Abstract
Severe respiratory syncytial virus (RSV) bronchiolitis in early life is a significant risk factor for future recurrent wheeze (RW) and asthma. The goal of the Azithromycin to Prevent Wheezing following severe RSV bronchiolitis II (APW-RSV II) clinical trial is to evaluate if azithromycin treatment in infants hospitalized with RSV bronchiolitis reduces the occurrence of RW during the preschool years.
The APW-RSV II clinical trial is a double-blind, placebo-controlled, parallel-group, randomized trial, including otherwise healthy participants, ages 30 days-18 months, who are hospitalized due to RSV bronchiolitis. The study includes an active randomized treatment phase with azithromycin or placebo for 2 weeks, and an observational phase of 18–48 months. Two hundred participants were enrolled during three consecutive RSV seasons beginning in the fall of 2016 and were randomized to receive oral azithromycin 10 mg/kg/day for 7 days followed by 5 mg/kg/day for an additional 7 days, or matched placebo. The study hypothesis is that in infants hospitalized with RSV bronchiolitis, the addition of azithromycin therapy to routine bronchiolitis care would reduce the likelihood of developing post-RSV recurrent wheeze (≥3 episodes). The primary clinical outcome is the occurrence of a third episode of wheezing, which is evaluated every other month by phone questionnaires and during yearly in-person visits.
A secondary objective of the APW-RSV II clinical trial is to examine how azithromycin therapy changes the upper airway microbiome composition, and to determine if these changes are related to the occurrence of post-RSV RW. Microbiome composition is characterized in nasal wash samples obtained before and after the study treatments.
This clinical trial may identify the first effective intervention applied during severe RSV bronchiolitis to reduce the risk of post-RSV RW and ultimately asthma.
Keywords: Respiratory syncytial virus (RSV) bronchiolitis, Azithromycin, Recurrent wheezing, Asthma, Microbiome
Abbreviations: APW, Azithromycin to Prevent Wheezing; RW, Recurrent wheezing; RSV, Respiratory syncytial virus; AE, Adverse events; AESI, AEs of Special Interest; AZM, Azithromycin; DSMB, Data safety and monitoring board; ED, Emergency department; ICS, Inhaled corticosteroids; IRB, Institutional review board; IL, Interleukin; LRTI, Lower respiratory tract infection; MMP-9, Matrix metallopeptidase-9; NHLBI, National Heart, Lung, and Blood Institute; PC, Phone call; RBEL, RSV Bronchiolitis in Early Life; RZ, Randomization; SAE, serious adverse events; SLCH, Saint Louis Children's Hospital; V, visit
1. Background
Childhood asthma is a significant health challenge today, without a known effective prevention [1,2]. Several studies have shown that severe respiratory syncytial virus (RSV) bronchiolitis in early life is strongly associated with the development of asthma in children [[3], [4], [5]]. We have previously demonstrated in a prospective cohort of 206 infants hospitalized for RSV bronchiolitis, that 48% of participants had a physician diagnosis of asthma and that 75% of participants experienced recurrent (at least 3) wheezing (RW) episodes by their 7th birthday (the RSV Bronchiolitis in Early Life (RBEL-I) study) [6]. As hospitalized infants have the greatest risk for post-RSV wheezing episodes and asthma, they represent the ideal population in which to explore intervention strategies for the prevention of post-RSV RW and asthma [7,8].
Numerous asthma-related therapies have been investigated and found to be unsuccessful in preventing post-RSV wheezing and asthma [9]. Airway inflammation associated with acute infectious bronchiolitis is typically neutrophil-rich, which may explain the lack of efficacy of asthma treatments, such as inhaled corticosteroids (ICS), systemic corticosteroids, and montelukast on the occurrence of post-bronchiolitis wheezing, as these medications typically target eosinophilic airway inflammation [[10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20]]. Therefore, a medication with anti-neutrophil properties, such as macrolides, may be effective in attenuating RSV-driven airway inflammation, and subsequently, post-RSV wheezing and potentially asthma.
Macrolides provide clinical benefits in other inflammatory airway diseases with a dominant neutrophilic airway inflammation component, such as cystic fibrosis and diffuse panbronchiolitis, potentially through anti-inflammatory activities [21,22]. Based on these reports, we previously investigated and revealed that azithromycin attenuated airway neutrophilic inflammation in a mouse model of bronchiolitis [23]. Based on our results from a mouse model, we conducted the azithromycin to prevent wheezing following RSV bronchiolitis I (APW-RSV I) trial [24]. This proof-of-concept trial in 40 infants hospitalized with RSV bronchiolitis revealed that azithromycin treatment for 2 weeks, when added to routine bronchiolitis care, significantly reduced the likelihood of RW over the following year without significant adverse events [24]. Based upon these findings, we are conducting the APW-RSV II clinical trial to investigate the effect of azithromycin on post-RSV recurrent (≥3 episodes) wheeze during the preschool years in a larger and longer clinical trial.
Studies from the past decade have suggested that the airway microbiome has an important role in asthma inception [[25], [26], [27]]. In our previous proof-of-concept trial in 40 children hospitalized with RSV bronchiolitis we detected that azithromycin therapy during severe RSV bronchiolitis reduced Moraxella abundance in the upper airway, and in-turn, lower Moraxella abundance was associated with lower odds of subsequent recurrent wheeze [28]. We concluded that additional studies should evaluate the role of the airway microbiome in the development of post-RSV recurrent wheeze [28]. In our previous studies we identified two potential pathways that may mediate the beneficial effects of azithromycin: anti-inflammatory effects and alterations of the airway microbiome [24,28]. Hence, in addition to the clinical outcome, we are now investigating potential mechanistic pathways.
2. Materials and methods
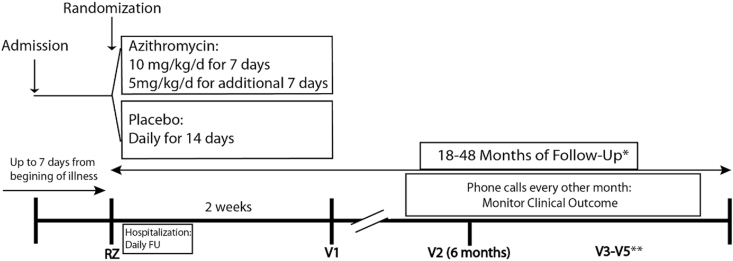
2.1. Study design overview (Fig. 1)
Fig. 1.
The APW-RSV II trial design
V: Visit, *Variable durations of follow-up periods are necessary, as patients were recruited over 3 consecutive RSV seasons. Therefore, not all patients will perform V4 and V5. **V3–V5 are conducted yearly. APW-RSV: The Azithromycin to Prevent Wheezing following severe RSV bronchiolitis, FU: Follow up.
The APW-RSV II clinical trial is a double blind, placebo-controlled, parallel-group, randomized trial, including otherwise healthy infants, ages 1 month (30 days) −18 months, who are hospitalized due to RSV bronchiolitis. The study includes an active treatment phase with azithromycin or placebo for 2 weeks, and an observational phase of 18–48 months (Fig. 1).
Study activities began in the fall of 2016 and, as of this writing (early-mid 2021), participant follow up continues. We enrolled study participants during three consecutive RSV seasons beginning in the fall of 2016. The duration of follow up is 18–48 months, which is determined based on the year in which the participants are recruited: first year recruits will be followed for up to 48 months, while the 3rd year recruits will be followed for at least 18 months (Fig. 1).
Study objectives:
-
1.Primary objectives: To determine if the addition of azithromycin therapy (compared to placebo) to routine bronchiolitis care in infants hospitalized with RSV bronchiolitis:
-
a)Reduces the occurrence of post-RSV RW (≥3 episodes) measured over a follow-up period of 18–48 months and
-
b)Changes airway bacterial microbiome community structure, and if that change is associated with a reduction in the occurrence of post-RSV RW.
-
a)
-
2.Secondary objectives: To determine if azithromycin therapy (compared to placebo) in infants hospitalized with RSV bronchiolitis will result in:
-
a.Improved asthma disease activity parameters such as days with respiratory symptoms, asthma medications use (controller and reliever use), oral corticosteroid use, and asthma related urgent visits.
-
b.Lower likelihood of developing parent-reported physician asthma diagnosis.
-
c.Lower mean total IgE levels and eosinophil counts measured at the last study visit.
-
d.Smaller proportion of children who will develop at least one positive specific IgE to inhalant allergens measured at the last study visits.
-
e.Changes in nasal wash levels of IL-8, and matrix metallopeptidase-9 (MMP-9) measured at randomization, on day 15 after randomization and 6 months after randomization.
-
f.Changes in the rates of upper airway colonization with macrolide-resistant bacteria measured at the end of the study treatments (day 15 after randomization) and at the 6-month clinic visit.
-
a.
2.2. Study sponsor
APW-RSV II is funded by the National Heart, Lung, and Blood Institute (NHLBI), R01HL130876 and is registered at Clinicaltrials.gov (NCT02911935). The study was approved by the Institutional Review Board (IRB) at Washington University School of Medicine.
The NHLBI selected the study Data and Safety Monitoring Board (DSMB) to monitor the study. All communication between the investigators and the DSMB courses through the staff of the NHLBI.
2.3. Intervention: Azithromycin (AZM)
The optimal dose and duration of AZM treatment needed to provide an anti-inflammatory effect is not established. Therefore, we utilize the exact dosing regimen used in our previous proof of concept APW-RSV I trial, which provided clinical benefit, was effective in exerting anti-inflammatory effects as evident by reduced nasal IL-8 levels, and was not associated with higher rates of adverse reactions compared to placebo [24]. Specifically, we randomized study participants to receive PO azithromycin 10 mg/kg/day for 7 days followed by 5 mg/kg/day for an additional 7 days, or matched placebo.
The duration of this treatment regimen was determined based upon previous studies in animal models of viral bronchiolitis that revealed immunologic events up to 21 days after infection that can lead to a Th2-prone phenotype [29]. As AZM has a very long half-life in lung tissue, 14 days of treatment with AZM should result in at least 23 days with effective anti-microbial concentrations in lung tissue and at least 35 days of measurable quantity in airway macrophages, which should provide coverage during recovery from bronchiolitis [30,31]. Moreover, we aimed to minimize potential adverse drug reactions by using a dosing regimen that was safe in our previous proof of concept APW-RSV I trial, and had an excellent safety profile in very-low birth-weight pre-term infants (n = 263) treated for up to 6 weeks [24,32,33].
2.4. Study population
The study population includes otherwise healthy infants with severe RSV bronchiolitis, defined as an episode that requires hospitalization. Severe RSV bronchiolitis is a major independent risk factor for RW episodes and asthma [6]. We focus on hospitalized infants since they have the highest risk to develop RW and asthma [7,8]. Our goal is to treat infants with azithromycin early in the disease process in order to provide the greatest opportunity to limit inflammatory damage to the airways and hence future wheezing. In order to treat early, infants were eligible to be enrolled in the study only if the duration of respiratory symptoms from onset until admission was 5 days or less, and if they could be randomized within 7 days from initiation of symptoms. Based on our power analysis, our initial goal was to recruit 188 infants over three consecutive RSV seasons. During the last year of recruitment, we increased our target to 200 infants for enhanced power after obtaining permission for this from the DSMB and IRB.
RSV infection was confirmed by positive nasal swab results by PCR assay (N = 95), direct antigen detection (N = 97) or by both tests (N = 7). Tests were performed by the St. Louis Children's Hospital (SLCH) virology laboratory, or from another Clinical Laboratory (for patients tested and transferred from an outside facility). Bronchiolitis was defined by the presence of at least two of the following: respiratory rate greater than 40 breaths/minute; cough; wheezing; audible rales, crackles, and/or rhonchi; paradoxical chest movements (retractions) [19]. The inclusion and exclusion criteria are listed in Table 1.
Table 1.
Inclusion/Exclusion criteria.
| Inclusion Criteria: |
| 1. Age 30 days-18 months. |
| 2. Duration of significant respiratory symptoms from onset of symptoms of current illness to admission is ≤ 120 h (5 days). |
| 3. Hospitalization in Saint Louis Children's Hospital for the first episode of RSV bronchiolitis. |
| 4. Parent/guardian available to provide informed consent. |
| 5. Randomization can be performed within 168 h (7 days) from onset of significant respiratory symptoms. |
| Exclusion Criteria: |
| 1. Diagnosis of asthma. |
| 2. Chronic treatment with any daily medication other than vitamins or nutritional supplements. |
| 3. Contraindication to the use of azithromycin or any other macrolide antibiotics. |
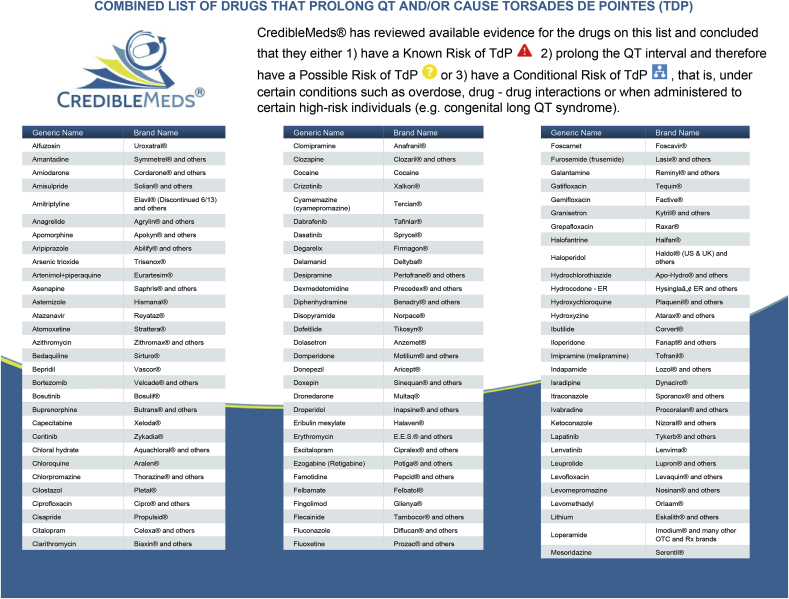
| 4. Current treatment with any medication that may cause QT interval prolongation (see Appendix 1. for a detailed list of these medications that we have utilized as an exclusion criteria). |
| 5. Failure to thrive (<3% for age). |
| 6. Gastroesophageal reflux requiring treatment with daily anti reflux medications (anti H2 or PPI). |
| 7. High dose vitamin D therapy (more than 400 IU per day). |
| 8. History of previous (before the current episode) wheeze or previous (before the current episode) treatment with albuterol. |
| 9. Ongoing need for invasive mechanical ventilation (intubation) or non-invasive mechanical ventilation (CPAP, BIPAP) due to RSV bronchiolitis. Can be enrolled after weaned from mechanical ventilation/BIPAP/CPAP if meets other criteria. |
| 10. Participation in another clinical trial. |
| 11. Prematurity (gestational age < 36 weeks). |
| 12. Presence or history of other significant disease (CNS, lung, cardiac, renal, GI, hepatic, hematologic, endocrine or immune disease). Children with atopic dermatitis and/or food allergy were not excluded from the study. |
| 13. Sibling enrolled in the clinical trial during the same RSV season. |
| 14. Significant developmental delay. |
| 15. The family has definitive plans to move from the clinical center area before trial completion. |
| 16. Treatment with any macrolide antibiotic (azithromycin, clarithromycin or erythromycin) over the past 4 weeks or current treatment with any macrolide antibiotic. Current or prior treatment with non-macrolide antibiotic was not an exclusion criterion. |
| 17. Treatment (past or present) with montelukast. |
| 18. History of previous treatment with corticosteroid (systemic or inhaled) for respiratory conditions. (This criterion was included to identify children who may have wheezed in the past.) |
| Post randomization exclusion criteria |
| Use of any medication that can cause QT prolongation during the first 14 days of the study (see Appendix 1.) |
* Significant respiratory symptoms defined as wheezing, significant cough, retractions. Time of admission is defined as the time that the child was seen in the ED for the visit that led to the hospitalization.
BIPAP: Bilevel Positive Airway Pressure, CPAP: Continuous positive airway pressure, CNS: Central nervous system, GI: Gastrointestinal, IU: International units, RSV: Respiratory syncytial virus, PPI: Proton pump inhibitor.
2.5. Specifics of study procedures
2.5.1. Randomization protocol
Eligible children were randomized to azithromycin or placebo groups in a 1:1 fashion based on a blocked randomization allocation sequence. Randomization was stratified by use of open-label non-macrolide antibiotic over the past 2 weeks prior to randomization. The investigators and study coordinators were blinded to treatment assignment and to the size of randomization blocks.
2.5.2. Recruitment and characteristics of enrolled patients
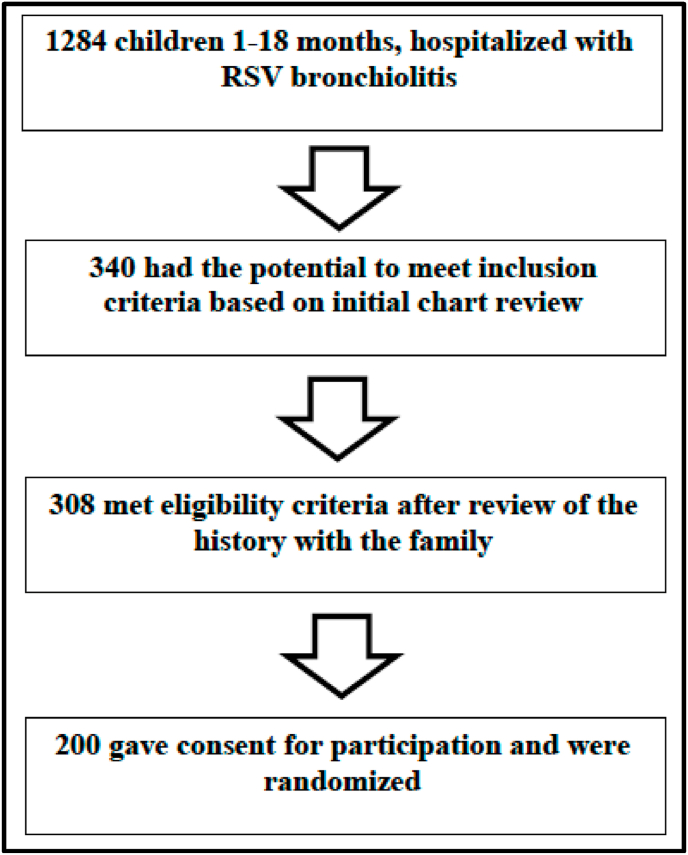
We identified 1284 children who were hospitalized with RSV bronchiolitis and were within the target age range during three consecutive RSV seasons (fall 2016–spring 2019). Out of these, 200 children met inclusion criteria, gave consent for participation, and were randomized (Fig. 2 (screening) and Table 2 (exclusions)).
Fig. 2.
Screening and enrollment of study participants.
Table 2.
Reasons for exclusion of children hospitalized with RSV bronchiolitis.
| Reason for exclusion | Number of children |
|---|---|
| Prior wheeze, bronchiolitis or asthma diagnosis | 298 |
| Significant other medical history or use of daily medications | 258 |
| Parents were not available for approach or refused participation | 228 |
| Prematurity (gestational age < 36 weeks) | 200 |
| Met other exclusion criteria* | 169 |
| Duration of respiratory symptoms longer than 5 days at the time of admission (or 7 days at randomization), or on mechanical ventilation at the time of screening | 157 |
| Did not meet protocol definition of bronchiolitis | 53 |
This table summarizes the reasons for exclusion of children aged 1–18 months that were hospitalized at St. Louis Children's Hospital due to RSV Bronchiolitis. Some children had more than one reason for exclusion.
* These include: Significant developmental delay, current use of macrolide or medication that may prolong QT interval, contraindication for azithromycin use, participation in another clinical trial or sibling enrolled in the clinical trial during the same RSV season, family is living out of the St. Louis metro area.
Enrollment seasons for these 200 children are as below:
-
1.
Fall 2016-Spring 2017: 39 participants were enrolled
-
2.
Fall 2017-Spring 2018: 96 participants were enrolled
-
3.
Fall 2018-Spring 2019: 65 participants were enrolled
The characteristics of enrolled children are described in Table 3. Median age of enrolled children was 3.3 months. The majority (72%) of enrolled children were Caucasian. 36% had parental history of asthma. Median duration of hospitalization was 52 hours.
Table 3.
Baseline characteristics of study population.
| Mean ± SD, median (IQR) or n (%) | |
|---|---|
| Age at enrollment (months) median (IQR) | 3.3 (2, 6.9) |
| Male | 109 (54.5%) |
| Race/Ethnicity* | |
| African American | 40 (20.1%) |
| Caucasian | 144 (72.4%) |
| More than one race | 15 (7.5%) |
| Birth Weight (kg) | 3.3 ± 0.5 |
| Birth by C-section | 57 (28.5%) |
| Gestational age at birth (weeks) | 38.8 ± 1.2 |
| Maternal smoking during pregnancy | 32 (16%) |
| History of breast feeding | 155 (77.5%) |
| History of eczema | 29 (14.5%) |
| Food allergy diagnosis | 8 (4%) |
| Parental history of asthma | 72 (36%) |
| Parental history of other atopic diseases | 129 (64.5%) |
| Pet exposure | 130 (65%) |
| Tobacco smoke exposure | 66 (33%) |
| Duration of Hospital Stay (hours) 1 | 52 (35, 85) |
| Duration of oxygen requirement, if required (hours) 2 | 47 (25, 68) |
| Lowest oxygen saturation on room air (%) 3 | 90.7 ± 4.8 |
| Need for BiPAP ventilation 1,4 | 13 (6.6%) |
IQR: Interquartile range, SD: Standard deviation.
Variables with a strongly skewed distribution are presented as median (IQR).
The total sample is 200 participants unless otherwise specified: 1. N=198. 2. N=114 (the other participants did not require oxygen). 3. N=197. 4. no child required invasive ventilation.
* One participant refused to answer this question.
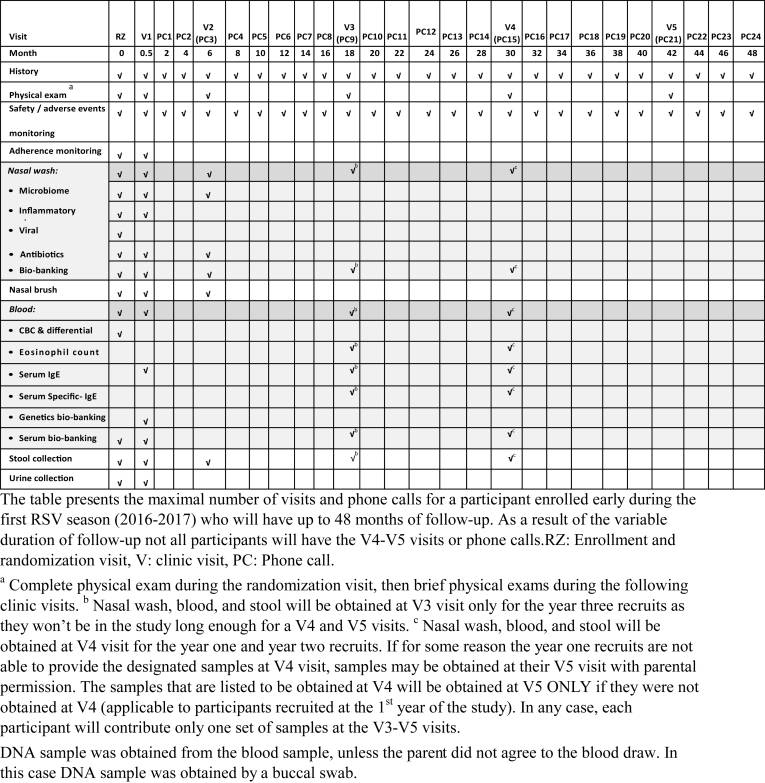
2.6. Enrollment and study visits (Table 4)
Table 4.
Study visits and study sample collections.
2.6.1. Enrollment and randomization visit (RZ) during the acute hospitalization (Table 4)
During enrollment and prior to randomization, the following activities were completed: parental consent, a physical examination, collection of patients’ medical and family history, including an environmental allergy questionnaire. Parents were educated about study medication administration and on the need to avoid any macrolide antibiotic or other QT prolonging medication during the active phase of the study. Baseline blood, stool, urine (if able to collect while the child is hospitalized), and nasal wash and nasal brush samples were collected. Nasal wash and nasal brush samples were obtained using the technique previously described [6,24].
2.6.2. Daily follow up during the acute hospitalization
Patients were followed daily while hospitalized by the study coordinators. We collected information on the use of supplemental oxygen, intravenous fluids, medications administered (albuterol or other bronchodilators, hypertonic saline inhalations, antibiotics, oral steroids, ICS, and montelukast), and length of hospitalization.
2.6.3. Weekly phone calls during the 2 weeks of active treatment phase of the study (MED 1, MED 2)
Weekly phone calls were made to review the study medication diary to monitor for adherence and adverse reactions.
2.6.4. Clinic visit: V1: 14 days after randomization (day 15 with a window of ±3 days)
The following procedures were performed: A brief history (including adverse drug reactions) and physical exam. The presence or absence of wheezing on examination was documented. The study medication diary was reviewed. Collection of blood, nasal wash and brush samples, urine, and stool samples (collected before or during visit). If a stool sample was not obtained, the family was given instructions to collect it at home and ship it to the study team by a courier service. If the family was unable to attend the clinic visit, a phone call was conducted in lieu of the clinic visit to collect clinical outcomes.
2.6.5. Follow up telephone calls (PC): 2 months after enrollment, and then every 2 months (these phone calls have a window of ±3 weeks)
Follow up phone calls are used to obtain the following information:
-
a)
Symptoms of wheezing (with and without a cold), use of rescue albuterol and oral corticosteroids, ED/unscheduled office visits, hospitalizations for respiratory symptoms, and parental absence from work due to the child's respiratory illnesses.
-
b)
Parent-reported physician diagnosis of asthma.
-
c)
Use of antibiotics, ICS, montelukast.
-
d)
Changes in pet exposure, day care status, and cigarette smoke exposure, and any unanticipated changes in medical status.
2.6.6. Yearly clinic visits (V2–V5): the first yearly visit was conducted 6 months after randomization, and then every 12 months (these yearly visits have a time window of ±1 month)
Participants recruited early during the first RSV season (2016–2017) may have up to four yearly visits (V2–V5). Participants recruited late during the last RSV season (2018–2019) may have only one yearly visit (V3). If any of these visits are conducted within the time frame of any of the phone calls, then the information from this visit is recorded in lieu of the phone call data. However, if the family cannot attend the yearly clinic visit (e.g., left the St. Louis metro area, Coronavirus disease of 2019 pandemic), a phone call is conducted in lieu of the clinic visit to collect clinical outcomes. The clinic visit includes the following:
-
a)
Brief physical exam (including weight and height) performed by the study coordinator.
-
b)
Monitoring for the same symptomatology, medication use, and parent-reported physician diagnosis of asthma as specified in the follow up phone call section.
-
c)
Obtaining nasal wash sample (V2 and V4 or last visit (V3) for year three recruits and nasal brush sample (V2 only)).
-
d)
Collecting stool sample that was obtained at home (V2 and V4 or last visit (V3) for year three recruits).
-
e)
Obtaining blood for V4, or last study visit (V3) for year three recruits.
2.7. Sample collection
2.7.1. Blood samples
Blood was collected at randomization and V1 for all enrolled patients whose parents consented to the blood collection. For the participants recruited at the first and second RSV season, blood samples were collected at V4. For the participants recruited at the third RSV season, blood samples were collected at V3 as they will not be in the study long enough for a V4 visit (Table 4). Baseline eosinophil count was obtained as a potentially relevant covariate for RW development. Eosinophil count and serum IgE levels to aeroallergens (house dust mite, cat, dog, mouse, and cockroach) were obtained as specified in Table 4 to evaluate the effect of azithromycin on atopy development.
2.7.2. Nasal wash
Nasal wash samples were obtained using the technique we utilized in our pilot APW-RSV study and in the RBEL study [6,24]. These samples will be utilized to measure a) inflammatory markers (IL-8 and, matrix metallopeptidase 9) in samples obtained at RZ, V1, and V2, b) upper airway microbiomes including antibiotic resistance studies in samples obtained at RZ, V1, and V2.
2.8. Study outcomes
The primary clinical outcome is the occurrence of a third episode of post-RSV wheezing measured over a follow up duration of 18–48 months.
2.8.1. Primary outcome selection rationale
Recurrent wheeze has been demonstrated to be a significant risk factor for physician-diagnosed asthma at seven years of age among children with RSV bronchiolitis in our previous RBEL study, and thus represents a robust intermediate outcome [6,34].
Defining the primary outcome as the “occurrence of a third episode of post-RSV wheezing”, which will be assessed using survival analysis methodology and not just “proportion of patients with recurrent wheeze”, will allow for the comparison of both the proportion of participants who develop recurrent wheeze and the time until this event between the treatment arms.
As the follow up period is 18–48 months, there is a 30-month variance in duration of follow up between the first and last recruit. This variance is related to recruitment over 3 RSV seasons and limitation of funding duration for up to 5 years. These 2 limitations did not allow for a uniform duration of follow up, unless each participant will be followed for no more than 18 months. Hence, we chose to use a variable duration of follow up in order to maximize follow up duration, and to increase our power to detect an effect of the intervention. Due to the variable duration of follow-up, we chose to analyze the primary outcome by survival analysis methodology, which fits this study design.
2.8.2. Episode of wheezing
The primary outcome is assessed during clinic visits that are conducted at 6, 18, 30, and 42 months from randomization, and by telephone call interviews that are conducted every two months (Table 4). Episode of wheezing is detected by the question “Has your child's chest sounded wheezy or whistling (with and without a cold)?” This question was adopted from the International Study of Asthma and Allergies in Childhood, and was previously utilized in our pilot APW-RSV study and in the RBEL study [6,24,35].
To define a new wheezing episode, a period of at least seven days between wheezing events must pass. If the time frame from the previous wheezing episode (last time that the child wheezed) to the onset of wheezing symptoms is shorter than seven days, these wheezing symptoms are attributed to the previous wheezing episode and not counted as a new event.
Wheezing detected during the initial RSV bronchiolitis is not included in the count of post-RSV wheezing. The time frame for the measurement of post-RSV wheezing (and other long-term outcomes) starts at the end of the treatment period (2 weeks after randomization) and ends at the last follow-up encounter. Therefore, a wheezing episode that occurs at the first 2 weeks of the study does not “count” toward the outcome of RW.
2.8.3. Secondary and exploratory outcomes
Secondary outcomes include: time to parent-reported physician diagnosis of asthma, annualized number of days of lower respiratory tract symptoms, rate of use of albuterol or corticosteroids (systemic and ICS), and the rates of drug related side effects and severe adverse reactions (SAE).
IgE levels and eosinophil counts measurements: Previous studies from in mouse models of viral bronchiolitis have shown that the viral bronchiolitis may lead to the development of type 2 airway inflammation [29,36]. In addition, a higher IGE level at the age of one year was detected in a cohort of infants hospitalized with RSV bronchiolitis in infancy [37]. Hence, we are aiming to characterize whether our study participants would develop characteristics of T2 inflammation and whether azithromycin may affect these markers.
Secondary and exploratory outcomes are listed in Table 5
Table 5.
Secondary and exploratory outcome variables.
| Secondary outcomes: |
|---|
| Annualized number of days with: any respiratory symptoms (wheezing, cough, or shortness of breath), or albuterol use. |
| Rate of oral corticosteroid courses. |
| Rate of antibiotic courses. |
| Rates of drug related side effects and severe adverse reactions*. |
| Time to parent-reported asthma diagnosis OR to the third episode of wheezing. |
| Time to parent-reported physician asthma diagnosis. |
|
Exploratory outcomes: |
| Annualized number of days with wheezing, and of days with nighttime awakening due to respiratory symptoms. |
| Cumulative number of wheezing episodes. |
| Annualized number of days with parental absence from work due to child's respiratory symptoms, and days with child absence from day-care. |
| Proportion of children prescribed asthma controller medications (ICS, LTRA). |
| Proportion of children with at least one positive serum specific IgE (SIgE) to inhalant allergen. |
| Rates of ED and urgent care visits, and of hospitalizations for respiratory symptoms. |
| Rates of upper respiratory tract infections. |
| Time to the fourth wheezing episode. |
| Total IgE level and eosinophil count**. |
ED: emergency department, ICS: inhaled corticosteroids; LTRA: leukotriene receptor antagonist.
* The monitoring of potential drug related side effects starts immediately after randomization. The time frame for measurement of all other long-term outcomes starts at the end of the treatment period (2 weeks from randomization) and ends at the end of the follow-up period.
These clinical outcomes are assessed during clinic visits that are conducted at 6, 18, 30, and 42 months from randomization, and by telephone call interviews that are conducted every 2 months (Table 4). These are measured as we previously described [6,24].
2.8.4. Impact of azithromycin on nasal wash IL-8 and MMP-9 levels
We will measure the effects of the intervention on IL-8 and MMP-9 nasal wash levels as markers for azithromycin's potential anti-inflammatory effects. Prior studies have shown that IL-8 and MMP-9 are biomarkers that may be affected by azithromycin therapy during RSV bronchiolitis and may predict future respiratory morbidity.
IL-8 is a neutrophil chemoattractant and elevated upper airway IL-8 levels have been associated with bronchiolitis severity [38,39]. We initially investigated the anti-inflammatory effects of azithromycin in a mouse model of viral bronchiolitis [23]. We reported that azithromycin decreased neutrophil accumulation in the lung and BAL and reduced the concentrations of CXCL1 (mouse equivalent of the human IL-8). Following this mouse study, we have performed the APW-RSV I proof of concept trial in 40 children hospitalized with RSV bronchiolitis. We reported that azithromycin decreased airway IL-8 levels in children with RSV bronchiolitis [24].
MMP-9 is a biomarker that correlates with the severity of lung injury and inversely correlates with lung function [40,41]. Hence, measuring its levels will complement the information we obtained from the IL-8 measurements. In a recent randomized phase 2 clinical trial that included children admitted to the pediatric intensive care unit with RSV bronchiolitis and required mechanical ventilation high dose azithromycin compared to placebo was associated with lower endotracheal MMP-9 levels on day 3 of treatment [42]. Future follow-up studies are required to evaluate if lower endotracheal MMP-9 levels would be associated with reduction in post-RSV wheezing.
Levels of both cytokines will be measured in nasal wash samples obtained at randomization, at the end of the study treatments (V1), and 6 months later (V2).
2.8.5. Microbiome outcomes
Airway microbiome has an important role in asthma inception [[25], [26], [27]]. Asymptomatic colonization of the hypopharynx with organisms such as Streptococcus pneumonia, Haemophilus influenzae, and Moraxella catarrhalis, was reported to be associated with a higher risk of developing bronchiolitis or pneumonia, persistent wheezing, and asthma during the preschool years [27,43]. Our preliminary data suggested that azithromycin treatment during RSV bronchiolitis modified the upper-airway microbiome composition, including a reduction in Moraxella abundance, which in turn was associated with lower odds of subsequent RW [28].
To evaluate the effect of AZM treatment on the upper airway microbiome, we compare the bacterial community structure between AZM and placebo groups at each of the following three time points: randomization, 2 weeks, and 6 months. Upper airway microbiome community structure is measured at the end of study treatments and is correlated with the occurrence of post–RSV RW using methods previously described [44].
The primary clinical outcome and the microbiome studies could be confounded by non-study antibiotic use, daycare attendance, breastfeeding and other infant feeding practices, upper respiratory infections (most commonly caused by rhinovirus), and indoor environmental exposures (allergens, tobacco smoke). We are systematically collecting data on these exposures during each encounter and will incorporate these into our analyses.
2.8.6. Antibiotic resistance studies
Macrolide antibiotic resistance patterns are being examined at samples taken at baseline, at the end of study treatments, and 6 months following randomization.
Nasal wash fluid was collected with Eswab and placed in Amies medium. After vortexing to remove specimen material from the swab, 100 μl of the eluate was inoculated onto two tryptic soy agar+5% sheep blood plates (BAP) and two chocolate agar plates (Hardy Diagnostics) which were streaked for isolation. Two azithromycin (15 μg) disks and one erythromycin (15 μg) disk (Hardy Diagnostics) were placed in the first quadrant of one BAP and one chocolate agar plates to screen for bacterial isolates with macrolide resistance. Plates were incubated at 35 °C in 5% CO2 and were examined for growth at 24 h and 48 h of incubation.
Upon examination of the plates, the presence or absence of upper respiratory microbiota was noted semi-quantitatively. On the agars containing no disks, all pathogenic organisms, including Streptococcus pneumoniae, beta-hemolytic Streptococcus species, Staphylococcus aureus, Staphylococcus intermedius, Moraxella catarrhalis, Enterococcus species, and Haemophilus species were isolated and identified. On the agars with disks, only isolates that were resistant to a macrolide (growing up to the disk) were isolated and identified. All isolates were identified using the VITEK MS MALDI-TOF (bioMerieux, Inc.). In addition, the identification of Streptococcus pneumoniae was confirmed using an optochin disk (Hardy Diagnostics).
Antimicrobial susceptibility testing (AST) was performed on Streptococcus pneumoniae, beta-hemolytic Streptococcus species, Staphylococcus aureus, Staphylococcus intermedius, and Enterococcus species recovered from the macrolide screening agars. On the non-screening agars, AST was performed on Staphylococcus aureus and Staphylococcus intermedius only. AST was performed using disk diffusion for cefoxitin, erythromycin, clindamycin, and azithromycin (Hardy Diagnostics) in accordance with and interpreted according to the Clinical and Laboratory Standards Institute guidelines. Inducible resistance to clindamycin was assessed using D-test when applicable.
2.9. Study medications
Generic azithromycin was purchased and dispensed by the investigational pharmacy at SLCH. We obtained an FDA's Investigational New Drug (IND) approval for using azithromycin as detailed in this protocol (IND: 112,359 (Exempt status)). The investigational pharmacy at SLCH also manufactured and dispensed the matching placebo using the following formulation for 30 ml of placebo:
-
1.
Mixed 31.5 g of SyrSpend SF Alka powder with 70 mg FD&C Red #3.
-
2.
Combined 2 g of SyrSpend SF Alka powder with 30 mg of powder obtained from Step #1.
Since azithromycin suspension, once reconstituted, is only stable for 10 days, we supplied parents with study medication for both the first and the second week of treatment.
Suspension for the first week of therapy was prepared (reconstituted with water) by the SLCH investigational pharmacy. The study medication powder was provided to the parents in the study medication bottle to be reconstituted by the parents for the second week of therapy. Before initiation of the second week of therapy, the parents were instructed to add sterile water to the study medication bottle based on our instruction (premeasured bottle of water was provided to the family). The study coordinator contacted the parents to assure the successful completion of this process.
2.10. Adherence
Parents were provided with a study medication diary at enrollment. Parents were instructed to write down medication administered in the diary. This was reviewed each day by the study coordinator during hospitalization and during the weekly phone call during the active treatment phase of the study. The diary was reviewed during the initial clinic visit (V1). Adherence assessment of the azithromycin versus placebo was based upon the medication diary entries.
2.11. Statistical design and analysis
The primary goal of this analysis is to test the null hypothesis that post- RSV RW is present at the same rate in the azithromycin group as compared to the placebo group. The primary analysis will be an intent-to-treat, comparing treatment groups using a Kaplan-Meier survival analysis. A Cox proportional hazards regression model will be used to estimate the magnitude of the treatment effect in terms of the hazard ratio for the occurrence of RW. In order to maximize the duration of follow-up in this trial which recruited subjects over 3 consecutive RSV seasons, participants have variable follow up durations determined by the year in which each participant is recruited.
2.11.1. Primary outcome analysis
The initial analytic strategy will be a log-rank test, which compares the Kaplan Meier survival curves describing the time to the third episode of wheezing. Then we will proceed to a multivariate Cox regression model that adjusts for potentially confounding variables. Race and parental history of asthma were significant predictors of childhood asthma development following severe RSV bronchiolitis in our RBEL cohort [6], and these covariates will be included in the multivariate model. We will assess other key (non-time-dependent) covariates using chi-square tests, t-tests, and potentially Wilcoxon's test to determine whether they will be included in subsequent multivariate models. Those fixed-time covariates that indicate a between-group difference (p < 0.1) will then be included in a stepwise multivariate Cox regression analysis along with the time dependent covariates (e.g., pet exposure). Additional analyses will include the use of an Anderson-Gill model in which wheezing episodes are treated as recurrent events when compared between groups [45]. In Cox models that do not contain time-dependent covariates, we will evaluate the proportional hazards assumption by assessing the parallelism of the log-log plots and using martingale residuals and the method of Lin and Ying [46]. The Cox models which contain time-dependent covariates will be assessed using Schoenfeld residuals [47].
The key statistic to be generated by the Cox regression analyses, for primary outcome reporting, will be covariate adjusted hazard rate ratios that compare the RW hazard rate in the azithromycin group with the corresponding hazard rate in the control group. Ninety-five percent confidence bounds will be generated for all calculated hazard rate ratios.
Finally, we will explore potential interactions between study treatment assignment and pre-specified covariates to evaluate whether these covariates are predictors of response to azithromycin therapy:
-
a.
Demographics: Sex, age, and race.
-
b.
Baseline asthma/allergy phenotypic characteristics: Personal history of eczema, parental history of asthma, and peripheral eosinophil count on enrollment.
-
c.
Environmental phenotypic characteristics: secondhand smoking, and pet exposure.
-
d.
Acute bronchiolitis characteristics: Duration of respiratory symptoms before enrollment, and the presence of co-infection with other respiratory viruses during RSV bronchiolitis (e.g. rhinovirus).
2.11.2. Censoring considerations
All subjects that do not attain the primary outcome (RW) will be censored at the time of the last contact with the subject. This applies whether the subject has dropped out or has completed the study without RW (administrative censoring).
2.11.3. Sensitivity analyses
Additional pre-specified statistical analyses will examine subset differences between children who were intubated during the acute RSV bronchiolitis and children who were not intubated to better interpret the results of the primary analysis based on the entire dataset. This will be performed by the following steps:
-
1.
We will examine potential differences in baseline covariates between children who were and were not intubated. If this analysis yields at least some suggestion of a between-group difference (p < 0.1), the presence/absence of intubation will be included as a covariate in the model that will be used for the primary outcome analysis.
-
2.
We will explore potential interaction between intubation and study treatment assignment although the power to detect such differences may be low (no preliminary data is available to project the expected power).
-
3.
We will repeat steps #1 and #2 for examining the effect of any mechanical ventilation (intubation, CPAP, BiPAP).
2.11.4. Secondary and exploratory outcomes analyses
Secondary outcome measures will be analyzed using a variety of statistical approaches. Biological outcome measures such as IL-8 levels measured at baseline and at two weeks after completion of the treatment will be evaluated using analysis of covariance with the post treatment measure as the dependent variable and with predictor variables that include the baseline value, the treatment group, and other pre-defined covariates.
Secondary outcome measures that are time-to-event variables such as asthma diagnosis will be compared across groups using survival and Cox regression approaches. Other secondary outcome measures are continuous measures that will be based on patient reports at phone calls that are made every two months. These include the number of days during the prior two months in which the subject experienced respiratory symptoms, used the rescue inhaler, or awakened at night due to such symptoms. These frequently occurring events will be evaluated using mixed model repeated measures analysis of variance in which the repeated outcome will be quantified every two months. We anticipate using an autoregressive or Toeplitz covariance structure in the repeated measures analyses because we expect within subject correlation coefficients to be smaller when data points are close together. However, the determination of the appropriate covariance structure will be based on more formal assessments of these correlation coefficients as well as on the Schwarz-Bayesian criterion and Akaike's information criterion.
Data regarding antibiotic use, systemic corticosteroids use, and the occurrence of upper respiratory infections will be also collected every two months. These data will be used to determine the rate of these events during each six months study interval, and these repeated measures data will be compared across groups using Generalized Estimating Equations.
2.11.5. Power calculation for the primary clinical outcome
Power calculations were performed using the program Power and Precision, Version 4 [48]. They accounted for the recruitment of patients over 3 consecutive RSV seasons (~5 months per year) with a follow up period of 18–48 months (Fig. 1: Study Design). Our power computations were based on an evaluation of the four year survival and hazard curves generated by 190 participants of the RBEL I cohort who share the same characteristics as the controls in this proposed research, and hence are used to estimate the RW rate in the control group [6]. The survival pattern observed in our randomized pilot study (APW-RSV) was utilized to estimate the effect of the intervention [24]. The RBEL survival curves showed a low placebo group hazard rate (HR) of about 2% during the first three months after hospitalization. This HR increased steadily to about 5% per month at the end of the first year. After that, HRs decreased steadily to 3% per month by the end of year two down to a monthly rate of 2% during year three, and 1% during year four. These assumptions yielded a four-year hazard rate of 71.6%, nearly identical to the actual rate of 70.4% observed in RBEL I. We used the HR as described as the basis for the placebo group in our power computation. These projected hazard rates yielded a four-year hazard rate of 55.1%. Thus, the power computations represented hazard rates of 71.6% in the placebo group and 55.1% in the azithromycin group over the follow-up period. Based on the parameters described above, two-sided tests at the 0.05 level of significance, and assuming a dropout rate of 20%, power computations yielded a requirement of 94 randomized subjects per group for a power of 0.9, and 70 per group for a power of 0.8. In evaluating these computations, it is important to note that we have assumed a hazard ratio during the first 18 months of at least two. Based on these considerations, we initially planned on randomizing a total of 188 subjects but for enhanced power, and with permission from the DSMB, we randomized a total of 200 subjects.
2.11.6. Microbiome outcomes
We will sequence all the nasal wash samples by 16S rRNA gene sequencing (V1-3 region) at Illumina MiSeq platform at Jackson Laboratory for Genomic Medicine [49,50]. 16S data are processed using USEARCH pipeline [51]. To evaluate the effect of azithromycin treatment on the upper airway and gut microbiome, we will compare the bacterial community structure between azithromycin and placebo groups at each of the following three time points: randomization, at two weeks and at six months, utilizing multivariate statistical testing that are widely used for the 16S microbiome analysis [52]. Data dimension reduction approaches will be used to identify the microbial clustering patterns and assess the similarity between the two groups in a reduced dimensional space at two individual time points. We will use PERMANOVA to test the significant differences of the overall bacterial community between the two groups [53]. To correct for potential confounding factors, we will test individual clinical variables in a PERMANOVA model. All the variables that show a trend towards an association with the treatment assignment will be included in the model, as described previously [54]. We will apply statistical approaches developed for differential bacteria feature identification to identify the signature bacteria taxa that contribute to the difference between two compared groups [55].
We will measure both alpha and beta diversity of the bacterial community in the two groups. Alpha diversity index such as Shannon diversity and richness will be used to evaluate the complexity of the whole microbial community; and Wilcox-sum-Rank testing will be used to test the difference diversities between compared groups. Beta diversity represented by Bray-Curtis dissimilarity will be used to indicate the inter-subject variation in the bacterial composition. The above analyses will allow us to characterize the microbial community at each individual sampling point, and to evaluate how it is affected by the azithromycin treatment.
To examine the relationship between microbiome changes and the diagnosis of RW, we will apply a linear mixed model to bacteria of interest such as the bacteria that are significantly different between the two groups identified at individual time points, as we have previously performed [56]. Microbiome data will be normalized to satisfy the assumption of normality by a logarithmic transformation. In the linear mixed model, transformed bacterial abundance will be modeled as the response, the sample collection time points, treatment groups, and RW outcome as fixed effects, and patients as random effect. P-values from multiple comparisons will be corrected by the false discovery rate (FDR) approach.
2.11.7. Power calculation for the microbiome outcomes
We utilized the whole study population (n = 200) for this aim. This sample size provided 90% power to detect the difference for a given taxa between the two groups, with effect size (5%) and standard deviation (SD1 = 2 and SD2 = 10) and alpha = 0.05. However, 141 patients are sufficient to provide 80% power to detect the same effect size. All sample size calculations assume 10% sample failed rate, which may be from different stages of the experiments, such as patient drop out, sample preparation and sequencing failure (the combined failure rate related to the last 2 reasons in our APW-RSV trial was less than 4%). These power calculations were performed based on the assumption that we will observe half of the effect size and standard deviation detected in our preliminary data, in which Moraxella decreased significantly after azithromycin treatment. We used a decreased effect size to infer the sample size as Moraxella was a relatively high abundance taxon in the data and was affected by the intervention in our preliminary studies. We are also interested in comprehensive detection of the difference of relatively rare taxa in those two groups.
2.12. Data and safety monitoring plan
The APW-RSV II clinical trial is overseen by the Washington University Human Research Protection Office (HRPO). We submitted an FDA Investigational New Drug (IND) application to allow the use of azithromycin in children 30 days-6 months of age, given the current indication for azithromycin is limited to children aged 6 months or older. The FDA reviewed our applications and concluded that an IND was not required to conduct this study (PIND 112359).
The PIs, Co-Investigators, the Washington University HRPO, and a National Heart, Lung, and Blood Institute (NHLBI) Data and Safety Monitoring Board (DSMB) monitor the study for adverse events, adherence to protocol, and patient accrual and withdrawal. All adverse events are reported to the Washington University School Medical Center IRB and the DSMB. In addition, we are following the NHLBI criteria for expedited reporting of severe adverse events (SAE) and unanticipated problems: these are reported to the IRB, NHLBI and if required will also be reported to the FDA.
2.12.1. Safety monitoring
The safety-reporting period began at the time of enrollment and ends with the completion of study participation (last visit or phone call). The intervention (study drug administration) ended 14 days after enrollment. Therefore, while reporting will continue for 48 months from enrollment, the study-related risks to the safety of children in the final 47 months of participation are limited to monitoring procedures at the four study visits (V2–V5). Therefore, all Severe Adverse Events (SAEs) and Adverse Events (AEs) were collected and reported from enrollment to time of Phone Call-1 (2 months following randomization). After Phone call 1, AE reporting is focused on the AEs of Special Interest (AESI; related to study procedures) for the remainder of the study period. SAEs will be continued to be reported until the end of study participation.
The PI or one of the other investigators, designated by the PI, decided if a SAE was related to the study medication. If it was determined that a SAE was possibly related, probably related, or definitely related to the study medication, then the study medication was discontinued. As with all AE/SAE, the participants will be followed until resolution of this event. We will not withdraw the participants from the study, as our analysis approach is an intention to treat approach.
3. Discussion
The APW-RSV II clinical trial is designed to investigate whether azithromycin therapy, an easily implemented intervention during RSV bronchiolitis, can reduce the risk of development of subsequent RW and asthma. This clinical trial has the potential to greatly impact pediatric health as up to 13% of new childhood asthma cases are attributable to RSV bronchiolitis [57].
Previous reviews and meta-analyses have established that early life RSV- lower respiratory tract infection (LRTI) is associated with future RW, asthma, and with impaired respiratory physiologic measurements such as reduced lung function and increased airway reactivity [[3], [4], [5]]. Since RSV-LRTI may have a causative role in asthma inception, primary prevention of RSV-LRTI may prevent this outcome. Indeed, a Dutch trial investigated this question and revealed that the prophylactic administration of the anti-RSV monoclonal antibody palivizumab to late pre-term infants resulted in approximately 50% reduction in wheezing days during the 1st year of life [58]. A follow-up study at the age of six years reported a reduction in parent-reported asthma, but there was no difference in physician-diagnosed asthma or lung function [59]. This Dutch trial focused on prevention of RSV-LRTI as an approach to prevent post-RSV RW. The attempt to prevent childhood asthma by RSV vaccinations is complicated by methodological issues resulting in inadequate power, as demonstrated by a recent analysis, which estimated that up to 30,000–100,000 participants are required to detect the effect of maternal RSV vaccines on RW/Asthma in their offspring [60]. In the APW-RSV II clinical trial, we have chosen a different approach as we are aiming to prevent wheezing episodes in children who already developed severe RSV- LRTI.
The target population of the APW-RSV II clinical trial includes children with severe RSV-LRTI (i.e., all required hospitalization). We decided to focus on this target population as not all RSV infections are the same in regards to the consequences of post-RSV RW and asthma. It has been reported that there is a gradient of increasing risk of asthma based upon bronchiolitis severity (as reflected by level of heath care utilization), with the greatest risk of asthma following bronchiolitis hospitalization [7,8].
There is an ongoing discussion on the question of whether early life RSV-LRTI is the cause of post-RSV RW and asthma, or just a marker of asthma tendency [61]. The APW-RSV II clinical trial may add new data on this important “cause and effect” question as we are investigating whether an intervention during the initial RSV-LRTI can prevent post-RSV RW. If RSV is indeed the cause of future wheezing and asthma, and since these consequences are associated with the RSV severity, then an intervention during the acute RSV-LRTI aiming to attenuate inflammatory response in this critical time may reduce the risk of developing future wheezing and asthma.
The APW-RSV II study has several unique characteristics: we applied a relatively short intervention (2 weeks) during the initial event (RSV-LRTI), and we are monitoring its effect for up to four years. A relatively long duration of follow-up is valuable, as it will allow us to evaluate whether the effect of the intervention is stable and does not subside with time. We have designed ancillary studies to investigate potential mechanisms that may mediate azithromycin effects: anti-inflammatory (nasal IL-8 measurements), prevention of lung tissue damage (nasal MMP-9 measurements), and airway microbiome modifications.
One limitation of azithromycin therapy is its potential to induce airway colonization with macrolide-resistant bacteria. Therefore, we are conducting an ancillary study investigating the short and long-term effect of azithromycin on the rate of airway colonization with macrolide-resistant bacteria. Our future recommendations for clinical management will depend not only on the efficacy of the intervention, but also on the magnitude of emergence and persistence of macrolide-resistant bacteria. In addition, azithromycin therapy during acute LRTI in preschool children was reported to be associated with short-term, but not long-term, changes to the gut microbiome [62]. Moreover, recent retrospective study revealed positive dose-dependent associations between the use of antibiotics, and mainly azithromycin, during acute bronchiolitis and future asthma [63]. This association was significant if the exposure was in the second or third year of life, but not if the exposure was in the first year of life. Based on the results of our previous pilot trial, and since the mean age of our cohort is 3 months, we do not believe this is a concern in our study population [24]. Nevertheless, azithromycin effect on the outcome of asthma will be evaluated in our current APW-RSV II clinical trial.
To conclude, if our hypothesis is confirmed, it will change the management of severe RSV bronchiolitis. The focus in the treatment will be shifted from the current approach of supportive care only to long-term prevention of post-RSV RW, potentially representing the first successful post-RSV asthma prevention modality.
Funding
The Azithromycin to Prevent Wheezing following severe RSV bronchiolitis-II clinical trial is funded by the National Heart, Lung, and Blood Institute (NHLBI), R01HL130876 and is registered at Clinicaltrials.gov (NCT02911935).
Contributor Information
Mythili Srinivasan, Email: srinivasanmythili@wustl.edu.
Leonard B. Bacharier, Email: leonard.bacharier@vumc.org.
Charles W. Goss, Email: cwgoss@wustl.edu.
Yanjiao Zhou, Email: Yazhou@uchc.esu.
Jonathan Boomer, Email: jboomer@kumc.edu.
Sarah Bram, Email: sarahnsmith@wustl.edu.
Carey-Ann Burnham, Email: cburnham@wustl.edu.
Timothy Casper, Email: timothy.casper@mercy.net.
Mario Castro, Email: mcastro2@kumc.edu.
Andrea Coverstone, Email: acoverstone@wustl.edu.
Matthew Haslam, Email: matthewdhaslam@gmail.com.
Watcharoot Kanchongkittiphon, Email: champw@hotmail.com.
Cadence Kuklinski, Email: ckuklin@wustl.edu.
Qinghua Lian, Email: lianq@wustl.edu.
Kenneth Schechtman, Email: kschechtman@wustl.edu.
Gregory A. Storch, Email: storch@wustl.edu.
Kelly True, Email: kelly.true@wustl.edu.
Meghan A. Wallace, Email: meghan.wallace@wustl.edu.
Huiqing Yin-DeClue, Email: hyindeclue@kumc.edu.
Elizabeth Ahrens, Email: ahrens.liz@wustl.edu.
Jinli Wang, Email: jinliwang@wustl.edu.
Avraham Beigelman, Email: beigelmana@wustl.edu.
Appendix 1.
Author Contribution
M. Srinivasan: Methodology, Validation, Formal analysis, Investigation, Resources, Writing – original draft, Writing – review & editing, Supervision, and Project administration. LB. Bacharier: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Supervision, Project administration, and Funding acquisition. CW. Goss: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Supervision, and Project administration. Y. Zhou: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Supervision, Project administration, and Funding acquisition. J. Boomer: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing – review & editing, Supervision, and Project administration. S. Bram: Methodology, Validation, Investigation, and Writing – review & editing. D. Burgdorf: Conceptualization, Methodology, Validation, Investigation, Data curation, Writing – original draft, Writing – review & editing, Supervision, and Project administration. C-AD. Burnham: Conceptualization, Methodology, Validation, Investigation, Data curation, Writing – original draft, Writing – review & editing, Supervision, and Project administration. T. Casper: Methodology, Validation, Investigation, and Writing – review & editing. M. Castro: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – review & editing, Supervision, and Project administration. A. Coverstone: Methodology, Validation, Investigation, and Writing – review & editing. M. Haslam: Conceptualization, Methodology, Validation, Investigation, Writing – review & editing, and Project administration. W. Kanchongkittiphon: Methodology, Validation, Investigation, and Writing – review & editing. C. Kuklinski: Methodology, Validation, Investigation, and Writing – review & editing. Q. Lian: Software, Validation, Formal analysis, Investigation, and Writing – review & editing. K. Schechtman: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Supervision, Project administration, and Funding acquisition. GA. Storch: Conceptualization, Methodology, Validation, Investigation, and Writing – review & editing. K. True: Conceptualization, Methodology, Validation, Investigation, Writing – review & editing, and Project administration. MA. Wallace: Conceptualization, Methodology, Validation, Formal analysis, Investigation, and Writing – original draft, Writing – review & editing. H. Yin-Declue: Conceptualization, Methodology, Validation, Investigation, Writing – review & editing, and Project administration. E. Ahrens: Methodology, Validation, Investigation, Writing – review & editing, and Project administration. J. Wang: Software, validation, formal analysis, investigation, and writing review and editing. A. Beigelman: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Supervision, Project administration, and Funding acquisition.
References
- 1.Akinbami L.J., Moorman J.E., Garbe P.L., Sondik E.J. Status of childhood asthma in the United States, 1980-2007. Pediatrics. 2009;123(Suppl 3):S131–S145. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- 2.Guilbert T.W., Morgan W.J., Zeiger R.S. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N. Engl. J. Med. 2006;354(19):1985–1997. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 3.Fauroux B., Simoes E.A.F., Checchia P.A. The burden and long-term respiratory morbidity associated with respiratory syncytial virus infection in early childhood. Infect. Dis. Ther. 2017;6(2):173–197. doi: 10.1007/s40121-017-0151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Regnier S.A., Huels J. Association between respiratory syncytial virus hospitalizations in infants and respiratory sequelae: systematic review and meta-analysis. Pediatr. Infect. Dis. J. 2013;32(8):820–826. doi: 10.1097/INF.0b013e31829061e8. [DOI] [PubMed] [Google Scholar]
- 5.Shi T., Ooi Y., Zaw E.M. Association between respiratory syncytial virus-associated acute lower respiratory infection in early life and recurrent wheeze and asthma in later childhood. J. Infect. Dis. 2019;222(Suppl 7):S628–S633. doi: 10.1093/infdis/jiz311. PMID: 31370064. [DOI] [PubMed] [Google Scholar]
- 6.Bacharier L.B., Cohen R., Schweiger T. Determinants of asthma after severe respiratory syncytial virus bronchiolitis. J. Allergy Clin. Immunol. 2012;130(1):91–100 e103. doi: 10.1016/j.jaci.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll K.N., Wu P., Gebretsadik T. The severity-dependent relationship of infant bronchiolitis on the risk and morbidity of early childhood asthma. J. Allergy Clin. Immunol. 2009;123(5):1055–1061. doi: 10.1016/j.jaci.2009.02.021. 1061 e1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Escobar G.J., Masaquel A.S., Li S.X., Walsh E.M., Kipnis P. Persistent recurring wheezing in the fifth year of life after laboratory-confirmed, medically attended respiratory syncytial virus infection in infancy. BMC Pediatr. 2013;13:97. doi: 10.1186/1471-2431-13-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beigelman A., Bacharier L.B. The role of early life viral bronchiolitis in the inception of asthma. Curr. Opin. Allergy Clin. Immunol. 2013;13(2):211–216. doi: 10.1097/ACI.0b013e32835eb6ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everard M.L., Swarbrick A., Wrightham M. Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Arch. Dis. Child. 1994;71(5):428–432. doi: 10.1136/adc.71.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marguet C., Bocquel N., Benichou J. Neutrophil but not eosinophil inflammation is related to the severity of a first acute epidemic bronchiolitis in young infants. Pediatr. Allergy Immunol. 2008;19(2):157–165. doi: 10.1111/j.1399-3038.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- 12.Blom D., Ermers M., Bont L., van Aalderen W.M., van Woensel J.B. Inhaled corticosteroids during acute bronchiolitis in the prevention of post-bronchiolitic wheezing. Cochrane Database Syst. Rev. 2007;(1):CD004881. doi: 10.1002/14651858.CD004881.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Ermers M.J., Rovers M.M., van Woensel J.B., Kimpen J.L., Bont L.J., Group R.S.V.C.S. The effect of high dose inhaled corticosteroids on wheeze in infants after respiratory syncytial virus infection: randomised double blind placebo controlled trial. BMJ. 2009;338:b897. doi: 10.1136/bmj.b897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zomer-Kooijker K., van der Ent C.K., Ermers M.J., Rovers M.M., Bont L.J., Group R.S.V.C.S. Lack of long-term effects of high-dose inhaled beclomethasone for respiratory syncytial virus bronchiolitis: a randomized placebo-controlled trial. Pediatr. Infect. Dis. J. 2014;33(1):19–23. doi: 10.1097/01.inf.0000437807.83845.d6. [DOI] [PubMed] [Google Scholar]
- 15.Bulow S.M., Nir M., Levin E. Prednisolone treatment of respiratory syncytial virus infection: a randomized controlled trial of 147 infants. Pediatrics. 1999;104(6):e77. doi: 10.1542/peds.104.6.e77. [DOI] [PubMed] [Google Scholar]
- 16.Jartti T., Lehtinen P., Vanto T. Evaluation of the efficacy of prednisolone in early wheezing induced by rhinovirus or respiratory syncytial virus. Pediatr. Infect. Dis. J. 2006;25(6):482–488. doi: 10.1097/01.inf.0000215226.69696.0c. [DOI] [PubMed] [Google Scholar]
- 17.Simoes E.A. Treatment and prevention of respiratory syncytial virus lower respiratory tract infection. Long-term effects on respiratory outcomes. Am. J. Respir. Crit. Care Med. 2001;163(3 Pt 2):S14–S17. doi: 10.1164/ajrccm.163.supplement_1.2011112. [DOI] [PubMed] [Google Scholar]
- 18.Peng W.S., Chen X., Yang X.Y., Liu E.M. Systematic review of montelukast's efficacy for preventing post-bronchiolitis wheezing. Pediatr. Allergy Immunol. 2014;25(2):143–150. doi: 10.1111/pai.12124. [DOI] [PubMed] [Google Scholar]
- 19.Bisgaard H., Flores-Nunez A., Goh A. Study of montelukast for the treatment of respiratory symptoms of post-respiratory syncytial virus bronchiolitis in children. Am. J. Respir. Crit. Care Med. 2008;178(8):854–860. doi: 10.1164/rccm.200706-910OC. [DOI] [PubMed] [Google Scholar]
- 20.Berry M., Morgan A., Shaw D.E. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax. 2007;62(12):1043–1049. doi: 10.1136/thx.2006.073429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedlander A.L., Albert R.K. Chronic macrolide therapy in inflammatory airways diseases. Chest. 2010;138(5):1202–1212. doi: 10.1378/chest.10-0196. [DOI] [PubMed] [Google Scholar]
- 22.Cameron E.J., McSharry C., Chaudhuri R., Farrow S., Thomson N.C. Long-term macrolide treatment of chronic inflammatory airway diseases: risks, benefits and future developments. Clin. Exp. Allergy. 2012;42(9):1302–1312. doi: 10.1111/j.1365-2222.2012.03979.x. [DOI] [PubMed] [Google Scholar]
- 23.Beigelman A., Mikols C.L., Gunsten S.P., Cannon C.L., Brody S.L., Walter M.J. Azithromycin attenuates airway inflammation in a mouse model of viral bronchiolitis. Respir. Res. 2010;11:90. doi: 10.1186/1465-9921-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beigelman A., Isaacson-Schmid M., Sajol G. Randomized trial to evaluate azithromycin's effects on serum and upper airway IL-8 levels and recurrent wheezing in infants with respiratory syncytial virus bronchiolitis. J. Allergy Clin. Immunol. 2015;135(5):1171–1178. doi: 10.1016/j.jaci.2014.10.001. e1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beigelman A., Weinstock G.M., Bacharier L.B. The relationships between environmental bacterial exposure, airway bacterial colonization, and asthma. Curr. Opin. Allergy Clin. Immunol. 2014;14(2):137–142. doi: 10.1097/ACI.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bisgaard H., Hermansen M.N., Bonnelykke K. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978. doi: 10.1136/bmj.c4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bisgaard H., Hermansen M.N., Buchvald F. Childhood asthma after bacterial colonization of the airway in neonates. N. Engl. J. Med. 2007;357(15):1487–1495. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y., Bacharier L.B., Isaacson-Schmid M. Azithromycin therapy during respiratory syncytial virus bronchiolitis: upper airway microbiome alterations and subsequent recurrent wheeze. J. Allergy Clin. Immunol. 2016;138(4):1215–1219. doi: 10.1016/j.jaci.2016.03.054. e1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grayson M.H., Cheung D., Rohlfing M.M. Induction of high-affinity IgE receptor on lung dendritic cells during viral infection leads to mucous cell metaplasia. J. Exp. Med. 2007;204(11):2759–2769. doi: 10.1084/jem.20070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danesi R., Lupetti A., Barbara C. Comparative distribution of azithromycin in lung tissue of patients given oral daily doses of 500 and 1000 mg. J. Antimicrob. Chemother. 2003;51(4):939–945. doi: 10.1093/jac/dkg138. [DOI] [PubMed] [Google Scholar]
- 31.Olsen K.M., San Pedro G., Gann L.P., Gubbins P.O., Halinski D.M., Campbell G.D., Jr. Intrapulmonary pharmacokinetics of azithromycin in healthy volunteers given five oral doses. Antimicrob. Agents Chemother. 1996;40(11):2582–2585. doi: 10.1128/aac.40.11.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ballard H.O., Shook L.A., Bernard P. Use of azithromycin for the prevention of bronchopulmonary dysplasia in preterm infants: a randomized, double-blind, placebo controlled trial. Pediatr. Pulmonol. 2011;46(2):111–118. doi: 10.1002/ppul.21352. [DOI] [PubMed] [Google Scholar]
- 33.Ballard H.O., Anstead M.I., Shook L.A. Azithromycin in the extremely low birth weight infant for the prevention of bronchopulmonary dysplasia: a pilot study. Respir. Res. 2007;8:41. doi: 10.1186/1465-9921-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Litonjua A.A., Carey V.J., Laranjo N. Effect of prenatal supplementation with vitamin D on asthma or recurrent wheezing in offspring by age 3 Years: the VDAART randomized clinical trial. J. Am. Med. Assoc. 2016;315(4):362–370. doi: 10.1001/jama.2015.18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asher M.I., Keil U., Anderson H.R. International study of asthma and Allergies in childhood (isaac) - rationale and methods. Eur. Respir. J. 1995;8(3):483–491. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 36.Park S.S., Ehlenbach S.J., Grayson M.H. Lung dendritic cells and IgE: the link between virus and atopy. Future Microbiol. 2008;3(3):241–245. doi: 10.2217/17460913.3.3.241. [DOI] [PubMed] [Google Scholar]
- 37.Sigurs N., Bjarnason R., Sigurbergsson F., Kjellman B., Bjorksten B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95(4):500–505. [PubMed] [Google Scholar]
- 38.Diaz P.V., Gaggero A.A., Pinto R.A., Mamani R., Uasapud P.A., Bono M.R. [Levels of inflammatory cytokines and plasma cortisol in respiratory syncytial virus bronchiolitis] Rev. Med. Chile. 2013;141(5):574–581. doi: 10.4067/S0034-98872013000500004. [DOI] [PubMed] [Google Scholar]
- 39.Smyth R.L., Mobbs K.J., O'Hea U., Ashby D., Hart C.A. Respiratory syncytial virus bronchiolitis: disease severity, interleukin-8, and virus genotype. Pediatr. Pulmonol. 2002;33(5):339–346. doi: 10.1002/ppul.10080. [DOI] [PubMed] [Google Scholar]
- 40.Sagel S.D., Kapsner R.K., Osberg I. Induced sputum matrix metalloproteinase-9 correlates with lung function and airway inflammation in children with cystic fibrosis. Pediatr. Pulmonol. 2005;39(3):224–232. doi: 10.1002/ppul.20165. [DOI] [PubMed] [Google Scholar]
- 41.Greenlee K.J., Werb Z., Kheradmand F. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol. Rev. 2007;87(1):69–98. doi: 10.1152/physrev.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kong M., Zhang W.W., Sewell K. Azithromycin treatment vs placebo in children with respiratory syncytial virus-induced respiratory failure: a phase 2 randomized clinical trial. JAMA Netw Open. 2020;3(4) doi: 10.1001/jamanetworkopen.2020.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vissing N.H., Chawes B.L., Bisgaard H. Increased risk of pneumonia and bronchiolitis after bacterial colonization of the airways as neonates. Am. J. Respir. Crit. Care Med. 2013;188(10):1246–1252. doi: 10.1164/rccm.201302-0215OC. [DOI] [PubMed] [Google Scholar]
- 44.Bacharier L.B., Guilbert T.W., Mauger D.T. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. J. Am. Med. Assoc. 2015;314(19):2034–2044. doi: 10.1001/jama.2015.13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson P.K., Gill R.D. Cox's regression model for counting processes: a large sample study. Ann. Stat. 1982;10:1100–1120. [Google Scholar]
- 46.Lin D.Y., Wei L.J., Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80(3):557–572. [Google Scholar]
- 47.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69(1):239–241. [Google Scholar]
- 48.Borenstein M., Rothstein H., Cohen J. Biostat Inc. 2001. Power and Precision-a stand-alone software program. Englewood, NJ. [Google Scholar]
- 49.Jumpstart consortium human microbiome project data generation working G. Evaluation of 16S rDNA-based community profiling for human microbiome research. PloS One. 2012;7(6) doi: 10.1371/journal.pone.0039315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edgar R.C., Flyvbjerg H. Error filtering, pair assembly and error correction for next-generation sequencing reads. Bioinformatics. 2015;31(21):3476–3482. doi: 10.1093/bioinformatics/btv401. [DOI] [PubMed] [Google Scholar]
- 52.Thomas T., Gilbert J., Meyer F. Metagenomics - a guide from sampling to data analysis. Microb. Inf. Exp. 2012;2(1):3. doi: 10.1186/2042-5783-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu G.D., Chen J., Hoffmann C. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Madan J.C., Koestler D.C., Stanton B.A. Serial analysis of the gut and respiratory microbiome in cystic fibrosis in infancy: interaction between intestinal and respiratory tracts and impact of nutritional exposures. mBio. 2012;3(4) doi: 10.1128/mBio.00251-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou Y.S.G., Sodergren E., Weinstock G., Walker W.A., Gregory K.E. Longitudinal analysis of the premature infant intestinal microbiome prior to necrotizing enterocolitis. PloS One. 2014 doi: 10.1371/journal.pone.0118632. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.James K.M., Gebretsadik T., Escobar G.J. Risk of childhood asthma following infant bronchiolitis during the respiratory syncytial virus season. J. Allergy Clin. Immunol. 2013;132(1):227–229. doi: 10.1016/j.jaci.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blanken M.O., Rovers M.M., Molenaar J.M. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N. Engl. J. Med. 2013;368(19):1791–1799. doi: 10.1056/NEJMoa1211917. [DOI] [PubMed] [Google Scholar]
- 59.Scheltema N.M., Nibbelke E.E., Pouw J. Respiratory syncytial virus prevention and asthma in healthy preterm infants: a randomised controlled trial. The Lancet Respiratory medicine. 2018;6(4):257–264. doi: 10.1016/S2213-2600(18)30055-9. [DOI] [PubMed] [Google Scholar]
- 60.Riddell C.A., Bhat N., Bont L.J. Informing randomized clinical trials of respiratory syncytial virus vaccination during pregnancy to prevent recurrent childhood wheezing: a sample size analysis. Vaccine. 2018;36(52):8100–8109. doi: 10.1016/j.vaccine.2018.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beigelman A., Bacharier L.B. Early-life respiratory infections and asthma development: role in disease pathogenesis and potential targets for disease prevention. Curr. Opin. Allergy Clin. Immunol. 2016;16(2):172–178. doi: 10.1097/ACI.0000000000000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei S., Mortensen M.S., Stokholm J. Short- and long-term impacts of azithromycin treatment on the gut microbiota in children: a double-blind, randomized, placebo-controlled trial. EBioMedicine. 2018;38:265–272. doi: 10.1016/j.ebiom.2018.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen IL HH, Chan YH, Huang HY, Yeh WJ, Wu TY, Suen JL, Yang SN, and Hung CH. Effect of antibiotic use for acute bronchiolitis on new-onset asthma in children. Sci. Rep..8(1):6090.. [DOI] [PMC free article] [PubMed]