must have had consumed at least 10 cigarettes per day at least 6 months prior to study inclusion. HTP users must have had consumed at least 10 ‘sticks’ per day at least 3 months prior to study inclusion. EC users, must have had taken at least 100 puffs per day of a nicotine-containing EC at least 6 months prior to study inclusion. OT users must have had regularly consumed oral tobacco (min. 1.5 g in prepacked portions (pouches) or 4 g as loose oral tobacco) at least 3 months prior to study inclusion.
NRT users must have had consumed a pre-approved quantity of one of the following NRT products: minimum 4 nicotine gums; minimum 3 nicotine patches; minimum 10 strokes of nicotine spray; minimum 8 nicotine lozenges per day for at least 30 days prior to study inclusion.
Non-users were subjects who had never smoked or who reported having smoked less than 99 cigarettes in their lifetime.
2.5. Exclusion criteria
Subjects were excluded from the enrolment if any of the following criteria were met:
-
•
Dual/multiple use of any other nicotine-containing product 4 weeks prior to the study;
-
•
BMI: < 18 and >33 kg/m2;
-
•
Pregnant and/or lactating women;
-
•
Drug abusers;
-
•
Chronic respiratory or cardiovascular disease like asthma, chronic obstructive pulmonary disease, chronic bronchitis, hypertension (self-reported or diagnosed);
-
•
Regular use of medication, excluding hormonal contraceptives and non-prescription pain medication, prior to study inclusion within the last three months or is intended to do so during the study conduct (definition of regular use of medication was based on individual physician discretion).
For conventional cigarette smokers, use of any other nicotine containing product, including the self-rolled cigarettes, was not permitted. The users from one of the other four user groups (HTP, Vapers, OT, NRT) must not had smoked a conventional cigarette in the last 4 weeks prior to study inclusion.
2.6. Study products
All study subjects were supplied with the usual amount of their commercially available products of choice, calculated for their use during the four-day confinement at the study site.
At any moment within the non-restriction periods, as defined in the Study Conduct, nicotine products were distributed by the study team upon request, retaining the consumption of nicotine products in an uncontrolled but recordable manner. Every nicotine product used, i.e. the number or amount consumed, as well as the rest of the product after consumption, was documented in the subject's diary and confirmed by an accompanying study nurse in the product-specific Consumption Protocol.
The compliance was ensured by means of the in-clinic setting throughout the entire study period. The clinical site has capabilities to separate different groups during their visits. Strict spatial separation was also ensured with respect to product use. This was of essence to avoid second-hand exposures, especially from CC smoke, but also from ECs and HTP aerosols. HTP consumption was strictly divided from other subjects at the site, in a well-ventilated separate room. CC and EC products were used outside of the clinic, in dedicated outdoor areas for this purpose.
2.7. Sample collections
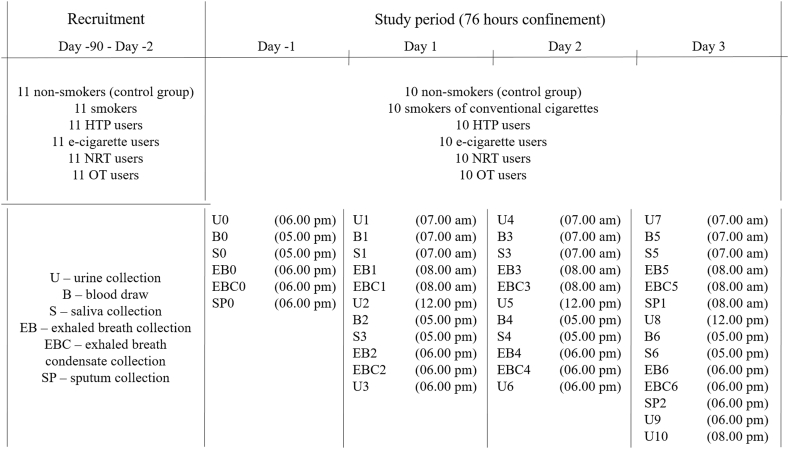
Urine (U), blood (B), saliva (S), exhaled breath (EB), exhaled breath condensate (EBC) and sputum (SP) were collected for the determination of biomarkers. The samples were collected in regular intervals from every participating subject, at the time points as indicated in Table 2, i.e. U-samples within the indicated time periods. In case of urine all voids were collected during the participants’ stay in the clinic. The voids were collected in several fractions defined by time of collection. Collected blood was further processed to receive plasma as well as washed erythrocytes.
Table 2.
Time schedule for the sample collections of the specimens.
| Time | Urine collection | Blood draw | Saliva collection | EB collection | EBC collection | Sputum collection |
|---|---|---|---|---|---|---|
| Day −1 | ||||||
| 04.00 p.m. | – | – | – | – | – | – |
| 05.00 p.m. | – | B0† | S0‡ | – | – | – |
| 06.00 p.m. | U0 * | – | – | EB0§ | EBC0¶ | SP0# |
| 07.00 p.m. | Dinner | |||||
| 08.00 p.m. | – | – | – | – | – | – |
| Day 1/Day 2/Day 3 | ||||||
| 07.00 a.m. | U1/U4/U7 | B1/B3/B5 | S1/S3/S5 | – | – | – |
| 08.00 a.m. | – | – | – | EB1/EB3/EB5 | EBC1/EBC3/EBC5 | SP1 (Day 3) |
| 09.00 a.m. | Breakfast | |||||
| 10.00 a.m. | – | – | – | – | – | – |
| 11.00 a.m. | – | – | – | – | – | – |
| 12.00 p.m. | U2/U5/U8 | – | – | – | – | – |
| 01.00 p.m. | Lunch | |||||
| 02.00 p.m. | – | – | – | – | – | – |
| 03.00 p.m. | – | – | – | – | – | – |
| 04.00 p.m. | – | – | – | – | – | – |
| 05.00 p.m. | – | B2/B4/B6 | S2/S4/S6 | – | – | – |
| 06.00 p.m. | U3/U6/U9 | – | – | EB2/EB4/EB6 | EBC2/EBC4/EBC6 | SP2 (Day 3) |
| 07.00 p.m. | Dinner | |||||
| 08.00 p.m. | U10 (Day 3) | – | – | – | – | – |
(*U – urine; †B – blood; ‡S – saliva; §EB – exhaled breath; ¶EBC – exhaled breath condensate; #SP – sputum).
For the breath samples' collection purposes, appropriate collection devices for the application within a clinical setting for EB and EBC were identified. Different thermodesorption (TD) systems were evaluated for trapping and analyzing EB, since TD has several advantages compared to other EB collection systems like Tedlar bags. Only low collected volumes are needed for sensitive EB determination using TD tubes due to the sample enrichment in the tube by adsorption to the sorbent material. This allows fast and robust sampling of EB in a clinical setting or large scale human biomonitoring. After comparing different TD collection systems, the BioVOC™ breath sampler from Markes (UK) was identified as the most suited set up. Its applicability was proven in a pilot experiment investigating EB of smokers and non-smokers by means of TD-GC-TOF-MS. Significant differences were observed, indicating the potential to identify biomarkers of exposure on EB also of further use groups included in the clinical study.
For EBC collection, two suitable collection systems for a clinical (large scale) application were identified. The RTube™, a breath condensate collection device developed by Respiratory Research (USA), and the SensAbues® device manufactured by SensAbues AB (Sweden) [45]. Based on the pilot analyses performed on the QExactive HFX Orbitrap LC-MS/(MS) system, with data processed by Compound Discoverer Software (Thermo Fisher Scientific), the SensAbues® was selected as EBC collection device for the clinical study. SensAbues® showed several advantages compared to the RTube™ with respect to the sensitivity, variability, the associated costs and detection rate of EBC components [59,60]. The SensAbues® showed higher efficiency of sample collection, as well as elevated analyte coverage and recovery. Additionally, it provides advantages in terms of ease of use – simple, straightforward and fast handling of the collection device with short sampling time of 5 min.
Due to the easy and non-invasive collection, urine is commonly used to access human exposure to chemical substances, i.e. pollutants and carcinogens [61,62]. It is a matrix of choice for distinguishing groups in tobacco-related biomarker studies [63], due to the presence of tobacco alkaloids, mercapturic acids, tobacco-specific nitrosamines (TSNAs), polycyclic aromatic hydrocarbons (PAHs), aromatic amines and volatile organic compounds (VOCs) [55,[64], [65], [66], [67]].
Blood contains high concentrations of tobacco-derived biomarkers of exposure and potential harm formed as a result of oxidative stress caused by smoking [68,69]. In particular, serum levels of cotinine, the major nicotine metabolite, make it a good biomarker for nicotine uptake and could be used to discriminate different user groups [[70], [71], [72], [73], [74]].
Parallel to its detection in blood, cotinine is also commonly detected in saliva as a result of tobacco smoke exposure [71,[75], [76], [77]]. Being in equilibrium with blood and collected in a non-invasive manner, saliva is an interesting matrix for biomarker research [[78], [79], [80]].
Induced sputum studies are performed to identify biomarkers of lung damage associated with tobacco smoke [[81], [82], [83]]. In this study, sputum samples were collected for exploratory purposes, i.e. as a trial comparison of biomarker profile between non-smokers and other use groups.
2.8. Study objectives
Several study objectives have been set, prior to the performed clinical trial, as follows:
-
•
Identification of suitable biomarkers and biomarker patterns for the evaluation of different nicotine delivery product groups (primary goal);
-
•
Assessment of the biomarkers in regard to their suitability for reliably discriminating between the different product categories (secondary goal);
-
•
Quantification of the identified biomarkers.
The set study goals will be accomplished mainly through the development of novel untargeted screening methods, by means of chromatographic methodology, coupled with high-resolution mass-spectrometric systems. Biological matrices to be analyzed encompass blood, urine, saliva, exhaled breath and exhaled breath condensate.
The quantification of the identified biomarkers will be performed using established instrumental methods. Several targeted methods have already been developed at our lab for the determination of biomarkers of exposure to cigarette smoke and tobacco use [[84], [85], [86], [87]].
The analytical strategy follows a top-down approach by making use of untargeted methods to decipher biomarkers specific for the different nicotine product users [75]. A combination of time-of-flight high-resolution mass spectrometry hyphenated to gas chromatography (GC-TOF-MS) with a TD unit for EB analysis and liquid chromatography coupled with high-resolution mass spectrometry (LC-Orbitrap-MS, QExactive) for EBC and analyses of other collected biological matrices will be applied.
The untargeted analysis of the breathome, encompassing the EB and EBC, may reveal suitable novel biomarkers of exposure specific to a certain nicotine delivery product category like EC or HTP. This approach will certainly promote extraction and detection of unknown metabolites and empower the untargeted analysis with the main goal of identifying new biomarkers of exposure specific for each of the examined study user groups.
2.9. HPLC-MS/MS exposomics
For the analysis of body fluids, encompassing urine, plasma, saliva and EBC, untargeted methods will be developed using high-resolution mass spectrometry (HRMS) hyphenated to liquid chromatography (LC). With the general goal to identify biomarkers and biomarker patterns specific to different nicotine product users, Vanquish UPLC system coupled with the QExactive HFX Orbitrap MS (Thermo Scientific) will be used.
For each matrix in which the untargeted biomarker identification will be performed, sample pretreatment is required to reduce the matrix effect on the analysis. Different sample pretreatment methods will be compared in order to identify the most suitable sample preparation, especially in terms of the signal intensity and numbers of potential hits detected.
Moreover, the effect of different mass spectrometric parameters will be investigated with the purpose of enhancing the sensitivity for a broad range of biomarkers. The chromatographic parameters will be optimized to achieve separation of the possible compounds of interest.
Compound search will be performed by Compound Discoverer (Thermo Fisher Scientific), a mass spectrometry data analysis software for untargeted methods which includes a tool for compound identification. After processing the data acquired by the QExactive HFX Orbitrap LC-MS/(MS) system, the dataset will be evaluated for compound identification based on the hits from the online platforms (e.g. ChemSpider, mzCloud, etc.), according to the optimized untargeted workflow parameters [88].
This kind of general untargeted approach is expected to allow reliable exposomics analyses and identification of specific biomarkers in the investigated biospecimens (e.g. urine, plasma, EBC, saliva).
2.10. TD-GC-TOF-MS breathomics
For EB exposomics analysis, a TD-GC-TOF-MS method will be optimized with regard to sensitivity and coverage of the expected analyte spectrum. The GC and TD method parameters will be adapted using non-smoker breath and EB samples of other experienced users from defined use groups as matrix samples. The method giving the highest compounds' abundance will be accepted to complete the development for untargeted analyses of EB samples.
For identification of compounds, the raw data obtained from the GC-TOF-MS will be processed by applying the workflow similar to an untargeted metabolomics analysis as established at ABF for various body fluids [75,79,84,89,90]. MassHunter Qualitative Analysis B.06.00 and Unknown Analysis B.07.01 (Agilent Technologies) will be used for data analysis and evaluation, based on the compound search and identification from the database of NIST 17 EI mass spectral library combined with Wiley Registry™ of mass spectral data, 11th edition.
2.11. GC-MS/MS adductomics
Various toxicants such as carbonyls or epoxides are metabolized to reactive intermediates which can form adducts with different proteins in vivo, e.g., hemoglobin (Hb) or human serum albumin (HSA). There are different nucleophilic sites of the proteins which bind these electrophiles. In HSA, Cys31 is the predominant site for adduct formation [91,92] while several toxicants are reported to add to the N-terminal valine of Hb [[93], [94], [95]]. Adducts with the N-terminal valine of erythrocyte globin can serve as individual biomarkers of systemic and cellular response to alkylating agents [96]. In order to investigate such adducts, washed erythrocytes were collected in our study. We aim to develop suitable sample purification strategies for the different adducts and subsequent analysis by GC-MS/MS in our untargeted approach.
2.12. Data analysis
Data analysis will be carried out with the obtained quantitative and semi-quantitative (untargeted) data obtained after the performed analyses on the samples, i.e. sample pooled groups, in accordance with the instrumental methodology used.
The data to be obtained from the LC-Orbitrap MS analyses, will be processed with the primary focus on the comparability of the abundance and number of detected compounds. For TD-GC-TOF-MS exposomics, data analysis will allow for automated peak detection after deconvolution in combination with a library search. For each quantitative analysis set, a corresponding software will be used to process the raw data and evaluate the final data obtained.
Statistical software tools and compound identification software will serve to compare the untargeted data between the examined user groups. Finally, a cross-comparison between the targeted and untargeted methodologies will be performed. Accordingly, correlations will be constructed between the user groups, to confirm the analogies, i.e. distinctions between groups' biomarker profiles. The most significant differences are expected to be obtained from comparing the non-smoker group with each of the user groups. Additionally, the smoker group is likely to show largest differences when compared to the data of other user groups. Significance of inter-group differences and correlations between specific biomarkers and biomarker profiles will be tested and evaluated appropriately.
Urine biomarkers will be expressed as amount of biomarkers excreted over 24-h periods. From the other matrices, all collected samples will be analyzed and evaluated as the individual collection time points.
For each collected matrix, the data of concentrations and profiles of detected biomarkers will be used for multiple comparisons between different study use groups within the time points, as well as comparisons between the collection time points.
Prior to the statistical evaluation of the obtained data, a representative data set will be investigated for normal distribution [75]. Assuming a normal distribution, parametric tests will be used for data assessment [97]. In case a non-normal distribution is observed, a non-parametric test (e.g. Mann-Whitney U Test) will be chosen accordingly for statistical evaluation [98,99].
3. Results and discussion
3.1. Expected results
The enrolment into this study started in September 2019 and the trial at the clinical site was finished in May 2020. With all collected biospecimens delivered and stored at our lab, sample management will follow, with a goal to perform all the scheduled quantitative and qualitative analyses in a timely fashion. Samples will be randomized for all MS analyses in order to overcome bias due to batch effects. For the same purpose, suitable quality control samples will be included in each batch. Accordingly, first results of this study with respect to the untargeted analyses are expected to be fully evaluated and presented in 2021.
The results of the untargeted analyses will be further substantiated by the quantification of a large set of biomarkers of exposure (BoE) in different matrices. These data should provide sufficient information to identify biomarker patterns which are able to differentiate the different nicotine product use groups.
3.2. Discussion
According to a review of the literature in preparation [100], average daily intake and uptake of nicotine and toxicants varies largely, depending on the user group. According to the review, 38 biomarkers, divided into 3 groups, were identified as the main intake and uptake chemicals of the user groups investigated hereby. As expected, conventional smokers were clearly differentiated from the other five groups. In addition, single biomarkers have also stood out as specific for vapers. The literature data analysis performed, allowed almost unequivocal identification of the product of use, based solely on the levels of 2–5 specific biomarkers. This approach is going to be used as a major guidance in biomarker pattern identification and distinction between specific product user groups.
However, in previously reported studies [12,101,102], the data is usually based on the self-reported surveys on single or dual use of nicotine products, which is often prone to bias, not being able to fully verify use compliance. Due to the ambulatory setup, such studies usually include a large number of participants, in effort to provide measurable evidence and statistical significance [12,101]. Control measures for second-hand exposure, as opposed to our study, are not monitored and can lead to false interpretation of the data [102]. Questionnaire contextual elements also mainly address the nicotine dependence and desire to quit or change product of use [101,102], while in our study the important factors in differential exposure are retained by strict consumption control. In addition, lack of clear dose-response relationship between biomarkers and reported exposure, e.g., no decrease of the biomarker concentration upon cessation, is a huge limitation in data evaluation [74,103]. To avoid this, in this trial, a strict consumption control was ensured during the separate confinement of groups at the study site.
Since differentiation between the groups based on the biomarker profiles can rely on very sensitive balance, i.e., the levels upon intake and uptake may vary due to various factors such as use behavior or food intake, a strong compliance including the documentation of the consumption is one of the prerequisites for the aim of our study. This is ensured through the controlled in-clinic confinement at the study site during the entire four-day study period.
Strict spatial separation to avoid possible cross-contaminations was provided and secondhand exposure between subjects of one group was minimized through use of a dedicated space for product consumption during the study. In order to reduce the bias between subjects due to different diet, all subjects were served standardized meals – identical in terms of quality, but based on their individual BMI indices in terms of quantity. During the confinement period subjects were free to drink water at any time, but alcohol or any other beverages were not permitted.
In this trial, urine-spot collection was performed within the defined time periods. Combining the collection points, urine pools will be created, which are considered to best reflect the biomarker profile during the controlled study confinement. Documentation of each urine sample amount collected allows pools to be created accordingly, taking the share of each aliquot into account. Alongside the established quantitation methods, LC-MS/MS exposomics will be carried out, after corresponding optimized sample preparation. It is expected to achieve certain equivalence between the biomarker profile from untargeted analysis and the abundant biomarkers which are to be quantified.
During this trial, a unique breath sampling was performed, with the purpose of collecting exhaled breath and exhaled breath condensate samples for the reliable exposomics analyses. The rapid, non-invasive collection, practically unlimited supply, and the possibility of real-time detection makes breath a very interesting candidate for the development of a rapid analysis system, once suitable biomarkers have been identified.
EBC has, so far, mainly been used as a matrix of biomarkers for lung disease [48]. In our approach, however, EBC is considered a matrix in which biomarkers can be identified, as an equivalent to other body fluids analyzed hereby.
EB is a matrix comprising breath of subjects directly adsorbed by the BIO-VOC device. In the pilot experimental setup, a total of 22 compounds were identified by GC-MS after sampling with TD-unit. Ten of the compounds were identified as significantly more abundant in smokers when compared to non-smokers, thus showing great potential to serve as a further biomarker-differentiating tool.
In addition, by analyzing other biospecimens and using all the available omics tools, it is expected to identify new biomarkers or biomarker patterns which could be designated as unique for single user groups. This would improve general distinction between the tested nicotine/tobacco user groups, with a special emphasis given to those use groups showing only little differences according to the review by Scherer et al. [100].
3.3. Conclusions
Considering the clear study compliance ensured in this trial, it is to be expected that, using various analytical techniques, a specific differentiation between distinct product use groups should be achieved. Particular matrices of interest that could specifically contribute to this are EB and EBC, which have not been used in nicotine and tobacco-product exposure studies so far. Moreover, based on the review by Scherer et al. [100], data provided from the collected urine samples are expected to provide most information when compared to the published data on the nicotine/tobacco user groups' biomarkers reported so far.
The main purpose of this work is to serve as a foundation for differentiating nicotine product user groups based on the biomarker profiles. Furthermore, data which will be generated through this study may have an outcome in developing diagnostic models for evaluation of chemically-derived health risks from individual nicotine-delivery products in further human studies. Finally, the identified biomarker patterns will be useful to establish robust compliance markers for long-term studies, for instance in product switching studies, in a free setting.
4. Compliance with ethical standards
4.1. Study limitations
All included participants were German citizens, recruited in one site in Hamburg, thus not reflecting the general population with respect to the different product use groups.
Due to the limited length of the study, no conclusions can be drawn on long-term changes of biomarker profiles in the nicotine product users.
Age, gender, and race of the included subjects were not considered in the evaluation of the biomarker profiles due to the small sample size in this study.
4.2. Risks and side effects
This clinical study is planned exclusively for research purposes. The subjects consumed the respective products in amounts equivalent to their normal daily consumption. Thus, the study did not pose any additional risks to the participants as they are exposed to the same risk while following their normal lifestyles.
Smoking causes many diseases, such as cancer, pulmonary and cardiovascular diseases and reduces overall general health. Quitting smoking reduces the risk of developing smoking-related disease and offers immediate and long-term benefits. The participants were encouraged explicitly to retain their sovereign attitude towards their products and consumption. The subjects were allowed to quit their habit (e.g. smoking or vaping) at any time during study conduct.
The planned actions in the study (in-patient stay for 76 h, blood draw, urine collection, saliva collection, sputum collection, exhaled breath collection and exhaled breath condensate collection) were performed by experienced, qualified medical staff at CTC North and did not represent a physical burden to health of the subjects.
A physician had explained to the subjects the nature, significance and implications, as well as possible risks and side effects, prior to the clinical examinations. Subjects were free to withdraw from the clinical study at any time, without providing any reason for doing so. All participants had signed an informed consent form.
Ownership of data and use of the study results
The authors are current employees of ABF GmbH, a certified bioanalytical contract-research laboratory with a sponsor role. All goods, materials, information (oral or written) and unpublished documentation provided to the investigators (or any company acting on their behalf), inclusive of this study, and the subjects’ case report forms are the exclusive property of the sponsor. ABF has the ownership of all data and results collected in this study.
Trial registration
German Clinical Trials Register (drks.de) ID: DRKS00022428.
Funding
This study was funded with a grant from the Foundation for a Smoke-Free World, a US nonprofit 501(c)(3) private foundation with a mission to end smoking in this generation. The Foundation accepts charitable gifts from PMI Global Services Inc. (PMI); under the Foundation’s Bylaws and Pledge Agreement with PMI, the Foundation is independent from PMI and the tobacco industry. The contents, selection, and presentation of facts, as well as any opinions expressed herein are the sole responsibility of the authors and under no circumstances shall be regarded as reflecting the positions of the Foundation for a Smoke-Free World, Inc.
Statement of human rights
All described procedures performed in the study involving human participants were in accordance with the ethical standards of the Ethics Commission of Hamburg Medical Association and the Good Clinical Practice (GCP). This article does not contain any studies with animals performed by any of the authors.
This clinical study was planned and performed in accordance with.
-
-
The Declaration of Helsinki in its version of Fortaleza, 2013;
-
-
§ 15 der Berufsordnung der Ärzte.
On behalf of the responsible investigator, the CTC North had submitted, among other documents, the study protocol, subject information and the informed consent form to the ‘Ethik-Kommission der Ärztekammer Hamburg’ and requested approval. The approval (favorable opinion) of the Ethics Committee (reference number: PV7084) was obtained prior to the clinical study start, on September 10, 2019.
CRediT authorship contribution statement
Filip Sibul: Project administration, Writing – original draft. Therese Burkhardt: Project administration, Writing – original draft. Alpeshkumar Kachhadia: Investigation, Writing – original draft. Fabian Pilz: Investigation, Writing – original draft. Gerhard Scherer: Conceptualization, Writing – review & editing. Max Scherer: Conceptualization, Supervision, Funding acquisition, Writing – review & editing. Nikola Pluym: Conceptualization, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare no conflict of interest.
References
- 1.Jha P., Ramasundarahettige C., Landsman V., Rostron B., Thun M., Anderson R.N., McAfee T., Peto R. 21st-century hazards of smoking and benefits of cessation in the United States. N. Engl. J. Med. 2013;368(4):341–350. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 2.Us Department of Health and Human Services The health consequences of smoking—50 years of progress: a report of the Surgeon General. 2014. https://www.cdc.gov/tobacco/data_statistics/sgr/50th-anniversary/index.htm
- 3.Us Department of Health and Human Services How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease- A report of the surgeon general, 2010. https://www.ncbi.nlm.nih.gov/books/NBK53017/ [PubMed]
- 4.Hajek P., Phillips-Waller A., Przulj D., Pesola F., Myers Smith K., Bisal N., Li J., Parrott S., Sasieni P., Dawkins L., Ross L., Goniewicz M., Wu Q., McRobbie H.J. A Randomized Trial of E-Cigarettes versus Nicotine-Replacement Therapy. 2019;380(7):629–637. doi: 10.1056/NEJMoa1808779. [DOI] [PubMed] [Google Scholar]
- 5.Halpern S.D., Harhay M.O., Saulsgiver K., Brophy C., Troxel A.B., Volpp K.G. A pragmatic trial of E-cigarettes, incentives. and Drugs for Smoking Cessation. 2018;378(24):2302–2310. doi: 10.1056/NEJMsa1715757. [DOI] [PubMed] [Google Scholar]
- 6.Hartmann-Boyce J., McRobbie H., Bullen C., Begh R., Stead L.F., Hajek P. Electronic cigarettes for smoking cessation. Cochrane Database Syst. Rev. 2016;9 doi: 10.1002/14651858.CD010216.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee S.-H., Ahn S.-H., Cheong Y.-S. Effect of electronic cigarettes on smoking reduction and cessation in Korean male smokers. A Randomized Controlled Study. 2019;32(4):567–574. doi: 10.3122/jabfm.2019.04.180384. [DOI] [PubMed] [Google Scholar]
- 8.Riahi F., Rajkumar S., Yach D. Tobacco smoking and nicotine delivery alternatives: patterns of product use and perceptions in 13 countries. F1000Research. 2019;8:80. doi: 10.12688/f1000research.17635.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shields P.G. Tobacco smoking, harm reduction, and biomarkers, JNCI. J. Natl. Cancer Inst. 2002;94(19):1435–1444. doi: 10.1093/jnci/94.19.1435. [DOI] [PubMed] [Google Scholar]
- 10.Stoklosa M., Cahn Z., Liber A., Nargis N., Drope J. Effect of IQOS introduction on cigarette sales: evidence of decline and replacement. Tobac. Contr. 2020;29:381–387. doi: 10.1136/tobaccocontrol-2019-054998. [DOI] [PubMed] [Google Scholar]
- 11.Walker N., Parag V., Verbiest M., Laking G., Laugesen M., Bullen C. Nicotine patches used in combination with e-cigarettes (with and without nicotine) for smoking cessation: a pragmatic, randomised trial. The Lancet Respiratory Medicine. 2020;8(1):54–64. doi: 10.1016/S2213-2600(19)30269-3. [DOI] [PubMed] [Google Scholar]
- 12.Caponnetto P., Caruso M., Maglia M., Emma R., Saitta D., Busa B., Polosa R., Prosperini U., Pennisi A., Benfatto F., Sartorio C., Guastella M., Mondati E. Non-inferiority trial comparing cigarette consumption, adoption rates, acceptability, tolerability, and tobacco harm reduction potential in smokers switching to Heated Tobacco Products or electronic cigarettes: study protocol for a randomized controlled trial. Contemporary clinical trials communications. 2020;17:100518. doi: 10.1016/j.conctc.2020.100518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song M.-A., Freudenheim J.L., Brasky T.M., Mathe E.A., McElroy J.P., Nickerson Q.A., Reisinger S.A., Smiraglia D.J., Weng D.Y., Ying K.L., Wewers M.D., Shields P.G. Biomarkers of exposure and effect in the lungs of smokers, nonsmokers. and Electronic Cigarette Users. 2020;29(2):443–451. doi: 10.1158/1055-9965.EPI-19-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goniewicz M.L., Smith D.M., Edwards K.C., Blount B.C., Caldwell K.L., Feng J., Wang L., Christensen C., Ambrose B., Borek N., van Bemmel D., Konkel K., Erives G., Stanton C.A., Lambert E., Kimmel H.L., Hatsukami D., Hecht S.S., Niaura R.S., Travers M., Lawrence C., Hyland A.J. Comparison of nicotine and toxicant exposure in users of electronic cigarettes and combustible cigarettes. JAMA network open. 2018;1(8) doi: 10.1001/jamanetworkopen.2018.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatsukami D., Meier E., Lindgren B.R., Anderson A., Reisinger S., Norton K., Strayer L., Jensen J., Dick L., Murphy S., Carmella S., Tang M.K., Chen M., Hecht S.S., O'Connor R.J., Shields P.G. A randomized clinical trial examining the effects of instructions for electronic cigarette use on smoking-related behaviors, and biomarkers of exposure, nicotine & tobacco research. official journal of the Society for Research on Nicotine and Tobacco. 2020;22(9):1524–1532. doi: 10.1093/ntr/ntz233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacob P., St Helen G., Yu L., Nardone N., Havel C., Cheung P., Benowitz N.L. Biomarkers of exposure for dual use of electronic cigarettes and combustible cigarettes: nicotelline, NNAL, and total nicotine equivalents, nicotine & tobacco research. official journal of the Society for Research on Nicotine and Tobacco. 2020;22(7):1107–1113. doi: 10.1093/ntr/ntz235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landmesser A., Scherer M., Pluym N., Sarkar M., Edmiston J., Niessner R., Scherer G. Biomarkers of exposure specific to E-vapor products based on stable-isotope labeled ingredients. Nicotine Tob. Res. : official journal of the Society for Research on Nicotine and Tobacco. 2019;21(3):314–322. doi: 10.1093/ntr/nty204. [DOI] [PubMed] [Google Scholar]
- 18.Flora J., Digard H., Sinclair C., Belushkin M. 2020. Heated Tobacco Products Task Force Technical Report Heated Tobacco Products (HTPs): Standardized Terminology and Recommendations for the Generation and Collection of Emissions. [Google Scholar]
- 19.Haziza C., de La Bourdonnaye G., Donelli A., Skiada D., Poux V., Weitkunat R., Baker G., Picavet P., Ludicke F. Favorable changes in biomarkers of potential harm to reduce the adverse health effects of smoking in smokers switching to the menthol tobacco heating system 2.2 for three months (Part 2), nicotine & tobacco research. official journal of the Society for Research on Nicotine and Tobacco. 2020;22(4):549–559. doi: 10.1093/ntr/ntz084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haziza C., de La Bourdonnaye G., Skiada D., Ancerewicz J., Baker G., Picavet P., Lüdicke F. Evaluation of the Tobacco Heating System 2.2. Part 8: 5-Day randomized reduced exposure clinical study in Poland, Regul. Toxicol. Pharmacol. 2016;81:S139–S150. doi: 10.1016/j.yrtph.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Lüdicke F., Ansari S.M., Lama N., Blanc N., Bosilkovska M., Donelli A., Picavet P., Baker G., Haziza C., Peitsch M., Weitkunat R. Effects of switching to a heat-not-burn tobacco product on biologically relevant biomarkers to assess a candidate modified risk tobacco product. A Randomized Trial. 2019;28(11):1934–1943. doi: 10.1158/1055-9965.EPI-18-0915. [DOI] [PubMed] [Google Scholar]
- 22.Pieper E., Mallock N., Henkler Stephani F., Luch A. Tabakerhitzer als neues produkt der Tabakindustrie: gesundheitliche risiken. Bundesgesundheitsblatt - Gesundheitsforsch. - Gesundheitsschutz. 2018;61(11):1422–1428. doi: 10.1007/s00103-018-2823-y. [DOI] [PubMed] [Google Scholar]
- 23.Simonavicius E., McNeill A., Shahab L., Brose L.S. Heat-not-burn tobacco products: a systematic literature review. Tobac. Contr. 2019;28(5):582–594. doi: 10.1136/tobaccocontrol-2018-054419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.St Helen G., Jacob P., Iii, Nardone N., Benowitz N.L. IQOS: examination of Philip Morris International's claim of reduced exposure. Tobac. Contr. 2018;27:s30–s36. doi: 10.1136/tobaccocontrol-2018-054321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leon M.E., Lugo A., Boffetta P., Gilmore A., Ross H., Schuz J., La Vecchia C., Gallus S. Smokeless tobacco use in Sweden and other 17 European countries. Eur. J. Publ. Health. 2016;26(5):817–821. doi: 10.1093/eurpub/ckw032. [DOI] [PubMed] [Google Scholar]
- 26.Furberg H., Lichtenstein P., Pedersen N.L., Bulik C., Sullivan P.F. Cigarettes and oral snuff use in Sweden: prevalence and transitions. Addiction. 2006;101(10):1509–1515. doi: 10.1111/j.1360-0443.2006.01550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lund K.E., McNeill A., Scheffels J. The use of snus for quitting smoking compared with medicinal products, Nicotine. Tob. Res. 2010;12(8):817–822. doi: 10.1093/ntr/ntq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borgerding M.F., Bodnar J.A., Curtin G.M., Swauger J.E. The chemical composition of smokeless tobacco: a survey of products sold in the United States in 2006 and 2007. Regul. Toxicol. Pharmacol. 2012;64(3):367–387. doi: 10.1016/j.yrtph.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Klus H., Kunze M., Koenig S., Pöschl E. Smokeless tobacco - an overview. Beitr. Tabakforsch. Int. 2009;23(5):248–276. [Google Scholar]
- 30.Rickert W.S., Joza P.J., Trivedi A.H., Momin R.A., Wagstaff W.G., Lauterbach J.H. Chemical and toxicological characterization of commercial smokeless tobacco products available on the Canadian market. Regul. Toxicol. Pharmacol. 2009;53(2):121–133. doi: 10.1016/j.yrtph.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Song M.-A., Marian C., Brasky T.M., Reisinger S., Djordjevic M., Shields P.G. Chemical and toxicological characteristics of conventional and low-TSNA moist snuff tobacco products. Toxicol. Lett. 2016;245:68–77. doi: 10.1016/j.toxlet.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hartmann-Boyce J., Chepkin S.C., Ye W., Bullen C., Lancaster T. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst. Rev. 2018;5(5) doi: 10.1002/14651858.CD000146.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stead L.F., Perera R., Bullen C., Mant D., Hartmann-Boyce J., Cahill K., Lancaster T. Nicotine replacement therapy for smoking cessation, Cochrane. Database.Syst.Rev. 2012;11:CD000146. doi: 10.1002/14651858.CD000146.pub4. [DOI] [PubMed] [Google Scholar]
- 34.Wadgave U., Nagesh L. Nicotine replacement therapy: an overview. Int. J. Health Sci. 2016;10(3):425–435. [PMC free article] [PubMed] [Google Scholar]
- 35.Jacob P., III, Hatsukami D., Severson H., Hall S., Yu L., Benowitz N.L. Anabasine and anatabine as biomarkers for tobacco use during nicotine replacement therapy, Cancer Epidemiology. Biomarkers & Prevention. 2002;11(12):1668–1673. [PubMed] [Google Scholar]
- 36.Round E.K., Chen P., Taylor A.K., Schmidt E. Biomarkers of tobacco exposure decrease after smokers switch to an E-cigarette or nicotine gum, nicotine & tobacco research. official journal of the Society for Research on Nicotine and Tobacco. 2019;21(9):1239–1247. doi: 10.1093/ntr/nty140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Food and Drug Administration (Fda) 2016. Scientific Review of Modified Risk Tobacco Product Application (MRTPA) under Section 911(d) of the FD&C Act. [Google Scholar]
- 38.Kwak J., Fan M., Harshman S.W., Garrison C.E., Dershem V.L., Phillips J.B., Grigsby C.C., Ott D.K. Evaluation of bio-VOC sampler for analysis of volatile organic compounds in exhaled breath. Metabolites. 2014;4(4):879–888. doi: 10.3390/metabo4040879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corradi M., Mutti A. Exhaled breath analysis: from occupational to respiratory medicine. Acta Biomed. : Atenei Parmensis. 2005;76(Suppl 2):20–29. Suppl 2. [PMC free article] [PubMed] [Google Scholar]
- 40.Neerincx A.H., Vijverberg S.J.H., Bos L.D.J., Brinkman P., van der Schee M.P., de Vries R., Sterk P.J., Maitland-van der Zee A.H. Breathomics from exhaled volatile organic compounds in pediatric asthma. Pediatr. Pulmonol. 2017;52(12):1616–1627. doi: 10.1002/ppul.23785. [DOI] [PubMed] [Google Scholar]
- 41.van der Schee M.P., Paff T., Brinkman P., van Aalderen W.M.C., Haarman E.G., Sterk P.J. Breathomics in lung disease. Chest. 2015;147(1):224–231. doi: 10.1378/chest.14-0781. [DOI] [PubMed] [Google Scholar]
- 42.Capone S., Tufariello M., Forleo A., Longo V., Giampetruzzi L., Radogna A.V., Casino F., Siciliano P. Chromatographic analysis of VOC patterns in exhaled breath from smokers and nonsmokers. Biomed. Chromatogr. : BMC (Biomed. Chromatogr.) 2018;32(4) doi: 10.1002/bmc.4132. [DOI] [PubMed] [Google Scholar]
- 43.Filipiak W., Ruzsanyi V., Mochalski P., Filipiak A., Bajtarevic A., Ager C., Denz H., Hilbe W., Jamnig H., Hackl M., Dzien A., Amann A. Dependence of exhaled breath composition on exogenous factors, smoking habits and exposure to air pollutants. J. Breath Res. 2012;6(3) doi: 10.1088/1752-7155/6/3/036008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jareno-Esteban J.J., Munoz-Lucas M.A., Carrillo-Aranda B., Maldonado-Sanz J.A., de Granda-Orive I., Aguilar-Ros A., Civera-Tejuca C., Gutierrez-Ortega C., Callol-Sanchez L.M. Volatile organic compounds in exhaled breath in a healthy population: effect of tobacco smoking. Arch. Bronconeumol. 2013;49(11):457–461. doi: 10.1016/j.arbres.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Mochalski P., King J., Klieber M., Unterkofler K., Hinterhuber H., Baumann M., Amann A. Blood and breath levels of selected volatile organic compounds in healthy volunteers. Analyst. 2013;138(7):2134–2145. doi: 10.1039/c3an36756h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castellanos M., Suñer R., Fernández-Real J.M., Sanchez J.M. 2,5-Dimethylfuran as a validated biomarker of smoking status, nicotine & tobacco research. official journal of the Society for Research on Nicotine and Tobacco. 2019;21(6):828–834. doi: 10.1093/ntr/nty078. [DOI] [PubMed] [Google Scholar]
- 47.Grob N.M., Aytekin M., Dweik R.A. Biomarkers in exhaled breath condensate: a review of collection, processing and analysis. J. Breath Res. 2008;2(3) doi: 10.1088/1752-7155/2/3/037004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hunt J. Exhaled breath condensate: an overview. Immunol. Allergy Clin. 2007;27(4):587–596. doi: 10.1016/j.iac.2007.09.001. (v) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dodig S., Cepelak I. Exhaled breath condensate--from an analytical point of view. Biochem. Med. 2013;23(3):281–295. doi: 10.11613/BM.2013.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brouwers J.F. Liquid chromatographic-mass spectrometric analysis of phospholipids. Chromatography, ionization and quantification. Biochim. Biophys. Acta. 2011;1811(11):763–775. doi: 10.1016/j.bbalip.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 51.Ecker J. Profiling eicosanoids and phospholipids using LC-MS/MS: principles and recent applications. J. Separ. Sci. 2012;35(10–11):1227–1235. doi: 10.1002/jssc.201200056. [DOI] [PubMed] [Google Scholar]
- 52.Forster M., Fiebelkorn S., Yurteri C., Mariner D., Liu C., Wright C., McAdam K., Murphy J., Proctor C. Assessment of novel tobacco heating product THP1.0. Part 3: comprehensive chemical characterisation of harmful and potentially harmful aerosol emissions. Regul. Toxicol. Pharmacol. 2018;93:14–33. doi: 10.1016/j.yrtph.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 53.Schaller J.-P., Keller D., Poget L., Pratte P., Kaelin E., McHugh D., Cudazzo G., Smart D., Tricker A.R., Gautier L., Yerly M., Reis Pires R., Le Bouhellec S., Ghosh D., Hofer I., Garcia E., Vanscheeuwijck P., Maeder S. Evaluation of the Tobacco Heating System 2.2. Part 2: chemical composition, genotoxicity, cytotoxicity, and physical properties of the aerosol. Regul. Toxicol. Pharmacol. 2016;81:S27–S47. doi: 10.1016/j.yrtph.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 54.Chang C.M., Edwards S.H., Arab A., Del Valle-Pinero A.Y., Yang L., Hatsukami D.K. Biomarkers of tobacco exposure: summary of an FDA-sponsored public workshop, cancer epidemiology, biomarkers & prevention : a publication of the American association for cancer research. cosponsored by the American Society of Preventive Oncology. 2017;26(3):291–302. doi: 10.1158/1055-9965.EPI-16-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gregg E.O., Minet E., McEwan M. Urinary biomarkers of smokers' exposure to tobacco smoke constituents in tobacco products assessment: a fit for purpose approach, Biomarkers : biochemical indicators of exposure, response. and susceptibility to chemicals. 2013;18(6):467–486. doi: 10.3109/1354750X.2013.821523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jay J., Pfaunmiller E.L., Huang N.J., Cohen G., Graff D.W. Five-day changes in biomarkers of exposure among adult smokers after completely switching from combustible cigarettes to a nicotine-salt pod system. Nicotine Tob. Res. 2019;22(8):1285–1293. doi: 10.1093/ntr/ntz206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peck M.J., Sanders E.B., Scherer G., Ludicke F., Weitkunat R. Review of biomarkers to assess the effects of switching from cigarettes to modified risk tobacco products. Biomarkers. 2018;23(3):213–244. doi: 10.1080/1354750X.2017.1419284. [DOI] [PubMed] [Google Scholar]
- 58.Shepperd C.J., Eldridge A., Camacho O.M., McAdam K., Proctor C.J., Meyer I. Changes in levels of biomarkers of exposure observed in a controlled study of smokers switched from conventional to reduced toxicant prototype cigarettes. Regul. Toxicol. Pharmacol. 2013;13:10. doi: 10.1016/j.yrtph.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 59.Garzinsky A.-M., Walpurgis K., Krug O., Thevis M. Does oral fluid contribute to exhaled breath samples collected by means of an electret membrane? 2019;11(11–12):1764–1770. doi: 10.1002/dta.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thevis M., Geyer H., Tretzel L., Schänzer W. Sports drug testing using complementary matrices: advantages and limitations. J. Pharmaceut. Biomed. Anal. 2016;130:220–230. doi: 10.1016/j.jpba.2016.03.055. [DOI] [PubMed] [Google Scholar]
- 61.Bjurlin M.A., Matulewicz R.S., Roberts T.R., Dearing B.A., Schatz D., Sherman S., Gordon T., Shahawy O.E. Carcinogen biomarkers in the urine of electronic cigarette users and implications for the development of bladder cancer: a systematic review. European urology oncology. 2020;S2588–9311(20) doi: 10.1016/j.euo.2020.02.004. 30029-8. [DOI] [PubMed] [Google Scholar]
- 62.Bocato M.Z., Bianchi Ximenez J.P., Hoffmann C., Barbosa F. An overview of the current progress, challenges, and prospects of human biomonitoring and exposome studies. J. Toxicol. Environ. Health B Crit. Rev. 2019;22(5–6):131–156. doi: 10.1080/10937404.2019.1661588. [DOI] [PubMed] [Google Scholar]
- 63.Habibagahi A., Alderman N., Kubwabo C. A review of the analysis of biomarkers of exposure to tobacco and vaping products. Analytical Methods. 2020 doi: 10.1039/d0ay01467b. [DOI] [PubMed] [Google Scholar]
- 64.Chen M., Carmella S.G., Sipe C., Jensen J., Luo X., Le C.T., Murphy S.E., Benowitz N.L., McClernon F.J., Vandrey R., Allen S.S., Denlinger-Apte R., Cinciripini P.M., Strasser A.A., al'Absi M., Robinson J.D., Donny E.C., Hatsukami D., Hecht S.S. Longitudinal stability in cigarette smokers of urinary biomarkers of exposure to the toxicants acrylonitrile and acrolein. PloS One. 2019;14(1) doi: 10.1371/journal.pone.0210104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Etemadi A., Poustchi H., Chang C.M., Blount B.C., Calafat A.M., Wang L., De Jesus V.R., Pourshams A., Shakeri R., Shiels M.S., Inoue-Choi M., Ambrose B.K., Christensen C.H., Wang B., Murphy G., Ye X., Bhandari D., Feng J., Xia B., Sosnoff C.S., Kamangar F., Brennan P., Boffetta P., Dawsey S.M., Abnet C.C., Malekzadeh R., Freedman N.D. Urinary biomarkers of carcinogenic exposure among cigarette, waterpipe and smokeless tobacco users and never users of tobacco in the golestan cohort study, cancer epidemiology, biomarkers & prevention : a publication of the American association for cancer research. cosponsored by the American Society of Preventive Oncology. 2019;28(2):337–347. doi: 10.1158/1055-9965.EPI-18-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hecht S.S. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002;23(6):907–922. doi: 10.1093/carcin/23.6.907. [DOI] [PubMed] [Google Scholar]
- 67.Scherer G., Urban M., Hagedorn H.W., Feng S., Kinser R., Sarkar M., Liang Q., Roethig H.J. Determination of two mercapturic acids related to crotonaldehyde in human urine: influence of smoking. Hum. Exp. Toxicol. 2007;26(1):37–47. doi: 10.1177/0960327107073829. [DOI] [PubMed] [Google Scholar]
- 68.Liu J., Liang Q., Frost-Pineda K., Muhammad-Kah R., Rimmer L., Roethig H., Mendes P., Sarkar M. Relationship between biomarkers of cigarette smoke exposure and biomarkers of inflammation, oxidative stress. and Platelet Activation in Adult Cigarette Smokers. 2011;20(8):1760–1769. doi: 10.1158/1055-9965.EPI-10-0987. [DOI] [PubMed] [Google Scholar]
- 69.Mahrous M.M., El-Barrany U.M., Ismail M.M.E.-D., Gaballah I.F., Rashed L.A. Blood biomarkers of nicotine-induced toxicity in healthy males. Egypt. J. Food Sci. 2019;9(1):28. [Google Scholar]
- 70.Benowitz N.L., Hukkanen J., Jacob P., 3rd Nicotine chemistry, metabolism, kinetics and biomarkers. Handb. Exp. Pharmacol. 2009;192:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Byrd G.D., Davis R.A., Ogden M.W. A rapid LC-MS-MS method for the determination of nicotine and cotinine in serum and saliva samples from smokers: validation and comparison with a radioimmunoassay method. J. Chromatogr. Sci. 2005;43(3):133–140. doi: 10.1093/chromsci/43.3.133. [DOI] [PubMed] [Google Scholar]
- 72.Lewis S.J., Cherry N.M., Mc L.N.R., Barber P.V., Wilde K., Povey A.C. Cotinine levels and self-reported smoking status in patients attending a bronchoscopy clinic. Biomarkers. 2003;8(3–4):218–228. doi: 10.1080/1354750031000120125. [DOI] [PubMed] [Google Scholar]
- 73.Ogden M.W., Marano K.M., Jones B.A., Morgan W.T., Stiles M.F. Switching from usual brand cigarettes to a tobacco-heating cigarette or snus: Part 2. Biomarkers of exposure. Biomarkers. 2015;20(6–7):391–403. doi: 10.3109/1354750X.2015.1094134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schick S.F., Blount B.C., 3rd P.J., Saliba N.A., Bernert J.T., Hellani A.E., Jatlow P., Pappas R.S., Wang L., Foulds J., Ghosh A., Hecht S.S., Gomez J.C., Martin J.R., Mesaros C., Srivastava S., Helen G.S., Tarran R., Lorkiewicz P.K., Blair I.A., Kimmel H.L., Doerschuk C.M., Benowitz N.L., Bhatnagar A. Biomarkers of exposure to new and emerging tobacco delivery products. 2017;313(3):L425–L452. doi: 10.1152/ajplung.00343.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goettel M., Niessner R., Mueller D., Scherer M., Scherer G., Pluym N. Metabolomic fingerprinting in various body fluids of a diet-controlled clinical smoking cessation study using a validated GC-TOF-MS metabolomics platform. J. Proteome Res. 2017;16(10):3491–3503. doi: 10.1021/acs.jproteome.7b00128. [DOI] [PubMed] [Google Scholar]
- 76.Jarvis M.J., Russell M.A., Benowitz N.L., Feyerabend C. Elimination of cotinine from body fluids: implications for noninvasive measurement of tobacco smoke exposure. Am. J. Publ. Health. 1988;78(6):696–698. doi: 10.2105/ajph.78.6.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scherer G., Richter E. Biomonitoring exposure to environmental tobacco smoke (ETS): a critical reappraisal. Hum. Exp. Toxicol. 1997;16:449–459. doi: 10.1177/096032719701600806. [DOI] [PubMed] [Google Scholar]
- 78.Bessonneau V., Pawliszyn J., Rappaport S.M. The saliva exposome for monitoring of individuals' health trajectories. Environ. Health Perspect. 2017;125(7) doi: 10.1289/EHP1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mueller D.C., Piller M., Niessner R., Scherer M., Scherer G. Untargeted metabolomic profiling in saliva of smokers and non-smokers by a validated GC-TOF-MS method. J. Proteome Res. 2013;13(3):1602–1613. doi: 10.1021/pr401099r. [DOI] [PubMed] [Google Scholar]
- 80.Panuwet P., D'Souza P.E., Phillips E.R., Ryan P.B., Barr D.B. Salivary bioscience and environmental exposure assessment. In: Granger D.A., Taylor M.K., editors. Salivary Bioscience: Foundations of Interdisciplinary Saliva Research and Applications. Springer International Publishing; Cham: 2020. pp. 349–370. [Google Scholar]
- 81.Bessa V., Tseliou E., Bakakos P., Loukides S. Noninvasive evaluation of airway inflammation in asthmatic patients who smoke: implications for application in clinical practice. Ann. Allergy Asthma Immunol. : official publication of the American College of Allergy, Asthma, & Immunology. 2008;101(3):226–232. doi: 10.1016/S1081-1206(10)60485-1. [DOI] [PubMed] [Google Scholar]
- 82.Comandini A., Rogliani P., Nunziata A., Cazzola M., Curradi G., Saltini C. Biomarkers of lung damage associated with tobacco smoke in induced sputum. Respir. Med. 2009;103(11):1592–1613. doi: 10.1016/j.rmed.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 83.Mazur W., Stark H., Sovijarvi A., Myllarniemi M., Kinnula V.L. Comparison of 8-isoprostane and interleukin-8 in induced sputum and exhaled breath condensate from asymptomatic and symptomatic smokers, respiration. international review of thoracic diseases. 2009;78(2):209–216. doi: 10.1159/000206010. [DOI] [PubMed] [Google Scholar]
- 84.Goettel M., Niessner R., Scherer M., Scherer G., Pluym N. Analysis of urinary eicosanoids by LC-MS/MS reveals alterations in the metabolic profile after smoking cessation. Chem. Res. Toxicol. 2018;31(3):176–182. doi: 10.1021/acs.chemrestox.7b00276. [DOI] [PubMed] [Google Scholar]
- 85.Wang Y., Wang C., Lin H., Liu Y., Li Y., Zhao Y., Li P., Liu J. Discovery of the potential biomarkers for discrimination between hedyotis diffusa and hedyotis corymbosa by UPLC-QTOF/MS metabolome analysis. Molecules. 2018;23(7):1525. doi: 10.3390/molecules23071525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pluym N., Gilch G., Scherer G., Scherer M. Analysis of 18 urinary mercapturic acids by two high-throughput multiplex-LC-MS/MS methods. Anal. Bioanal. Chem. 2015;407(18):5463–5476. doi: 10.1007/s00216-015-8719-x. [DOI] [PubMed] [Google Scholar]
- 87.Piller M., Gilch G., Scherer G., Scherer M. Simple, fast and sensitive LC-MS/MS analysis for the simultaneous quantification of nicotine and 10 of its major metabolites. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2014;951–952:7–15. doi: 10.1016/j.jchromb.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 88.Ribbenstedt A., Ziarrusta H., Benskin J.P. Development, characterization and comparisons of targeted and non-targeted metabolomics methods. PloS One. 2018;13(11) doi: 10.1371/journal.pone.0207082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goettel M., Niessner R., Pluym N., Scherer G., Scherer M. A fully validated GC-TOF-MS method for the quantification of fatty acids revealed alterations in the metabolic profile of fatty acids after smoking cessation. 2017;1041–1042:141–150. doi: 10.1016/j.jchromb.2016.12.035. [DOI] [PubMed] [Google Scholar]
- 90.Mueller D.C., Degen C., Scherer G., Jahreis G., Niessner R., Scherer M. Metabolomics using GC-TOF-MS followed by subsequent GC-FID and HILIC-MS/MS analysis revealed significantly altered fatty acid and phospholipid species profiles in plasma of smokers. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2014;966:117–126. doi: 10.1016/j.jchromb.2014.02.044. [DOI] [PubMed] [Google Scholar]
- 91.Carlsson H., Rappaport S.M., Törnqvist M. Protein adductomics: methodologies for untargeted screening of adducts to serum albumin and hemoglobin in human blood samples. High-throughput. 2019;8(1):6. doi: 10.3390/ht8010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sabbioni G., Turesky R.J. Biomonitoring human albumin adducts: the past, the present, and the future. Chem. Res. Toxicol. 2017;30(1):332–366. doi: 10.1021/acs.chemrestox.6b00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carlsson H., von Stedingk H., Nilsson U., Törnqvist M. LC-MS/MS screening strategy for unknown adducts to N-terminal valine in hemoglobin applied to smokers and nonsmokers. Chem. Res. Toxicol. 2014;27(12):2062–2070. doi: 10.1021/tx5002749. [DOI] [PubMed] [Google Scholar]
- 94.Mowrer J., Törnqvist M., Jensen S., Ehrenberg L. Modified Edman degradation applied to hemoglobin for monitoring occupational exposure to alkylating agents. Toxicol. Environ. Chem. 1986;11:215–231. [Google Scholar]
- 95.Schettgen T., Müller J., Ferstl C., Angerer J., Göen T., Hartwig A. Hämoglobinaddukte von Ethylenoxid (N-(2-Hydroxyethyl)valin), Propylenoxid (N-(2-Hydroxypropyl)valin), Acrylnitril (N-(2-Cyanoethyl)valin), Acrylamid (N-(2-Carbonamidethyl)valin) und Glycidamid (N-(2-Hydroxy-2-carbonamidethyl)valin) [Biomonitoring Methods in German Language, 2015] The MAK‐Collection for Occupational Health and Safety. 2016:2221–2253. [Google Scholar]
- 96.Lewalter J. N-alkylvaline levels in globin as a new type of biomarker in risk assessment of alkylating agents. Int. Arch. Occup. Environ. Health. 1996;68(6):519–530. doi: 10.1007/BF00377881. [DOI] [PubMed] [Google Scholar]
- 97.Chin R., Lee B.Y. Chapter 15 - analysis of data. In: Chin R., Lee B.Y., editors. Principles and Practice of Clinical Trial Medicine. Academic Press; New York: 2008. pp. 325–359. [Google Scholar]
- 98.Beukelman T., Brunner H.I. Chapter 6 - trial design, measurement, and analysis of clinical investigations. In: Petty R.E., Laxer R.M., Lindsley C.B., Wedderburn L.R., editors. Textbook of Pediatric Rheumatology. seventh ed. W.B. Saunders; Philadelphia: 2016. pp. 54–77.e2. [Google Scholar]
- 99.DePoy E., Gitlin L.N. Chapter 20 - statistical analysis for experimental-type designs. In: DePoy E., Gitlin L.N., editors. Introduction to Research. fifth ed. Mosby; St. Louis: 2016. pp. 282–310. [Google Scholar]
- 100.G. Scherer, N. Pluym, M. Scherer, Are There Typical Exposure Patterns for Users of Various Nicotine/tobacco Products? an Evaluation of the Literature, (Personal Communication).
- 101.Piper M.E., Smith S.S., Schlam T.R., Fiore M.C., Jorenby D.E., Fraser D., Baker T.B. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Arch. Gen. Psychiatr. 2009;66(11):1253–1262. doi: 10.1001/archgenpsychiatry.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goniewicz M.L., Smith D.M., Edwards K.C., Blount B.C., Caldwell K.L., Feng J., Wang L., Christensen C., Ambrose B., Borek N., van Bemmel D., Konkel K., Erives G., Stanton C.A., Lambert E., Kimmel H.L., Hatsukami D., Hecht S.S., Niaura R.S., Travers M., Lawrence C., Hyland A.J. Comparison of nicotine and toxicant exposure in users of electronic cigarettes and combustible cigarettes. JAMA network open. 2018;1(8) doi: 10.1001/jamanetworkopen.2018.5937. e185937-e185937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Peck M.J., Sanders E.B., Scherer G., Lüdicke F., Weitkunat R. Review of biomarkers to assess the effects of switching from cigarettes to modified risk tobacco products. Biomarkers. 2018;23(3):213–244. doi: 10.1080/1354750X.2017.1419284. [DOI] [PubMed] [Google Scholar]