Highlights
-
•
Ten percentage of patient with RE has adult onset of symptoms.
-
•
Experience with IL-1Ra like anakinra in RE patients is limited.
-
•
26 years after RE onset anakinra was introduced and led to complete seizure control.
-
•
Premature withdrawal of anakinra (2 months) caused relapse of seizures.
-
•
Our patient remains seizure free 13 months after 2nd course (7 months) of anakinra.
Abbreviations: ASM, antiseizure medication; FBTC, Focal to bilateral tonic-clonic; FIRES, febrile infection-related epilepsy syndrome; GTC, Generalized tonic-clonic; IVIG, Intravenous immunoglobulins
Keywords: Anakinra, Drug resistant epilepsy, Hemiatrophy, Neuroinflammation, Rasmussen’s encephalitis
Abstract
Neuroinflammation has been considered an important pathophysiological process involved in epileptogenesis and may provide possibilities for new treatment possibilities. We present the case of a 45-year-old female with drug resistant epilepsy and progressive right-sided cerebral hemiatrophy associated with adult onset Rasmussen’s encephalitis. Over a period of 26 years, she was treated with 14 different antiseizure medications, intravenous immunoglobulins, glucocorticosteroids, underwent two operations with focal resection and subpial transections, and tried out trigeminal nerve stimulation. Extensive blood tests, including antibodies relevant for autoimmune encephalitis, and brain biopsy did not show any signs of neuroinflammation.
Eventually, the patient received the interleukin-1 receptor antagonist, anakinra. Within 1–2 days after injection, seizure frequency decreased significantly, and, after one week, the seizures stopped completely. Anakinra treatment was continued for 2 months. Stopping medication led to a relapse of seizures after 2 weeks, with a frequency of up to 45 seizures per day. Reintroduction of anakinra led to rapid recovery. Treatment with anakinra was continued for 7 months. The treatment was discontinued in April 2020, and the patient has been completely seizure free since then. There have been no other changes in antiseizure medication.
Introduction
Inflammation is increasingly recognized as a key contributor to epilepsy [1]. Although the involvement of inflammatory processes in seizure generation and maintenance has been established, their role in progressive epilepsies is still unknown [2]. As epilepsy may originate from a large variety of brain pathologies, inflammatory processes may be distinctive regarding where, when, and how they are involved, and the battery of inflammation mediators and pathways that they contribute may also be characteristic. Rasmussen’s encephalitis (RE) is a slowly progressive disease characterized by drug-resistant focal epilepsy often in the form of Epilepsia Partialis Continua, hemiparesis and progressive cognitive decline with cerebral hemiatrophy [3], [4], [5]. Neuropathological and immunological studies support the notion that RE is an immune-mediated disease associated with both adaptative immune reactions, with T-lymphocyte responses, and microglia-induced degeneration [4], [6].
An increasing body of evidence indicates a potential effect of immunosuppressive therapy in RE, including high-dose corticosteroids, intravenous immunoglobulin (IVIG), and plasmapheresis, but also immunomodulatory agents like tacrolimus, and azathioprine, rituximab, and natalizumab [4], [7], [8], [9]. However, it is unclear if immunotherapy can modify the long-term outcome like cognitive decline and neurological deterioration [5], [10]. Generally, knowledge is sparse regarding the most appropriate choice and timing of anti-inflammatory therapy.
Anakinra is a recombinant and slightly modified form of the endogenously expressed interleukin-1(IL-1) receptor antagonist (IL-1Ra) that blocks the activity of both IL-1α and IL-1β. IL-1α and IL-1β are expressed in neurons and glial cells, and both receptors trigger inflammation upon stimulation. IL-1 is a proinflammatory cytokine implicated in the pathogenesis of several autoinflammatory disorders [11]. IL-1β has ictogenic properties in various seizure models and has been shown to contribute to the generation of febrile seizures in rodents [12], [13]. Further, IL-1 receptor antagonists have been shown to have pronounced anticonvulsant activity in mice [14], [15] and inhibition of IL-1β synthesis, even after epilepsy onset, may prevent epileptogenesis [16]. Thus, as an IL-1Ra, anakinra is an attractive new treatment option. The drug is currently used for rheumatic diseases in adults where disease-modifying anti-rheumatic drugs have failed, and in rare diseases like macrophage activation syndrome [11].
There are a handful of case reports of patients with febrile infection-related epilepsy syndrome (FIRES) or other autoimmune status epilepticus syndromes being treated successfully with anakinra [17], [18], [19]. Here we present a 45-year-old female patient, with adult onset Rasmussen’s encephalitis, showed a significant response following late-introduced treatment with anakinra.
Case report
Our patient is a previously healthy 45-year-old female who presented with her first epileptic seizure, a focal seizure with impaired awareness (FIAS) followed by focal to bilateral tonic-clonic seizure (FBTCS) at the age of 17 years. A timeline is shown in the Fig. 1. At the age of 24 years, she was hospitalized after a FBTCS. Investigation of the cerebrospinal fluid (CSF) showed a slightly increased cell count indicating a possible viral infection, and the patient was therefore treated with acyclovir. A cerebral MRI scan was normal, while EEG showed sharp activity in the right frontal region. Subsequent CSF investigations have been negative for encephalitic antibodies, and repetitive tests have shown normal CSF cell count (Table 1).
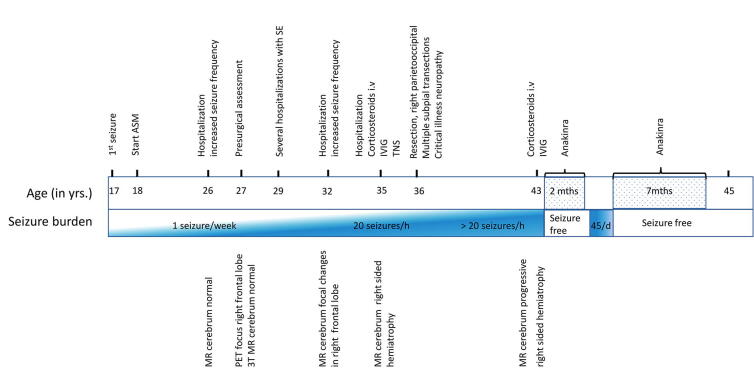
Fig. 1.
A timeline presenting the medical history including seizure burden, neuroimaging findings and most important treatment.
Table 1.
Supplementary examinations.
| Test | Date of test | Result |
|---|---|---|
| MRI | 1992 | Normal |
| 2003 | 3 Tesla MRI normal | |
| 2007 | Slightly atrophy over the right frontoparietal region | |
| 2012 | More marked atrophy, most prominent in the right frontal lobe. Increased enhancement in different areas in the right hemisphere, some resembling edema | |
| 2016 | Increased atrophy in the right frontoparietal region | |
| 2019-April | Considerable loss of brain substance in the right hemisphere and in mesencephalon, pons and diffuse symmetrical atrophy of cerebellum. | |
| 2019- June | New edema with diffusion restriction in the right cortical parietooccipital region and in the right thalamus, representing ictal/ post ictal activity | |
| 2019-Septembre | Considerable loss of brain substance in the right hemisphere and in mesencephalon, pons and cerebellum - unchanged from April 2019 | |
| Brain pathology | 2012 | Brain biopsy: reactive gliosis and nerve cell loss in the gray matter of the brain, slight lymphocyte infiltration. Immunostaining for B and T cells did not show any significant changes except for a few CD3 positive T-cells perivascularly. No increase in CD20 positive B-cells. |
| Cerebrospinal fluid | 2000 | Slight increase in leucocytes |
| 2012/ 2013/2019 | Normal with regards to cell count, protein, glucose ratio. Encephalitis antibodies negative for: anti-NMDA, AMPA1, AMPA 2, Gabareceptor B1, Contactin-associated protein 2, Leucin-rich glioma- inactivated protein 1, anti-CASPR2, anti-DPPX | |
| Blood tests | 1992–2020 | SR, CRP, white blood count: normal; encephalitis antibodies: Negative |
| EEG | 2002 | Epileptiform discharges were present and most prominent in the right frontal region, but also seen in in the temporal region |
| 2019-June | Focal status epilepticus, with some clinical seizures starting in the right occipital region | |
| 2019-September | Slow (theta and delta) activity bilaterally, and additionally more focal slowing over the right frontotemporal region. Sharp waves were also seen in this region, but were less frequent than in July |
Between the ages of 18 and 26 years the patient had sporadic seizures of different semiology, including: 1) focal aware seizures (FAS), typically starting with jerking in the left shoulder, arm, and face, sometimes followed by head rotation, 2) FAS evolving to FIAS with speech arrest, and 3) sporadic FBTCS. At that time, repetitive EEG examinations showed epileptic activity in the right frontotemporal region without clinical correlation.
At the age of 27 years, a presurgical assessment was performed. It was not possible to precisely locate the epileptic focus other than to the right hemisphere and surgery was rejected. In the following years, she was hospitalized for weeks to months several times per year due to serial attacks or focal status epilepticus with FIAS/FAS.
Four years after presurgical assessment, a cerebral MRI showed focal frontal right-sided cortical edema and a somewhat more marked Sylvian fissure (Fig. 2).
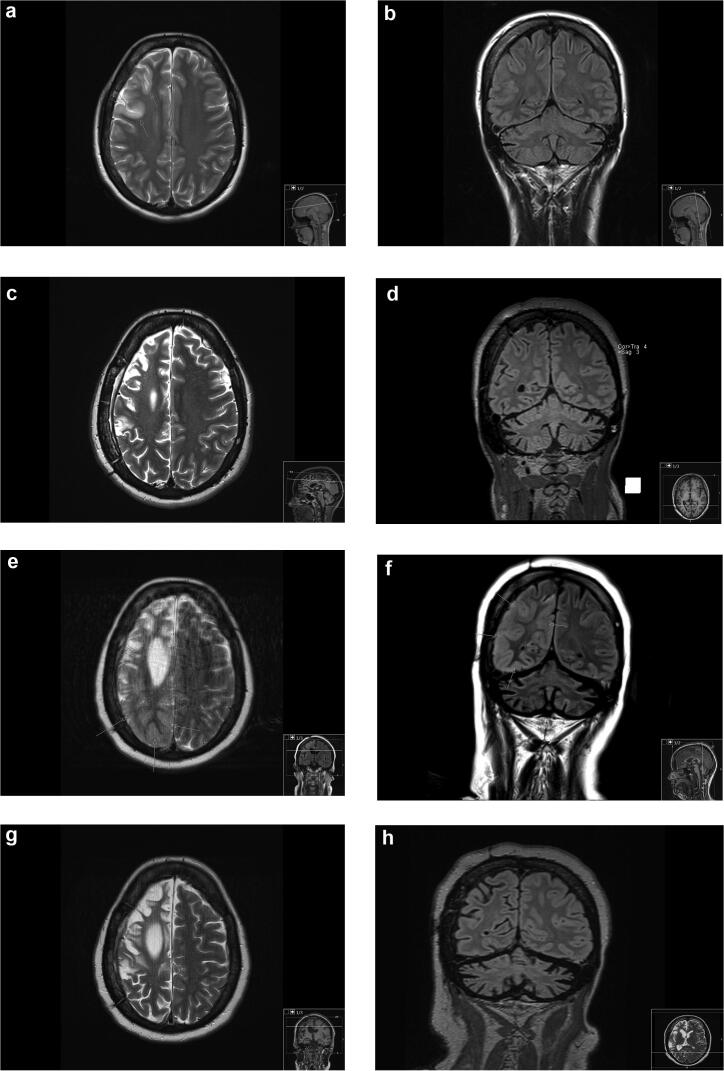
Fig. 2.
Axial T2 and coronal FLAIR MR images 2008 (age 32 years)(a and b), 2013 (age 37 years)(c and d), June 2019 (e and f) and September 2019 (age 43 years)(g and h). First examination (a and b) showed focal frontal right-sided cortical edema (arrows), otherwise normal. Second examination in 2013 (c and d) revealed right sided hemiatrophy and status after subpial resection. In June 2019 (e and f) there was progressive hemiatrophy and extensive parietooccipital cortical edema (arrows). The edema had subsided in September 2019 (g and h).
At 35 years of age, the patient was hospitalized due to severe clinical deterioration, with 10–20 FAS and FIAS per hour. She was treated with a multitude of different antiseizure medications (ASMs) at high dosages, including i.v. valproate, fos-phenytoin, levetiracetam, clonazepam, and lacosamide. Immunoglobulins and high-dose i.v. steroids were ineffective. Non-invasive trigeminal nerve stimulation was also tried without success. In total, she was continuously hospitalized for 8 months.
At that time, MRI showed atrophic involvement of the entire right hemisphere and several smaller areas of gliotic/edematous changes cortically, frontally and related to the insula, concurrent with post-epileptic changes.
One year later at the age of 36 years, she was operated twice, first with a resection of focal epileptic tissue in sensory cortex of the right parietooccipital region (histopathology results are presented in Table 1). Due to lack of seizure control, she was re-operated with multiple subpial resections in the right motor cortex. Gradually her epilepsy improved and, 3 months after her second operation, she was discharged from hospital. At that time, she was treated with phenobarbital, phenytoin, and clonazepam.
In the following years, the patient was hospitalized several times annually. Clinically, she gradually deteriorated with a progressive left-sided spastic hemiparesis and a moderate cognitive impairment.
In June 2019, the patient was again admitted to our hospital with increasing seizure frequency. On admission she had more than 20 seizures per hour. No ASM regimen was effective and seizure frequency was unaltered for several weeks. At that time, the patient had tried 14 different ASM (briviact, carbamazepine, clobazam, clonazepam, gabapentin, lacosamide, levetiracetam, lamotrigine, perampanel, phenobarbital, phenytoin, topiramate, valproate, zonisamide). A high dose pulsed i.v. corticosteroid therapy with methylprednisolone 1 g/day for 5 days did not lead to any improvement, nor did IVIG treatment.
An MRI scan of the brain showed seizure-related signal changes consistent with edema in the right parietotemporooccipital region and posteriomedially in the right thalamus in addition to progressive hemiatrophy (Fig. 2). EEG now showed occasional independent seizures arising from the left frontal region, in addition to focal status epilepticus in the right temporoparietal region (Fig. 3a).
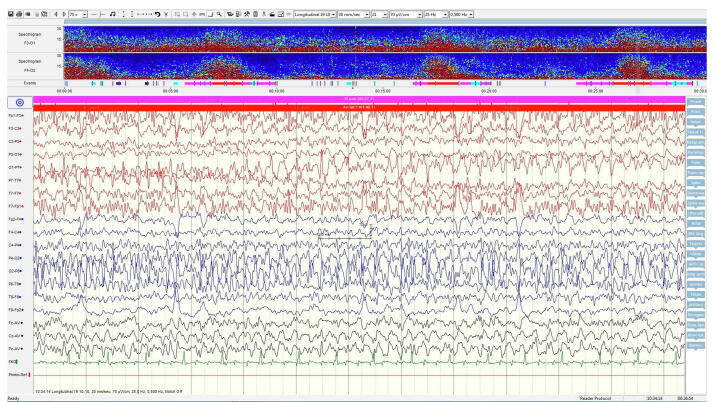
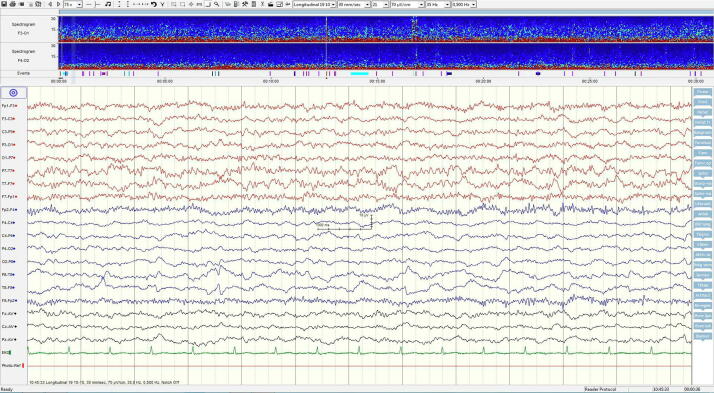
Fig. 3.
A) Longitudal EEG recording demonstrating marked epileptic activity during a seizure shortly before treatment with anakinra. The trend analysis (spectrogram) shown on top shows four epileptic seizures within the 30 min registration period. B) Longitudinal EEG recording 6 weeks after start of anakinra with no ongoing epileptic activity. No seizures are now seen in the 30 min trend analysis.
On July 11th 2019, anakinra (Kineret) 100 mg sc. × 1 was introduced. Within 1–2 days after injection, seizure frequency decreased significantly, and, after one week, the seizures stopped completely followed by a marked improvement in EEG (Fig. 3b). After 2 months, the treatment with anakinra was stopped, which led to a relapse of seizures after 2 weeks, with a frequency of up to 45/day. Reintroduction of anakinra led to rapid recovery with an approximate latency to control of 10 days. Treatment with anakinra was further continued for 7 months. The patient has been seizure free since then (13 months). No definite side effects occurred, but the patient experienced pneumonia on one occasion after introduction of anakinra, and was prone to urinary infections. While she still presents with a spastic paralysis of her left arm. Her cognitive function has slightly improved.
Discussion
This report demonstrates a dramatic effect of treatment with anakinra in a patient with adult onset RE. Clinically, the epilepsy started during adolescence with low frequency focal seizures and few episodes of FBTCS. Over time, seizure frequency and severity increased, with increasingly long periods of hospitalization and progressive neurological and cognitive deficits. Radiologically, the patient developed progressive loss of brain volume, starting with localized cortical thinning evolving to extensive brain hemiatrophy (Fig. 2). Based on reports from case series it is estimated that 10% of all RE patients have late-onset of symptoms [4], [5], [20]. The clinical course of adult onset RE seems to be slower and less severe than in children. It is reported more frequent FIAS and less likely Epilepsia Partialis Continua. Patients suffer from mild cognitive deficit and have better outcome [20]. Autoantibodies are rarely described [4], [20]. The typically protracted evolution of symptoms can delay correct diagnosis, as in our patient. We did not find any evidence of an ongoing inflammatory process in CSF analyses, PET, numerous MRI scans, and brain biopsy. No autoantibodies were detected in our patient.
Before introduction of anakinra, the patient’s condition had worsened with further progression, involving also the ipsilateral occipital region, which was visible in both EEG and MRI. In this clinically very difficult situation, anakinra treatment was tried despite any evidence of inflammation and earlier poor responses to IVIG and corticosteroids. The response to anakinra was almost immediate, with seizure freedom within approximately a week. The fact that seizures returned upon cessation of anakinra after two months, and that a reintroduction of the drug once again initiated immediate improvement with seizures control after 10 days, leaves us feeling convinced that the effect is induced by the medication.
Inflammation, and particularly pro-inflammatory cytokines such as IL-1, are increasingly recognized as being involved in the pathogenesis of seizures and epilepsy [21], [22]. In several different animal models of epileptogenesis, pharmacologic blockade of IL-1 through the use of IL-1Ra, resulted in the reduction of seizures and cellular injury [15], [23], [24], [25]. Taken together these findings encourage further studies, including clinical trials with specific anti-inflammatory medication including IL-1R blockers [23].
A recent publication from Liba et al., reported on seven patients with RE who were treated with different immunosuppressive agents [6], but none of medications was targeting IL-1R. Although T cell-targeted therapy substantially reduced inflammation in brain tissues, intractable epilepsy persisted clinically in these patients. This result suggests a relative independence of seizure activity and the presence of T- cell inflammation in the brain. This is interesting because neuropathological and immunological studies support the theory that RE is probably driven by a response to one or more antigenic epitopes, with potential additional contribution by autoantibodies [4]. The mode of action of anakinra differs from those of the substances used in the study by Liba et al. [6]. IL-1Ra has been used in the treatment of FIRES. To date, there are no reports describing use of IL-1Ra in RE. Our case shows that anakinra can be considered a possible treatment alternative in drug-resistant epilepsies with a proposed immunological background like RE.
So far, we do not have an algorithm for treating adult patients with epilepsy with anakinra. The treatment regime for the present patient was adjusted based on response and side effects. Our patient experienced pneumonia on one occasion after introduction of anakinra, and was prone to urinary infections, which could indicate some caution in treatment. Our experience from treating this patient was that two months of treatment was insufficient. However, after an additional seven months of treatment, the seizures have apparently stopped.
In conclusion, inflammation may be either the cause or the driver in the epileptogenic process in some patients, despite common inflammation biomarkers being absent. In selected drug-resistant epilepsy patients anakinra could therefore be a treatment option.
Ethical statement
The authors ensure that this work has been carried out in accordance with the Declaration of Helsinki.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Vezzani A., Balosso S., Ravizza T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol. 2019;15:459–472. doi: 10.1038/s41582-019-0217-x. [DOI] [PubMed] [Google Scholar]
- 2.Vezzani A. Epilepsy and Inflammation in the Brain: Overview and Pathophysiology: Epilepsy and Inflammation in the Brain. Epilepsy Currents. 2014;14:3–7. doi: 10.5698/1535-7511-14.s2.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pardo C.A., Nabbout R., Galanopoulou A.S. Mechanisms of epileptogenesis in pediatric epileptic syndromes: Rasmussen encephalitis, infantile spasms, and febrile infection-related epilepsy syndrome (FIRES) Neurotherapeutics. 2014;11:297–310. doi: 10.1007/s13311-014-0265-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varadkar S., Bien C.G., Kruse C.A., Jensen F.E., Bauer J., Pardo C.A. Rasmussen's encephalitis: clinical features, pathobiology, and treatment advances. Lancet Neurol. 2014;13:195–205. doi: 10.1016/S1474-4422(13)70260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bien C.G., Granata T., Antozzi C., Cross J.H., Dulac O., Kurthen M. Pathogenesis, diagnosis and treatment of Rasmussen encephalitis: A European consensus statement. Brain. 2005;128:454–471. doi: 10.1093/brain/awh415. [DOI] [PubMed] [Google Scholar]
- 6.Liba Z., Vaskova M., Zamecnik J., Kayserova J., Nohejlova H., Ebel M. An immunotherapy effect analysis in Rasmussen encephalitis. BMC Neurol. 2020;20:359. doi: 10.1186/s12883-020-01932-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Granata T., Fusco L., Gobbi G., Freri E., Ragona F., Broggi G. Experience with immunomodulatory treatments in Rasmussen's encephalitis. Neurology. 2003;61:1807–1810. doi: 10.1212/01.wnl.0000099074.04539.e0. [DOI] [PubMed] [Google Scholar]
- 8.Thilo B., Stingele R., Knudsen K., Boor R., Bien C.G., Deuschl G. A case of Rasmussen encephalitis treated with rituximab. Nat Rev Neurol. 2009;5:458–462. doi: 10.1038/nrneurol.2009.98. [DOI] [PubMed] [Google Scholar]
- 9.Bittner S., Simon O.J., Gobel K., Bien C.G., Meuth S.G., Wiendl H. Rasmussen encephalitis treated with natalizumab. Neurology. 2013;81:395–397. doi: 10.1212/WNL.0b013e31829c5ceb. [DOI] [PubMed] [Google Scholar]
- 10.Orsini A., Foiadelli T., Carli N., Costagliola G., Masini B., Bonuccelli A. Rasmussen's encephalitis: From immune pathogenesis towards targeted-therapy. Seizure. 2020;81:76–83. doi: 10.1016/j.seizure.2020.07.023. [DOI] [PubMed] [Google Scholar]
- 11.Dinarello C.A., Simon A., van der Meer J.W. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vezzani A., Maroso M., Balosso S., Sanchez M.A., Bartfai T. IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav Immun. 2011;25:1281–1289. doi: 10.1016/j.bbi.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 13.Dube C., Vezzani A., Behrens M., Bartfai T., Baram T.Z. Interleukin-1beta contributes to the generation of experimental febrile seizures. Ann Neurol. 2005;57:152–155. doi: 10.1002/ana.20358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vezzani A., Conti M., De Luigi A., Ravizza T., Moneta D., Marchesi F. Interleukin-1beta immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: functional evidence for enhancement of electrographic seizures. J Neurosci. 1999;19:5054–5065. doi: 10.1523/JNEUROSCI.19-12-05054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vezzani A., Moneta D., Conti M., Richichi C., Ravizza T., De Luigi A. Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc Natl Acad Sci U S A. 2000;97:11534–11539. doi: 10.1073/pnas.190206797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iori V., Iyer A.M., Ravizza T., Beltrame L., Paracchini L., Marchini S. Blockade of the IL-1R1/TLR4 pathway mediates disease-modification therapeutic effects in a model of acquired epilepsy. Neurobiol Dis. 2017;99:12–23. doi: 10.1016/j.nbd.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Kenney-Jung D.L., Vezzani A., Kahoud R.J., LaFrance-Corey R.G., Ho M.L., Muskardin T.W. Febrile infection-related epilepsy syndrome treated with anakinra. Ann Neurol. 2016;80:939–945. doi: 10.1002/ana.24806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeSena A.D., Do T., Schulert G.S. Systemic autoinflammation with intractable epilepsy managed with interleukin-1 blockade. J Neuroinflammation. 2018;15:38. doi: 10.1186/s12974-018-1063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shukla N., Risen S., Erklauer J., Lai Y.-C., Riviello J., Muscal E. Anakinra(IL-1 blockade) Use in Children with Suspected FIRES: A Single Institution Experience (P4.346) Neurology. 2018;90 P4.346. [Google Scholar]
- 20.Dupont S., Gales A., Sammey S., Vidailhet M., Lambrecq V. Late-onset Rasmussen Encephalitis: A literature appraisal. Autoimmun Rev. 2017;16:803–810. doi: 10.1016/j.autrev.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 21.Kolosowska K., Maciejak P., Szyndler J., Turzynska D., Sobolewska A., Plaznik A. The role of IL-1beta and glutamate in the effects of lipopolysaccharide on the hippocampal electrical kindling of seizures. J Neuroimmunol. 2016;298:146–152. doi: 10.1016/j.jneuroim.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 22.Arisi G.M., Foresti M.L., Katki K., Shapiro L.A. Increased CCL2, CCL3, CCL5, and IL-1beta cytokine concentration in piriform cortex, hippocampus, and neocortex after pilocarpine-induced seizures. J Neuroinflammation. 2015;12:129. doi: 10.1186/s12974-015-0347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marchi N., Fan Q., Ghosh C., Fazio V., Bertolini F., Betto G. Antagonism of peripheral inflammation reduces the severity of status epilepticus. Neurobiol Dis. 2009;33:171–181. doi: 10.1016/j.nbd.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Librizzi L., Noe F., Vezzani A., de Curtis M., Ravizza T. Seizure-induced brain-borne inflammation sustains seizure recurrence and blood-brain barrier damage. Ann Neurol. 2012;72:82–90. doi: 10.1002/ana.23567. [DOI] [PubMed] [Google Scholar]
- 25.Noe F.M., Polascheck N., Frigerio F., Bankstahl M., Ravizza T., Marchini S. Pharmacological blockade of IL-1beta/IL-1 receptor type 1 axis during epileptogenesis provides neuroprotection in two rat models of temporal lobe epilepsy. Neurobiol Dis. 2013;59:183–193. doi: 10.1016/j.nbd.2013.07.015. [DOI] [PubMed] [Google Scholar]