Abstract
Arguably, the most important discovery in the biology of aging to date was that simply reducing food intake extended life and improved many aspects of health in a diversity of animal species. The conventional wisdom that emerged from first 50 years of rodent food restriction studies included (1) that the longevity impact of restriction was greater the longer restriction was imposed, and (2) that restricting calories rather than any specific macronutrient was critical to its health and longevity benefits. However these assumptions began to crumble as more and more restriction research was performed on other species besides laboratory rodents. Recent investigations of flies, rodents, monkeys, and increasingly humans, has begun to parse how calorie restriction, protein restriction, intermittent fasting, and the temporal pattern of eating all impact the health benefits of food restriction. Fly research continues to inform, as it has repeatedly shown that genotype, age, sex, duration, and tempo restriction all affect the health impact. Ultimately, optimizing human diets will require a personalized approach using omics approaches.
Introduction
Arguably, the most important discovery in the biology of aging to date – and also one of the oldest discoveries – was that simply reducing food intake extended life and improved many aspects of health in a diversity of animal species [1,2]. The field of what was most often called food restriction or dietary restriction (DR) was dominated for many decades by a relatively small group of researchers using laboratory rodents in long-term survival studies. Following McCay’s original research paradigm using rats [3], one key research assumption was that the health and longevity effects of food restriction, which we will call the DR effect, required chronic, long-term restriction. Another assumption based on rodent studies was that restricting calories, rather than specific macronutrients was responsible for the DR effect [1,4]. As a consequence, DR was more and more frequently called caloric restriction. In the late 20th century as invertebrate models such as Drosophila melanogaster flies and Caenorhabditis elegans nematodes became more and more influential in aging studies, researchers using those models did not automatically accept these assumptions. Largely because of research initiated in invertebrate models, these assumptions have begun to crumble. Also, as research attention has shifted from focusing mainly on lifespan to focusing on healthspan, and as humans have become more eager for evidence-based nutritional advice on healthy diets for themselves, macronutrient composition of the diet, as well as the length and timing of restriction have emerged as important contributors to the healthful impact of DR. In this review, we will highlight some of these recent variations on an old paradigm (Figure 1).
Figure 1.
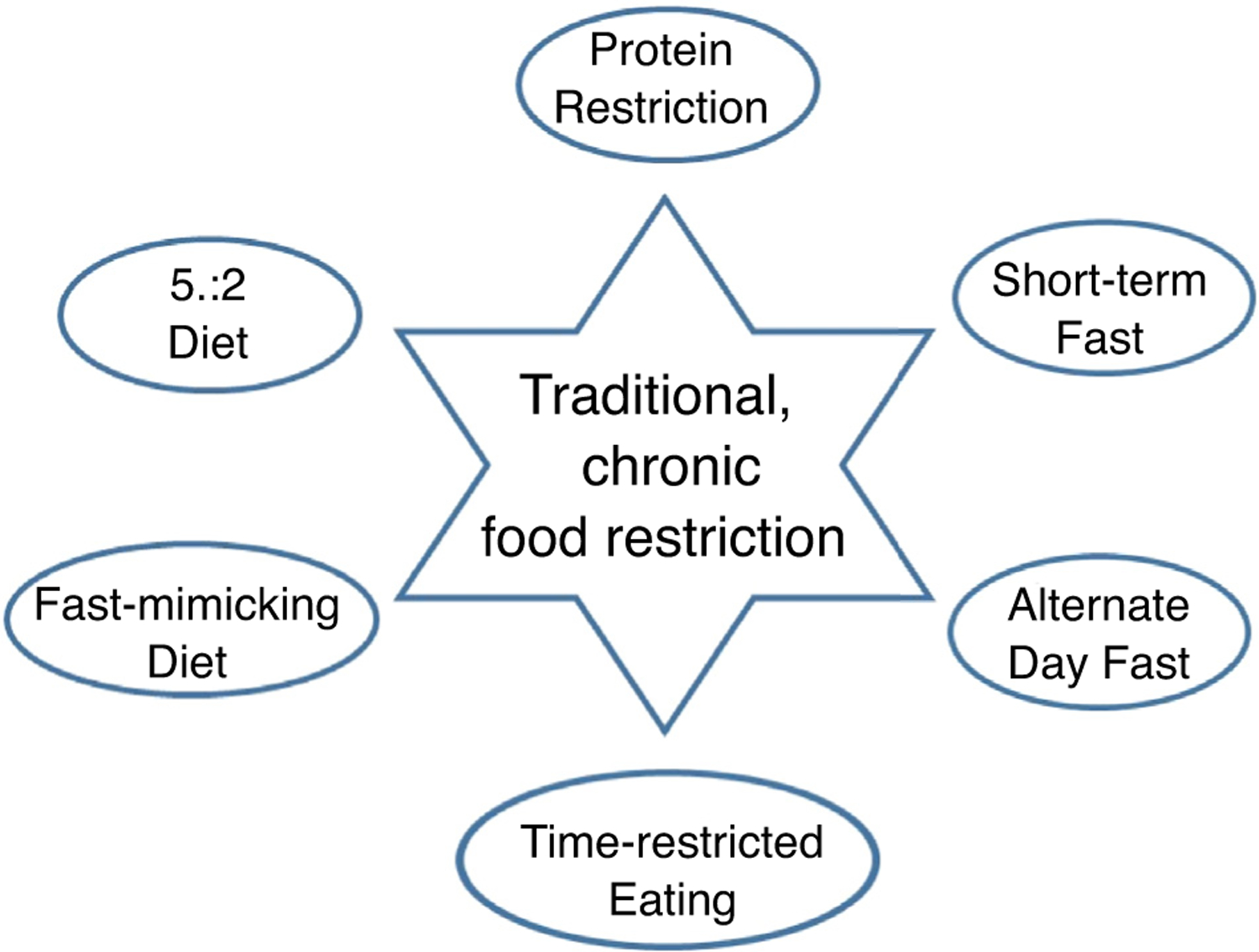
Some currently experimental variations on traditional daily food restriction as experimentally investigated by early food restriction researchers. All of these have shown some health benefits in short-term human or life-long rodent studies. Protein restriction is self-explanatory, short-term fast refers to 2–3 day water only fasts, alternate day fast is self-explanatory, time-restricted eating describes confining one’s eating to a restricted number of hours (usually 4–6) during the day [45], the fast-mimicking diet is consumption of a ‘fast mimicking,’ that is, low protein, low calorie, diet for 5 consecutive days every month [46], and the 5:2 diet is an intermittent restriction diet in which practitioners severely restrict food intake for two consecutive days each week and eat normally the other five days [42].
Translation, short-term fasting and protein restriction
Two long-term DR studies in nonhuman primates revealed that the translation of DR rodent studies to humans was anything but straightforward. Laboratory rodents spend their lives in cages only a few times bigger than their body length so have little opportunity for physical activity. They have food available 24 hours per day, and not surprisingly are significantly more obese than rodents living in the field [5]. When we restrict their diets are we returning them to a body composition more like they evolved with, or are we reducing them from a ‘normal’ weight to an emaciated but healthier state? The two nonhuman primate studies illustrate the translational issue this poses. Both studies used rhesus monkeys. Both restricted their animals’ calories by 30% from control levels. The University of Wisconsin study allowed their control animals to eat ad libitum and become obese as captive monkeys invariably do. They found 30% DR improved multiple aspects of health and significantly increased survival [6]. The National Institute of Aging study fed their control animals a regulated amount to achieve a ‘healthy body weight’ and restricted from there. Although they found modest improvements in several aspects of health, there was no vestige of a DR effect on longevity [7]. Either of these studies may be relevant to different subgroups of modern humans. Short-term human studies have also been of both types. The CALERIE study was a controlled clinical trial on healthy young to middle-age non-obese people with a target of 25% reduction in energy intake over two years. Confirming that most humans are not capable of sustained major reduction in energy intake, the achieved level of energy reduction over the two years was 11.7 ± 0.7% (mean ± standard error) with a 10.4 ± 0.6% weight loss, less than half the targeted value. However, this modest reduction of Body Mass Index (BMI: kg/m2) from marginally overweight 25.1 (range: 21.9–28.0 kg/m2) at the beginning of the study to 22.5 ± 0.1%, the middle of WHO’s ‘healthy weight’ range, was attended by improvements in multiple cardiovascular risk factors [8]. A more extreme, although less controlled, food restriction regime is practiced by members of the Calorie Restriction Optimal Nutrition Society, or CRONies) [9], whose level of motivation inspired by the prospect of longer life has allowed them to maintain a high level of self-imposed restriction for years. The CRONies studied by Holloszy and Fontana had mean BMIs of 19.6 after 6 years of self-imposed restriction. They displayed excellent values for a range of cardiovascular risk factors. However, these risk factors are not substantially better than those people who achieve less extreme leanness by other means such as a vegan diet or long-term endurance exercise [10]. On the negative side, DR but not exercise, was associated with reduced lower extremity strength and aerobic capacity, as well as reduced bone mineral density [11,12]. On balance, it still is not clear what level of DR might be generally beneficial to humans under what conditions, even if more people were capable of doing it.
Drosophila research first showed that short-term food restriction, rather than life-long restriction, could alter mortality rate in as little as 1–3 days [13]. Also, studies by toxicologists and others had shown that DR protected animals against a variety of stressors [14]. These observations led researchers to investigate whether short-term fasting might protect against the stress of surgically induced ischemia injury and the side-effects of cancer chemotherapy using mouse models, which indeed it did [15–17]. These discoveries were followed up by investigations into the molecular basis of these benefits which led to investigation on what aspect of the fasting diet was important. Was it specific macronutrients or fasting itself?
The evidence that calorie, rather than some specific macronutrient, restriction was responsible for the DR effect had always been somewhat equivocal. Even early rodent studies found that restricting protein while keeping calories constant had salubrious effects [18]. Indeed, restricting even a single amino acid had been shown to extend rodent life [19]. Yet protein restriction for extended health received little attention until researchers showed repeatedly that protein, not calorie, restriction was the driving force for extending Drosophila lifespan [20–22]. This led to a revived interest in protein and even specific amino acid restriction in mammals. Further research on recovery from surgical ischemia/reperfusion injury paradigm described earlier showed that complete food-free, short-term fasting was not required for the observed protective effect. It could also be achieved by depleting dietary protein or even by depleting a single amino acid [23]. Recently a more sophisticated approach to investigating macronutrient composition in diets has emerged. ‘Nutritional geometry’ manipulates dietary macronutrients in varying combinations rather than individually and is revealing fascinating patterns in nutritional physiology [24–26]. With respect to DR, this work found that reducing calorie intake by food dilution did not extend life in mice as standard DR did, raising the possibility that the neurobiology of satiety may be involved in the DR effect somehow. Other fly and rodent restriction research now began to raise cautionary flags about any single dietary studies focused on single genotypes or ages of experimental animals by showing that genetic background, including sex, could have a major impact on the health impact of either general food restriction or the restriction of specific macronutrients [27–31]. Currently, it seems unlikely that any one dietary formulation will be universally healthful. It will depend on all the variables mentioned above. Human studies combined with extensive ‘omics’ analysis should open the way to more precise and predictable dietary interventions into health.
Other forms of restriction
Another revision to the traditional DR regime came from the observation that because restricted rodents were hungry they ate their food quickly, usually in less than an hour, so that the standard research paradigm could be re-interpreted as a 23 hour daily fast. Modern research on the physiological and molecular biology of fasting shows that fasting can quickly and profoundly alter cellular fuel use as well as gene expression in organ-specific ways [32]. For instance, fasting rodents show a significant elevation in plasma ketones, indicative of a switch from carbohydrate to fatty acid metabolism, within 4–8 hours. Humans display a similar pattern after 8–12 hours of fasting [33]. Also, for years, some rodent studies had restricted food intake by feeding their animals ad lib on an every other day schedule. This research approach had similar health and survival effects to daily restriction, yet when food consumption was eventually measured, it sometimes turned out that the animals were gorging sufficiently on the days they ate that they consumed on average as many calories as the control animals eating every day. These observations focused attention on whether the timing of eating or restricting may be as important as the amount eaten or its macronutrient composition [34]. Consequently, a variety of timed fasting or restriction regimes have emerged. These diets include intermittent fasting, typically eating every other day [33], which has been shown to confer health benefits such as improved insulin sensitivity and resistance to renal ischemia-reperfusion injury in mice [35] and improved cardiometabolic risk markers and reduced fat mass in humans [36]. Also, time-restricted eating, typically eating ad lib but restricting daily food consumption to a time window, typically 4–6 hours has been found to decrease 24 hour glucose levels and glycemic excursions. This approach is based on chronobiology which finds that more than 10% of expressed genes have circadian expression patterns, combined with the knowledge that in our evolutionary past before artificial sources of light existed, meals would necessarily be eaten during daylight hours [37]. Numerous studies have found that circadian misalignment of feeding behavior has been shown to have deleterious effects such as increased blood pressure, serum glucose and insulin levels and decreased sleep efficiency [38] and circadian alignment has shown beneficial effects, such as improved glucose control, decreased appetite and fat, and inflammatory markers in humans [39,40]. Another incarnation of intermittent restriction is the ‘fast-mimicking,’ that is, low protein, low calorie, diet. In this approach, the low protein/calorie diet is eaten for 5 consecutive days per month with normal food consumption the other days. This diet has shown a variety of beneficial effects in mice such as increased longevity, reduced visceral fat and cancer incidence, and improved immune system markers, and in short-term human studies decreased risk factors for diabetes, cardiovascular disease, and cancer [41]. A somewhat different version of this is the 5:2 diet in which a normal diet is eaten five days per week with a severely restricted number of calories eaten on two consecutive days. That diet has also shown short-term health benefits, including lowering total and visceral adiposity and may have improved liver function in human studies [42]. The tremendous advantage of these new, restriction-based, dietary regimes are they can easily be modeled laboratory species but more importantly, unlike chronic calorie restriction, people actually have a chance of adopting them if the evidence on their health benefits warrants [40]. However, once again research in flies showed the complexity that could be found within these research paradigms. Specifically, the age and duration of intermittent fasting have both been found to affect its health impact [43,44] and presumably so will sex and genotype.
Conclusion
The amount, composition, and timing of nutrient intake are all turning out to be important in the preservation of human health. Now that more translational dietary and nutritional regimes have been developed, along with ‘omic’ tools to assess their impact, we expect rapid expansion in our knowledge of optimized, personalized diets in the coming years.
Acknowledgements
Our research is funded by N.I.H. grants R01 AG057434, R21 AG058811, P30 AG050886 (S.N.A.) and K99AG059920 (J.M.H.) and the Glenn Foundation for Medical Research.
Footnotes
Conflict of interest statement
Nothing declared.
References
- 1.Weindruch R, Walford RL: The Retardation of Aging and Disease by Dietary Restriction. Springfield, IL: Charles C. Thomas; 1988. [Google Scholar]
- 2.Swindell WR: Dietary restriction in rats and mice: a meta-analysis and review of the evidence for genotype-dependent effects on lifespan. Ageing Res Rev 2012, 11:254–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCay CM, Crowell MF, Maynard LA: The effect of retarded growth upon the length of the life span and upon ultimate body size. J Nutr 1935, 13:669–679. [PubMed] [Google Scholar]
- 4.Masoro EJ: Overview of caloric restriction and ageing. Mech Ageing Dev 2005, 126:913–922. [DOI] [PubMed] [Google Scholar]
- 5.Austad SN, Kristan DM: Are mice calorically restricted in nature? Aging Cell 2003, 2:201–207. [DOI] [PubMed] [Google Scholar]
- 6.Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM: Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun 2014, 5:3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M et al. : Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 2012, 489:318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kraus WE, Bhapkar M, Huffman KM, Pieper CF, Krupa Das S, Redman LM, Villareal DT, Rochon J, Roberts SB, Ravussin E et al. : 2 years of calorie restriction and cardiometabolic risk (calerie): exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol 2019, 7:673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fontana L, Meyer TE, Klein S, Holloszy JO: Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A 2004, 101:6659–6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fontana L, Meyer TE, Klein S, Holloszy JO: Long-term low-calorie low-protein vegan diet and endurance exercise are associated with low cardiometabolic risk. Rejuvenation Res 2007, 10:225–234. [DOI] [PubMed] [Google Scholar]
- 11.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Ehsani AA, Holloszy JO: Lower extremity muscle size and strength and aerobic capacity decrease with caloric restriction but not with exercise-induced weight loss. J Appl Physiol 2007, 102:634–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villareal DT, Fontana L, Weiss EP, Racette SB, Steger-May K, Schechtman KB, Klein S, Holloszy JO: Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: a randomized controlled trial. Arch Intern Med 2006, 166:2502–2510. [DOI] [PubMed] [Google Scholar]
- 13.Mair W, Goymer P, Pletcher SD, Partridge L: Demography of dietary restriction and death in Drosophila. Science 2003, 301:1731–1733. [DOI] [PubMed] [Google Scholar]
- 14.Hart RW, Chou MW, Feuers RJ, Leakey JE, Duffy PH, Lyn-Cook B, Turturro A, Allaben WT: Caloric restriction and chemical toxicity/carcinogenesis. Qual Assur 1992, 1:120–131. [PubMed] [Google Scholar]
- 15.Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, Longo VD: Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci U S A 2008, 105:8215–8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell JR, Verweij M, Brand K, van de Ven M, Goemaere N, van den Engel S, Chu T, Forrer F, Muller C, de JM et al. : Short-term dietary restriction and fasting precondition against ischemia reperfusion injury in mice. Aging Cell 2010, 9:40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verweij M, van Ginhoven TM, Mitchell JR, Sluiter W, van den Engel S, Roest HP, Torabi E, Ijzermans JN, Hoeijmakers JH, de Bruin RW: Preoperative fasting protects mice against hepatic ischemia/reperfusion injury: mechanisms and effects on liver regeneration. Liver Transpl 2011, 17:695–704. [DOI] [PubMed] [Google Scholar]
- 18.Iwasaki K, Gleiser CA, Masoro EJ, McMahan CA, Seo EJ, Yu BP: Influence of the restriction of individual dietary components on longevity and age-related disease of fischer rats: the fat component and the mineral component. J Gerontol 1988, 43: B13–B21. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerman JA, Malloy V, Krajcik R, Orentreich N: Nutritional control of aging. Exp Gerontol 2003, 38:47–52. [DOI] [PubMed] [Google Scholar]
- 20.Mair W, Piper MD, Partridge L: Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol 2005, 3:e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruce KD, Hoxha S, Carvalho GB, Yamada R, Wang HD, Karayan P, He S, Brummel T, Kapahi P, Ja WW: High carbohydrate-low protein consumption maximizes Drosophila lifespan. Exp Gerontol 2013, 48:1129–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tatar M, Post S, Yu K: Nutrient control of Drosophila longevity. Trends Endocrinol Metab TEM 2014, 25:509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brandhorst S, Harputlugil E, Mitchell JR, Longo VD: Protective effects of short-term dietary restriction in surgical stress and chemotherapy. Ageing Res Rev 2017, 39:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JW, Taylor PW, Soran N, Raubenheimer D: Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc Natl Acad Sci U S A 2008, 105:2498–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG et al. : The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab 2014, 19:418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Senior AM, Solon-Biet SM, Cogger VC, Le Couteur DG, Nakagawa S, Raubenheimer D, Simpson SJ: Dietary macronutrient content, age-specific mortality and lifespan. Proc Biol Sci R Soc 2019, 286 20190393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim K, Jang T, Min K-J, Lee KP: Effects of dietary protein: carbohydrate balance on life-history traits in six laboratory strains of Drosophila melanogaster. Entomol Exp Appl 2019:1–10.
- 28.Liao CY, Rikke BA, Johnson TE, Diaz V, Nelson JF: Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell 2010, 9:92–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell SJ, Madrigal-Matute J, Scheibye-Knudsen M, Fang E, Aon M, Gonzalez-Reyes JA, Cortassa S, Kaushik S, Gonzalez-Freire M, Patel B et al. : Effects of sex, strain, and energy intake on hallmarks of aging in mice. Cell Metab 2016, 23:1093–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagarajan-Radha V, Rapkin J, Hunt J, Dowling DK: Interactions between mitochondrial haplotype and dietary macronutrient ratios confer sex-specific effects on longevity in Drosophila melanogaster. J Gerontol Ser A Biol Sci Med Sci 2019, 74:1573–1581. [DOI] [PubMed] [Google Scholar]
- 31.Wu Q, Yu G, Cheng X, Gao Y, Fan X, Yang D, Xie M, Wang T, Piper MDW, Yang M: Sexual dimorphism in the nutritional requirement for adult lifespan in Drosophila melanogaster. Aging Cell 2020, 19:e13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mattson MP, Moehl K, Ghena N, Schmaedick M, Cheng A: Intermittent metabolic switching, neuroplasticity and brain health. Nat Rev Neurosci 2018, 19:63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Cabo R, Mattson MP: Effects of intermittent fasting on health, aging, and disease. New Engl J Med 2019, 381:2541–2551. [DOI] [PubMed] [Google Scholar]
- 34.Anson RM, Guo Z, de CR, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, Mattson MP: Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A 2003, 100:6216–6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reynolds JS, Peng W, Chu T, Mitchell JR: Effects of timing of food intake and fat/carbohydrate ratio on insulin sensitivity and preconditioning against renal ischemia reperfusion injury by calorie restriction. Nutr Healthy Aging 2019, 5:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stekovic S, Hofer SJ, Tripolt N, Aon MA, Royer P, Pein L, Stadler JT, Pendl T, Prietl B, Url J et al. : Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell Metab 2020, 31:878–881. [DOI] [PubMed] [Google Scholar]
- 37.de Goede P, Foppen E, Ritsema W, Korpel NL, Yi CX, Kalsbeek A: Time-restricted feeding improves glucose tolerance in rats, but only when in line with the circadian timing system. Front Endocrinol (Lausanne) 2019, 10:554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheer FA, Hilton MF, Mantzoros CS, Shea SA: Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A 2009, 106:4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM: Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients 2019, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ravussin E, Beyl RA, Poggiogalle E, Hsia DS, Peterson CM: Early time-restricted feeding reduces appetite and increases fat oxidation but does not affect energy expenditure in humans. Obesity 2019, 27:1244–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, Dubeau L, Yap LP, Park R, Vinciguerra M et al. : A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab 2015, 22:86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Panizza CE, Lim U, Yonemori KM, Cassel KD, Wilkens LR, Harvie MN, Maskarinec G, Delp EJ, Lampe JW, Shepherd JA et al. : Effects of intermittent energy restriction combined with a Mediterranean diet on reducing visceral adiposity: a randomized active comparator pilot study. Nutrients 2019, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Catterson JH, Khericha M, Dyson MC, Vincent AJ, Callard R, Haveron SM, Rajasingam A, Ahmad M, Partridge L: Short-term, intermittent fasting induces long-lasting gut health and tor-independent lifespan extension. Curr Biol CB 2018, 28:1714–1724 e1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang S, Ratliff EP, Molina B, El-Mecharrafie N, Mastroianni J, Kotzebue RW, Achal M, Mauntz RE, Gonzalez A, Barekat A et al. : Aging and intermittent fasting impact on transcriptional regulation and physiological responses of adult Drosophila neuronal and muscle tissues. Int J Mol Sci 2018, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mattson MP, Allison DB, Fontana L, Harvie M, Longo VD, Malaisse WJ, Mosley M, Notterpek L, Ravussin E, Scheer FA et al. : Meal frequency and timing in health and disease. Proc Natl Acad Sci U S A 2014, 111:16647–16653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei M, Brandhorst S, Shelehchi M, Mirzaei H, Cheng CW, Budniak J, Groshen S, Mack WJ, Guen E, Di Biase S et al. : Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci Transl Med 2017, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]


