Abstract
Environmental exposures, psychosocial stressors and nutrition are all potentially important influences that may impact health outcomes directly or via interactions with the genome or epigenome over generations. While there have been clear successes in large-scale human genetic studies in recent decades, there is still a substantial amount of missing heritability to be elucidated for complex childhood disorders. Mounting evidence, primarily in animals, suggests environmental exposures may generate or perpetuate altered health outcomes across one or more generations. One putative mechanism for these environmental health effects is via altered epigenetic regulation. This review highlights the current epidemiologic literature and supporting animal studies that describe intergenerational and transgenerational health effects of environmental exposures. Both maternal and paternal exposures and transmission patterns are considered, with attention paid to the attendant ethical, legal and social implications.
Subject terms: Epigenetics, Population genetics
Carrie Breton and colleagues review the literature supporting evidence for transgenerational health effects of environmental exposures by epigenetic mechanisms. This Review summarizes current knowledge based on animal and human cohort studies, and discusses the ethical, legal, and social implications of epigenetic research in humans
Introduction
Heritability of health and disease risk in humans and other species involves influences beyond the genetic code. While many successes in large-scale human genetic studies exist, there remains a substantial amount of missing heritability in complex childhood disorders. That is, genetic variants have often explained a small fraction of the overall heritability of these diseases and their effect sizes are small. Environmental exposures, including chemical toxicants, psychosocial stressors, behaviors, and nutrition, are all potentially important influences that may impact health outcomes directly or via interactions with the genome. Such processes may explain some of the missing heritability for conditions that repeat across generations. Nonetheless, the hypothesis that factors other than the DNA sequence can be molecularly transmitted across one or more generations is contentious1, eliciting well-worn Lamarck-versus-Darwin or nature-versus-nurture debates as well as raising concerns about epigenetic determinism, the risk of stigmatization or discrimination, and protection of privacy.
Some effects of environmental exposures can be transmitted across generations via changes to the sequence of germline DNA. It is also hypothesized that environmental exposure effects can be transmitted across generations via epigenetic modifications of germline DNA. For the purposes of this discussion, we will use the term intergenerational transmission to refer to transmission theoretically occurring when an exposure simultaneously affects the biology of an individual and its progeny, either by causing genetic or epigenetic changes to the individual and its germ cells or, in the case of pregnant females, causing genetic or epigenetic changes to the individual, its developing offspring, and the offspring’s fetal germ cells. We will use the term transgenerational transmission to refer to transmission occurring when genetic or epigenetic changes are passed on to a subsequent generation that did not experience direct exposure either preconceptionally or prenatally (Box 1). When nonpregnant individuals are exposed, the earliest transgenerational effects that could be assessed are in their grandchildren; when pregnant females are exposed, the earliest transgenerational effects that could be assessed are in their great-grandchildren2.
While evidence in support of transgenerational transmission exists in animal models3–9, evidence in humans is virtually nonexistent, in part because of logistic, financial, and ethical hurdles that limit epidemiologic studies spanning multiple generations. Many common environmental exposures are not restricted to a single-generation in humans, making it difficult to distinguish between inherited and current effects10. Major challenges to demonstrating that epigenetic mechanisms are specifically responsible for transgenerational effects include the need to evaluate epigenetic marks across multiple tissues, the difficulties in ruling out confounding effects of genetic, ecological, and sociocultural factors, and the inability to control completely the effects of other environmental influences11. Additionally, for feasibility and ethical reasons, human studies often require minimally invasive biomarker collection, such as through saliva or blood, which is insufficient to capture the complex dynamics of epigenetic changes that may occur in specific tissues throughout development12. Last, there are ethical, legal, and social implications to this work that should be considered particularly in the context of structural inequities13–15.
This review highlights the current epidemiologic literature and supporting animal studies that describe intergenerational and/or transgenerational health effects of environmental exposures. Both maternal and paternal exposures and transmission patterns are considered, with an emphasis on studies showing epigenetic modification as a potential mediator. Evidence for these processes in humans is drawn from the literature addressing a variety of exposures and health outcomes, including respiratory health, birth weight, obesity, cardiovascular health, and neurodevelopmental disorders. Last, we discuss the unique opportunities and challenges for investigating generational influences on child health in the Environmental influences on Child Health Outcomes (ECHO) study, which will integrate the study of genetics and epigenetics with exposures and health outcomes across diverse human populations in the United States.
Box 1 Definitions of terms used.
Epigenetics
The study of heritable changes in gene function not due to changes in DNA sequence
Epigenetic inheritance vs genetic inheritance
The transmission of epigenetic marks across generations compared to the transmission of DNA sequences across generations
Intergenerational transmission
Transmission theoretically occurring when an exposure simultaneously affects the biology of an individual and its progeny, either by causing genetic or epigenetic changes to the individual and its germ cells or, in the case of pregnant females, causing genetic or epigenetic changes to the individual, its developing offspring, and the offspring’s fetal germ cells.
Transgenerational transmission
Transmission occurs when genetic or epigenetic changes are passed on to a subsequent generation that did not experience direct exposure either preconceptionally or prenatally.2
DNA methylation
The addition of a methyl group (CH3) to a cytosine base in DNA.
Genetic and epigenetic mechanisms
Mechanisms of genetic inheritance include the following: germline chromosomal aneuploidies, germline DNA sequence variations such as single nucleotide polymorphisms, small segments of nucleotide insertions and deletions, and larger structural variants, as well as tandem repeats and retrotransposons (reviewed in Jackson et al. 201816). Epigenetic inheritance involves mechanisms that do not change the DNA sequence but can still alter phenotypes and be passed on through mitosis or meiosis, depending on whether one is evaluating somatic or germline cells. Epigenetic modifications may include the attachment or removal of molecules to DNA itself or to the proteins (histones) around which DNA is wrapped when chromatin is bundled, the positioning of those bundles (nucleosomes), or the interplay of transcription factors and other enzymes within gene-regulatory networks. DNA methylation occurs when a methyl group is added to the 5-carbon of a cytosine ring, resulting in 5-methylcytosine (5-mC). This addition occurs most often in the context of a CpG site, which is a cytosine nucleotide located proximal to a guanidine nucleotide. Throughout the genome, the majority of CpG sites are methylated, with the exception of CpG islands in the germline and near promoter regions, which are largely unmethylated and associated with gene expression. MicroRNA (miRNA), small noncoding RNA (ncRNA), and transfer RNA (tRNA) also can exert posttranscriptional control over gene expression. Modifications of N-terminal tail amino acids in histones H3 and H4, such as methylation, acetylation, phosphorylation, and ubiquitination, are associated with open- and closed- chromatin states. Depending on location, histone methylation may enhance or suppress gene expression17.
Higher levels of concordance in genome-wide methylation profiles between monozygotic vs. dizygotic twins suggest an interplay between genetics and epigenetics in the early years of life18. Using methylation arrays, McRae and colleagues19 evaluated the role of genetic heritability in DNA methylation patterns across generations among families that included twin pairs, as well as their siblings and parents. Inherited similarities in DNA methylation profiles were largely due to genetic effects, and non-CpG sequence variation only accounted for 20% of interindividual differences. However, whole-genome bisulfite sequencing approaches for assessing DNA methylation in multiple tissues have demonstrated that population-variable CpGs are depleted in promoter regions, and interindividual DNA methylation differences are primarily nongenetic in origin, with a nonshared environment accounting for most of the variance20. More recently, next-generation sequencing-based approaches have identified regions of systemic interindividual variation that are inter-correlated over long genomic distances, conserved across diverse human ethnic groups, sensitive to the periconceptional environment, and associated with genes implicated in a broad range of human disorders and phenotypes21. Together, these investigations suggest that the combined role of environmental exposures and genetic variation on inherited DNA methylation patterns may depend on the genomic context.
Germline changes associated with environmental exposures
Transgenerational effects, which are hypothesized to occur across multiple generations and transcend direct exposure, require modification of the genome and/or epigenome of germline cells. For practical reasons, epidemiologic studies have primarily assessed outcomes of maternal prenatal exposure in the gametes of male offspring, using semen quality as an indicator of potential heritability. In one analysis, sons of overweight mothers were found to have higher sperm DNA fragmentation compared with sons of normal weight mothers22. Some investigations have found associations of birthweight, a proxy for the fetal environment, with sperm concentration, motility, and morphology23–26. Human studies have reported prenatal exposure to both persistent27 and nonpersistent28 environmental chemicals to be associated with altered semen parameters, findings that are supported by links between endocrine-disrupting chemicals (EDCs) and semen quality in the animal literature29,30. Taken together, these results suggest that in utero exposures may program male fetal germ cells and/or Sertoli and Leydig cells, which are laid down in the fetal testes and are responsible for future sperm production31–33. The consequences of such effects on future progeny are largely unknown.
While germline cells may be impacted by environmental exposures in utero, during childhood, and throughout reproductive life, the embryonic period of gonadal sex determination is the most sensitive to environmental insults influencing the epigenome, as this is when human primordial germ cells undergo extensive epigenetic modification34. Epigenetic changes to the male and female germlines may occur during gametogenesis in the adult gonads, as well35. In females, two major functions of the ovary are the production of germ cells and sex steroid hormones, chiefly 17β-estradiol and progesterone. At birth, the mammalian ovary contains a finite number of primordial follicles36. These follicles constitute the ovarian reserve from which preovulatory follicles develop. Postnatal exposure to environmental, industrial, chemotherapeutic, and xenoestrogenic chemicals can diminish ovarian follicles and alter steroidogenesis, resulting in impaired ovarian function and infertility in animal studies37. Animal and human studies have shown environmental conditions to affect oocyte maturation, potentially resulting in long-term developmental consequences. For example, psychological stress may reduce oocyte competence, and bisphenol A (BPA) exposure has been associated with adverse effects on oocyte maturation and embryo quality and implantation38,39. Even low concentrations of BPA alter the epigenome of mammalian female germ cells, impacting gene expression, chromosome dynamics, and oocyte development40. Within in vivo animal models, components of tobacco smoke have been shown to alter gonadotropin-releasing hormone 3 (gnrh3) DNA methylation, global 5mC levels, and global histone H3 lysine 4 dimethylation (H3K4me2) in zebrafish brains and oocytes, reducing their development and maturation capability41,42.
In males, because sperm are produced continuously in the testes from puberty onward, current exposures are often considered as potential triggers of both genetic and epigenetic changes. A number of health conditions, behaviors, and environmental exposures in men have been associated with sperm DNA fragmentation, including obesity43, smoking44–46, varicocele47,48, sexually transmitted infection49–51, chemotherapy or radiotherapy52, paracetamol53, air pollution54–57, and a variety of EDCs58–61. In turn, sperm DNA fragmentation has been linked to male factor infertility62, miscarriage63,64, and reduced live birth rate after in vitro fertilization65. Male offspring of subfertile fathers who used intracytoplasmic sperm injection to conceive were at increased risk of poor semen quality66 and likely to have genetic abnormalities related to azoospermia67, indicating the potential heritability of subfertility that may result from environmentally-induced alterations to germline DNA. Epigenetic mechanisms have also been proposed to explain the transmission of obesity from father to child43,68. Rodent studies indicate that, in addition to diet and exercise69,70, paternal stress71 and exposure to EDCs72 may affect offspring health and behavior via epigenetic pathways. One of the most notable causes of sperm DNA fragmentation is oxidative stress (OS)73,74. Sperm are particularly vulnerable to genetic damage due to OS because sperm heads, which are filled with tightly packed chromatin, lack cytoplasm that contains the enzymes necessary for DNA repair75. OS may also affect the sperm epigenome, as some studies have shown hypoxia to be associated both with impairments in spermatogenesis and alterations in DNA methylation76–81. In general, it is theorized that OS-mediated sperm DNA fragmentation and/or epigenetic changes may underlie observed associations of a wide variety of paternal environmental and lifestyle factors with birth defects and childhood diseases82,83.
Rationale for epigenetic effects
Single-generation epigenetic effects on children
A growing body of literature supports associations between prenatal exposures and epigenetic modifications in children84,85. Epigenetic processes, such as CpG methylation and chromatin remodeling, are highly regulated during embryogenesis to ensure proper development86,87. Disruption of these mechanisms is of interest to child health researchers because they facilitate key developmental events, including placental and fetal growth, genomic imprinting, and cell differentiation88. While epigenetic modifications demonstrate plasticity during development, they also can remain stable, biologically embedding the effects of periconceptional exposures within offspring89. Environmental perturbation of epigenetic mechanisms can therefore change gene expression and result in altered outcomes, whether beneficial or adverse, at birth or later in life89,90. Nonetheless, studies that demonstrate epigenetic modifications that mediate the relationship between periconceptional exposures and child health using formal or causal mediation frameworks are generally lacking.
Epigenetic modifications may be induced by prenatal exposures and can be inherited intergenerationally, escaping the major waves of epigenetic reprogramming that occur during fertilization and gametogenesis86,87,91–106. One hypothesized molecular mechanism for bypassing the DNA methylation reprogramming wave is through small regulatory RNAs, sequentially generated in parental somatic tissues, packaged in extracellular vesicles (ECVs), and delivered to early embryos, where they ultimately drive a global reprogramming of genome expression107. Other means of escaping the early embryonic reprogramming are evidenced by CpG loci adjacent to intracisternal-A-particle elements or telomeric regions106. Single-generation epigenetic effects may also occur when exposures directly affect the developing somatic tissue postfertilization. Single-generation effects have been investigated primarily in relation to maternal factors, including diet, environmental chemical exposure, cardiometabolic disorders, gut microbiota, and mental health90,108–113 Paternal preconception diet and exposure to drugs, toxicants, and EDCs have also been associated with epigenetic modifications among children114,115.
Epidemiologic studies have often been limited in their ability to conclude causal effects of prenatal exposures on epigenetic modifications in children due to cross-sectional designs, underpowered analyses, cell-type heterogeneity, and other limitations common to epigenomic studies104,116,117. While increasing evidence supports that single-generation effects are likely to occur in humans, the extent to which these effects persist across multiple generations remains unclear.
Animal models of epigenetic inheritance
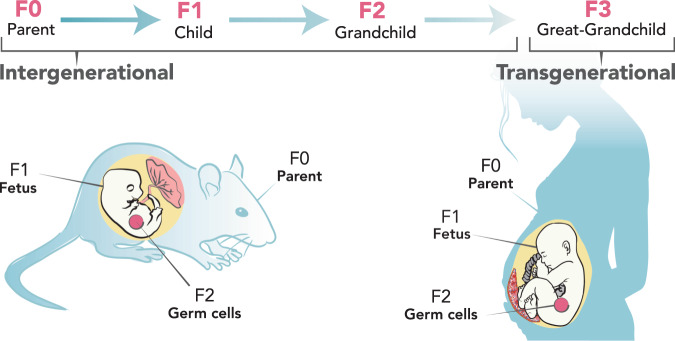
Numerous animal studies have described non-genetic inheritance of phenotypes, including the ones discussed below and others including eye color, cancer, and prostate and kidney diseases3–9,118–123. Often, these phenotypes result from environmental exposures during the parent generation, such as to particulate matter, diet, stress, and EDCs. This section discusses current evidence supporting both intergenerational and transgenerational effects on various health outcomes relevant to humans. In animal studies, generations are labeled as F0 (the exposed original generation), followed by F1, F2, F3… for subsequent generations. Because maternal F0 exposures that occur during pregnancy have the potential to affect the F1 fetus as well as the developing F1 gametes that give rise to the F2 grand offspring, only patterns of maternal transmission that extend to the F3 generation meet the stricter criteria for transgenerational inheritance (Fig. 1).
Fig. 1. Intergenerational and transgenerational inheritance.
Depiction of inheritance patterns from the parent (F0) generation to the child (F1), grandchild (F2), and great-grandchild (F3) in humans and animals. An exposure in F0 can directly affect the developing fetus (F1) and the germ cells in F2; therefore, both routes of transmission are considered intergenerational. Transgenerational effects may be observed beginning with the F3 generation.
Respiratory health
A number of rodent studies have focused on maternal prenatal environmental exposure and offspring respiratory outcomes. Some have investigated prenatal exposure to bacteria components, tobacco, nicotine, fungi, air pollutants, diet, maternal stress, and allergens in models of allergic asthma124–133 (detailed models reviewed in Kraus-Etschmann et al. 2015120). Others have linked prenatal exposures such as environmental tobacco smoke, maternal immunization, and traffic-derived particulate matter to impaired lung function and/or structure134–137, altered expression of asthma-related genes138,139, or reduced allergen-specific IgE production140. A few studies included exposure during the preconception period, and two studies focused exclusively on this period141,142.
Evidence for transmission of exposure effects on respiratory health-relevant outcomes beyond the F1 generation is emerging. Preconceptional and late-pregnancy sensitization to fungi was associated with lower IgE, altered airway eosinophilia, and differences in DNA methylation of IL-4 and IFN-g promoters in the F2 generation128. In utero exposure to environmental tobacco smoke altered lung function, increased inflammation, and was associated with changes in microRNA in both F1 and F2 generations133. In a study of maternal prenatal and perinatal exposure to phthalates in mice, increased allergic airway inflammation was observed in both F1 and F2 generations. A rat model of prenatal nicotine exposure demonstrated transmission of asthma phenotypes to the F1, F2, and F3 generation in males143,127 and an association with epigenetic changes in reproductive and lung tissues in the F1 generation127. Exposure to environmental particles during mouse embryonic development increased asthma-like phenotypes in the F1, F2, and F3 generation in offspring144. Despite all of these findings, the underlying mechanisms for the inheritance of asthma risk via epigenetic processes remain poorly understood120,145,146. Causal links between transgenerational epigenetic changes and disease development have yet to be established.
Obesity
In animal models of rats and mice, maternal prenatal nutrition has been shown to influence offspring development and long-term health, including body size, obesity, and related metabolic disorders147–149, although the mechanisms remain unclear10,150,151. Consumption of a high-fat diet during pregnancy has been shown to increase rates of obesity and metabolic syndrome in rats by predisposing offspring to an obesogenic phenotype152, providing some evidence of developmental programming that has been shown to persist across generations in studies of other rodents and mammals153–155. Few studies have examined F3 generation phenotypes, although increased body size and related phenotypes have been shown to persist across two generations of mice after prenatal exposure to a maternal high-fat diet156, with sex-specific and parental lineage effects in the F3 offspring157.
As previously reviewed in Vickers 2014119, there is additional evidence that ancestral exposure to obesogenic chemicals results in the transmission of obesity-related phenotypes through at least three generations158. Exposure of dams to EDCs such as the pesticide dichlorodiphenyltrichloroethane (DDT), a mixed hydrocarbon jet fuel (JP-8)159, the herbicide glyphosate160, and common plastic ingredients such as BPA, diethylhexyl phthalate, and dibutyl phthalate72, has led to increased obesity in F3 rats161. Prenatal exposure to environmental obesogens, including tributyltin162,163, heavy metals164, and the pesticide methyoxychlor165, has been shown to produce lasting effects to at least the F4 generation.
There is also some evidence that inherited sperm epigenetic mutations (epimutations) underlie transgenerational obesity as well as other outcomes in the F3 generation following F0 exposure during pregnancy72,166,167. Observed alterations to IGF-2 and H19 expression in F1 sperm and F1 and F2 offspring suggest their involvement, along with other sperm epimutations168, in the transmission of the obesity phenotype169. Transgenerational effects of obesogenic exposures could also occur through alterations in chromatin structure and accessibility163,170 or other mechanisms171.
Neurological/behavioral outcomes
As previously reviewed118,121,122, many animal studies provide evidence for epigenetic and long-term neurodevelopmental and behavioral consequences of maternal prenatal stress172, nutrition, obesity173, and other exposures174 in the developing offspring. Other studies suggest that paternal exposures can alter offspring neurodevelopment, potentially through epigenetic changes in sperm172,175–179. Fewer studies have investigated whether the effects of parental exposure are passed on to F2 generations and beyond. In zebrafish, prenatal exposure to contaminants, including low-dose inorganic arsenic180 and lead181, produced neurobehavioral, epigenetic, and brain transcriptomic alterations in the F2 generation. In mice, early postnatal stress transmitted depressive-like behaviors up to the third-generation (F1, F2, and F3). Behavioral traits co-segregated with altered DNA methylation in the male germ line182,183, and there was evidence for microRNA involvement176,177, suggesting that transgenerational inheritance could occur through the transmission of acquired epigenetic marks. Evidence in C. elegans suggests that small RNAs in neurons control chemotaxis behavior of the progeny for at least three generations184. Prenatal infections and immune challenges that produce intergenerational neurodevelopmental and neurobehavioral disorders have also been shown to transmit transgenerational behavioral abnormalities to F3 offspring, primarily through the paternal lineage and potentially through overlapping epigenetic mechanisms185. Rodent studies of impaired maternal care and social context produced altered behaviors, including neophobia in F1186 that persisted to the F2 and F3 generations through epigenetic mechanisms187. Maternal high-fat diet led to cognitive disabilities and an altered response to a noncompetitive receptor antagonist of the N-methyl-D-aspartate receptor in adult mouse F2 and F3 offspring in a sex-specific manner188. Maternal BPA exposure disrupted social interactions in F3 mice and dysregulated normal expression of postsynaptic density genes during neural development189. Similarly, chronic paternal adolescent stress produced transgenerational behavior and amygdala transcriptomic changes in F1 and F2 mice190.
Evidence in humans
Human studies that document both intergenerational and transgenerational effects of environmental exposures on health outcomes are relatively rare. This section presents available evidence, covering a range of environmental exposures and health outcomes. The underlying mechanisms that might explain these effects have been neither uniformly nor comprehensively tested and are, in fact, the subject of some debate1,2,11. While the focus here is on hypothesized epigenetic mechanisms of intergenerational and transgenerational transmission of environmental exposure effects, we acknowledge and discuss the evidence and the challenges in disentangling potential epigenetic mechanisms from concurrent genetic, ecological, and sociocultural factors that may track across generations.
Inter- and transgenerational effects on respiratory health
Moderate evidence exists for transmission of asthma, allergy, and other respiratory diseases through the third-generation. In the nationally representative Child Development Supplement to the Panel Study of Income Dynamics cohort of more than 2500 children, children with a grandparent with asthma had 1.5 times the odds of reported asthma. Children with a parent and grandparent with asthma had over four times the odds of reported asthma compared with those without a parental or grandparental history of asthma191. Studies such as these have implicated genetic risk factors or gene-environment interactions for inherited asthma risk across a generation;192–195 however, nonbiological explanations and interactions between genetic and non-genetic risk factors are also feasible. For example, multiple generations of families living in low-socioeconomic neighborhoods with higher exposure burden and greater poverty may also explain or exacerbate asthma risk196,197.
Perhaps, the most well-studied environmental risk factor for asthma is tobacco smoke. A number of cohort studies have begun to evaluate inter- and transgenerational asthma risk to cigarette-smoking exposure. In a case-control study nested within the Children’s Health Study in Southern California, grandmaternal smoking during pregnancy was associated with a 2-fold increased odds of asthma in the grandchild198. If both the child’s mother and grandmother smoked during pregnancy, the child had even higher odds of developing asthma compared with no exposure198. In the Norwegian Mother and Child Cohort Study, grandmaternal smoking during pregnancy was associated with 15% and 21% greater relative risk of child asthma at ages 3 and 7 years, respectively, and 15% greater relative risk for dispensed asthma medications at age 7 years199. Data from the Ageing Lungs in the European Cohorts Study found that grandmaternal smoking during pregnancy was associated with child asthma with nasal allergies200. In comparison to these studies based on retrospective reporting, prospectively collected data from the Swedish National Board of Health and Welfare and Statistics Sweden registries demonstrated that children of age 1 to 6 years had increased odds of asthma or of wheeze or asthma if their grandmothers smoked during weeks 10–12 of pregnancy, regardless of maternal smoking history. The odds of asthma were higher with greater cigarette exposure (10 + cigarettes/day vs. none: OR 1.23, 1.17–1.30). Grandmaternal smoking was associated most robustly with child early persistent asthma compared with early transient and late-onset asthma201. In contrast, the English population-based Avon Longitudinal Study of Parents and Children did not find evidence of transmission of effects of grandmaternal smoking during pregnancy on child lung function, bronchial responsiveness, or doctor-diagnosed asthma202. Overall, these studies provide relatively strong epidemiological support for an association between grandmaternal smoking and child asthma outcomes. Notably, because most of this research relied on retrospective reporting of exposures, unmeasured residual confounding, and potential recall bias may have influenced results. For example, differences in maternal education, geographical and temporal trends in smoking patterns, income level, and race and ethnicity not sufficiently included in study samples or completely accounted for in covariate-adjusted models may contribute to some of the discrepancies in the results.
A few studies also have compared differences in child asthma risk depending on maternal versus paternal line of transmission to examine whether fathers’ smoking may influence risk through, presumably, heritable epigenetic alterations in sperm precursor cells prior to conception; however, the results are mixed202,203. While some emerging evidence implicates epigenetic regulation as the underlying observed transgenerational health effects, the findings have been inconsistent204,205. Cross-generational effects in the absence of direct exposures may induce apparent changes in disease risk among generations through several mechanisms beyond epigenetic ones, including shared familial environment or other cultural effects146,204.
Inter- and transgenerational effects on birthweight
A few studies have examined birthweight across three generations. In the Uppsala Birth Cohort Multigenerational Study in Sweden, correlations between grandparent and grandchild birthweight were stronger along the maternal line; however, this finding did not account for maternal birthweight206. In the Aberdeen cohort, grandmother’s birthweight was associated with child birth weight independent of maternal birth weight as well as prenatal and sociodemographic factors207. Overall, the data from these and other cohorts suggest that social, environmental, and metabolic factors experienced by grandparents influence their grandchildren’s birthweight. Whether these effects are mediated by epigenetic mechanisms is unknown. In some cases, epigenetic modifications, such as DNA methylation, have been associated with birthweight in the F1 generation, but their persistence to the F2 generation or beyond has not been assessed100,208–210. Although the methylation patterns that are associated with birthweight persist into childhood, there is evidence that they may not persist into adulthood100,211. Therefore, transfer to the F2 generation is less likely, although possible, since these human studies do not assess the methylation of gamete cell DNA. Methylation patterns at the PBX1 locus have been associated with birthweight in more than one cohort, but are not associated with others210–212. This discrepancy in the results is unfortunately common in intergenerational epigenetic studies and may be due to differences in the environmental exposures that establish the epigenetic patterns.
Current data suggest that some of the intergenerational social influences on birth weight include education, geographical location, and other sociodemographic characteristics. For example, grandparents’ educational attainment and residential environment have been associated with grandchildren’s birthweight213,214. In the Fragile Families and Child Wellbeing Study, having a grandfather with less than a high school education was associated with a 93-gram reduction in birth weight and a 59% increase in odds of low birthweight214. An examination of multiple social and biological factors within the Aberdeen Children of the 1950s study revealed that both distal and proximal grandparental and parental life-course biological and social factors predicted child size at birth. Inequalities in size at birth appeared to be generated largely via continuity of the social environment across generations, with inequalities in maternal early-life growth predicted by the grandparental social environment during the mother’s early life215. Studies of the social influences of intergenerational effects on birthweight rarely address potential epigenetic mechanisms by which these exposures affect outcomes.
BMI and smoking history across generations have also been examined for their relations to grandchildren’s birth weight, but studies have largely failed to find direct associations. Some data suggest that associations between grandparents’ BMI and grandchildren’s birthweight are mediated by maternal BMI216,217. There is evidence that smoking may have intergenerational effects on birthweight. One study, which accounted for family clustering, found that birth weight was higher in children whose grandmother and mother both smoked during their pregnancies relative to children whose grandmother and mother both did not smoke during pregnancy218. This association was dependent on the grandmother’s birth cohort: infants whose grandmothers were born between 1929 and 1945 experienced this effect, but infants whose grandmothers were born between 1904 and 1928 did not. Other studies have also shown that the relation between grandmothers’ smoking and children’s birthweight is mediated by maternal smoking217,219,220. However, the pattern of the results of these studies is not consistent. Notably, these analyses have been conducted across a number of different time periods, when the content of cigarettes and the intensity of their use were changing frequently. Therefore, the transgenerational influence of this environmental exposure on birthweight, and any role for epigenetics, remains unclear.
Inter- and transgenerational effects on cardiovascular health
Changes in parents’ and grandparents’ food access have been associated with children’s cardiovascular health, sometimes with sex-specific patterns. Analysis of data from three cohorts born in 1890, 1905, and 1920 in Overkalix Municipality in northern Sweden revealed that if the food was not readily available during the father’s slow growth period (ages 9 to 12 years), then the child’s cardiovascular disease mortality was low; diabetes mortality increased if the paternal grandfather was exposed to a surfeit of food during his slow-growth period (OR 4.1, 95% CI 1.33–12.93)221. Cardiovascular mortality in the grandchild was not associated with sharp changes in food access experienced by maternal grandparents or paternal grandfather. However, if the paternal grandmother lived through a sharp change in food supply from year to year prior to puberty, her sons’ daughters had an excess risk for cardiovascular mortality (OR 2.69, 95% CI 1.05–6.92)222. Other studies have noted associations between grandmother’s exposure to famine during pregnancy and grandchild’s development of poor cardiometabolic outcomes by adulthood, including hyperglycemia and type 2 diabetes223. In the case of the Dutch Hunger Winter, individuals prenatally exposed to famine also had less DNA methylation of the imprinted IGF2 gene compared with their unexposed, same-sex siblings six decades later. These data suggested that very early mammalian development is a crucial period for establishing and maintaining epigenetic marks224.
Inter- and transgenerational effects on neurodevelopmental outcomes
Numerous grandparental exposures have been linked to grandchild neurodevelopmental outcomes. This section summarizes studies that have examined third-generation effects of tobacco and alcohol use, and stress/trauma on child neurodevelopmental outcomes, including cognitive abilities, emotional and behavioral symptoms/diagnoses, neurodevelopmental disorders, and substance-use disorders.
As was the case for respiratory health, grandparental smoking has also been associated with neurodevelopmental effects in the grandchildren. Existing studies have focused largely on associations with child-externalizing problems (e.g., aggression, rule-breaking). In at least two studies, the association between grandparental smoking history or substance use and child-externalizing behaviors was mediated by parental psychological symptoms and tobacco use225–227. Other studies have examined transgenerational effects of smoking on attention-deficit/hyperactivity disorder and an autism-spectrum disorder. An analysis of the Norwegian Mother and Child Cohort found that grandparental smoking during pregnancy had a similar magnitude of association with child attention-deficit/hyperactivity disorder diagnosis as maternal smoking during pregnancy228. These findings suggest that the association between maternal smoking during pregnancy and child attention-deficit/hyperactivity disorder may not be due to causal intrauterine effects, but rather reflect unmeasured confounding factors. In the Avon Longitudinal Study of Parents and Children, maternal grandmaternal smoking during pregnancy was associated with elevated granddaughter scores on autism spectrum traits and increased risk for autism-spectrum disorder diagnosis229. Notably, the effects were magnified for children whose mothers did not smoke during pregnancy. In contrast, paternal grandmaternal smoking was not related to these outcomes in grandchildren229.
Fewer studies exist on the effects of alcohol across generations. Available evidence suggests that grandparental and parental alcohol use have independent, cumulative effects on child risk for alcohol-use problems230,231. A large survey of college students revealed that problem drinking was greater among students reporting a parent or grandparent diagnosed with or treated for alcoholism, with the highest rates among those for whom both a parent and grandparent were affected230. Grandparental alcohol-use problems appear to have unique effects on child alcohol use problems, even when the parent is apparently unaffected231. In a nationally-representative sample of adults in Sweden born between 1980 and 1990, stability in alcohol-use disorders (AUD) declined by ~50% between the first and second generations. However, grandchildren with two or more grandparents affected by AUD had 40% greater AUD risk relative to grandchildren with only one affected grandparent231. Limited evidence suggests that the effects may be moderated by sex, with stronger associations of grandparent and parent AUD with child AUD when generations are of the same sex231. Across studies, the cross-generational patterns suggest a possible role for genetic transmission of risk for AUD; however, studies that consider the degree of exposure to alcohol-abusing family members indicate that risk is at least partially socially mediated232. Grandparent alcohol use also may influence grandchild behavioral precursors known to increase the risk for alcohol-use problems, such as aggression and inhibitory control. For example, one study found that grandparent alcohol use was associated with child aggression, with the effect mediated by parental AUD and by marital and parent–child aggression233. However, another study failed to find an association between grandparent alcohol use and child inhibitory control234. More research in this area is needed, including designs that can disentangle the independent and joint influences of genetic, epigenetic, social, and environmental factors on cross-generational associations. Notably, when interpreting findings in this area, consideration of the impact of changing norms about alcohol use across generations, including types of alcohol consumed and motivation for drinking, is important235.
In addition to the effects of chemical exposures, stress and trauma have also been evaluated across generations for their effects on neurodevelopment. The majority of third-generation studies examining the putative effects of grandparental stress and trauma exposures have been conducted among Holocaust survivors. In a meta-analysis of 13 community samples representing 1012 children with or without a grandparent who was a Holocaust survivor, Sagi-Schwartz and colleagues concluded that there was no evidence for tertiary traumatization236. Specifically, there were no differences in the psychological well-being and adaptation between grandchildren of Holocaust survivors and comparison groups; this result held regardless of the manner of participant recruitment. The results highlight the notion that Holocaust survivors may represent a group of resilient individuals without a genetic bias to developing posttraumatic stress reactions; some suggest such a characteristic helped promote survival (consistent with work on Rwandan survivors described by de Quervian et al. 2007237) and, subsequently, facilitated the well-being of their children and grandchildren238. Consideration of other contributing factors among Holocaust survivors is important given evidence that they tend to have fewer children per family than comparison groups239. Finally, one study has examined tertiary trauma risk in a non-Holocaust survivor sample of 2,282 first-grade children from seven districts in Shanghai, China240. Analyses showed that children whose parents and/or grandparents experienced traumatic events had higher scores on parent-reported behavioral and emotional problems compared with children with no family trauma history. However, no differences were observed when teacher reports were examined. The likely influence of parental characteristics on report of child behavior was further indicated in analyses that accounted for parental depressive symptoms, physical health, and parenting factors; in these adjusted models, any effect of grandparent trauma on child emotional and behavioral outcomes became negligible.
A handful of studies also show alterations to DNA methylation from parental-exposed trauma. For example, post-traumatic stress disorder from exposure to the Tutsi genocide has been associated with NRC31 epigenetic modifications in both mothers and their offspring, suggesting alterations to the HPA axis241. Adult offspring of Holocaust survivors show reduced DNA methylation and increased gene expression in FKBP5, a gene associated with indices of glucocorticoid sensitivity242.
To date, current evidence does not provide consistent support for third-generation effects of stress exposures on child neurodevelopmental outcomes. However, caution is warranted in making firm conclusions from the extant literature. Studies are relatively few in number and vary widely in sampling design, life stage of assessment, and type of outcomes measured. Retrospective and proxy reporting of exposures by parent or offspring may also be subject to bias.
Current operational and analytical challenges in the field
Inter- and transgenerational epidemiological studies face several obstacles to their success (Box 2). One of the greatest operational challenges to performing intergenerational and transgenerational investigations in human populations is the lengthy time (20–40 years) between generations. As a result, there is a paucity of data on the fourth generation in humans. This inherent limitation poses challenges to cohort retention and creates problems in terms of technological changes related to biospecimen storage and medical record keeping. Retrospective collection of information about experiences, exposures, and medical conditions is subject to both ascertainment and recall biases, particularly if a child or grandchild has a medical condition. Furthermore, parental age can be highly variable within and between families and generations of a human study, meaning that generations being compared within large human studies may span multiple decades. Methods for controlling for these inherent time variables are being developed, but this is an area where future research could be beneficial. Animal models that test specific hypotheses related to human intergenerational and transgenerational studies are clearly important to verify any conclusions because they can be conducted faster and can control for generation time, age, genetic background, and environment.
A second major operational challenge is that existing studies vary widely in the specific exposures and outcomes assessed across generations and geographical locations, and in the study designs used to collect and analyze the data. Clinical assessments and phenotyping of outcome measures need to be standardized with respect to the developmental stage within a study when possible. For many environmental chemical exposures, the most valid, uniform method may be to measure them in biospecimens; however, this requires that cohorts banked relevant biospecimens generations ago, that the biospecimens were stored in a manner consistent with current best practices for given assay requirements, and that the exposures of interest do not degrade over such a long period in storage. Moreover, there may be ethical concerns about using samples from subjects whose informed consent was provided years or decades ago and may not directly address these types of assays in biospecimens. Furthermore, different study designs and/or analysis strategies can lead to different conclusions from the data243.
Epigenetic marks are potentially uniquely poised to serve as the interface between genetics and environment, but careful interpretation of epigenetic results from intergenerational and transgenerational studies is critical. Evidence that an epigenetic mark is inherited within a family does not necessarily mean that it is purely environmentally induced and epigenetically transmitted. For example, some single nucleotide polymorphisms may determine DNA methylation if they cause a gain or loss of a CpG dinucleotide. These are expected to be rare (< 1%), based on a study of neonatal genotype-methylation comparisons244. However, even when genetic analyses of common single nucleotide polymorphisms are included in cross-generational epigenetic study designs, genetic effects from rare variants or copy number variants may remain that can impact the interpretation of the results. Ideal intergenerational and transgenerational epigenetic study designs would include the integration of multiple ‘omic layers of information to obtain the most holistic investigation of disease risk inheritance within families. Multi-omic study designs offer many benefits, including increased confidence in the results when specific genes or gene pathways are identified in more than one layer of information. In addition to shared genetics, families tend to have shared living environments, socioeconomic status, diet, tobacco and alcohol use, and other cultural behaviors. Structural inequities may persist within families across generations, raising concerns that the epigenetic marks may be erroneously considered heritable if inequities are not adequately captured or measured. For example, socioeconomic status may be a predictor of certain environmental exposures as well as many health outcomes and can persist across generations245. Controlling for such confounding requires appropriate study designs that can mitigate the problem (e.g., sibling designs) or adequate measurement of potential confounders. Second, the timing of environmental exposures in the grandparent’s generation relative to their own and their children’s gamete development must be considered.
A remaining challenge is the choice of tissues for collection and investigation. Unlike DNA, which is consistent across tissue sources, epigenetic marks can vary by cell type or subtype. Because most human epigenetic studies collect biological samples from living individuals, tissue sources are predominantly blood, saliva, buccal, and semen collections. Determining whether these surrogate tissues are relevant for investigations of less accessible tissues, such as brain or other internal organs, may require comparisons of existing databases of epigenetic roadmaps of multiple human tissues and cell types relevant to the disease of interest246. In addition, correction for cell-type composition is important in human epigenomic studies to determine if the epigenetic changes being investigated are a result of a cell composition change, as opposed to changes within all cell types or a specific cell lineage247. Because environmental exposures and gene-by-environment interactions that occur in utero have a particular impact on long-term health outcomes, samples obtained from women and offspring during pregnancy and at birth, including maternal and cell-free fetal DNA from maternal prenatal plasma, as well as the placenta, cord blood, and newborn blood spots, should be particularly useful for intergenerational epigenetic studies involving childhood diseases248.
Box 2 Major challenges for epidemiologic studies or inter- and transgenerational effects.
A long time period is needed to observe changes across generations in human studies, during which time study leadership, methods, staffing, and funding may change.
Methods for phenotyping health outcomes or exposure assessment are often not standardized, or standards may evolve over time, making it hard to compare across cohorts and over time.
In addition to shared genetics, families tend to have shared living environments and shared environments such as socioeconomic status, diet, tobacco and alcohol use, and other cultural behaviors. This makes disentangling shared genetics from a shared environment a challenge.
There is a need to balance individual responsibility for health and structural or societal responsibility for health. Epigenetic programming raises concerns over the risk of stigmatization or discrimination broadly as well as specifically regarding parenting and reproduction, legal proceedings, political theory, and privacy concerns.
The level of privacy protection needed for epigenetic research is currently debatable.
Integrating data from multiple ‘omics layers across multiple generations would help to obtain the most holistic investigation of disease risk inheritance within families, but statistical methods for doing so are complicated, computationally challenging, and continually evolving.v
Epigenetic marks can vary by cell type or subtype. Because most human epigenetic studies collect biological samples from living individuals, tissue sources are predominantly blood, saliva, buccal, and semen collections. Care must be taken to interpret the relevance of changes in these surrogate tissues to human health.
Ethical, legal, and social implications of epigenetic research
The complexity of epigenetic programming of health highlights the inherent tension in the balance between individual responsibility for health and structural or societal responsibility for health15. The current field of epigenetics lies at the crossroads of ethics, law, and society, having stimulated much discussion in recent years and yielding diverse opinions regarding the balance between risks and rewards of epigenetic studies13–15. Epigenetics blurs the line between the interconnectedness of nature and nurture in the inheritance of traits and disease susceptibilities within families. This leads to concerns about new forms of epigenetic determinism or environmental determinism based on the possibility of transgenerational epigenetic effects on child development. Scientists have raised concerns about the “biologization of social space and time” that is, that environmental and socio-cultural circumstances conceptualized as external factors can be internalized into the body. Adverse effects or unintended consequences may arise “from translating social disparities into biological inequalities and reducing complex social problems to molecular codes”13. However, a potential advantage of understanding biological correlates of social inequities is that the impact of these inequities on health could be measured before and after interventions, opening up the possibility for epigenetic biomarkers to become an important part of medical care in the future.
Other areas of concern involve the risk of stigmatization or discrimination broadly as well as specifically regarding parenting and reproduction, legal proceedings, political theory, and privacy concerns. The focus on developmental origins of adult disease potentially places undue scrutiny on the behavior of pregnant women, which could lead to greater control over the female body249. For instance, could epigenetic data be used in legal proceedings to support claims of negligent parenting?250 Challenges also arise when defining a reference epigenome which epigenome should be considered the healthy reference? Will a new and greater epigenetic understanding of social disparities help target interventions or will it give rise to increased racialization without structural determinants of inequities being addressed? An additional concern is that newly developed epigenetic norms may unduly burden the already-marginalized groups by placing more intense scrutiny and blame for sociocultural practices or behaviors that might affect the new epigenetic norm13, rather than focusing on upstream, structural factors that produce health disparities.
Last, there is an ongoing debate over the level of privacy protection needed for epigenetic research. On the one hand, the genetic privacy framework may be adequate and translatable to epigenetics, while on the other hand epigenetic data may be more sensitive and require greater protection14. At the heart of the epigenetic debate lies the varied attempts by scientists and bioethicists to account for the social, political, economic, and cultural context of our biology15.
An opportunity: the ECHO study
The Environmental influences on Child Health Outcomes (ECHO) program, created in 2016, is an NIH-funded national program that supports existing observational and intervention studies to answer important questions about key developmental areas, including pre-, peri-, and early postnatal outcomes, airway conditions, obesity, and neurodevelopment251. The program consists of 72 cohorts (https://www.nih.gov/echo/pediatric-cohorts), with an estimated sample size exceeding 50,000 children from diverse populations across the United States. The program leverages extant data along with newly collected data from primary and secondary sources along with biospecimens collected across key developmental periods252. The structure of ECHO is such that the principal investigators of each cohort form a steering committee designed to guide the research program. The ECHO program is also supported by a funded coordinating center, data analysis center, children’s health exposure analysis resource, patient-reported outcome core, and genetics core253. ECHO forms a large multidisciplinary network of researchers focused on solution-oriented research among a heterogeneous set of existing cohorts. This structure differs from other large-cohort studies (e.g., Avon Longitudinal Study of Parents and Children) in that the extant cohorts that are a part of the ECHO program vary significantly in their study design, year of initiation, research domain focus, and study population. This heterogeneity offers unique challenges related to the harmonization of extant data, but also provides researchers with the diversity needed to answer important questions regarding the impact of biology, structural inequities, changing policy, and socio-cultural context on child health.
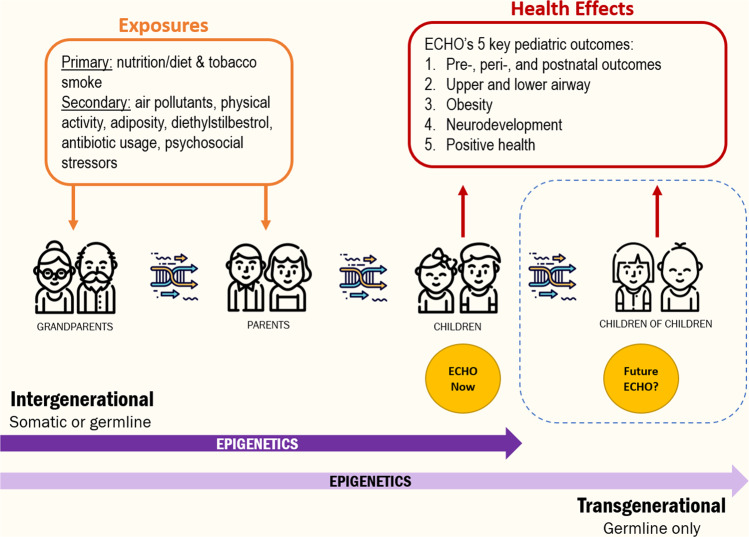
While inter- and transgenerational data collection remains challenging and the ethical, legal, and social implications of such research must be considered, the ECHO program offers a unique platform and opportunity in which to explore the health effects of exposures, including more contemporaneous ones, that are present or have far-reaching consequences across generations (Fig. 2). These exposures, which are extensively measured within the ECHO cohorts, are hypothesized to impact a number of health outcomes in children. As a part of ECHO, biological specimens, which can be used for genetic and epigenetic analyses, are being collected and analyzed. When considering maternal–paternal–child trios for intergenerational hypotheses, we estimate that approximately 10,000 will be available in the ECHO program. Further, the possibility of extending the ECHO program to include a second-generation expands the potential reach of ECHO in a way few studies have been able to accomplish. Indeed, several cohorts within ECHO are already recruiting and collecting data on this third-generation. With the availability of these large numbers of dyads and triads and the potential for the collection and analysis of epigenetic, genetic, and ‘omics data in combination with socio-economic and cultural data, the ECHO program is poised to answer key questions regarding the mechanisms through which environmental exposures impact child health across multiple generations.
Fig. 2. Opportunities in ECHO for understanding the influences of the environment on child health across generations.
The Environmental influences on Child Health Outcomes (ECHO) program, created in 2016, is an NIH-funded national program that supports existing observational and intervention studies to answer important questions about key developmental areas, including pre-, peri-, and early postnatal outcomes, airway conditions, obesity, and neurodevelopment. Exposures of interest include nutrition, tobacco smoke, pollutants, physical activity, drug usage, and stress. The ECHO program offers a unique platform and opportunity in which to explore the health effects of exposures, including more contemporaneous ones, that are present or have far-reaching consequences across generations.
Supplementary information
Acknowledgements
The authors wish to thank our Environmental influences on Child Health Outcomes (ECHO) colleagues, the medical, nursing, and program staff, as well as the children and families participating in the ECHO cohorts. We also acknowledge the contribution of the following ECHO program collaborators: Coordinating Center: Duke Clinical Research Institute, Durham, North Carolina: Smith PB, Newby KL, and Benjamin DK. Research reported in this publication was supported by the Environmental influences on Child Health Outcomes (ECHO) program, Office of The Director, National Institutes of Health, under Award Numbers U2COD023375 (Coordinating Center), U24OD023382 (Data Analysis Center), and UH3OD023287, UH3OD023305, UH3OD023337, UH3OD023313, UH3OD023285, UH3OD023328, UH3OD023244, UH3OD023342, UH3OD023365, UH3OD023282, UH3OD023289, and UH3OD023348 This work was also supported by the following NIH grants: 5K99ES030403, R01MH121070, R01AI141569-1A1, P30ES023513-supported EHSC scholar fund, R01ES029213, R01HD093643, R01HL125761, T32AA00745, and K01DK120807 The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author contributions
CVB conceived of the concept for the review paper, facilitated the organization of review sections and coordination of the writing team, and helped write the introduction, ethics, challenges, and conclusion sections. RL facilitated organization of review sections and coordination of the writing team and served as editor. LGK wrote the section on germline changes associated with environmental exposures and overall editing. MBE provided overall editing and streamlining. AKP wrote the introduction. TB provided overall editing. JB wrote the section on neurodevelopmental outcomes in humans. SSC wrote the section on inter- and transgenerational effects on birthweight. CSD contributed to overall editing and the neurodevelopment section. AH wrote the section on neurodevelopmental outcomes in humans. HJ wrote the section on evidence in animals. JML wrote the introduction and the section on challenges to the field. RLM wrote the section on respiratory health in humans. RM wrote the section on opportunities in ECHO. JP provided overall editing. RS wrote the section on evidence in animals and on opportunities in ECHO. SFS wrote the section on birthweight in humans and cardiovascular effects. IT wrote the section on neurodevelopmental outcomes in humans. DW wrote the section on rationale for epigenetic effects. YZ provided overall editing. RF wrote the sections on germline changes and single-generation effects in humans, created Fig. 1, and provided overall editing.
Competing interests
Joseph M. Braun’s institution was financially compensated for his services as an expert witness for plaintiffs in litigation related to PFAS-contaminated drinking water; these funds were not paid to him directly. All other authors declare no competing interests.
Footnotes
Peer review information Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Brooke LaFlamme. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-021-02316-6.
References
- 1.Heard E, Martienssen RA. Transgenerational epigenetic inheritance: myths and mechanisms. Cell. 2014;157:95–109. doi: 10.1016/j.cell.2014.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagy C, Turecki G. Transgenerational epigenetic inheritance: an open discussion. Epigenomics. 2015;7:781–790. doi: 10.2217/epi.15.46. [DOI] [PubMed] [Google Scholar]
- 3.Whitelaw NC, Whitelaw E. Transgenerational epigenetic inheritance in health and disease. Curr. Opin. Genet. Dev. 2008;18:273–279. doi: 10.1016/j.gde.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Pang S, Curran SP. Longevity and the long arm of epigenetics: acquired parental marks influence lifespan across several generations. Bioessays. 2012;34:652–654. doi: 10.1002/bies.201200046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benayoun BA, Brunet A. Epigenetic memory of longevity in caenorhabditis elegans. Worm. 2012;1:77–81. doi: 10.4161/worm.19157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger SL. Transgenerational inheritance of longevity: epigenetic mysteries abound. Cell Metab. 2012;15:6–7. doi: 10.1016/j.cmet.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Rankin CH. A review of transgenerational epigenetics for RNAi, longevity, germline maintenance and olfactory imprinting in Caenorhabditis elegans. J. Exp. Biol. 2015;218:41–49. doi: 10.1242/jeb.108340. [DOI] [PubMed] [Google Scholar]
- 8.Aiken CE, Tarry-Adkins JL, Ozanne SE. Transgenerational effects of maternal diet on metabolic and reproductive ageing. Mamm. Genome. 2016;27:430–439. doi: 10.1007/s00335-016-9631-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaiserman AM, Koliada AK, Jirtle RL. Non-genomic transmission of longevity between generations: potential mechanisms and evidence across species. Epigenetics Chromatin. 2017;10:38. doi: 10.1186/s13072-017-0145-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huypens P, et al. Epigenetic germline inheritance of diet-induced obesity and insulin resistance. Nat. Genet. 2016;48:497–499. doi: 10.1038/ng.3527. [DOI] [PubMed] [Google Scholar]
- 11.Horsthemke B. A critical view on transgenerational epigenetic inheritance in humans. Nat. Commun. 2018;9:2973. doi: 10.1038/s41467-018-05445-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martos SN, Tang WY, Wang Z. Elusive inheritance: transgenerational effects and epigenetic inheritance in human environmental disease. Prog. Biophys. Mol. Biol. 2015;118:44–54. doi: 10.1016/j.pbiomolbio.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dupras C, Saulnier KM, Joly Y. Epigenetics, ethics, law and society: a multidisciplinary review of descriptive, instrumental, dialectical and reflexive analyses. Soc. Stud. Sci. 2019;49:785–810. doi: 10.1177/0306312719866007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang JY, King NB. Epigenetics changes nothing: what a new scientific field does and does not mean for ethics and social justice. Public Health Ethics. 2018;11:69–81. doi: 10.1093/phe/phx013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiapperino L. Epigenetics: ethics, politics, biosociality. Br. Med. Bull. 2018;128:49–60. doi: 10.1093/bmb/ldy033. [DOI] [PubMed] [Google Scholar]
- 16.Jackson M, Marks L, May GHW, Wilson JB. The genetic basis of disease. Essays Biochem. 2018;62:643–723. doi: 10.1042/EBC20170053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammoud SS, et al. Distinctive chromatin in human sperm packages genes for embryo development. Nature. 2009;460:473–478. doi: 10.1038/nature08162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraga MF, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc. Natl Acad. Sci. USA. 2005;102:10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McRae AF, et al. Contribution of genetic variation to transgenerational inheritance of DNA methylation. Genome Biol. 2014;15:R73. doi: 10.1186/gb-2014-15-5-r73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Busche S, et al. Population whole-genome bisulfite sequencing across two tissues highlights the environment as the principal source of human methylome variation. Genome Biol. 2015;16:290. doi: 10.1186/s13059-015-0856-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunasekara CJ, et al. A genomic atlas of systemic interindividual epigenetic variation in humans. Genome Biol. 2019;20:105. doi: 10.1186/s13059-019-1708-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hakonsen LB, et al. Exposures that may affect sperm DNA integrity: two decades of follow-up in a pregnancy cohort. Reprod. Toxicol. 2012;33:316–321. doi: 10.1016/j.reprotox.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 23.Auger J, et al. Sperm morphological defects related to environment, lifestyle and medical history of 1001 male partners of pregnant women from four European cities. Hum. Reprod. 2001;16:2710–2717. doi: 10.1093/humrep/16.12.2710. [DOI] [PubMed] [Google Scholar]
- 24.Boeri L, et al. Low birth weight is associated with a decreased overall adult health status and reproductive capability - results of a cross-sectional study in primary infertile patients. PLoS ONE. 2016;11:e0166728. doi: 10.1371/journal.pone.0166728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahn LG, et al. The relation of birth weight and adiposity across the life course to semen quality in middle age. Epidemiology. 2019;30:S17–s27. doi: 10.1097/EDE.0000000000001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olsen J, et al. Birthweight and semen characteristics. Int. J. Androl. 2000;23:230–235. doi: 10.1046/j.1365-2605.2000.00239.x. [DOI] [PubMed] [Google Scholar]
- 27.Guo YL, Hsu PC, Hsu CC, Lambert GH. Semen quality after prenatal exposure to polychlorinated biphenyls and dibenzofurans. Lancet. 2000;356:1240–1241. doi: 10.1016/S0140-6736(00)02792-6. [DOI] [PubMed] [Google Scholar]
- 28.Axelsson J, et al. Prenatal phthalate exposure and reproductive function in young men. Environ. Res. 2015;138:264–270. doi: 10.1016/j.envres.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 29.Barakat R, Seymore T, Lin PP, Park CJ, Ko CJ. Prenatal exposure to an environmentally relevant phthalate mixture disrupts testicular steroidogenesis in adult male mice. Environ. Res. 2019;172:194–201. doi: 10.1016/j.envres.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faqi AS, Dalsenter PR, Merker HJ, Chahoud I. Reproductive toxicity and tissue concentrations of low doses of 2,3,7,8-tetrachlorodibenzo-p-dioxin in male offspring rats exposed throughout pregnancy and lactation. Toxicol. Appl Pharm. 1998;150:383–392. doi: 10.1006/taap.1998.8433. [DOI] [PubMed] [Google Scholar]
- 31.Byskov AG. Differentiation of mammalian embryonic gonad. Physiol. Rev. 1986;66:71–117. doi: 10.1152/physrev.1986.66.1.71. [DOI] [PubMed] [Google Scholar]
- 32.Prince FP. The triphasic nature of Leydig cell development in humans, and comments on nomenclature. J. Endocrinol. 2001;168:213–216. doi: 10.1677/joe.0.1680213. [DOI] [PubMed] [Google Scholar]
- 33.Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125:769–784. doi: 10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- 34.Surani MA. Reprogramming of genome function through epigenetic inheritance. Nature. 2001;414:122–128. doi: 10.1038/35102186. [DOI] [PubMed] [Google Scholar]
- 35.Zamudio NM, Chong S, O’Bryan MK. Epigenetic regulation in male germ cells. Reproduction. 2008;136:131–146. doi: 10.1530/REP-07-0576. [DOI] [PubMed] [Google Scholar]
- 36.Hirshfield AN. Development of follicles in the mammalian ovary. Int. Rev. Cytol. 1991;124:43–101. doi: 10.1016/S0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- 37.Hoyer PB, Sipes IG. Assessment of follicle destruction in chemical-induced ovarian toxicity. Annu Rev. Pharm. Toxicol. 1996;36:307–331. doi: 10.1146/annurev.pa.36.040196.001515. [DOI] [PubMed] [Google Scholar]
- 38.Meldrum DR, et al. Aging and the environment affect gamete and embryo potential: can we intervene? Fertil. Steril. 2016;105:548–559. doi: 10.1016/j.fertnstert.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Machtinger R, Orvieto R. Bisphenol A, oocyte maturation, implantation, and IVF outcome: review of animal and human data. Reprod. Biomed. Online. 2014;29:404–410. doi: 10.1016/j.rbmo.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 40.Eichenlaub-Ritter U, Pacchierotti F. Bisphenol A effects on mammalian oogenesis and epigenetic integrity of oocytes: a case study exploring risks of endocrine disrupting chemicals. Biomed. Res. Int. 2015;2015:698795. doi: 10.1155/2015/698795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao D, et al. Embryonic exposure to benzo(a)pyrene inhibits reproductive capability in adult female zebrafish and correlation with DNA methylation. Environ. Pollut. 2018;240:403–411. doi: 10.1016/j.envpol.2018.04.139. [DOI] [PubMed] [Google Scholar]
- 42.Liu H, et al. Toxic effects of 1-(N-methyl-N-nitrosamino)-1-(3-pyridinyl)-4-butanal on the maturation and subsequent development of murine oocyte. Ecotoxicol. Environ. Saf. 2019;181:370–380. doi: 10.1016/j.ecoenv.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 43.Du Plessis SS, Cabler S, McAlister DA, Sabanegh E, Agarwal A. The effect of obesity on sperm disorders and male infertility. Nat. Rev. Urol. 2010;7:153–161. doi: 10.1038/nrurol.2010.6. [DOI] [PubMed] [Google Scholar]
- 44.Esakky P, Moley KH. Paternal smoking and germ cell death: a mechanistic link to the effects of cigarette smoke on spermatogenesis and possible long-term sequelae in offspring. Mol. Cell Endocrinol. 2016;435:85–93. doi: 10.1016/j.mce.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gunes S, Metin Mahmutoglu A, Arslan MA, Henkel R. Smoking-induced genetic and epigenetic alterations in infertile men. Andrologia. 2018;50:e13124. doi: 10.1111/and.13124. [DOI] [PubMed] [Google Scholar]
- 46.Sepaniak S, et al. The influence of cigarette smoking on human sperm quality and DNA fragmentation. Toxicology. 2006;223:54–60. doi: 10.1016/j.tox.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Dieamant F, et al. Semen parameters in men with varicocele: DNA fragmentation, chromatin packaging, mitochondrial membrane potential, and apoptosis. JBRA Assist Reprod. 2017;21:295–301. doi: 10.5935/1518-0557.20170053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roque M, Esteves SC. Effect of varicocele repair on sperm DNA fragmentation: a review. Int. Urol. Nephrol. 2018;50:583–603. doi: 10.1007/s11255-018-1839-4. [DOI] [PubMed] [Google Scholar]
- 49.Cunningham KA, Beagley KW. Male genital tract chlamydial infection: implications for pathology and infertility. Biol. Reprod. 2008;79:180–189. doi: 10.1095/biolreprod.108.067835. [DOI] [PubMed] [Google Scholar]
- 50.Gallegos G, et al. Sperm DNA fragmentation in infertile men with genitourinary infection by chlamydia trachomatis and mycoplasma. Fertil. Steril. 2008;90:328–334. doi: 10.1016/j.fertnstert.2007.06.035. [DOI] [PubMed] [Google Scholar]
- 51.Moazenchi M, et al. The impact of Chlamydia trachomatis infection on sperm parameters and male fertility: a comprehensive study. Int J. STD AIDS. 2018;29:466–473. doi: 10.1177/0956462417735245. [DOI] [PubMed] [Google Scholar]
- 52.Morris ID. Sperm DNA damage and cancer treatment. Int. J. Androl. 2002;25:255–261. doi: 10.1046/j.1365-2605.2002.00372.x. [DOI] [PubMed] [Google Scholar]
- 53.Smarr MM, Kannan K, Chen Z, Kim S, Buck Louis GM. Male urinary paracetamol and semen quality. Andrology. 2017;5:1082–1088. doi: 10.1111/andr.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bosco L, et al. Sperm DNA fragmentation: An early and reliable marker of air pollution. Environ. Toxicol. Pharm. 2018;58:243–249. doi: 10.1016/j.etap.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 55.Jurewicz J, Dziewirska E, Radwan M, Hanke W. Air pollution from natural and anthropic sources and male fertility. Reprod. Biol. Endocrinol. 2018;16:109. doi: 10.1186/s12958-018-0430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lafuente R, Garcia-Blaquez N, Jacquemin B, Checa MA. Outdoor air pollution and sperm quality. Fertil. Steril. 2016;106:880–896. doi: 10.1016/j.fertnstert.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 57.Rubes J, et al. Episodic air pollution is associated with increased DNA fragmentation in human sperm without other changes in semen quality. Hum. Reprod. 2005;20:2776–2783. doi: 10.1093/humrep/dei122. [DOI] [PubMed] [Google Scholar]
- 58.Goldstone AE, Chen Z, Perry MJ, Kannan K, Louis GM. Urinary bisphenol A and semen quality, the LIFE Study. Reprod. Toxicol. 2015;51:7–13. doi: 10.1016/j.reprotox.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harchegani AB, et al. Mechanisms of diazinon effects on impaired spermatogenesis and male infertility. Toxicol. Ind. Health. 2018;34:653–664. doi: 10.1177/0748233718778665. [DOI] [PubMed] [Google Scholar]
- 60.Pan Y, et al. Association between phthalate metabolites and biomarkers of reproductive function in 1066 Chinese men of reproductive age. J. Hazard Mater. 2015;300:729–736. doi: 10.1016/j.jhazmat.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 61.Zamkowska D, Karwacka A, Jurewicz J, Radwan M. Environmental exposure to non-persistent endocrine disrupting chemicals and semen quality: An overview of the current epidemiological evidence. Int. J. Occup. Med. Environ. Health. 2018;31:377–414. doi: 10.13075/ijomeh.1896.01195. [DOI] [PubMed] [Google Scholar]
- 62.Santi D, Spaggiari G, Simoni M. Sperm DNA fragmentation index as a promising predictive tool for male infertility diagnosis and treatment management - meta-analyses. Reprod. Biomed. Online. 2018;37:315–326. doi: 10.1016/j.rbmo.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 63.Coughlan C, et al. Sperm DNA fragmentation, recurrent implantation failure and recurrent miscarriage. Asian J. Androl. 2015;17:681–685. doi: 10.4103/1008-682X.144946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robinson L, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum. Reprod. 2012;27:2908–2917. doi: 10.1093/humrep/des261. [DOI] [PubMed] [Google Scholar]
- 65.Osman A, Alsomait H, Seshadri S, El-Toukhy T, Khalaf Y. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: a systematic review and meta-analysis. Reprod. Biomed. Online. 2015;30:120–127. doi: 10.1016/j.rbmo.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 66.Belva F, et al. Semen quality of young adult ICSI offspring: the first results. Hum. Reprod. 2016;31:2811–2820. doi: 10.1093/humrep/dew245. [DOI] [PubMed] [Google Scholar]
- 67.Practice Committee of American Society for Reproductive Medicine; Practice Committee of Society for Assisted Reproductive Technology. Genetic considerations related to intracytoplasmic sperm injection (ICSI) Fertil. Steril. 2008;90:S182–184. doi: 10.1016/j.fertnstert.2008.08.048. [DOI] [PubMed] [Google Scholar]
- 68.Raad G, et al. Paternal obesity: how bad is it for sperm quality and progeny health? Basic Clin. Androl. 2017;27:20. doi: 10.1186/s12610-017-0064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fullston T, et al. Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F2 generation and alters the transcriptional profile of testis and sperm microRNA content. FASEB J. 2013;27:4226–4243. doi: 10.1096/fj.12-224048. [DOI] [PubMed] [Google Scholar]
- 70.McPherson NO, Owens JA, Fullston T, Lane M. Preconception diet or exercise intervention in obese fathers normalizes sperm microRNA profile and metabolic syndrome in female offspring. Am. J. Physiol. Endocrinol. Metab. 2015;308:E805–E821. doi: 10.1152/ajpendo.00013.2015. [DOI] [PubMed] [Google Scholar]
- 71.Dias BG, Ressler KJ. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat. Neurosci. 2014;17:89–96. doi: 10.1038/nn.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS ONE. 2013;8:e55387. doi: 10.1371/journal.pone.0055387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aitken RJ, De Iuliis GN. On the possible origins of DNA damage in human spermatozoa. Mol. Hum. Reprod. 2010;16:3–13. doi: 10.1093/molehr/gap059. [DOI] [PubMed] [Google Scholar]
- 74.Sakkas D, Alvarez JG. Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil. Steril. 2010;93:1027–1036. doi: 10.1016/j.fertnstert.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 75.Gonzalez-Marin C, Gosalvez J, Roy R. Types, causes, detection and repair of DNA fragmentation in animal and human sperm cells. Int. J. Mol. Sci. 2012;13:14026–14052. doi: 10.3390/ijms131114026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Delbes G, Hales BF, Robaire B. Toxicants and human sperm chromatin integrity. Mol. Hum. Reprod. 2010;16:14–22. doi: 10.1093/molehr/gap087. [DOI] [PubMed] [Google Scholar]
- 77.Donkin I, Barres R. Sperm epigenetics and influence of environmental factors. Mol. Metab. 2018;14:1–11. doi: 10.1016/j.molmet.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Godmann M, Lambrot R, Kimmins S. The dynamic epigenetic program in male germ cells: Its role in spermatogenesis, testis cancer, and its response to the environment. Microsc Res. Tech. 2009;72:603–619. doi: 10.1002/jemt.20715. [DOI] [PubMed] [Google Scholar]
- 79.Muratori, M. & De Geyter, C. Chromatin condensation, fragmentation of DNA and differences in the epigenetic signature of infertile men. Best Pract. Res. Clin. Endocrinol Metab10.1016/j.beem.2018.10.004 (2018). [DOI] [PubMed]
- 80.Wyck S, et al. Oxidative stress in sperm affects the epigenetic reprogramming in early embryonic development. Epigenetics Chromatin. 2018;11:60. doi: 10.1186/s13072-018-0224-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang SY, et al. Hypoxia causes transgenerational impairments in reproduction of fish. Nat. Commun. 2016;7:12114. doi: 10.1038/ncomms12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gavriliouk D, Aitken RJ. Damage to sperm DNA mediated by reactive oxygen species: its impact on human reproduction and the health trajectory of offspring. Adv. Exp. Med. Biol. 2015;868:23–47. doi: 10.1007/978-3-319-18881-2_2. [DOI] [PubMed] [Google Scholar]
- 83.Ji BT, et al. Paternal cigarette smoking and the risk of childhood cancer among offspring of nonsmoking mothers. J. Natl Cancer Inst. 1997;89:238–244. doi: 10.1093/jnci/89.3.238. [DOI] [PubMed] [Google Scholar]
- 84.Kundakovic, M. & Jaric, I. The epigenetic link between prenatal adverse environments and neurodevelopmental disorders. Genes8, 10.3390/genes8030104 (2017). [DOI] [PMC free article] [PubMed]
- 85.Perera F, Herbstman J. Prenatal environmental exposures, epigenetics, and disease. Reprod. Toxicol. 2011;31:363–373. doi: 10.1016/j.reprotox.2010.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ho L, Crabtree GR. Chromatin remodelling during development. Nature. 2010;463:474–484. doi: 10.1038/nature08911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leitch HG, Tang WW, Surani MA. Primordial germ-cell development and epigenetic reprogramming in mammals. Curr. Top. Dev. Biol. 2013;104:149–187. doi: 10.1016/B978-0-12-416027-9.00005-X. [DOI] [PubMed] [Google Scholar]
- 88.Kiefer JC. Epigenetics in development. Dev. Dyn. 2007;236:1144–1156. doi: 10.1002/dvdy.21094. [DOI] [PubMed] [Google Scholar]
- 89.Heindel JJ, Vandenberg LN. Developmental origins of health and disease: a paradigm for understanding disease cause and prevention. Curr. Opin. Pediatr. 2015;27:248–253. doi: 10.1097/MOP.0000000000000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rojas D, et al. Prenatal arsenic exposure and the epigenome: identifying sites of 5-methylcytosine alterations that predict functional changes in gene expression in newborn cord blood and subsequent birth outcomes. Toxicol. Sci. 2015;143:97–106. doi: 10.1093/toxsci/kfu210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Breton CV, et al. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am. J. Respir. Crit. Care Med. 2009;180:462–467. doi: 10.1164/rccm.200901-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Felix JF, et al. Cohort profile: pregnancy and childhood epigenetics (PACE) consortium. Int. J. Epidemiol. 2018;47:22–23u. doi: 10.1093/ije/dyx190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fry RC, et al. Activation of inflammation/NF-kappaB signaling in infants born to arsenic-exposed mothers. PLoS Genet. 2007;3:e207. doi: 10.1371/journal.pgen.0030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gruzieva O, et al. Epigenome-wide meta-analysis of methylation in children related to prenatal NO2 air pollution exposure. Environ. Health Perspect. 2017;125:104–110. doi: 10.1289/EHP36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gruzieva O, et al. Prenatal particulate air pollution and DNA methylation in newborns: an epigenome-wide meta-analysis. Environ. Health Perspect. 2019;127:57012. doi: 10.1289/EHP4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Herbstman J, Tang D, Zhu D, Perera F. Prenatal exposure to polycyclic aromatic hydrocarbons and CPG methylation. Epidemiology. 2009;20:S93. doi: 10.1097/01.ede.0000362991.36685.80. [DOI] [Google Scholar]
- 97.Howe CG, et al. Maternal gestational diabetes mellitus and newborn DNA methylation: findings from the pregnancy and childhood Epigenetics Consortium. Diabetes Care. 2020;43:98–105. doi: 10.2337/dc19-0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Joubert BR, et al. DNA methylation in newborns and maternal smoking in pregnancy: genome-wide consortium meta-analysis. Am. J. Hum. Genet. 2016;98:680–696. doi: 10.1016/j.ajhg.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kazmi N, et al. Hypertensive disorders of pregnancy and DNA methylation in newborns. Hypertension. 2019;74:375–383. doi: 10.1161/HYPERTENSIONAHA.119.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Küpers LK, et al. Meta-analysis of epigenome-wide association studies in neonates reveals widespread differential DNA methylation associated with birthweight. Nat. Commun. 2019;10:1893. doi: 10.1038/s41467-019-09671-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Merid SK, et al. Epigenome-wide meta-analysis of blood DNA methylation in newborns and children identifies numerous loci related to gestational age. Genome Med. 2020;12:25. doi: 10.1186/s13073-020-0716-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reese SE, et al. Epigenome-wide meta-analysis of DNA methylation and childhood asthma. J. Allergy Clin. Immunol. 2019;143:2062–2074. doi: 10.1016/j.jaci.2018.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sharp GC, et al. Maternal alcohol consumption and offspring DNA methylation: findings from six general population-based birth cohorts. Epigenomics. 2018;10:27–42. doi: 10.2217/epi-2017-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sharp GC, et al. Maternal BMI at the start of pregnancy and offspring epigenome-wide DNA methylation: findings from the pregnancy and childhood epigenetics (PACE) consortium. Hum. Mol. Genet. 2017;26:4067–4085. doi: 10.1093/hmg/ddx290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sikdar S, et al. Comparison of smoking-related DNA methylation between newborns from prenatal exposure and adults from personal smoking. Epigenomics. 2019;11:1487–1500. doi: 10.2217/epi-2019-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hackett JA, et al. Germline DNA demethylation dynamics and imprint erasure through 5-hydroxymethylcytosine. Science. 2013;339:448–452. doi: 10.1126/science.1229277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Spadafora C. Transgenerational epigenetic reprogramming of early embryos: a mechanistic model. Environ. Epigenet. 2020;6:dvaa009. doi: 10.1093/eep/dvaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Banik, A. et al. Maternal factors that induce epigenetic changes contribute to neurological disorders in offspring. Genes10.3390/genes8060150 (2017). [DOI] [PMC free article] [PubMed]
- 109.Bellinger DC. Prenatal exposures to environmental chemicals and Children’s Neurodevelopment: An Update. Saf. Health Work. 2013;4:1–11. doi: 10.5491/SHAW.2013.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Indrio F, et al. Epigenetic matters: the link between early nutrition, microbiome, and long-term health development. Front Pediatr. 2017;5:178. doi: 10.3389/fped.2017.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ma RC, Tutino GE, Lillycrop KA, Hanson MA, Tam WH. Maternal diabetes, gestational diabetes and the role of epigenetics in their long term effects on offspring. Prog. Biophys. Mol. Biol. 2015;118:55–68. doi: 10.1016/j.pbiomolbio.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 112.Nemoda Z, Szyf M. Epigenetic Alterations and Prenatal Maternal Depression. Birth Defects Res. 2017;109:888–897. doi: 10.1002/bdr2.1081. [DOI] [PubMed] [Google Scholar]
- 113.Sanders AP, et al. Cadmium exposure and the epigenome: exposure-associated patterns of DNA methylation in leukocytes from mother-baby pairs. Epigenetics. 2014;9:212–221. doi: 10.4161/epi.26798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Curley JP, Mashoodh R, Champagne FA. Epigenetics and the origins of paternal effects. Horm. Behav. 2011;59:306–314. doi: 10.1016/j.yhbeh.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schmidt CW. Chips off the old block: how a father’s preconception exposures might affect the health of his children. Environ. Health Perspect. 2018;126:022001. doi: 10.1289/EHP2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bakulski KM, Fallin MD. Epigenetic epidemiology: promises for public health research. Environ. Mol. Mutagen. 2014;55:171–183. doi: 10.1002/em.21850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ladd-Acosta C, Fallin MD. The role of epigenetics in genetic and environmental epidemiology. Epigenomics. 2016;8:271–283. doi: 10.2217/epi.15.102. [DOI] [PubMed] [Google Scholar]
- 118.Marques AH, O’Connor TG, Roth C, Susser E, Bjorke-Monsen AL. The influence of maternal prenatal and early childhood nutrition and maternal prenatal stress on offspring immune system development and neurodevelopmental disorders. Front Neurosci. 2013;7:120. doi: 10.3389/fnins.2013.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vickers MH. Early life nutrition, epigenetics and programming of later life disease. Nutrients. 2014;6:2165–2178. doi: 10.3390/nu6062165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Krauss-Etschmann S, Meyer KF, Dehmel S, Hylkema MN. Inter- and transgenerational epigenetic inheritance: evidence in asthma and COPD? Clin. Epigenetics. 2015;7:53. doi: 10.1186/s13148-015-0085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Babenko O, Kovalchuk I, Metz GA. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci. Biobehav Rev. 2015;48:70–91. doi: 10.1016/j.neubiorev.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 122.Borge TC, Aase H, Brantsaeter AL, Biele G. The importance of maternal diet quality during pregnancy on cognitive and behavioural outcomes in children: a systematic review and meta-analysis. BMJ Open. 2017;7:e016777. doi: 10.1136/bmjopen-2017-016777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Norouzitallab P, Baruah K, Vanrompay D, Bossier P. Can epigenetics translate environmental cues into phenotypes? Sci. Total Environ. 2019;647:1281–1293. doi: 10.1016/j.scitotenv.2018.08.063. [DOI] [PubMed] [Google Scholar]
- 124.Blumer N, Herz U, Wegmann M, Renz H. Prenatal lipopolysaccharide-exposure prevents allergic sensitization and airway inflammation, but not airway responsiveness in a murine model of experimental asthma. Clin. Exp. Allergy. 2005;35:397–402. doi: 10.1111/j.1365-2222.2005.02184.x. [DOI] [PubMed] [Google Scholar]
- 125.Blacquiere MJ, et al. Maternal smoking during pregnancy induces airway remodelling in mice offspring. Eur. Respir. J. 2009;33:1133–1140. doi: 10.1183/09031936.00129608. [DOI] [PubMed] [Google Scholar]
- 126.Wongtrakool C, Wang N, Hyde DM, Roman J, Spindel ER. Prenatal nicotine exposure alters lung function and airway geometry through alpha7 nicotinic receptors. Am. J. Respir. Cell Mol. Biol. 2012;46:695–702. doi: 10.1165/rcmb.2011-0028OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rehan VK, et al. Perinatal nicotine exposure induces asthma in second generation offspring. BMC Med. 2012;10:129. doi: 10.1186/1741-7015-10-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Niedzwiecki M, et al. Prenatal exposure to allergen, DNA methylation, and allergy in grandoffspring mice. Allergy. 2012;67:904–910. doi: 10.1111/j.1398-9995.2012.02841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Manners S, Alam R, Schwartz DA, Gorska MM. A mouse model links asthma susceptibility to prenatal exposure to diesel exhaust. J. Allergy Clin. Immunol. 2014;134:63–72. doi: 10.1016/j.jaci.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lim R, Fedulov AV, Kobzik L. Maternal stress during pregnancy increases neonatal allergy susceptibility: role of glucocorticoids. Am. J. Physiol. Lung Cell Mol. Physiol. 2014;307:L141–L148. doi: 10.1152/ajplung.00250.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Richgels PK, Yamani A, Chougnet CA, Lewkowich IP. Maternal house dust mite exposure during pregnancy enhances severity of house dust mite-induced asthma in murine offspring. J. Allergy Clin. Immunol. 2017;140:1404–1415 e1409. doi: 10.1016/j.jaci.2016.12.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Singh SP, et al. Prenatal secondhand cigarette smoke promotes Th2 polarization and impairs goblet cell differentiation and airway mucus formation. J. Immunol. 2011;187:4542–4552. doi: 10.4049/jimmunol.1101567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Singh SP, et al. Gestational Exposure to Sidestream (Secondhand) Cigarette Smoke Promotes Transgenerational Epigenetic Transmission of Exacerbated Allergic Asthma and Bronchopulmonary Dysplasia. J. Immunol. 2017;198:3815–3822. doi: 10.4049/jimmunol.1700014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Joad JP, Ji C, Kott KS, Bric JM, Pinkerton KE. In utero and postnatal effects of sidestream cigarette smoke exposure on lung function, hyperresponsiveness, and neuroendocrine cells in rats. Toxicol. Appl Pharm. 1995;132:63–71. doi: 10.1006/taap.1995.1087. [DOI] [PubMed] [Google Scholar]
- 135.Singh SP, et al. Prenatal cigarette smoke decreases lung cAMP and increases airway hyperresponsiveness. Am. J. Respir. Crit. Care Med. 2003;168:342–347. doi: 10.1164/rccm.200211-1262OC. [DOI] [PubMed] [Google Scholar]
- 136.Ji CM, Plopper CG, Witschi HP, Pinkerton KE. Exposure to sidestream cigarette smoke alters bronchiolar epithelial cell differentiation in the postnatal rat lung. Am. J. Respir. Cell Mol. Biol. 1994;11:312–320. doi: 10.1165/ajrcmb.11.3.8086168. [DOI] [PubMed] [Google Scholar]
- 137.Ji CM, et al. Maternal exposure to environmental tobacco smoke alters Clara cell secretory protein expression in fetal rat lung. Am. J. Physiol. 1998;275:L870–L876. doi: 10.1152/ajpcell.1998.275.5.C1182. [DOI] [PubMed] [Google Scholar]
- 138.Blacquiere MJ, et al. Maternal smoking during pregnancy decreases Wnt signalling in neonatal mice. Thorax. 2010;65:553–554. doi: 10.1136/thx.2009.120154. [DOI] [PubMed] [Google Scholar]
- 139.Rouse RL, Boudreaux MJ, Penn AL. In utero environmental tobacco smoke exposure alters gene expression in lungs of adult BALB/c mice. Environ. Health Perspect. 2007;115:1757–1766. doi: 10.1289/ehp.10358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nygaard UC, Hansen JS, Groeng EC, Melkild I, Lovik M. Suppression of allergen-specific IgE in offspring after preconceptional immunisation: maternal, paternal and genetic influences. Scand. J. Immunol. 2013;77:92–103. doi: 10.1111/sji.12017. [DOI] [PubMed] [Google Scholar]
- 141.Fedulov AV, Kobzik L. Allergy risk is mediated by dendritic cells with congenital epigenetic changes. Am. J. Respir. Cell Mol. Biol. 2011;44:285–292. doi: 10.1165/rcmb.2009-0400OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lenberg J, Qian Q, Sun Z, Alam R, Gorska MM. Pre-pregnancy exposure to diesel exhaust predisposes offspring to asthma through IL-1beta and IL-17A. J. Allergy Clin. Immunol. 2018;141:1118–1122 e1113. doi: 10.1016/j.jaci.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rehan VK, Liu J, Sakurai R, Torday JS. Perinatal nicotine-induced transgenerational asthma. Am. J. Physiol. Lung Cell Mol. Physiol. 2013;305:L501–L507. doi: 10.1152/ajplung.00078.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gregory DJ, Kobzik L, Yang Z, McGuire CC, Fedulov AV. Transgenerational transmission of asthma risk after exposure to environmental particles during pregnancy. Am. J. Physiol. Lung Cell Mol. Physiol. 2017;313:L395–l405. doi: 10.1152/ajplung.00035.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Perez MF, Lehner B. Intergenerational and transgenerational epigenetic inheritance in animals. Nat. Cell Biol. 2019;21:143–151. doi: 10.1038/s41556-018-0242-9. [DOI] [PubMed] [Google Scholar]
- 146.Morkve Knudsen T, et al. Transgenerational and intergenerational epigenetic inheritance in allergic diseases. J. Allergy Clin. Immunol. 2018;142:765–772. doi: 10.1016/j.jaci.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mahmood S, Smiraglia DJ, Srinivasan M, Patel MS. Epigenetic changes in hypothalamic appetite regulatory genes may underlie the developmental programming for obesity in rat neonates subjected to a high-carbohydrate dietary modification. J. Dev. Orig. Health Dis. 2013;4:479–490. doi: 10.1017/S2040174413000238. [DOI] [PubMed] [Google Scholar]
- 148.Segovia SA, Vickers MH, Zhang XD, Gray C, Reynolds CM. Maternal supplementation with conjugated linoleic acid in the setting of diet-induced obesity normalises the inflammatory phenotype in mothers and reverses metabolic dysfunction and impaired insulin sensitivity in offspring. J. Nutr. Biochem. 2015;26:1448–1457. doi: 10.1016/j.jnutbio.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 149.Camilleri-Carter TL, Dowling DK, R LR, Piper MDW. Transgenerational obesity and healthy aging in drosophila. J. Gerontol. A Biol. Sci. Med. Sci. 2019;74:1582–1589. doi: 10.1093/gerona/glz154. [DOI] [PubMed] [Google Scholar]
- 150.Lagisz M, et al. Transgenerational effects of caloric restriction on appetite: a meta-analysis. Obes. Rev. 2014;15:294–309. doi: 10.1111/obr.12138. [DOI] [PubMed] [Google Scholar]
- 151.Vickers MH. Developmental programming and transgenerational transmission of obesity. Ann. Nutr. Metab. 2014;64:26–34. doi: 10.1159/000360506. [DOI] [PubMed] [Google Scholar]
- 152.Wu Q, Suzuki M. Parental obesity and overweight affect the body-fat accumulation in the offspring: the possible effect of a high-fat diet through epigenetic inheritance. Obes. Rev. 2006;7:201–208. doi: 10.1111/j.1467-789X.2006.00232.x. [DOI] [PubMed] [Google Scholar]
- 153.Masuyama H, Mitsui T, Nobumoto E, Hiramatsu Y. The effects of high-fat diet exposure in utero on the obesogenic and diabetogenic traits through epigenetic changes in adiponectin and leptin gene expression for multiple generations in female mice. Endocrinology. 2015;156:2482–2491. doi: 10.1210/en.2014-2020. [DOI] [PubMed] [Google Scholar]
- 154.Jimenez-Chillaron JC, Ramon-Krauel M, Ribo S, Diaz R. Transgenerational epigenetic inheritance of diabetes risk as a consequence of early nutritional imbalances. Proc. Nutr. Soc. 2016;75:78–89. doi: 10.1017/S0029665115004231. [DOI] [PubMed] [Google Scholar]
- 155.Zambrano E, et al. Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J. Physiol. 2005;566:225–236. doi: 10.1113/jphysiol.2005.086462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dunn GA, Bale TL. Maternal high-fat diet effects on third-generation female body size via the paternal lineage. Endocrinology. 2011;152:2228–2236. doi: 10.1210/en.2010-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dunn GA, Bale TL. Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice. Endocrinology. 2009;150:4999–5009. doi: 10.1210/en.2009-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chamorro-Garcia R, Blumberg B. Transgenerational effects of obesogens and the obesity epidemic. Curr. Opin. Pharm. 2014;19:153–158. doi: 10.1016/j.coph.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tracey R, Manikkam M, Guerrero-Bosagna C, Skinner MK. Hydrocarbons (jet fuel JP-8) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. Reprod. Toxicol. 2013;36:104–116. doi: 10.1016/j.reprotox.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kubsad D, et al. Assessment of glyphosate induced epigenetic transgenerational inheritance of pathologies and sperm epimutations: generational toxicology. Sci. Rep. 2019;9:6372. doi: 10.1038/s41598-019-42860-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Skinner MK, et al. Ancestral dichlorodiphenyltrichloroethane (DDT) exposure promotes epigenetic transgenerational inheritance of obesity. BMC Med. 2013;11:228. doi: 10.1186/1741-7015-11-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chamorro-Garcia R, et al. Transgenerational inheritance of increased fat depot size, stem cell reprogramming, and hepatic steatosis elicited by prenatal exposure to the obesogen tributyltin in mice. Environ. Health Perspect. 2013;121:359–366. doi: 10.1289/ehp.1205701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chamorro-Garcia R, et al. Ancestral perinatal obesogen exposure results in a transgenerational thrifty phenotype in mice. Nat. Commun. 2017;8:2012. doi: 10.1038/s41467-017-01944-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Camsari C, et al. Transgenerational effects of periconception heavy metal administration on adipose weight and glucose homeostasis in mice at maturity. Toxicol. Sci. 2019;168:610–619. doi: 10.1093/toxsci/kfz008. [DOI] [PubMed] [Google Scholar]
- 165.Manikkam M, Haque MM, Guerrero-Bosagna C, Nilsson EE, Skinner MK. Pesticide methoxychlor promotes the epigenetic transgenerational inheritance of adult-onset disease through the female germline. PLoS ONE. 2014;9:e102091. doi: 10.1371/journal.pone.0102091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Guerrero-Bosagna C, Weeks S, Skinner MK. Identification of genomic features in environmentally induced epigenetic transgenerational inherited sperm epimutations. PLoS ONE. 2014;9:e100194. doi: 10.1371/journal.pone.0100194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.King SE, et al. Sperm epimutation biomarkers of obesity and pathologies following DDT induced epigenetic transgenerational inheritance of disease. Environ. Epigenet. 2019;5:dvz008. doi: 10.1093/eep/dvz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ben Maamar M, King SE, Nilsson E, Beck D, Skinner MK. Epigenetic transgenerational inheritance of parent-of-origin allelic transmission of outcross pathology and sperm epimutations. Dev. Biol. 2020;458:106–119. doi: 10.1016/j.ydbio.2019.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ding GL, et al. Transgenerational glucose intolerance with Igf2/H19 epigenetic alterations in mouse islet induced by intrauterine hyperglycemia. Diabetes. 2012;61:1133–1142. doi: 10.2337/db11-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lee MK, Blumberg B. Transgenerational effects of obesogens. Basic Clin. Pharm. Toxicol. 2019;125(Suppl 3):44–57. doi: 10.1111/bcpt.13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Drake AJ, Liu L, Kerrigan D, Meehan RR, Seckl JR. Multigenerational programming in the glucocorticoid programmed rat is associated with generation-specific and parent of origin effects. Epigenetics. 2011;6:1334–1343. doi: 10.4161/epi.6.11.17942. [DOI] [PubMed] [Google Scholar]
- 172.Bohacek J, et al. Pathological brain plasticity and cognition in the offspring of males subjected to postnatal traumatic stress. Mol. Psychiatry. 2015;20:621–631. doi: 10.1038/mp.2014.80. [DOI] [PubMed] [Google Scholar]
- 173.Cirulli, F., Musillo, C. & Berry, A. Maternal obesity as a risk factor for brain development and mental health in the offspring. Neuroscience, 10.1016/j.neuroscience.2020.01.023 (2020). [DOI] [PubMed]
- 174.Buck JM, O’Neill HC, Stitzel JA. Developmental nicotine exposure engenders intergenerational downregulation and aberrant posttranslational modification of cardinal epigenetic factors in the frontal cortices, striata, and hippocampi of adolescent mice. Epigenetics Chromatin. 2020;13:13. doi: 10.1186/s13072-020-00332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rodgers AB, Morgan CP, Leu NA, Bale TL. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc. Natl Acad. Sci. USA. 2015;112:13699–13704. doi: 10.1073/pnas.1508347112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gapp K, et al. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci. 2014;17:667–669. doi: 10.1038/nn.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gapp K, et al. Early life stress in fathers improves behavioural flexibility in their offspring. Nat. Commun. 2014;5:5466. doi: 10.1038/ncomms6466. [DOI] [PubMed] [Google Scholar]
- 178.Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J. Neurosci. 2013;33:9003–9012. doi: 10.1523/JNEUROSCI.0914-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Holloway ZR, et al. Paternal factors in neurodevelopmental toxicology: THC exposure of male rats causes long-lasting neurobehavioral effects in their offspring. Neurotoxicology. 2020;78:57–63. doi: 10.1016/j.neuro.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 180.Valles S, et al. Exposure to low doses of inorganic arsenic induces transgenerational changes on behavioral and epigenetic markers in zebrafish (Danio rerio) Toxicol. Appl Pharm. 2020;396:115002. doi: 10.1016/j.taap.2020.115002. [DOI] [PubMed] [Google Scholar]
- 181.Meyer DN, et al. Developmental exposure to Pb(2+) induces transgenerational changes to zebrafish brain transcriptome. Chemosphere. 2020;244:125527. doi: 10.1016/j.chemosphere.2019.125527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Franklin TB, et al. Epigenetic transmission of the impact of early stress across generations. Biol. Psychiatry. 2010;68:408–415. doi: 10.1016/j.biopsych.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 183.Dietz DM, et al. Paternal transmission of stress-induced pathologies. Biol. Psychiatry. 2011;70:408–414. doi: 10.1016/j.biopsych.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Posner R, et al. Neuronal small rnas control behavior transgenerationally. Cell. 2019;177:1814–1826 e1815. doi: 10.1016/j.cell.2019.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Weber-Stadlbauer U. Epigenetic and transgenerational mechanisms in infection-mediated neurodevelopmental disorders. Transl. Psychiatry. 2017;7:e1113. doi: 10.1038/tp.2017.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Champagne FA, Meaney MJ. Transgenerational effects of social environment on variations in maternal care and behavioral response to novelty. Behav. Neurosci. 2007;121:1353–1363. doi: 10.1037/0735-7044.121.6.1353. [DOI] [PubMed] [Google Scholar]
- 187.Curley JP, Davidson S, Bateson P, Champagne FA. Social enrichment during postnatal development induces transgenerational effects on emotional and reproductive behavior in mice. Front Behav. Neurosci. 2009;3:25. doi: 10.3389/neuro.08.025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sarker, G. & Peleg-Raibstein, D. Maternal overnutrition induces long-term cognitive deficits across several generations. Nutrients11, 10.3390/nu11010007 (2018). [DOI] [PMC free article] [PubMed]
- 189.Wolstenholme JT, et al. Transgenerational bisphenol a causes deficits in social recognition and alters postsynaptic density genes in mice. Endocrinology. 2019;160:1854–1867. doi: 10.1210/en.2019-00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Manners MT, et al. Transgenerational inheritance of chronic adolescent stress: effects of stress response and the amygdala transcriptome. Genes Brain Behav. 2019;18:e12493. doi: 10.1111/gbb.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Valerio MA, Andreski PM, Schoeni RF, McGonagle KA. Examining the association between childhood asthma and parent and grandparent asthma status: implications for practice. Clin. Pediatr. 2010;49:535–541. doi: 10.1177/0009922809356465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Garcia-Sanchez A, et al. Genome-wide association studies (GWAS) and their importance in asthma. Allergol. Immunopathol. 2015;43:601–608. doi: 10.1016/j.aller.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 193.Hemminki K, Li X, Sundquist K, Sundquist J. Familial risks for asthma among twins and other siblings based on hospitalizations in Sweden. Clin. Exp. Allergy. 2007;37:1320–1325. doi: 10.1111/j.1365-2222.2007.02737.x. [DOI] [PubMed] [Google Scholar]
- 194.Toskala E, Kennedy DW. Asthma risk factors. Int Forum Allergy Rhinol. 2015;5(Suppl 1):S11–S16. doi: 10.1002/alr.21557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ober C. Asthma Genetics in the Post-GWAS Era. Ann. Am. Thorac. Soc. 2016;13(Suppl 1):S85–S90. doi: 10.1513/AnnalsATS.201507-459MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Koinis Mitchell D, Murdock KK. Identifying risk and resource factors in children with asthma from urban settings: the context-health-development model. J. Asthma. 2005;42:425–436. doi: 10.1081/JAS-200067936. [DOI] [PubMed] [Google Scholar]
- 197.Kravitz-Wirtz, N. et al. Early-life air pollution exposure, neighborhood poverty, and childhood asthma in the united states, 1990(-)2014. Int J. Environ. Res. Public Health15, 10.3390/ijerph15061114 (2018). [DOI] [PMC free article] [PubMed]
- 198.Li YF, Langholz B, Salam MT, Gilliland FD. Maternal and grandmaternal smoking patterns are associated with early childhood asthma. Chest. 2005;127:1232–1241. doi: 10.1378/chest.127.4.1232. [DOI] [PubMed] [Google Scholar]
- 199.Magnus MC, et al. Grandmother’s smoking when pregnant with the mother and asthma in the grandchild: the Norwegian Mother and Child Cohort Study. Thorax. 2015;70:237–243. doi: 10.1136/thoraxjnl-2014-206438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Accordini S, et al. A three-generation study on the association of tobacco smoking with asthma. Int J. Epidemiol. 2018;47:1106–1117. doi: 10.1093/ije/dyy031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lodge CJ, et al. Grandmaternal smoking increases asthma risk in grandchildren: A nationwide Swedish cohort. Clin. Exp. Allergy. 2018;48:167–174. doi: 10.1111/cea.13031. [DOI] [PubMed] [Google Scholar]
- 202.Miller LL, Henderson J, Northstone K, Pembrey M, Golding J. Do grandmaternal smoking patterns influence the etiology of childhood asthma? Chest. 2014;145:1213–1218. doi: 10.1378/chest.13-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Braback L, et al. Childhood asthma and smoking exposures before conception-A three-generational cohort study. Pediatr. Allergy Immunol. 2018;29:361–368. doi: 10.1111/pai.12883. [DOI] [PubMed] [Google Scholar]
- 204.Arshad SH, Karmaus W, Zhang H, Holloway JW. Multigenerational cohorts in patients with asthma and allergy. J. Allergy Clin. Immunol. 2017;139:415–421. doi: 10.1016/j.jaci.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Joubert BR, et al. Maternal smoking and DNA methylation in newborns: in utero effect or epigenetic inheritance? Cancer Epidemiol. Biomark. Prev. 2014;23:1007–1017. doi: 10.1158/1055-9965.EPI-13-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.De Stavola BL, Leon DA, Koupil I. Intergenerational correlations in size at birth and the contribution of environmental factors: The Uppsala Birth Cohort Multigenerational Study, Sweden, 1915-2002. Am. J. Epidemiol. 2011;174:52–62. doi: 10.1093/aje/kwr032. [DOI] [PubMed] [Google Scholar]
- 207.Lahti-Pulkkinen M, et al. Intergenerational transmission of birth weight across 3 generations. Am. J. Epidemiol. 2018;187:1165–1173. doi: 10.1093/aje/kwx340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Agha G, et al. Birth weight-for-gestational age is associated with DNA methylation at birth and in childhood. Clin. Epigenetics. 2016;8:118. doi: 10.1186/s13148-016-0285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tuscher JJ, Day JJ. Multigenerational epigenetic inheritance: one step forward, two generations back. Neurobiol. Dis. 2019;132:104591. doi: 10.1016/j.nbd.2019.104591. [DOI] [PubMed] [Google Scholar]
- 210.Lin X, et al. Developmental pathways to adiposity begin before birth and are influenced by genotype, prenatal environment and epigenome. BMC Med. 2017;15:50. doi: 10.1186/s12916-017-0800-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Simpkin AJ, et al. Longitudinal analysis of DNA methylation associated with birth weight and gestational age. Hum. Mol. Genet. 2015;24:3752–3763. doi: 10.1093/hmg/ddv119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Cardenas A, et al. Mediation by placental dna methylation of the association of prenatal maternal smoking and birth weight. Am. J. Epidemiol. 2019;188:1878–1886. doi: 10.1093/aje/kwz184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Collins JW, Jr., David RJ, Rankin KM, Desireddi JR. Transgenerational effect of neighborhood poverty on low birth weight among African Americans in Cook County, Illinois. Am. J. Epidemiol. 2009;169:712–717. doi: 10.1093/aje/kwn402. [DOI] [PubMed] [Google Scholar]
- 214.McFarland MJ, McLanahan SS, Goosby BJ, Reichman NE. Grandparents’ education and infant health: pathways across generations. J. Marriage Fam. 2017;79:784–800. doi: 10.1111/jomf.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Morton SM, De Stavola BL, Leon DA. Intergenerational determinants of offspring size at birth: a life course and graphical analysis using the aberdeen children of the 1950s study (ACONF) Int J. Epidemiol. 2014;43:749–759. doi: 10.1093/ije/dyu028. [DOI] [PubMed] [Google Scholar]
- 216.Agius R, Savona-Ventura C, Vassallo J. Transgenerational metabolic determinants of fetal birth weight. Exp. Clin. Endocrinol. Diabetes. 2013;121:431–435. doi: 10.1055/s-0033-1345121. [DOI] [PubMed] [Google Scholar]
- 217.Shen Y, et al. Maternal birth weight and bmi mediate the transgenerational effect of grandmaternal bmi on grandchild’s birth weight. Obesity. 2020;28:647–654. doi: 10.1002/oby.22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rillamas-Sun E, Harlow SD, Randolph JF., Jr. Grandmothers’ smoking in pregnancy and grandchildren’s birth weight: comparisons by grandmother birth cohort. Matern Child Health J. 2014;18:1691–1698. doi: 10.1007/s10995-013-1411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ding M, et al. Smoking during pregnancy in relation to grandchild birth weight and BMI trajectories. PLoS ONE. 2017;12:e0179368. doi: 10.1371/journal.pone.0179368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hypponen E, Smith GD, Power C. Effects of grandmothers’ smoking in pregnancy on birth weight: intergenerational cohort study. BMJ. 2003;327:898. doi: 10.1136/bmj.327.7420.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kaati G, Bygren LO, Edvinsson S. Cardiovascular and diabetes mortality determined by nutrition during parents’ and grandparents’ slow growth period. Eur. J. Hum. Genet. 2002;10:682–688. doi: 10.1038/sj.ejhg.5200859. [DOI] [PubMed] [Google Scholar]
- 222.Bygren LO, et al. Change in paternal grandmothers’ early food supply influenced cardiovascular mortality of the female grandchildren. BMC Genet. 2014;15:12. doi: 10.1186/1471-2156-15-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Li J, et al. Prenatal exposure to famine and the development of hyperglycemia and type 2 diabetes in adulthood across consecutive generations: a population-based cohort study of families in Suihua, China. Am. J. Clin. Nutr. 2017;105:221–227. doi: 10.3945/ajcn.116.138792. [DOI] [PubMed] [Google Scholar]
- 224.Heijmans BT, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl Acad. Sci. USA. 2008;105:17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Brook JS, Zhang C, Balka EB, Brook DW. Pathways to children’s externalizing behavior: a three-generation study. J. Genet. Psychol. 2012;173:175–197. doi: 10.1080/00221325.2011.594821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bailey JA, Hill KG, Oesterle S, Hawkins JD. Linking substance use and problem behavior across three generations. J. Abnorm Child Psychol. 2006;34:263–292. doi: 10.1007/s10802-006-9033-z. [DOI] [PubMed] [Google Scholar]
- 227.Bailey JA, Hill KG, Oesterle S, Hawkins JD. Parenting practices and problem behavior across three generations: monitoring, harsh discipline, and drug use in the intergenerational transmission of externalizing behavior. Dev. Psychol. 2009;45:1214–1226. doi: 10.1037/a0016129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Gustavson, K. et al. Smoking in pregnancy and child ADHD. Pediatrics10.1542/peds.2016-2509 (2017). [DOI] [PMC free article] [PubMed]
- 229.Golding J, et al. Grand-maternal smoking in pregnancy and grandchild’s autistic traits and diagnosed autism. Sci. Rep. 2017;7:46179. doi: 10.1038/srep46179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Perkins HW, Berkowitz AD. Collegiate COAs and alcohol abuse: problem drinking in relation to assessments of parent and grandparent alcoholism. J. Counseling Dev. 1991;69:237–240. doi: 10.1002/j.1556-6676.1991.tb01495.x. [DOI] [Google Scholar]
- 231.Kendler KS, Ohlsson H, Sundquist J, Sundquist K. Transmission of alcohol use disorder across three generations: a Swedish National Study. Psychol. Med. 2018;48:33–42. doi: 10.1017/S0033291717000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Brown SA, Tate SR, Vik PW, Haas AL, Aarons GA. Modeling of alcohol use mediates the effect of family history of alcoholism on adolescent alcohol expectancies. Exp. Clin. Psychopharmacol. 1999;7:20–27. doi: 10.1037/1064-1297.7.1.20. [DOI] [PubMed] [Google Scholar]
- 233.Fuller BE, et al. Predictors of aggression across three generations among sons of alcoholics: relationships involving grandparental and parental alcoholism, child aggression, marital aggression and parenting practices. J. Stud. Alcohol. 2003;64:472–483. doi: 10.15288/jsa.2003.64.472. [DOI] [PubMed] [Google Scholar]
- 234.Pears K, Capaldi DM, Owen LD. Substance use risk across three generations: the roles of parent discipline practices and inhibitory control. Psychol. Addict. Behav. 2007;21:373–386. doi: 10.1037/0893-164X.21.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Valentine G, Holloway SL, Jayne M. Generational patterns of alcohol consumption: continuity and change. Health Place. 2010;16:916–925. doi: 10.1016/j.healthplace.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 236.Sagi-Schwartz A, van IJzendoorn MH, Bakermans-Kranenburg MJ. Does intergenerational transmission of trauma skip a generation? No meta-analytic evidence for tertiary traumatization with third generation of Holocaust survivors. Attachment Hum. Dev. 2008;10:105–121. doi: 10.1080/14616730802113661. [DOI] [PubMed] [Google Scholar]
- 237.de Quervain DJ, et al. A deletion variant of the α2b-adrenoceptor is related to emotional memory in Europeans and Africans. Nat. Neurosci. 2007;10:1137. doi: 10.1038/nn1945. [DOI] [PubMed] [Google Scholar]
- 238.van IJzendoorn MH, Bakermans-Kranenburg MJ, Sagi-Schwartz A. Are children of Holocaust survivors less well-adapted? A meta-analytic investigation of secondary traumatization. J. Trauma Stress. 2003;16:459–469. doi: 10.1023/A:1025706427300. [DOI] [PubMed] [Google Scholar]
- 239.Eaton WW, Sigal JJ, Weinfeld M. Impairment in Holocaust survivors after 33 years: data from an unbiased community sample. Am. J. Psychiatry. 1982;139:773–777. doi: 10.1176/ajp.139.12.1646-c. [DOI] [PubMed] [Google Scholar]
- 240.Siegel J, Han WJ. Family exposure to potentially traumatic events and chinese children’s psychological adjustment: a transgenerational study. J. Child Fam. Stud. 2017;27:431–442. doi: 10.1007/s10826-017-0894-2. [DOI] [Google Scholar]
- 241.Perroud N, et al. The Tutsi genocide and transgenerational transmission of maternal stress: epigenetics and biology of the HPA axis. World J. Biol. Psychiatry. 2014;15:334–345. doi: 10.3109/15622975.2013.866693. [DOI] [PubMed] [Google Scholar]
- 242.Bierer LM, et al. Intergenerational effects of maternal holocaust exposure on fkbp5 methylation. Am. J. Psychiatry. 2020;177:744–753. doi: 10.1176/appi.ajp.2019.19060618. [DOI] [PubMed] [Google Scholar]
- 243.McGee, G. et al. Methodological issues in population-based studies of multigenerational associations. American Journal of Epidemiology, 10.1093/aje/kwaa125 (2020). [DOI] [PMC free article] [PubMed]
- 244.Teh AL, et al. The effect of genotype and in utero environment on interindividual variation in neonate DNA methylomes. Genome Res. 2014;24:1064–1074. doi: 10.1101/gr.171439.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Cheng, T. L., Johnson, S. B. & Goodman, E. Breaking the intergenerational cycle of disadvantage: the three generation approach. Pediatrics137, 10.1542/peds.2015-2467 (2016). [DOI] [PMC free article] [PubMed]
- 246.Kundaje A, et al. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518:317–330. doi: 10.1038/nature14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Birney E, Smith GD, Greally JM. Epigenome-wide association studies and the interpretation of disease -omics. PLoS Genet. 2016;12:e1006105. doi: 10.1371/journal.pgen.1006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Hertz-Picciotto I, et al. A prospective study of environmental exposures and early biomarkers in autism spectrum disorder: design, protocols, and preliminary data from the MARBLES study. Environ. Health Perspect. 2018;126:117004. doi: 10.1289/EHP535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.S, R. in Postgenomics Perspective on Biology after the Genome (ed Richardson S. and Stevens H.) 210–231 (Duke University Press, 2015).
- 250.CJ W. Transgenerational tort liability for epigenetic disease. DePaul J. Health Care Law. 2010;13:319–338. [Google Scholar]
- 251.Gillman MW, Blaisdell CJ. Environmental influences on child health outcomes, a research program of the national institutes of health. Curr. Opin. Pediatr. 2018;30:260–262. doi: 10.1097/MOP.0000000000000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Blackwell CK, Wakschlag LS, Gershon RC, Cella D, with the, E. P. R. O. C. Measurement framework for the environmental influences on child health outcomes research program. Curr. Opin. Pediatr. 2018;30:276–284. doi: 10.1097/MOP.0000000000000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Jacobson LP, Lau B, Catellier D, Parker CB. An Environmental influences on child health outcomes viewpoint of data analysis centers for collaborative study designs. Curr. Opin. Pediatr. 2018;30:269–275. doi: 10.1097/MOP.0000000000000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.