Abstract
Background: The ketogenic diet (KD) has been reported to play an important role in the development of cancer by an abundance of pre-clinical experiments; however, their conclusions have been controversial. We therefore aimed to perform a systematic review and meta-analysis of animal studies evaluating the effects of KD on cancer.
Methods: Relevant studies were collected by searching PubMed, Embase, and Web of Science. Outcome measures comprised tumor weight, tumor volume, and survival time. Meta-analysis was performed using the random-effect model according to heterogeneity.
Results: The search resulted in 1,254 references, of which 38 were included in the review and 17 included in the meta-analysis. Pooled results indicated that KD supplementation significantly prolonged survival time [standardized mean difference (SMD) = 1.76, 95% CI (0.58, 2.94), p = 0.003], and reduced tumor weight [SMD = −2.459, 95% CI (−4.188, −0.730), p = 0.027] and tumor volume [SMD = −0.759, 95% CI (−1.349, −0.168), p = 0.012]. Meta-regression and subgroup analysis results suggested that KD supplementation at a ratio of 4:1 was associated with remarkable prolongation of survival time in animals with limited tumor types.
Conclusion: In summary, the pre-clinical evidence pointed toward an overall anti-tumor effect of the KD in animals studies currently available with limited tumor types.
Keywords: ketogenic diet, tumor, meta-analysis, survival time, animal studies
Introduction
Cancer is one of the major problems worldwide and is grievously harmful to human health (1). Recently, it has been found that tumor metabolic reprogramming is a central feature of tumors (2). The Warburg effect, as the core of tumor metabolic reprogramming, indicates that tumor cells tend to undergo aerobic glycolysis to metabolize glucose (3). Thus, reducing glucose supply and selectively cutting off the energy source of tumor cells could inhibit tumor growth (4). The ketogenic diet (KD), characterized by a high-fat, low-carbohydrate, and adequate-protein diet, can meet such demand. Therefore, ketogenic therapy for cancer has emerged and become an area of wide discussion in tumor research in recent years.
A great number of pre-clinical studies have suggested that KD is a potent anticancer therapy when used separately or as an adjuvant (5). It has been reported that KD not only slowed tumor growth and delayed the initiation of tumor development, but also prolonged survival time (6, 7). In addition, some studies have demonstrated that KD could increase the sensitivity of tumor cells to classic chemotherapy and radiotherapy when used in combination (8–10). Furthermore, KD has been reported to enhance the efficacy of targeted therapy and overcome drug resistance in several tumor models when using PI3K inhibitors (11), as well as reduce metastatic potential (12, 13). On the contrary, pro-tumor effects or severe side effects have been found in certain cancer models. For instance, such effects have been described in a rat model of tuberous sclerosis complex when investigating the long-term KD treatment effects on kidney cancer (14), while another study observed that tumor growth has significantly increased with KD supplementation in a mouse model of BRAF V600E-positive melanoma (15). Therefore, it is controversial whether KD has shown anti-tumor effects in pre-clinical studies.
To date, clinical evidence from randomized controlled clinical trials is still lacking, and available evidence is mostly from case reports and pilot/feasibility studies. To better understand the anti-tumor effects of KD and to pave the way for further prospective clinical studies, we performed a systematic review and meta-analysis of current available data on animal tumor models treated with KD alone or in combination with classic therapy and/or caloric restriction.
Materials and Methods
Literature Search
A comprehensive, computerized literature search was performed in PubMed, Embase, and Web of Science up to April, 2020 using the following key words: “ketogenic,” “caloric restriction” paired with the following: “glioma,” “glioblastoma,” “tumor,” “cancer,” “neuroblastoma,” “carcinoma” (see Supplementary Table 1). References of the identified publications were then reviewed to further identify potentially relevant articles.
Study Selection and Inclusion Criteria
Studies were included in our article if the following criteria were met: (1) published as full-length articles in English; (2) reported as animal studies; (3) the exposure of interest was KD alone or in combination with classic therapy and/or caloric restriction; and (4) reported data on at least one of the following: survival time, tumor volume, or tumor weight. The following additional exclusion criteria were used for full-text screening: (1) full text not available, (2) double publication, (3) conference abstracts, (4) review, (5) editorials, and (6) comments.
Data Extraction and Quality Assessment
Literature search, data extraction, and quality assessment were completed independently by two authors (J.L. and H.Y.Z.) according to the inclusion criteria. In cases of disagreement between the authors, consensus was reached. The following information were extracted: the first author's name, published year, tumor type, animal species, cell strain, the ketogenic ratio, the composition of KD, whether KD was accompanied with caloric restriction, study groups, animal number of each group, survival time, tumor weight, tumor volume, the levels of glucose and β-hydroxybutyrate, the changes of body weight and conclusion. Outcome measures, including tumor weight, tumor volume, and survival time were included in the meta-analysis. The mean value, standard deviation (SD), and number of animals per group were extracted. For studies with multiple intervention groups [e.g., KD and KD + chemotherapy (CT)], the shared control group was split into 2 or more groups of smaller sample sizes to overcome unit-of-analysis errors, and these multiple comparisons were included into the meta-analysis according to instructions of the Cochrane's Handbook.
Data Synthesis and Statistical Analysis
Given that various measurements have been applied in the included studies, the pooled effects are presented as standardized mean difference (SMD) with 95% confidence intervals (CI). The Cochrane's Q-test was performed to assess inter-study heterogeneity, and significant heterogeneity was considered when p-value was < 0.10. The I2 statistic was also examined, and an I2 value of > 50% indicated significant heterogeneity among the studies. A random effects model or fixed effects model was used according to the heterogeneity.
To explore the potential causes of heterogeneity, meta-regression analysis and pre-defined subgroup analysis were performed. Furthermore, potential publication bias was assessed using the Egger regression asymmetry test and funnel plots. All meta-analyses and statistical analyses were performed using the Stata software (version 12.0; Stata Corporation, College Station, TX, USA).
Results
Description of the Included Studies
The comprehensive search strategy on the effects of KD on tumors resulted in 1,254 records. After removal of duplicates, 673 studies remained. After title and abstract screening, the full texts of 110 studies were screened. Ultimately, 38 studies were included in our systematic review, of which 17 studies were included in the meta-analysis (Figure 1).
Figure 1.
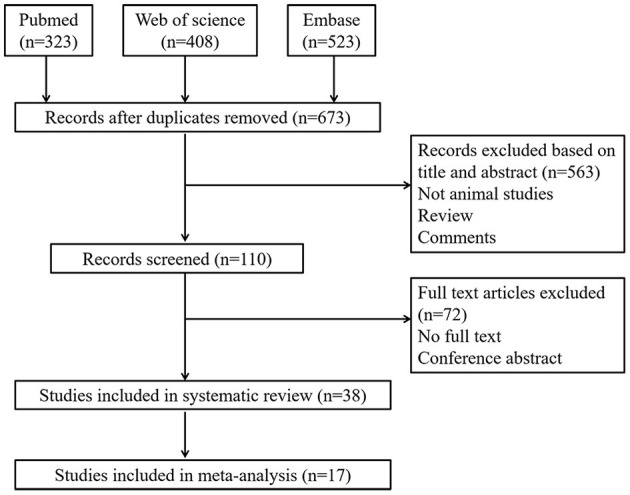
Flow diagram showing literature search and selection results.
The characteristics of all included studies are described in Table 1 and the detailed composition of KD involved in meta-analysis are listed in Supplementary Table 2. Different mouse cancer models have been used to evaluate the anti-tumor effects of KD, including glioblastoma, breast cancer, lung cancer, melanoma, liver cancer, colon cancer, prostate cancer, pancreatic cancer, kidney cancer, neuroblastoma, anaplastic thyroid cancer, medulloblastoma, gastric cancer, and systemic metastatic cancer. The animal species included C57BL/6, BALB/c, VM/Dk, nu/nu, NMR1, CDF1, SCID, athymic nude mice, CD-1 nude mice, Ptch1+/−Trp53−/− mice, Eker (Tsc2+/−) rats and so on. On the other hand, many cell strains were involved, including U87-MG, GL261-Luc2, VM-M3, 4T1, ES272, NCI-H292, A549, LLC1, SH-SY5Y, 786-O, 8505C, LNCaP LAPC-4, et al. KD ratios ranging from 1:1 to 8:1 were widely used across studies. Although some studies reported pro-tumor effects or no effects, most studies suggested that dietary interventions with KD constituted potent anti-cancer therapy. Besides, as a monotherapy, KD has also been used as an adjuvant for chemotherapy, radiotherapy or hyperbaric oxygen therapy, or in combination with metformin.
Table 1.
Animal studies reporting the effect of the KD on tumor growth and survival.
| Tumor | References | Animal species | Cell strain | KD ratio | CR | Study groups | Animal number | Tumor growth | Survival time | G, β-HB and BW | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Glioblastoma | Zhou et al. (16) | C57BL/6J; BALBc/J-SCID mice | U87-MG | 4:1 | Yes | SD; KD; KD-CR | n = 12–14 | KD vs. SD: ns | KD vs. SD: ns | KD-CR reduces G and BW; KD-CR elevates β-HB. | KD-CR has anti-tumor effect. |
| KD-CR vs. SD: inhibition | KD-CR vs. SD: increase | ||||||||||
| Stafford et al. (17) | C57BL/6 mice | GL261 | 8:1 | No | SD; KD | n = 20 | – | KD vs. SD: increase | G: ns; β-HB: increase; BW: ns. | KD improves survivability | |
| Abdelwahab et al. (8) | Albino C57BL/6 mice | GL261-Luc2 | 4:1 | No | SD; KD; SD + RT; KD + RT | n = 11–19 | Inhibition | KD vs. SD: increase; KD + RT vs. SD + RT: no data | G: decrease; β-HB: increase; BW: reduce. | KC enhances survival and slows tumor growth; additive effect of KD + RT | |
| Rieger et al. (18) | Athymic nude mice | U87MG | 3.14:1 | No | SD; KD; SD + CT; KD + CT | n = 5–7 | KD vs. SD: ns; KD + CT vs. SD + CT: inhibition | KD + CT vs. SD + CT: increase | G: ns; β-HB: increase; BW: ns. | No effect of KD alone; enhanced survival of KD + CT vs. SD + CT | |
| Woolf et al. (19) | Albino C57BL/6 mice | GL261-Luc2 | 4:1 | No | SD; KD | n = 6 | – | – | G: decrease; β-HB: increase; BW: reduce. | Alters the hypoxic | |
| Response, reduced tumor microvasculature | |||||||||||
| Lussier et al. (7) | Albino C57BL/6 mice | GL261-Luc2 | 4:1 | No | SD; KD | n = 12 | – | KD vs. SD: increase | G: decrease; β-HB: increase; BW: ns. | KD enhances survival | |
| De Feyter et al. (20) | Fischer rats | RG2, 9L | 4:1 | Yes | SD-CR; KD-CR | n = 10–11 | ns | ns | G: decrease; β-HB: increase; BW: reduce. | No effect | |
| Augur et al. (21) | VM/Dk mice | VM-M3 | 2.7:1 | No | SD; KD | n = 4–9 | – | ns | G: decrease; β-HB: increase; BW: reduce. | No effect | |
| Mukherjee et al. (22) | VM/Dk mice | VM-M3 | 4:1 | Yes | SD; KD-CR; SD + DON; KD-CR + DON | n = 10–15 | – | Increase | CR-KD ± DON decreases G and increases β-HB; BW: reduce. | KD-CR + DON prolong survival time | |
| Breast | Gluschnaider (23) | Transgenic FVB MMTV-PyMT mice | Spontaneous tumor development | 4:1 | No | SD; HFD; KD; MEDICA | n = 8–10 | KD vs. SD: inhibition | – | BW: ns. | Anti-tumor |
| Zhuang et al. (24) | BALB/c mice | 4T1 | 6:1 | Yes | SD; SD + Metformin; KD-CR; KD-CR + Metformin | n = 10 | Inhibition | – | G: decrease | KD-CR have anti-tumor effects; additive effect of KD + Metformin | |
| Hopkins et al. (11) | C57BL/6 mice | ES272 | 6:1 | No | SD; SD + PI3K inhibitors; KD; KD + PI3K inhibitors | n = 5 | Inhibition | – | – | No effect of KD alone, additive effect of KD + PI3K inhibitors | |
| Lung | Allen et al. (9) | nu/nu mice | NCI-H292, A549 | 4:1 | No | SD; KD; IR; IR + KD; CT; CT + KD; IR + CT; IR + CT + KD | n = 5–16 | Inhibition | KD + RT: prolongs survival; KD + RT/CT: prolongs survival | β-HB: increases | No effect of KD alone, enhanced anti-tumor effects of KD + CT/RT |
| Stemmer et al. (25) | C57BL/6 (Fgf21 WT and KO) mice | LLC1 | 3:1; 8:1 | No | SD; RP-KD; LP-KD | n = 8–15 | Inhibition | – | Low protein KD decreases G and increases β-HB. BW: ns. | LP-KD has anti-tumor effect | |
| Liver | Healy et al. (26) | C57BL/6N mice | DEN-induced hepatocellular carcinoma | 5:1 | No | SD; WD-L; WD-C; FD; KD | n = 6–12 | – | – | G: ns; BW: ns. | Anti-tumor |
| Byrne et al. (27) | C57BL/6N mice | DEN-induced hepatocellular carcinoma | 4:1 | No | SD; KD | n = 7 | ns | - | β-HB: increase; BW: increase. | No effect | |
| BRAF V600E-positive A375 melanoma | Xia et al. (15) | nu/nu mice | A375, A2058 (BRAF V600E) | 4:1; 6:1 | No | SD; KD | n = 8 | Increase | – | G: decreases; β-HB: ns; BW: ns. | Pro-tumor |
| SKMEL-2 cells melanoma | Xia et al. (15) | nu/nu mice | SK-MEL-2 (NRAS Q61R) | 4:1 | No | SD; KD | n = 7 | ns | - | G: decreases; β-HB: ns; BW: ns. | No effect |
| Colon | Tisdale et al. (28) | NMR1 mice | MAC16 | 1:1; 2:1 | No | SD; 68%fat KD ± 3-hydroxybutyrate; 80% fat KD ± 3-hydroxybutyrate | ns | 80% fat KD ± 3-hydroxybutyrate decreases tumor weight | - | G: ns; β-HB: increase; BW: reduce. | Anti-tumor |
| Beck and Tisdale (29) | NMR1 mice | MAC16 | 2:1 | No | SD; KD | n = 6–12 | KD decreases tumor weight | – | G: ns; β-HB: increase; BW: reduce. | Anti-tumor | |
| Hao et al. (30) | BALB/c nude mice | HCT-116 | 3:1 | No | SD; MKD; LKD | n = 12 | KD inhibits tumor growth | KD prolongs survival time | G: ns; β-HB: increase; BW: increase. | Anti-tumor | |
| Nakamura et al. (31) | CDF1 mice | Colon 26 | 3:1 | No | SD; KD | n = 5–10 | KD decrease tumor weight | – | β-HB: increase; BW: reduce. | Anti-tumor | |
| Kasumi and Sato (32) | BALB/c mice | Colon 26 | 4:1 | No | SD; KD | n = 6–8 | ns | KD prolongs survival time | G: ns; β-HB: increase; BW: reduce. | KD improves the prognosis | |
| Prostate | Freedland et al. (33) | SCID mice | LAPC-4 | 2:1 | No | Low fat; Western diet; no-carbohydrate ketogenic diet (NCKD) | n = 11 | ns | NCKD prolong survival time | G: increase; β-HB: increase; BW: increase. | Anti-tumor |
| Mavropoulos et al. (34) | Fox chase SCID mice | LNCaP | 2:1 | No | NCKD; LFD; MCD | n = 11 | NCKD decreases tumor volume | NCKD prolongs survival time | G: ns; β-HB: increase; BW: decrease. | Anti-tumor | |
| Kim et al. (35) | Athymic nude mice | LAPC-4 | 2:1 | No | SD ± MCT1 inhibitor; KD ± MCT1 inhibitor | ns | KD decreases tumor volume | KD prolongs survival time | G: ns; BW: ns. | Anti-tumor | |
| Pancreatic cancer | Shukla et al. (36) | Athymic nude mice | S2-013 | 2:1 | No | SD; KD | n = 9 | KD decreases tumor weight and tumor volume | – | G: decrease; β-HB: increase; BW: reduce. | Anti-tumor |
| Zahra et al. (10) | Athymic nude mice | MIA PaCa-2 | 4:1 | No | SD; KD; RT; KD + RT | n = 9–16 | KD + RT decreases tumor volume | KD + RT prolongs survival time | G: decrease; β-HB: increase; BW: reduce. | No effect of KD alone, additive anti-tumor effect of KD + CT | |
| Zhang et al. (37) | nu/nu mice | PANC-1 | 3:1 | No | SD; KD | n = 8 | KD decreases tumor weight | KD prolong survival time | G: decrease; β-HB: increase; BW: reduce. | Anti-tumor | |
| Kidney cancer | Liskiewicz et al. (14) | Eker (Tsc2+/−) rats | Spontaneous tumor development | 8:1 | No | SD; KD | n = 1–34 | KD increases renal tumor growth | – | G: decrease; β-HB: increase | Pro-tumor |
| Vidali et al. (38) | CD-1 nude mice | 786-O | 8:1 | No | SD; LCT-KD; MCT-KD | n = 5–6 | KD tends to slow down tumor growth, but with no significance. | KD reduces the overall survival | LCT-KD increases β-HB; BW: reduce. | KD might be contraindicated in the treatment of RCC patients presenting with Stauffer's syndrome | |
| Anaplastic thyroid cancer | Aggarwal et al. (39) | CD-1 nude mice | 8505C | No | SD; KD; SD + N AC; KD + NAC | n = 6 | KD or KD + NAC | – | G: decrease; β-HB: increase | Anti-tumor | |
| Neuroblastoma | Morscher et al. (40) | CD-1 nude mice | SH-SY5Y (non-NMYC amplified); SK-N-BE(2) (NMYC amplified) | 2:1 | Yes | SD; CR-SD; KD; CR-KD | n = 8–11 | CR-SD, CR-KD decrease tumor volume | CR-SD, CR-KD prolong survival time | CR-KD decreases G and BW; CR-KD increases β-HB. | No effect of KD alone; enhanced effects of CR-KD |
| Morscher et al. (41) | CD-1 nude mice | SH-SY5Y (non-NMYC amplified); | 2:1 | Yes | Cell line: SH-SY5Y (non-NMYC amplified) | n = 8–12 | KD + CT, CR-KD + CT decrease tumor volume | KD + CT, CR-KD + CT prolong survival time | CR-KD decreases G and increases β-HB; BW: reduce. | Antitumor effects of KD + CT; additive effect of CR on KD + CT | |
| SD; SD + CT; CR-SD + CT; KD + CT; KD-CR + CT | |||||||||||
| Morscher et al. (41) | CD-1 nude mice | SK-N-BE(2) (NMYC amplified) | 2:1 | Yes | Cell line: SK-N-BE(2) (NMYC amplified) | n = 8–12 | KD + CT: ns; CR-KD + CT decreases tumor volume | KD + CT: ns; CR-KD + CT prolongs survival time | CR-KD decreases G and increases β-HB; BW: reduce. | No effect of KD + CT; enhanced anti-tumor of CR on KD + CT | |
| SD; SD + CT; CR-SD + CT; KD + CT; KD-CR + CT | |||||||||||
| Aminzadeh-Gohari et al. (42) | CD-1 nude mice | SH-SY5Y (non-NMYC amplified); SK-N-BE(2) (NMYC amplified) | 8:1 | No | SD; LCT-KD; MCT-KD | n = 10–12 | LCT-KD and MCT-KD decrease tumor volume | MCT-KD prolongs survival time | G: decrease; β-HB: increase; BW: ns. | Anti-tumor | |
| Medulloblastoma | Dang et al. (43) | Ptch1+/−Trp53−/−mice | Spontaneous tumor development | 4:1 | No | SD; KD | n = 4 | ns | ns | BW: reduce. | No effect |
| Dang et al. (43) | NOD SCID mice | Medulloblastoma from Ptch1+/−Trp53−/− mice | 6:1 | No | SD; KD | n = 4 | ns | ns | G: decrease; β-HB: increase; BW: reduce. | No effect | |
| Gastric cancer | Otto et al. (6) | NMRI nude mice | 23132/87 | 2.7:1 | No | SD; KD | n = 12 | KD inhibits tumor growth | KD prolongs survival time | G: ns; β-HB: increase; BW: ns. | Anti-tumor |
| Systemic metastatic cancer | Poff et al. (12) | VM/Dk mice | VM-M3 | 4:1 | No | SD; SD + HBO2T; KD; KD + HBO2T | n = 8–13 | KD, KD + HBO2T inhibit tumor growth | KD, KD + HBO2T prolong survival time | G: decrease; β-HB: ns; BW: reduce. | Anti-tumor; enhanced antitumor effects of HBO2T on KD |
| Poff et al. (13) | VM/Dk mice | VM-M3 | 1.5:1 | No | SD; KD; KD + KE:KD + KE + HBO2T | n = 7–17 | KD, KD + KE, KD + KE + HBO2T inhibit tumor growth | KD, KD + KE, KD + KE + HBO2T prolong survival time | KD + KE, KD + KE + HBO2T decrease G and increase β-HB; BW: reduce. | Anti-tumor |
KD, ketogenic diet; CR, calorie-restricted; RT, radiotherapy; CT, chemotherapy; DON, 6-diazo-5-oxo-L-norleucine; RP, regular protein; LP, low- protein; WD-L, Western diet based on lard; WD-C, Western diet based on coconut oil; FD, fructose diet; NCKD, high-fat/no-carbohydrate ketogenic diet; LFD, low-fat/high-carbohydrate diet; MCD, high-fat/moderate-carbohydrate diet; NAC, N-acetylcysteine; HBO2T, hyperbaric oxygen therapy; KE, ketone ester; G, glucose; β-HB, b-hydroxybutyrate; BW, body weight.
Effects of KD on Survival Time in Animal Models
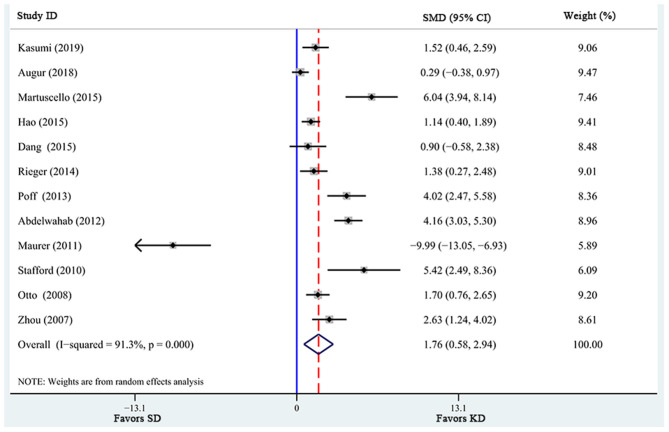
There were a total of 12 studies investigating the effects of KD supplementation on survival time (Table 2). Significant heterogeneity was found among these studies (I2 = 91.3%, p = 0.000). Pooled analysis of the overall effects suggested that KD significantly prolonged survival time [SMD = 1.76, 95% CI (0.58, 2.94), p = 0.003; Figure 2] in animal models.
Table 2.
Studies fulfilling all inclusion criteria for the meta-analysis on survival time: outcome data.
| References | KD ratio | SD group | KD group | ||
|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | ||
| Kasumi and Sato (32) | 4 | 9 | 19.8, 1.7 | 9 | 23.7, 3.2 |
| Augur et al. (21) | 3 | 17 | 15.2, 2.6 | 17 | 16.1, 3.5 |
| Martuscello et al. (44) | 6 | 11 | 38, 1 | 10 | 56, 4.2 |
| Hao et al. (30) | 3 | 12 | 24.8, 3.1 | 24 | 34.51, 0.1 |
| Dang et al. (43) | 4 | 4 | 16.3, 2.3 | 4 | 17.8, 0.5 |
| Rieger et al. (18) | 3 | 8 | 33.9, 1.6 | 8 | 35.6, 0.7 |
| Poff et al. (12) | 4 | 13 | 31.2, 4.4 | 8 | 48.9, 4.4 |
| Abdelwahab et al. (8) | 4 | 19 | 23.3, 1.1 | 20 | 28.8, 1.5 |
| Maurer et al. (45) | 3 | 12 | 94.9, 1.3 | 12 | 24, 1.2 |
| Stafford et al. (17) | 6 | 5 | 19, 0.7 | 5 | 34.2, 1.1 |
| Otto et al. (6) | 3 | 12 | 23.3, 3.1 | 12 | 19.7, 8.5 |
| Zhou et al. (16) | 4 | 7 | 16.7, 1.4 | 9 | 23.7, 0.9 |
Figure 2.
Forest plot from meta-analysis of standardized mean difference in survival time of animal tumor models randomized to ketogenic diet (KD) or standard diet (SD). The effect size of each study is proportional to the statistical weight. The diamond indicates the overall summary estimate for the analysis; the width of the diamond represents the 95% CI. SMD, standardized mean difference; CI, confidence interval.
Meta-Regression Analysis and Subgroup Analysis
In view of the fact that statistical heterogeneity existed across the included studies, meta-regression analysis was performed by including several pre-defined covariates to explore the potential sources of heterogeneity. The results indicated that KD ratio was positively related to effect size [regression coefficient = 1.69, 95% CI (0.86, 2.52), p = 0.02]. Furthermore, animal number was not a significant modifier to the effects of KD supplementation on survival time (p = 0.655).
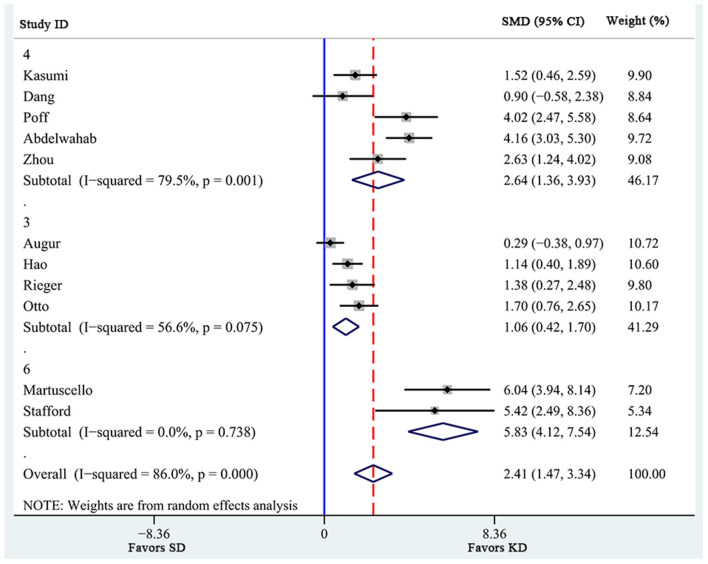
Additionally, a pre-defined subgroup analysis was conducted to observe the influence of study characteristics on the effects of KD supplementation on survival time. First, 3 subgroups were obtained according to KD ratio. As shown in Figure 3, even though all 3 subgroups showed significantly prolonged survival time, the effects of KD ratios of 4 [SMD 2.64 (1.36, 3.93), n = 5] seemed to be larger than those of 3 [SMD 1.06 (0.42, 1.70), n = 3]. In addition, heterogeneity levels significantly decreased in the subgroup analysis of KD ratios of 6 (I2 = 0.0%), while high heterogeneity levels were still observed in subgroups with ratios of 4 (I2 = 79.5%) and of 3 (I2 = 56.6%). Specifically, KD supplementation with a ratio of 4 seemed to be associated with more remarkable prolongation of survival time in animals (p = 0.001).
Figure 3.
Subgroup estimation of the effects of ketogenic diet supplementation on survival time. The numbers 3, 4, and 6 represent the KD ratio. The effect size of each study is proportional to the statistical weight. The diamond indicates the overall summary estimate for the analysis; the width of the diamond represents the 95% CI. SMD, standardized mean difference; CI, confidence interval.
Effects of KD on Tumor Weight and Tumor Volume
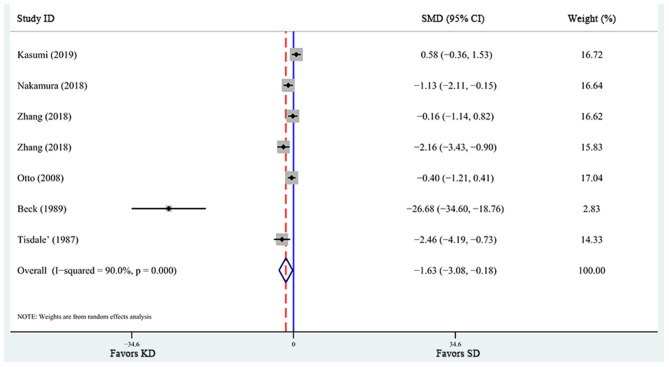
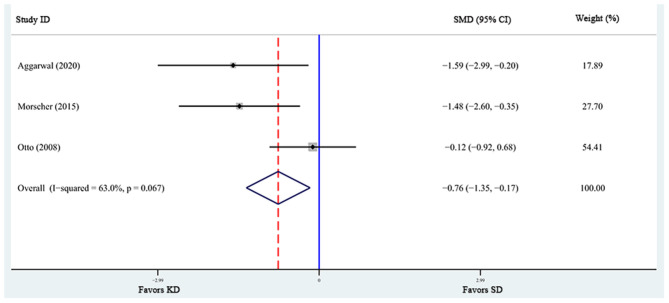
A total of 6 articles (6, 28, 29, 31, 32, 37), including 7 studies, reported the effects of KD supplementation on tumor weight in animal models. The overall effects were estimated using a random-effect model because significant heterogeneity was found (I2 = 90%, p = 0.000). Meanwhile, significant heterogeneity also existed in tumor volume (6, 39, 40) (I2 = 63%, p = 0.067). The pooled results indicated that KD significantly reduced tumor weight and tumor volume [tumor weight: SMD = −2.459, 95% CI (−4.188, −0.730), p = 0.027, Figure 4; tumor volume: SMD = −0.759, 95% CI (−1.349, −0.168), p = 0.012, Figure 5]. Meta-regression and subgroup analyses were not performed for these outcomes because of the limited number of studies included.
Figure 4.
Forest plot from meta-analysis of standardized mean difference in tumor weight of animal tumor models randomized to ketogenic diet (KD) or standard diet (SD). The effect size of each study is proportional to the statistical weight. The diamond indicates the overall summary estimate for the analysis; the width of the diamond represents the 95% CI. SMD, standardized mean difference; CI, confidence interval.
Figure 5.
Forest plot from meta-analysis of standardized mean difference in tumor volume of animal tumor models randomized to ketogenic diet (KD) or standard diet (SD). The effect size of each study is proportional to the statistical weight. The diamond indicates the overall summary estimate for the analysis; the width of the diamond represents the 95% CI. SMD, standardized mean difference; CI, confidence interval.
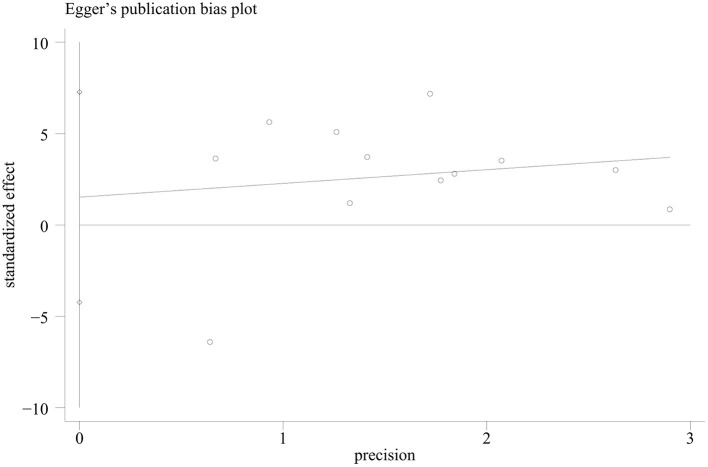
Publication Bias
Publication bias was assessed for the outcome of overall survival time, since this outcome has been analyzed in the highest number of studies. The Egger regression asymmetry test of the 12 studies suggested no significant publication bias for survival time [p = 0.569, 95% CI (−4.23, 7.27), Figure 6].
Figure 6.
The Egger's test regression plot in the meta-analysis of survival time of animal tumor models randomized to ketogenic diet (KD) or standard diet (SD).
Discussion
In this meta-analysis, we summarized evidence from 17 published animal studies that investigated the effects of KD supplementation on anti-tumor effects. Consistent with the previous meta-analysis which reported that unrestricted KD delayed tumor growth in mice (46), our results showed that KD alone or in combination with caloric restriction significantly reduced tumor weight and volume as well as prolonged survival time. Results of our meta-regression and subgroup analyses suggested that KD supplementation with a ratio of 4 seemed to associate with remarkable prolongation of survival time in animals with limited tumor types.
Traditional KD consisted of a 4:1 ratio of fat to carbohydrate plus protein, with calories from fat, protein, and carbohydrate being 90, 8, and 2%, respectively. Other alternatives to traditional KD include the medium-chain triglyceride (MCT)-based KD, the Atkins diet, and a low glycemic index diet (47). In order to enhance the anti-tumor effects of KD, several studies have either increased the proportion of fat, or supplemented KD with MCTs, omega-3 fatty acids or ketone esters (6, 13, 30, 42, 44). For example, Aminzadeh-Gohari et al. found that KD (8:1) with a fat content of 25% MCTs and 75% long-chain triglycerides (LCTs) produced a stronger anti-tumor effect compared to that with only LCTs (42). The reason may be that MCTs are more rapidly absorbed into the bloodstream and oxidized for energy because of their ability to passively diffuse through membranes (48). In addition, MCTs have the unique ability to promote ketone body synthesis in the liver (49). Tisdale et al.'s study indicated that high fat KD (2:1) showed a significant reduction in tumor size when compared with normal diet and low fat KD (1:1) (28). These results demonstrated that it is important to optimize KD compositions to suppress tumor growth.
Totally, KDs have revealed the potential anti-tumor effects, which is correlated with the restricted glucose and induction ketone bodies (e.g., β-hydroxybutyrate) (50). Ketone bodies are suitable energy replacements for normal cells with functional mitochondria, but unsuitable for tumor cells, as tumor cell mitochondrial functions are dysregulated (51). Indeed, most animal tumor models report decrease of glucose and increase of ketone bodies (Table 1). On the other hand, KDs are known to have an appetite suppressing effect which may contribute to body weight loss (52), while some studies report no significant effect or increase of body weight. The discrepancy may be caused by the animal species and growth stage, or the composition the KD.
Caloric restriction (CR) has been reported to prevent tumorigenesis by decreasing metabolic rate and oxidative damage (53). Morscher et al. found that the growth of neuroblastoma xenografts was significantly reduced by KD (2:1) when combined with CR (40). Another study indicated that anti-tumor and anti-angiogenic effects were revealed in experimental mouse and human brain tumors at a 4:1 KD ratio (16). It is reported that tumor growth is more strongly correlated with circulating glucose levels than with circulating ketone body levels (51). The reduction in glucose levels following CR largely accounts for why tumors grow minimally on either restricted KD or on restricted high carbohydrate standard diets. Although CR exhibited good anti-tumor effects and the potential to sensitize cancer cells to chemotherapy, CR has been considered to be contraindicated in a range of cancer patients, particularly those with cachexia (5). Thus, more attention is required on optimizing KD compositions to enhance the anti-tumor effects.
The efficacy of KD may also be influenced by cancer type or even subtype, genetic background, and tumor-associated syndromes. KD with a ratio of 4:1 did not slow the growth of spontaneous medulloblastoma tumors or allograft flank tumors (43), while it was reported to be anti-tumor in other cancer models, including glioblastoma (7) and colon cancer (32). Meanwhile, one study indicated that the anti-neuroblastoma effects of KD were considerably attenuated in SKN-BE(2) neuroblastoma xenografts, which carried MYCN amplification, TP53 mutation (p.C135F), and chromosome 1p loss of heterozygosity, compared to SH-SY5Y xenografts which are TP53 wild-type and non-MYCN amplified (42). Another report indicated that mice bearing renal cell carcinoma xenografts with signs of Stauffer's syndrome experienced dramatic weight loss and liver dysfunction when treated with KD (38). Additionally, Maurer et al. found that a KD did not alter tumor growth or extend the life of mice given an orthotopic injection of LNT-229 glioma cells when compared to mice maintained on SD (45). This is in contrast to the study using a rodent KD (17). This discrepancy may be related, in part, to the cell line and/or model system used. Therefore, it is necessary to evaluate the effects of KD in pre-clinical studies for every specific type of tumor before its application to cancer patients. Furthermore, genetic alterations, tumor-associated syndromes, and anti-tumor mechanisms of KD should also be considered.
To date, human data on KD and cancer are mostly single case reports (54, 55) or pilot/feasibility studies (56–58), which have mostly focused on the safety and tolerability of KD. Only 3 randomized controlled trials are available. Two of them involved ovarian and endometrial cancer, and mainly focused on safety, adherence, and the mental and physical functions (59, 60). The other trial evaluated the safety, tolerability, and beneficial effects of KD on body composition, blood parameters, and survival in breast cancer (61), which suggested that chemotherapy combined with KD can improve the biochemical parameters, body composition, and overall survival with no substantial side effects in breast cancer patients. Thus, it is still necessary for more randomized controlled trials to explore the benefits of adjuvant KD in specific cancers.
Several potential limitations should be addressed in the present meta-analysis. First, we did not have complete access to every full text papers, resulting in a small number of studies included in this meta-analysis; results of some of the estimations, such as those for the effects of KD supplementation on tumor weight and tumor volume, should therefore be interpreted with caution. In addition, despite the attempts to explore the potential causative factors of heterogeneity, high heterogeneity was found among the studies. In addition, the number of included studies in the subgroup analysis was relatively small.
Conclusion
In summary, the pre-clinical evidence pointed toward an overall anti-tumor effect of the KD in animals studies currently available with limited tumor types. The efficacy of KD on tumor influenced by many factors, including cancer type or even subtype, genetic background, cell line and/or model system, the composition of KD and tumor-associated syndromes. Therefore, more pre-clinical studies should be performed to elaborate the anti-tumor effect of KD in the future.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
ZD conceived of the study idea. JL and HZ conducted the literature review and performed the data extraction. JL drafted the manuscript. All authors were involved in consensus agreements concerning data discrepancies, involved in revising the article for important intellectual content, interpreting the data, and approved the final version to be published.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Professor Kentaro Nakamura, Eiji Kasumi, Thomas Seyfried, and Jie Zhang for providing us with the relevant study data.
Footnotes
Funding. This work was financially supported by the Natural Science Foundation of Hubei Province (No. 2020CFB500).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.594408/full#supplementary-material
References
- 1.Siegel RL. Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. (2019) 69:7–34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.Vander Heiden MG, DeBerardinis RJ. Understanding the Intersections between metabolism and cancer biology. Cell. (2017) 168:657–69. 10.1016/j.cell.2016.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vidali S, Aminzadeh S, Lambert B, Rutherford T, Sperl W, Kofler B, et al. Mitochondria: the ketogenic diet–A metabolism-based therapy. Int J Biochem Cell Biol. (2015) 63:55–9. 10.1016/j.biocel.2015.01.022 [DOI] [PubMed] [Google Scholar]
- 4.Simone BA, Champ CE, Rosenberg AL, Berger AC, Monti DA, Dicker AP, et al. Selectively starving cancer cells through dietary manipulation: methods and clinical implications. Future Oncol. (2013) 9:959–76. 10.2217/fon.13.31 [DOI] [PubMed] [Google Scholar]
- 5.Weber DD, Aminazdeh-Gohari S, Kofler B. Ketogenic diet in cancer therapy. Aging. (2018) 10:164–5. 10.18632/aging.101382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otto C, Kaemmerer U, Illert B, Muehling B, Pfetzer N, Wittig R, et al. Growth of human gastric cancer cells in nude mice is delayed by a ketogenic diet supplemented with omega-3 fatty acids and medium-chain triglycerides. BMC Cancer. (2008) 8:122. 10.1186/1471-2407-8-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lussier DM, Woolf EC, Johnson JL, Brooks KS, Blattman JN, Scheck AC. Enhanced immunity in a mouse model of malignant glioma is mediated by a therapeutic ketogenic diet. BMC Cancer. (2016) 16:310. 10.1186/s12885-016-2337-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdelwahab MG, Fenton KE, Preul MC, Rho JM, Lynch A, Stafford P, et al. The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma. PLoS ONE. (2012) 7:e36197. 10.1371/journal.pone.0036197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen BG, Bhatia SK, Buatti JM, Brandt KE, Lindholm KE, Button AM, et al. Ketogenic diets enhance oxidative stress and radio-chemo-therapy responses in lung cancer xenografts. Clin Cancer Res. (2013) 19:3905–13. 10.1158/1078-0432.CCR-12-0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zahra A, Fath MA, Opat E, Mapuskar KA, Bhatia SK, Ma DC, et al. Consuming a ketogenic diet while receiving radiation and chemotherapy for locally advanced lung cancer and pancreatic cancer: the University of Iowa experience of two phase 1 clinical trials. Radiat Res. (2017) 187:743–54. 10.1667/RR14668.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hopkins BD, Pauli C, Du X, Wang DG, Li X, Wu D, et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature. (2018) 560:499–503. 10.1038/s41586-018-0343-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poff AM, Ari C, Seyfried TN, D'Agostino DP. The ketogenic diet and hyperbaric oxygen therapy prolong survival in mice with systemic metastatic cancer. PLoS ONE. (2013) 8:e65522. 10.1371/journal.pone.0065522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poff AM, Ward N, Seyfried TN, Arnold P, D'Agostino DP. Non-toxic metabolic management of metastatic cancer in VM mice: novel combination of ketogenic diet, ketone supplementation, and hyperbaric oxygen therapy. PLoS ONE. (2015) 10:e0127407. 10.1371/journal.pone.0127407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liskiewicz AD, Kasprowska D, Wojakowska A, Polanski K, Lewin-Kowalik J, Kotulska K, et al. Long-term high fat ketogenic diet promotes renal tumor growth in a rat model of tuberous sclerosis. Sci Rep. (2016) 6:21807. 10.1038/srep21807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia S, Lin R, Jin L, Zhao L, Kang HB, Pan Y, et al. Prevention of dietary-fat-fueled ketogenesis attenuates BRAF V600E tumor growth. Cell Metab. (2017) 25:358–73. 10.1016/j.cmet.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou WH, Mukherjee P, Kiebish MA, Markis WT, Mantis JG, Seyfried TN. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab. (2007) 4:5. 10.1186/1743-7075-4-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stafford P, Abdelwahab MG, Kim DY, Preul MC, Rho JM, Scheck AC. The ketogenic diet reverses gene expression patterns and reduces reactive oxygen species levels when used as an adjuvant therapy for glioma. Nutr Metab (Lond). (2010) 7:74. 10.1186/1743-7075-7-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rieger J, Bahr O, Maurer GD, Hattingen E, Franz K, Brucker D, et al. ERGO: a pilot study of ketogenic diet in recurrent glioblastoma. Int J Oncol. (2014) 44:1843–52. 10.3892/ijo.2014.2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woolf EC, Curley KL, Liu QW, Turner GH, Charlton JA, Preul MC, et al. The ketogenic diet alters the hypoxic response and affects expression of proteins associated with angiogenesis, invasive potential and vascular permeability in a mouse glioma model. PLoS ONE. (2015) 10:e0130357. 10.1371/journal.pone.0130357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Feyter HM, Behar KL, Rao JU, Madden-Hennessey K, Ip KL, Hyder F, et al. A ketogenic diet increases transport and oxidation of ketone bodies in RG2 and 9L gliomas without affecting tumor growth. Neuro Oncol. (2016) 18:1079–87. 10.1093/neuonc/now088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Augur ZM, Doyle CM, Li MY, Mukherjee P, Seyfried TN. Nontoxic targeting of energy metabolism in preclinical VM-M3 experimental glioblastoma. Front Nutr. (2018) 5:91. 10.3389/fnut.2018.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukherjee P, Augur ZM, Li MY, Hill C, Greenwood B, Domin MA, et al. Therapeutic benefit of combining calorie-restricted ketogenic diet and glutamine targeting in late-stage experimental glioblastoma. Commun Biol. (2019) 2:1–14. 10.1038/s42003-019-0455-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gluschnaider U. Long-chain fatty acid analogues suppress breast tumorigenesis and progression. Cancer Res. (2014) 74:6991–7002. 10.1158/0008-5472.CAN-14-0385 [DOI] [PubMed] [Google Scholar]
- 24.Zhuang Y, Chan DK, Haugrud AB, Miskimins WK. Mechanisms by which low glucose enhances the cytotoxicity of metformin to cancer cells both in vitro and in vivo. PLoS ONE. (2014) 9:e108444. 10.1371/journal.pone.0108444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stemmer K, Zani F, Habegger KM, Neff C, Kotzbeck P, Bauer M, et al. FGF21 is not required for glucose homeostasis, ketosis or tumour suppression associated with ketogenic diets in mice. Diabetologia. (2015) 58:2414–23. 10.1007/s00125-015-3668-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Healy ME, Chow JD, Byrne FL, Breen DS, Leitinger N, Li C, et al. Dietary effects on liver tumor burden in mice treated with the hepatocellular carcinogen diethylnitrosamine. J Hepatol. (2015) 62:599–606. 10.1016/j.jhep.2014.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Byrne FL, Hargett SR, Lahiri S, Roy RJ, Berr SS, Caldwell SH, et al. Serial MRI imaging reveals minimal impact of ketogenic diet on established liver tumor growth. Cancers (Basel). (2018) 10:312. 10.3390/cancers10090312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tisdale MJ, Brennan RA, Fearon KC. Reduction of weight loss and tumour size in a cachexia model by a high fat diet. Br J Cancer. (1987) 56:39–43. 10.1038/bjc.1987.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beck SA, Tisdale MJ. Effect of insulin on weight loss and tumour growth in a cachexia model. Br J Cancer. (1989) 59:677–81. 10.1038/bjc.1989.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao GW, Chen YS, He DM, Wang HY, Wu GH, Zhang B. Growth of human colon cancer cells in nude mice is delayed by ketogenic diet with or without omega-3 fatty acids and medium-chain triglycerides. Asian Pac J Cancer Prev. (2015) 16:2061–8. 10.7314/APJCP.2015.16.5.2061 [DOI] [PubMed] [Google Scholar]
- 31.Nakamura K, Tonouchi H, Sasayama A, Ashida K. A ketogenic formula prevents tumor progression and cancer cachexia by attenuating systemic inflammation in colon 26 tumor-bearing mice. Nutrients. (2018) 10:206. 10.3390/nu10020206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasumi E, Sato N. A ketogenic diet improves the prognosis in a mouse model of peritoneal dissemination without tumor regression. J Clin Biochem Nutr. (2019) 64:201–8. 10.3164/jcbn.18-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freedland SJ, Mavropoulos J, Wang A, Darshan M, Demark-Wahnefried W, Aronson WJ, et al. Carbohydrate restriction, prostate cancer growth, and the insulin-like growth factor axis. Prostate. (2008) 68:11–9. 10.1002/pros.20683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mavropoulos JC, Buschemeyer WC, Tewari AK, Rokhfeld D, Pollak M, Zhao Y, et al. The effects of varying dietary carbohydrate and fat content on survival in a murine LNCaP prostate cancer xenograft model. Cancer Prev Res (Phila). (2009) 2:557–65. 10.1158/1940-6207.CAPR-08-0188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim HS, Masko EM, Poulton SL, Kennedy KM, Pizzo SV, Dewhirst MW, et al. Carbohydrate restriction and lactate transporter inhibition in a mouse xenograft model of human prostate cancer. BJU Int. (2012) 110:1062–9. 10.1111/j.1464-410X.2012.10971.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shukla SK, Gebregiworgis T, Purohit V, Chaika NV, Gunda V, Radhakrishnan P, et al. Metabolic reprogramming induced by ketone bodies diminishes pancreatic cancer cachexia. Cancer Metab. (2014) 2:18. 10.1186/2049-3002-2-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J, Jia PP, Liu QL, Cong MH, Gao Y, Shi HP, et al. Low ketolytic enzyme levels in tumors predict ketogenic diet responses in cancer cell lines in vitro and in vivo. J Lipid Res. (2018) 59:625–34. 10.1194/jlr.M082040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vidali S, Aminzadeh-Gohari S, Feichtinger RG, Vatrinet R, Koller A, Locker F, et al. The ketogenic diet is not feasible as a therapy in a CD-1 nu/nu mouse model of renal cell carcinoma with features of Stauffer's syndrome. Oncotarget. (2017) 8:57201–15. 10.18632/oncotarget.19306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aggarwal A, Yuan ZL, Barletta JA, Lorch JH, Nehs MA. Ketogenic diet combined with antioxidant N-acetylcysteine inhibits tumor growth in a mouse model of anaplastic thyroid cancer. Surgery. (2020) 167:87–92. 10.1016/j.surg.2019.06.042 [DOI] [PubMed] [Google Scholar]
- 40.Morscher RJ, Aminzadeh-Gohari S, Feichtinger R, Mayr JA, Lang R, Neureiter D, et al. Inhibition of neuroblastoma tumor growth by ketogenic diet and/or calorie restriction in a CD1-nu mouse model. PLoS ONE. (2015) 10:e0129802. 10.1371/journal.pone.0129802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morscher RJ, Aminzadeh-Gohari S, Hauser-Kronberger C, Feichtinger RG, Sperl W, Kofler B. Combination of metronomic cyclophosphamide and dietary intervention inhibits neuroblastoma growth in a CD1-nu mouse model. Oncotarget. (2016) 7:17060–73. 10.18632/oncotarget.7929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aminzadeh-Gohari S, Feichtinger RG, Vidali S, Locker F, Rutherford T, O'Donnel M, et al. A ketogenic diet supplemented with medium-chain triglycerides enhances the anti-tumor and anti-angiogenic efficacy of chemotherapy on neuroblastoma xenografts in a CD1-nu mouse model. Oncotarget. (2017) 8:64728–44. 10.18632/oncotarget.20041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dang MT, Wehrli S, Dang CV, Curran T. The ketogenic diet does not affect growth of hedgehog pathway medulloblastoma in mice. PLoS ONE. (2015) 10:e0133633. 10.1371/journal.pone.0133633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martuscello RT, Vedam-Mai V, McCarthy DJ, Schmoll ME, Jundi MA, Louviere CD, et al. A supplemented high-fat low-carbohydrate diet for the treatment of glioblastoma. Clin Cancer Res. (2016) 22:2482–95. 10.1158/1078-0432.CCR-15-0916 [DOI] [PubMed] [Google Scholar]
- 45.Maurer GD, Brucker DP, Bahr O, Harter PN, Hattingen E, Walenta S, et al. Differential utilization of ketone bodies by neurons and glioma cell lines: a rationale for ketogenic diet as experimental glioma therapy. BMC Cancer. (2011) 11:315. 10.1186/1471-2407-11-315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klement RJ, Champ CE, Otto C, Kammerer U. Anti-tumor effects of ketogenic diets in mice: a meta-analysis. PLoS ONE. (2016) 11:e0155050. 10.1371/journal.pone.0155050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ricka D., Messer EHK. Ketogenic diets for the treatment of epilepsy. In: Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease. Elsevier Inc. (2015). p. 441–8. 10.1016/B978-0-12-411462-3.00046-1 [DOI] [Google Scholar]
- 48.Shah ND, Limketkai BN. The use of medium-chain triglycerides in gastrointestinal disorders. Pract Gastroenterol. (2017) 41:20–8. [Google Scholar]
- 49.Page KA, Williamson A, Yu N, McNay EC, Dzuira J, McCrimmon RJ, et al. Medium-chain fatty acids improve cognitive function in intensively treated type 1 diabetic patients and support in vitro synaptic transmission during acute hypoglycemia. Diabetes. (2009) 58:1237–44. 10.2337/db08-1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seyfried TN, Sanderson TM, El-Abbadi MM, McGowan R, Mukherjee P. Role of glucose and ketone bodies in the metabolic control of experimental brain cancer. Br J Cancer. (2003) 89:1375–82. 10.1038/sj.bjc.6601269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids. (2004) 70:309–19. 10.1016/j.plefa.2003.09.007 [DOI] [PubMed] [Google Scholar]
- 52.Johnstone A, Horgan G, Murison S, Bremner D, Lobley G. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am J Clin Nutr. (2008) 87:44–55. 10.1093/ajcn/87.1.44 [DOI] [PubMed] [Google Scholar]
- 53.Martin-Montalvo A, Villalba JM, Navas P, de Cabo R. NRF2, cancer and calorie restriction. Oncogene. (2011) 30:505–20. 10.1038/onc.2010.492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwartz K, Chang HT, Nikolai M, Pernicone J, Rhee S, Olson K, et al. Treatment of glioma patients with ketogenic diets: report of two cases treated with an IRB-approved energy-restricted ketogenic diet protocol and review of the literature. Cancer Metab. (2015) 3:3. 10.1186/s40170-015-0129-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zuccoli G, Marcello N, Pisanello A, Servadei F, Vaccaro S, Mukherjee P, et al. Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: case report. Nutr Metab (Lond). (2010) 7:33. 10.1186/1743-7075-7-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rieger J, Baehr O, Hattingen E, Maurer G, Coy J, Weller M, et al. The ERGO trial: A pilot study of a ketogenic diet in patients with recurrent glioblastoma. J Clin Oncol. (2010) 28. 10.1200/jco.2010.28.15_suppl.e12532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin-McGill KJ, Marson AG, Tudur Smith C, Jenkinson MD. Ketogenic diets as an adjuvant therapy in glioblastoma (the KEATING trial): study protocol for a randomised pilot study. Pilot Feasibility Stud. (2017) 3:67. 10.1186/s40814-017-0209-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin-McGill KJ, Marson AG, Tudur Smith C, Young B, Mills SJ, Cherry MG, et al. Ketogenic diets as an adjuvant therapy for glioblastoma (KEATING): a randomized, mixed methods, feasibility study. J Neuro Oncol. (2020) 147:213–27. 10.1007/s11060-020-03417-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cohen CW, Fontaine KR, Arend RC, Soleymani T, Gower BA. Favorable effects of a ketogenic diet on physical function, perceived energy, and food cravings in women with ovarian or endometrial cancer: a randomized, controlled trial. Nutrients. (2018) 10:1187. 10.3390/nu10091187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cohen CW, Fontaine KR, Arend RC, Gower BA. A ketogenic diet is acceptable in women with ovarian and endometrial cancer and has no adverse effects on blood lipids: a randomized, controlled trial. Nutr Cancer. (2020) 72:584–94. 10.1080/01635581.2019.1645864 [DOI] [PubMed] [Google Scholar]
- 61.Khodabakhshi A, Akbari ME, Mirzaei HR, Mehrad-Majd H, Kalamian M, Davoodi SH. Feasibility, safety, and beneficial effects of MCT-based ketogenic diet for breast cancer treatment: a randomized controlled trial study. Nutr Cancer. (2020) 72:627–34. 10.1080/01635581.2019.1650942 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.