Abstract
The C-type lectin receptor langerin plays a vital role in the mammalian defense against invading pathogens. Langerin requires a Ca2+ cofactor, the binding affinity of which is regulated by pH. Thus, Ca2+ is bound when langerin is on the membrane but released when langerin and its pathogen substrate traffic to the acidic endosome, allowing the substrate to be degraded. The change in pH is sensed by protonation of the allosteric pH sensor histidine H294. However, the mechanism by which Ca2+ is released from the buried binding site is not clear. We studied the structural consequences of protonating H294 by molecular dynamics simulations (total simulation time: about 120 μs) and Markov models. We discovered a relay mechanism in which a proton is moved into the vicinity of the Ca2+-binding site without transferring the initial proton from H294. Protonation of H294 unlocks a conformation in which a protonated lysine side chain forms a hydrogen bond with a Ca2+-coordinating aspartic acid. This destabilizes Ca2+ in the binding pocket, which we probed by steered molecular dynamics. After Ca2+ release, the proton is likely transferred to the aspartic acid and stabilized by a dyad with a nearby glutamic acid, triggering a conformational transition and thus preventing Ca2+ rebinding. These results show how pH regulation of a buried orthosteric binding site from a solvent-exposed allosteric pH sensor can be realized by information transfer through a specific chain of conformational arrangements.
Keywords: pH regulation, allosteric regulation, conformational change, calcium-binding protein, pattern recognition receptor (PRR), molecular dynamics, computer modeling
Abbreviations: ITC, isothermal titration calorimetry; MD, molecular dynamics; tIC, time-independent component
When pathogens invade a mammal (or more specifically: a human), Langerhans cells capture some of the pathogens, process them, and present antigens to the adaptive immune system. The swift activation of the adaptive immune system is critical for the survival of the mammal, and langerin plays a vital role in this process. Langerin is a transmembrane carbohydrate receptor, which is expressed by Langerhans cells of mammalian skin and mucosa (1, 2). It belongs to the class of type II C-type lectin receptors (3, 4). It detects pathogens such as influenza virus (5), measles virus (6), HIV (7), fungi (8), mycobacteria (9), and bacteria (10).
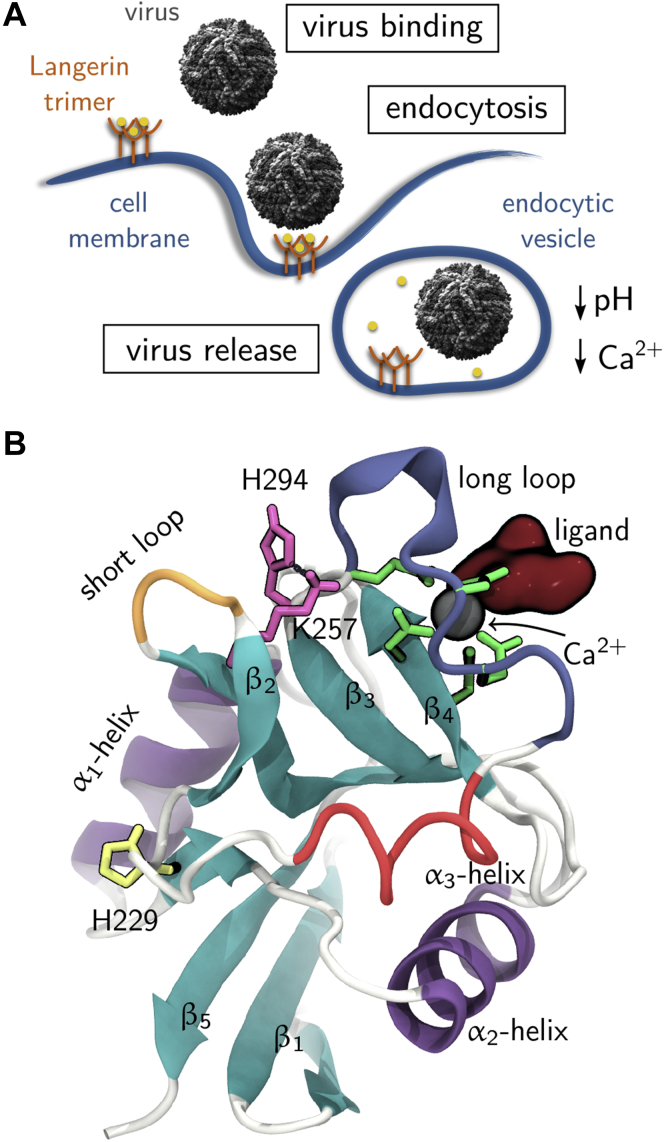
Langerin recognizes these pathogens by binding to carbohydrates on the pathogen surface. Its carbohydrate-binding pocket contains a Ca2+ cation as cofactor that is essential for carbohydrate binding, and thus for the capture of pathogens. After the initial binding event, the pathogen is captured in an endocytic vesicle, and langerin releases the pathogen into the endosome (Fig. 1A) (1, 2, 7, 11). This cargo release is triggered by a drop of pH from 7 in the extracellular medium to 5.5 to 6 in the early endosome (12) and by a substantial drop in the Ca2+ concentration from about 1 to 2 mM to a value in the micromolar range (13, 14, 15).
Figure 1.
C-type lectin langerin.A, langerin’s function as an endocytic pattern recognition receptor (www.scistyle.com; https://creativecommons.org/licenses/by-sa/4.0/). B, langerin carbohydrate recognition domain (Protein Data Bank ID 3p5g (16)).
The pH-dependent cargo release is accomplished by a fascinating mechanism in which various chemical equilibria are carefully balanced. To be able to release the cargo into the more acidic endosome, the carbohydrate affinity of langerin needs to be pH dependent. However, the change in pH does not affect the carbohydrate binding itself. Instead, langerin depends on a Ca2+ cofactor for carbohydrate binding, and the observed pH dependence of the carbohydrate affinity is caused by an underlying pH dependence of the Ca2+ affinity (17). We previously showed that the Ca2+ affinity is lower at pH 6 than at pH 7. The pH sensitivity, measured as the difference in the Ca2+ binding free energies, is ΔΔG = 5.1 kJ mol−1 (17). At high Ca2+ concentrations (10 mM) the carbohydrate affinity ceases to be pH dependent, because the excess in Ca2+ outweighs any change in Ca2+ affinity due to a change in pH. However, in the endosome the Ca2+ concentration is low. Thus, the drop in pH from the extracellular medium to the endosome causes a decrease in Ca2+ affinity, and the unbinding of the Ca2+ cofactor leads to the dissociation of the carbohydrate ligand and to the release of the pathogen. Similarly, pH sensitivities of either ligand or Ca2+ affinities have been observed for several other C-type lectins (18), including ASGPR (14, 19, 20) the macrophage mannose receptor (21), DC-SIGN and DC-SIGNR (22, 23, 24, 25), and LSECtin (26) (example structures in Fig. S32). In DC-SIGNR and LSECtin, which have a different biological role than langerin, a drop in pH causes an increase in ligand affinity. The mechanisms underlying the regulation by the pH in C-type lectins are highly diverse and not yet studied in detail.
The observation that the Ca2+ affinity in C-type lectins is pH dependent is surprising. First, when a carbohydrate (and attached to it an entire virus) is bound to a C-type lectin, the Ca2+-binding site is almost certainly not solvent exposed. Second, the Ca2+ in C-type lectins is coordinated by either aspartate or glutamate side chains, whose reference pKa values (27) (in water at 25 °C) are 3.71 (aspartate) and 4.15 (glutamate). By themselves, these residues are not sensitive to a change in pH from 7 to 6. Pairs of acidic residues can in principle form a protonated dyad, which is the close arrangement of two residues with acidic side chains such that protonation of their carboxyl groups is coupled. This results in an increased pKa of the protonated residue, stabilized by the unprotonated form of the other group. Prominent examples of this effect are found in the proteins HIV-1 protease (28, 29), BACE-1 (30), BACE-2, and CatD, where it can increase the pKa of aspartic acid from its reference value to 5.2 (31). The presence of organic ligands can increase these values further (32). However, a protonated dyad can only form if Ca2+ has already left the binding pocket. So, the question arises: how do C-type lectins sense a change in pH, and how does this lead to the release of Ca2+?
For langerin we previously identified the histidine residue H294 as a partial pH sensor that regulates the Ca2+ affinity (17). The reference pKa of histidine is 6.04 (in water at 25 °C) (27), which makes it sensitive to a pH change from 7 to 6. When H294 is mutated to A294, the pH sensitivity is about 40% smaller than in the wildtype (ΔΔG = 3.1 kJ mol−1 upon a change in pH from 7 to 6). Because the histidine side chain points away from the Ca2+-binding site, it is unlikely that the decrease in Ca2+ affinity is caused by electrostatic repulsion between the protonated histidine and the Ca2+. This mechanism has been suggested for the C-type lectin ASGPR, in which, however, the histidine pH sensor is located directly underneath the Ca2+-binding pocket (Fig. S32D) (20). Instead, we showed—by combining NMR experiments, site-directed point mutations, and molecular dynamics simulations—that H294 is at the center of an allosteric network that contains the Ca2+-binding site. More specifically, in its unprotonated form H294 forms a hydrogen bond with lysine K257, which is also present in the known crystal structures of langerin (16). This hydrogen bond cannot be formed if H294 is protonated, and the allosteric mechanism that regulates the Ca2+ affinity likely hinges on this hydrogen bond.
Yet, protonation of H294 is only the initial detection that the surrounding medium has changed. Even though we identified the residues that are involved in the allosteric network, we do not yet understand how the protonation of H294 could ultimately affect the Ca2+-binding pocket. Several allosteric effects have been reported for C-type lectins (see ref. (33) for a recent review), but little is known about their underlying molecular mechanisms that could be applied to the situation in langerin. The goal of this study is to elucidate how the protonation of H294 changes the conformational ensemble of langerin and to investigate the effect these conformational changes have on the Ca2+-binding pocket. A model of how the signal, that the pH has changed, traverses the allosteric network to the buried Ca2+-binding site and triggers the Ca2+ release might serve as a blueprint for understanding how pH-sensitive ligand binding is achieved in C-type lectins and other proteins.
Results and discussion
Structure of the langerin carbohydrate recognition domain
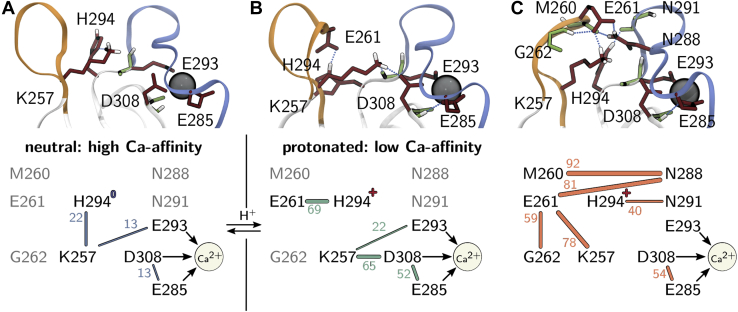
Langerin forms a homotrimer. The monomers consist of a short cytoplasmic tail, a transmembrane region, and a long alpha-helical neck (residues 56–197) extending into the extracellular milieu, which carries the C-terminal carbohydrate recognition domain (16, 18). The carbohydrate recognition domain has the typical C-type lectin domain fold (Fig. 1B) (4), which consists of two extended β sheets (turquoise), each composed of three single strands. The two β sheets are flanked by three α helices (purple, α3 in red). The carbohydrate-binding pocket, which contains the Ca2+-binding site, is located on top of the β4 strand. One residue from this β sheet directly binds to the Ca2+: D308. In addition, the Ca2+ is held in place by E293 and E285 in the long-loop (blue), which coordinate to Ca2+ from the side. E285 is part of a conserved EPN-motif (E285, P286, N287 in langerin), which determines the selectivity for mannose, fucose, and glucose over galactose (18, 34, 35). The pH sensor H294 (pink) is located at the end of the long-loop. If its side chain is unprotonated, it forms a hydrogen bond to K257 (also pink) in the short-loop (orange). The allosteric network that regulates the Ca2+ affinity comprises the long- and the short-loop (17). H229 (yellow) is the only other histidine residue in the langerin carbohydrate recognition domain. A pathogen would bind via a carbohydrate ligand (dark red) to langerin and would be separated from the pH sensor by the long-loop. If Ca2+ is bound to langerin, we will call the system holo-langerin, otherwise apo-langerin.
The effect of H294 protonation on the conformational ensemble
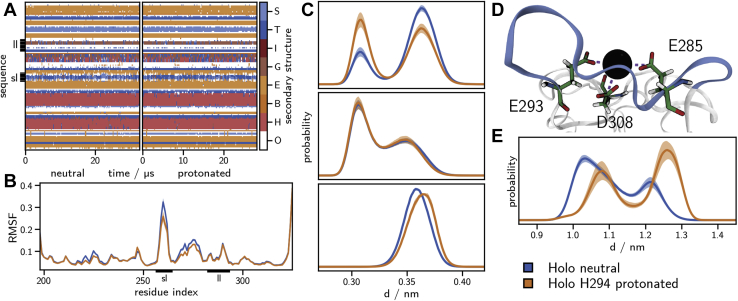
We conducted 31 μs of molecular dynamics simulations of holo-langerin, in which all residues were protonated according to their default protonation state at pH 7, i.e., H294 was unprotonated, and the overall protein was neutral (neutral state). We compare these simulations with 27 μs of holo-langerin, in which H294 was protonated (protonated state). Protonation of H294 has no influence on the secondary structure of langerin (Fig. 2A, Fig. S1). Thus, any conformational change due to the protonation of H294 affects the side chains, or those residues that are not assigned to a specific secondary structure, i.e., the loop regions.
Figure 2.
Structural consequences of H294 protonation.A, analysis by the hydrogen bond estimation algorithm DSSP of the secondary structure in the neutral (left) and the protonated holo-state (right). Legend: S, bend; T, hydrogen bonded turn; I, 5-helix; G, 3-helix; E, extended strand, part of β-ladder; B, isolated β-bridge; H, α helix; O, unassigned. B, Cα-root-mean-square fluctuation (RMSF). C, carboxyl carbon–Ca2+ distance histograms for E285 (upper graph), E293 (middle graph), and D308 (lower graph). D, structure of the Ca2+-binding site showing the distances plotted in C with dashed lines. E, histogram of the minimum distance between Ca2+ and the side-chain N atoms (Nδ and Nε) of H294. Solid lines, mean of the histograms calculated for each simulation replica. Shaded area, 95% confidence interval of the mean obtained by bootstrapping (1000 samples). ll, long-loop; sl, short-loop.
One way a conformational change in the loop regions could manifest itself, is by a change of the loop flexibility. This is, however, not corroborated by the root-mean-square fluctuations of the individual residues (Fig. 2B). The short-loop (sharp peak around residue 260) and the α3 helix (broad peak around residue 275) are more rigid in the protonated state, but the difference is very small. The flexibility in all other regions of the protein, and in particular the long-loop region, does not change upon protonation.
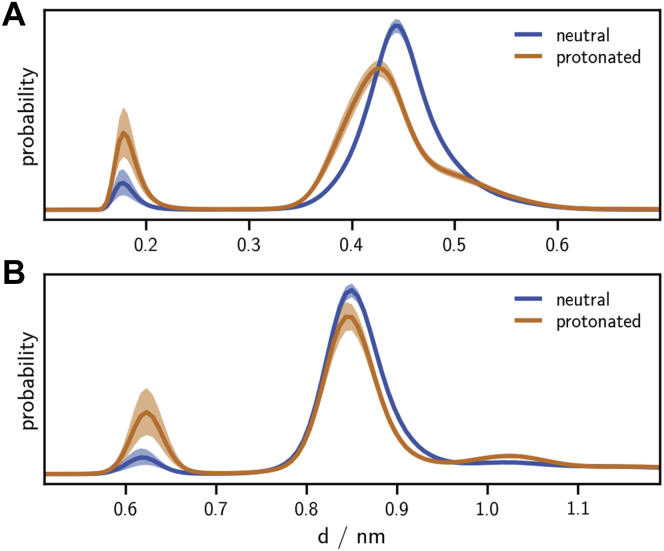
To gauge whether protonation of H294 has an influence on the conformation of the Ca2+-binding site, we measured the distance distribution between the carboxyl group of the Ca2+-coordinating residues—E285, E293, and D308—and the Ca2+ (Fig. 2, C and D). For E293 and D308 the differences are too minor to explain the observed difference in Ca2+ affinity. For E285 the distribution shifts slightly to lower distances and thus to a potentially tightly bound Ca2+, not explaining it either. The distance difference between the two populated states is about 0.05 nm.
Yet, we know from our previous analyses (17) that protonation of H294 causes a significant shift in the conformational ensemble, and this is again confirmed by the distance distributions between the H294 side chain and the Ca2+ in the neutral and the protonated state (Fig. 2E). In the protonated state the distribution shifts to larger distances, well beyond 1 nm. At this distance, we do not expect a significant influence of the positively charged H294 side chain on the Ca2+, considering that H294 is located on the protein surface and that the dielectric constant between the two interacting groups is relatively high (see Figs. S20 and S21 for an assessment of the Coulomb interaction) (36). Thus, we can rule out that the decrease in Ca2+ affinity is caused by direct Coulomb repulsion between the protonated H294 and the Ca2+.
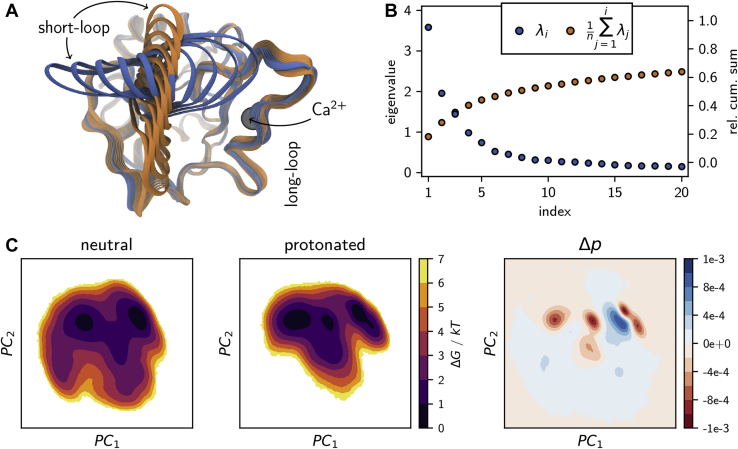
To uncover which residues besides H294 are involved in the conformational shift, one needs to compare the two conformational ensembles. This cannot be accomplished in the full high-dimensional conformational space. Instead, one needs to project the two ensembles into a low-dimensional space that is representative of both systems. Principal component analysis (37) identifies low-dimensional spaces that preserve the directions of the largest conformational variance (38). To be able to directly compare the neutral and the protonated ensemble, we combined the simulations in the two protonation states to obtain a joint principal component space. The principal component with the largest variance represents the opening and closing of the gap between the short-loop and long-loop (blue sequence of structures in Fig. 3A). The second principal component represents a sideways shear motion of the short-loop (orange sequence of structures in Fig. 3A). This is in line with our previous finding that the allosteric network is centered on these two loops (17). Even though the two principal components cover only about 28% of the total structural variance (Fig. 3B), they represent the conformational fluctuations that are most sensitive to a protonation of H294. Separate principal component analyses of the two protonation states yielded principal components that were almost identical, indicating that the joint principal components are a faithful representation of the largest variances for both protonation states. Figure 3C shows the free energy surface of the two systems in the space of the first two joint principal components. The free-energy surface of the unprotonated system is shallow with two minima corresponding to the open and closed states of the short- and long-loop. Upon protonation, the free energy surface becomes much steeper and more structured. One can discern at least three minima. The difference plot of the probability densities in the neutral and protonated states (Fig. 3C to the right) shows these emerging conformations in red.
Figure 3.
Principle component analysis.A, structural interpolations along the first two principal components. B, Eigenvalue spectrum of the principal component analysis (blue dots) and the cumulative sum normalized by the total sum of all N eigenvalues (orange dots). C, free-energy surfaces from the 2D projections of the individual holo-langerin trajectories onto principal components 1 and 2 and difference plot of the underlying probability distributions (neutral – protonated).
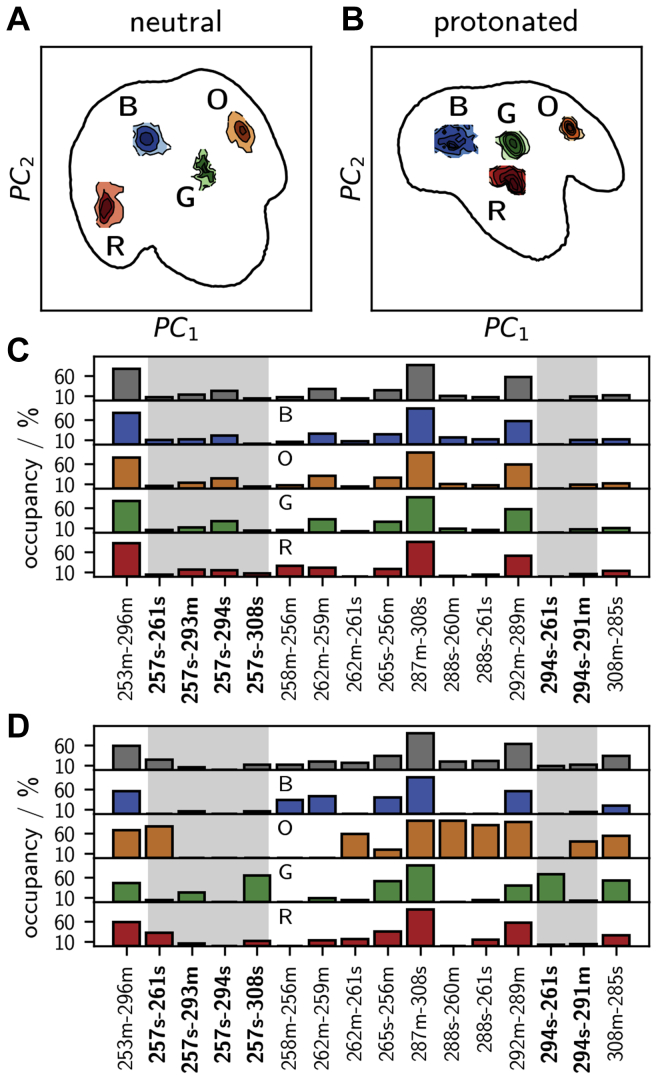
We extracted the highly populated regions by clustering in the space of the first two principal components using the density-based common-nearest-neighbors cluster algorithm (39, 40, 41) and characterized the hydrogen bond pattern of the short- and long-loop residues in each of the clusters (Fig. 4). Figure 4, C and D show a subset of the full analysis (see Fig. S10) focusing on fluctuating hydrogen bonds. In the neutral state, the clusters have essentially the same hydrogen bond populations as the total ensemble, which is consistent with the shallow free-energy surface in Figure 3C.
Figure 4.
Characterization of conformational states via hydrogen bonds. Four most populated clusters in the principal-component free-energy surface of A, the neutral and B, the protonated holo-langerin. Per cluster hydrogen bond occupancy in C, neutral and D, protonated holo-langerin (populations in the full ensemble in gray). Hydrogen bonds involving K257 or H294 are highlighted by a gray background.
The situation is different in the protonated state. Here, each of the four clusters is stabilized by a hydrogen bond pattern that is distinctively different from the hydrogen bond pattern of the total distribution (Fig. 4B). This indicates that, upon protonation of H294 several distinct short-loop/long-loop conformations emerge.
The most striking change arises in the green (G) cluster: the hydrogen bond between the side chain of K257 and the side chain of D308, which is barely populated in the unprotonated state (4.2%), is populated to 65.4% in this cluster and 12.9% in the ensemble. In parallel, the side chain of the now protonated H294 forms a hydrogen bond with the carboxyl group of E261. The structure is further stabilized by a hydrogen bond between the side chain of S265 and the main chain of T256. Note the significance of this finding: the K257 side chain, which is no longer engaged in a hydrogen bond with H294, forms a new hydrogen bond with the Ca2+-coordinating residue D308 and thereby moves a proton into the vicinity of the Ca2+-binding pocket.
The conformation of the orange (O) cluster is complementary to that of the green cluster. The side chain of K257 forms a hydrogen bond with the carboxyl group of E261, while H294 engages in a hydrogen bond to the backbone carbonyl oxygen of N291. The conformation is stabilized by hydrogen bonds between the side chain of N288 and the backbone carbonyl oxygen of M260 and the side chain of E261. N288 is located in the center of the long-loop, and E261 is located in the center of the short-loop. Thus, these two hydrogen bonds closely connect the two loops explaining why this structure appears in the closed-loop region of the free-energy surface. The main chain–main chain hydrogen bond between N292 and A289 additionally stabilizes this structure.
The blue (B) cluster is an open-loop structure in which neither K257 nor H294 is engaged in one of the considered hydrogen bonds. It features the 258m–256m and 262m–259m hydrogen bonds within the short-loop. The red (R) cluster is a slightly sheared structure in which the K257 side chain partly forms a hydrogen bond to the carboxyl group of E261 and partly to the carboxyl group of D308.
Three hydrogen bonds in Figure 4 directly involve Ca2+-coordinating residues. First, we already discussed the hydrogen bond K257–D308. Second, the hydrogen bond between the main chain of N287 and the side chain of D308 is important for the stability of the long-loop fold. It is occupied to about 90% in both protonation states. Third, population of the hydrogen bond between the main chain amid group of D308 and the carboxyl group of E285 is increased in the protonated state. This is particularly true for cluster G (green) and O (orange). This hydrogen bond might compete with the coordination of E285 to Ca2+ and thereby might contribute to the observed decrease in Ca2+ affinity. In both the neutral and the protonated systems, the bonds N288s–M260m, N288s–E261s, K257s–E261s, and G262m–E261s are strongly correlated (see Fig. S10). In the protonated state a strong correlation between K257s–D308s and H294s–E261s arises, indicating that these two hydrogen bonds are formed and broken simultaneously.
A mechanism for the pH-sensitive Ca2+ affinity in langerin
We are now ready to propose a mechanism that explains how protonation of H294 can lead to a decrease in Ca2+ affinity. In the neutral state, K257 and H294 form a hydrogen bond that is populated over a wide range of conformations. We also observe a weak hydrogen bond of the K257 side chain to the main chain of the Ca2+-coordinating residue E293, but direct hydrogen bonds to the Ca2+-coordinating carboxyl groups are hardly ever formed (Fig. 5A). Upon a drop of pH from 7 to 6, the side chain of H294 is protonated in accordance with its pKa: H294 is the initial pH sensor. The protonation of H294 changes the hydrogen bond pattern between the short- and the long-loops. In particular, the side chains of H294 and K257 form new contacts, which gives rise to previously inaccessible conformations. Cluster O (orange) and cluster G (green) exhibit mutually exclusive hydrogen bond patterns. In cluster O, multiple hydrogen bonds connect the short- and the long-loops causing a closed loop conformation. The positively charged side chain of K257 forms a hydrogen bond to the negatively charged side chain of E261. But similar to the neutral state, there is no direct hydrogen bond to the Ca2+-coordinating carboxyl groups (Fig. 5C). This is different in cluster G. Here the positively charged side chain of H294 forms a hydrogen bond with the negatively charged carboxyl group of E261. Simultaneously, the positively charged side chain of K257 forms a hydrogen bond with the carboxyl group of D308 (Fig. 5B). This hydrogen bond withdraws electron density from the coordinative bond between D308 and Ca2+ and thereby reduces the Ca2+ affinity. It is even conceivable that the proton is transferred entirely to the carboxyl group of D308 (42). We thus propose that cluster G (green) is responsible for the decrease in Ca2+ affinity at pH 6.
Figure 5.
Allosteric mechanism for the pH-sensitive Ca2+affinity in langerin.A, neutral state, B, cluster G (green) in the protonated state, and C, cluster O (orange) in the protonated state. Lines, hydrogen bonds with population in percent. Arrows, coordination between carboxyl groups and Ca2+.
In this mechanism, K257 acts as a proton reservoir. The initial detection of a pH change via protonation of H294 leads to the cluster G, in which K257 moves a proton into the vicinity of the Ca2+-binding site and locally increases the proton concentration. Thus, the signal that the pH has changed is allosterically transferred to the Ca2+-binding pocket without transferring the actual proton that triggered the mechanism.
A crucial assertion in the proposed mechanism is that the life time of cluster G (green) represents a distinct conformation that is stable enough for the Ca2+ to leave the binding pocket. The fact that cluster G corresponds to a free-energy minimum in the space of the principal components hints at a stable conformation. But because the principal components maximize the spatial variance and not the variance in time, this is not sufficient to be certain.
Figure 6A shows the distance distribution between the K257 and D308 side chain for the neutral and the protonated states. In both protonation states, the maximum at short distances around 0.2 nm is well separated from the maximum at larger distances.
Figure 6.
Frequency of K257 interaction with the Ca2+-binding site.A, K257–D308 side-chain distance distribution. B, K257 side-chain amine – Ca2+distance distribution. Solid lines, mean of the histograms calculated for each simulation replica. Shaded area, 95% confidence interval of the mean obtained by bootstrapping (1000 samples).
In the neutral state, the short distances are populated only in 4.3% of all simulated conformations, which increases to 13.2% when H294 is protonated. This is in line with the increase of population in the K257–D308 hydrogen bond from 4.2% to 12.9%. We obtain the same results, when plotting the distance between the K257 side-chain amine and the Ca2+ in Figure 6B. Thus, cluster G (green) indeed represents a distinct conformation.
To assess the stability of conformations in cluster G (green), and to relate its formation to other dynamic processes in the protein, we constructed a core-set Markov model of the conformational dynamics (43, 44, 45). In Markov models, the conformational space is discretized into states and the conformational dynamics are modeled as Markov transitions within a lag time τ between pairs of these states, where the transition probabilities are obtained from molecular dynamics simulations. From the eigenvectors and eigenvalues of the Markov-model transition matrix one obtains long-lived conformations as well as the hierarchy of the free-energy barriers separating them. The special feature of core-set Markov models is that the states are confined to the regions close to the minima of the free-energy surface, i.e., so-called core sets, whereas the regions between these minima are modeled by committor functions. This reduces the discretization error of the model considerably.
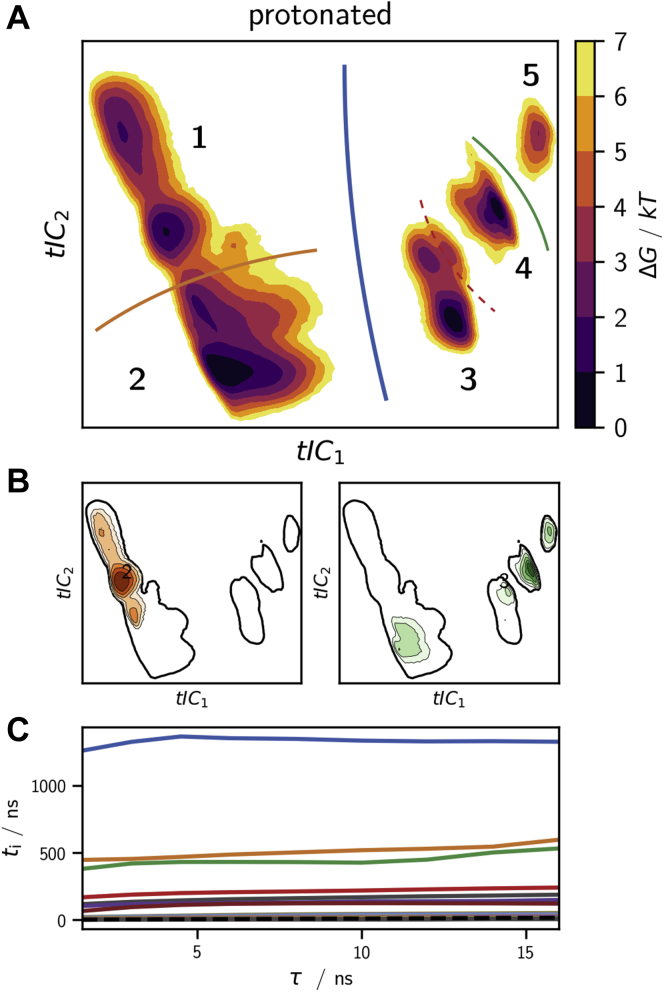
The Markov model construction is preceded by a dimensionality reduction of the conformational space using the time-independent component analysis (46, 47). Time-independent components (tICs) maximize the variance within lag time τ rather than the instantaneous variance maximized by principal components. A projection into a low-dimensional tIC space can thus be interpreted as projection into the space of the slowly varying coordinates of the system. Figure 7A shows the free-energy surface of the protonated system projected into the space of the first and the second tICs (see Supporting information for other projections), and Figure 7B shows the projection of cluster G (green) and O (orange) into this space.
Figure 7.
Core-set Markov model of the conformational dynamics of protonated holo-langerin.A, free energy surfaces from the 2D projections of protonated holo-langerin trajectories onto the first two time-independent components (tICs). Solid lines, transition regions between the five metastable states connected by the four slowest dynamic processes. B, projections of cluster G (green) and O (orange) into the space of the first two tICs. C, implied time scales of the core-set Markov model. The colors of the processes match the transition regions drawn into (A).
We then identified 22 core sets in the space of the first six tICs using common-nearest-neighbors clustering (39, 41) and used them to construct a core-set Markov model. The implied timescale test shows that the timescales of our core-set Markov model are independent of the lag time τ indicating a very small discretization error and thus a high-quality Markov model (Fig. 7C). The slowest dynamic process occurs on a timescale of about 1.3 μs and corresponds to changes in the local conformations of E261 and its hydrogen bond pattern. It thus separates the conformations of cluster G (green) and cluster O (orange) along the blue barrier in Figure 7A. Note that all conformations in which the K257s–D308s hydrogen bond is formed alongside H294s–E261s are located on the right-hand side of this barrier (see Supporting information). The fact that we find some structures that have originally been assigned to the G (green) conformation on the left-hand side of the barrier is likely due to the insufficient separation of long-lived conformations in the principal component space (Fig. 7B). Next, protonated langerin has two slow timescales that occur at about 500 ns. One process describes transitions between the closed loop conformations in region 1 and conformations in which the distance between the long- and the short-loop is larger in region 2. The other process represents a transition between conformations in which the backbone orientation of N291 forbids the N292m–A289m hydrogen bond giving rise to a distortion of the long-loop (region 5) and the conformations in which the N292m–A289m hydrogen bond is possible (regions 3 and 4). The dashed barrier marks transitions to more open short-loop forms occurring on a timescale of 210 ns.
In summary, conformations in which the K257–D308 hydrogen bond is formed are separated from the alternative O (orange) conformation by a rare transition that occurs on a timescale of 1.3 μs. Within the right-hand side of the barrier in Figure 7A the G (green) conformation is at least stable on a timescale of 200 ns. This is likely sufficient to enable the escape of the Ca2+ from the binding pocket. A core-set Markov model of neutral holo-langerin is reported in the Supporting information.
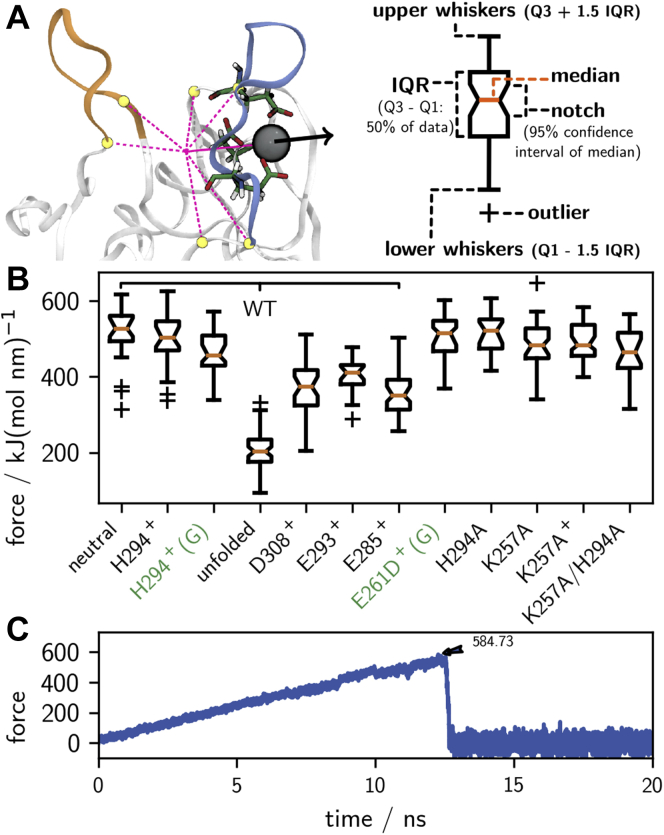
To directly probe how the stability of the Ca2+-bound state of the protein depends on the protonation state and on the conformation of langerin, we used constant-velocity steered–molecular dynamics (MD) experiments (48, 49, 50). In these simulations, a force that increases linearly with time is applied to the Ca2+ atom (Fig. 8A), and the opposing force (i.e., the resistance against this pulling force) is measured. At a certain maximum force the ionic bonds between the Ca2+ atom and the coordinating residues rupture and the Ca2+ leaves the binding pocket. In the computer experiment, this is marked by a sudden drop in the opposing force (Fig. 8C). The rupture force is a rough measure for the free-energy difference to the transition state ΔG‡. The rational is that a deeper free-energy minimum of the Ca2+-bound state is associated with a steeper slope to the transition state, and the rupture force, reflecting the maximal slope, reports on the stability of the Ca2+-bound state (51, 52). We chose the pulling rate such that the rupture events are observed after several nanoseconds. This ensures that the system has enough time to adjust to the pulling and also that the initial starting conformation is preserved to some degree.
Figure 8.
Constant-velocity steered-MD.A, pull coordinate defined as the distance vector between Ca2+ and the center of mass of the Cα-atoms of residues 257, 264, 281, 282, 293, and 294. B, maximal pulling force observed acting on Ca2+ during simulations of langerin in various states as notched box representation. The orange line represents the median, while the box enframes the interquartile range. The box notches indicate the 95% confidence interval on the median. Points lying beyond 1.5 times the edges of the box are regarded as outliers (+), and the whiskers mark the data range without outliers. C, example for a force trajectory with a rupture event at about 12 ns. Maximum force indicated by an arrow.
For each system, we conducted 40 steered-MD simulations and report the data as notched boxplot in Figure 8B. Overall, the plot shows that we could determine the median of rupture force with high confidence and hardly any outliers. The rupture force decreases from the neutral to the protonated system (H294+) and then further to simulations of the protonated system started in the G (green) conformation, in which the K257 amine forms a hydrogen bond with the D308 carboxyl group. This decrease is predicted by our mechanism. Note that classical force fields cannot model instantaneous shifts in the electron density due to the formation of hydrogen bonds. Thus, the rupture force in the G (green) conformation might actually be somewhat lower. If the Ca2+-coordinating residue D308 is protonated, corresponding to a situation in which the proton is transferred from K257 to D308, the rupture force is about 150 kJ/(mol nm) lower than in the neutral system.
The same is observed when one of the other two Ca2+-coordinating residues is protonated. A drastic reduction in the rupture force is observed, when the experiment is started from a state where the long-loop is unfolded. This is expected, as one of the Ca2+-coordinating residues E285 is removed from the cage of the binding site in this arrangement. The rupture force for the mutant E261D (started from an analogon of the G conformation) and the mutant H294A are in the same range as for the neutral wild-type langerin.
Of note, the rupture forces for K257A mutants are insensitive toward the modeled state of H294. The binding capability is virtually the same, no matter if H294 is neutral, protonated, or mutated. This substantiates the importance of K257 to transport a protonation signal to the Ca2+-binding site.
Comparison with experimental data
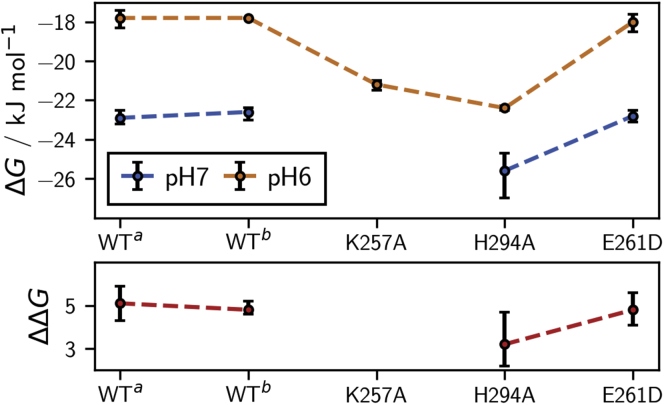
The Ca2+-dissociation constant of wildtype langerin at pH 7 is Kd = 105 ± 15 μM and increases to Kd = 800 ± 150 μM at pH 6 (17), as determined by isothermal titration calorimetry (ITC). These dissociation constants correspond to binding free energies of ΔGpH 7 = −22.9 kJ/mol, and ΔGpH 6 = −17.8 kJ/mol at T = 300 K, yielding a pH sensitivity of ΔΔG = ΔGpH 6 − ΔGpH 7 = 5.1 kJ/mol (Fig. 9). By contrast the dissociation constants of the H294A mutant, in which the pH sensor H294 is removed, are Kd = 35 ± 15 μM at pH 7 (ΔGpH 7 = −25.6 kJ/mol) and Kd = 125 ± 5 μM at pH 6 (ΔGpH 6 = −22.4 kJ/mol), corresponding to a reduced pH sensitivity of ΔΔG = 3.2 kJ/mol (17) (Fig. 9). Our mechanism so far explains the pH sensitivity due to the pH sensor H294. The fact that the H294A mutant exhibits a residual pH sensitivity indicates that langerin has a second pH sensor.
Figure 9.
Ca2+binding free energies under standard conditions in kJ/mol. Calculated as ΔG = −RTln (Kd), where R = 8.314 J/(K mol) is the gas constant, T = 300 K is the temperature, and Kd in units of mol/l are the experimentally determined dissociation constants. Measurements at pH 6 (blue) and pH 7 (orange) with experimental uncertainties indicated with error bars and pH sensitivities in kJ/mol calculated as ΔΔG = ΔGpH 6 − ΔGpH 7 (red).
To convince ourselves of the robustness of these results, we remeasured the dissociation constants of wildtype langerin (see Supporting information). We obtained Kd = 113 ± 14 μM at pH 7 (ΔGpH 7 = −22.6 kJ/mol) and Kd = 802 ± 150 μM at pH 6 (ΔGpH 6 = −17.8 kJ/mol), yielding a pH sensitivity of ΔΔG = 4.8 kJ/mol (Fig. 9). This is in excellent agreement with our previous results.
Four residues are central to our mechanism: H294, K257, D308, and E261. D308 directly coordinates to Ca2+and is therefore not a suitable candidate for site-directed mutagenesis. In contrast to H294A, the pH sensitivity of K257A could not be determined because the protein precipitated at pH 7. However, both mutants have a higher Ca2+ affinity than wildtype langerin at pH 6, which previously could not be explained. The overall higher Ca2+ affinity in the K257A mutant is predicted by our mechanism, because the K257–D308 hydrogen bond that destabilizes the Ca2+ coordination cannot be formed in the absence of the K257 side chain. The H294A mutant has the K257 side chain, and the conformation in which K257 is in the vicinity of D308 (Fig. 6) can in principle be formed. However, in our simulations of H294A we find that the K257 side chain is in the vicinity of the D308 side chain in only 1.7% of the simulated structures, which might explain the higher Ca2+ affinity of the H294A mutant (see Supporting information).
Besides H294 and K257, residue E261 is important for the stabilization of the G (green) conformation, which is responsible for lowering the Ca2+ affinity. However, it also stabilizes the cluster O (orange), which is not expected to increase the Ca2+ affinity, because K257 forms a hydrogen bond with E261 rather than with D308 in this conformation. We therefore predicted that mutating E261 has little effect on the pH sensitivity. We measured the Ca2+-dissociation constants for the E261D mutant at pH 6 and pH 7 by ITC (see Supporting information), and the results confirm our prediction. The dissociation constants of the E261D mutant are Kd = 108 ± 11 μM at pH 7 (ΔGpH 7 = −22.8 kJ/mol) and Kd = 742 ± 141 μM at pH 6 (ΔGpH 6 = −18.0 kJ/mol), yielding a pH sensitivity of ΔΔG = 4.8 kJ/mol (Fig. 9).
Long-loop unfolding
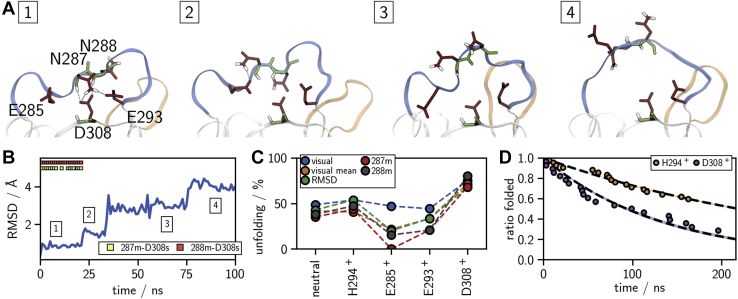
So far, our mechanism explains how Ca2+ is destabilized in the binding pocket of holo-langerin. However, if the proton is transferred from K257 to D308, the mechanism also has profound effects on apo-langerin. In holo-langerin the long-loop is stabilized in a well-defined conformation (folded long-loop conformation) by E285, which coordinates to Ca2+. In apo-langerin this interaction is not possible, and the long-loop spontaneously unfolds in our simulations as shown by the RMSD evolution in Figure 10B. Similar long-loop unfolding has been observed in the crystal structures of other C-type lectins, like tetranectin (53), TC14 (54), or MBP (55). To estimate the unfolding rate, we conducted 30 to 60 simulations (see Supporting information) for each of the following protonation states of apo-langerin: neutral, H294 protonated, H294 and E285 protonated, H294 and E293 protonated, and H294 and D308 protonated, each of them started in the folded conformation. In four of the five protonation states 44% to 54% of all trajectories unfold within 220 ns simulation time, as determined by visual inspection (Fig. 10C, blue dots). The carboxyl group D308 is critical for the stabilization of the folded loop conformation in the absence of Ca2+ by forming hydrogen bonds with the N287 side chain, as well as with the backbone amide-hydrogen of N287 and N288 (Fig. 10A). If D308 is protonated, all three hydrogen bonds are much weaker, and consequently the long-loop unfolds at a higher rate (75% within 220 ns).
Figure 10.
Long-loop unfolding in apo-langerin.A, example structures for a fully folded (A1), intermediate (A2), partially unfolded (A3), and fully unfolded (A4) state. B, example trajectory of the long-loop Cα-RMSD; 22 ns, intermediate state; 30 ns, unfolding event. C, percentage of unfolded trajectories within 220 ns determined by: last folded frame (visual), mean of last folded and first unfolded frame (visual mean), RMSD > 0.2 nm, and hydrogen bonds N287m–D308s (287m) and N288m–D308s (288m). D, decay plot of folded trajectories (last folded frame) and exponential fit (dashed line ±σ), H294+: H294 protonated, D308+: H294 and D308 protonated.
Long-loop unfolding often occurs via an intermediate conformation, in which the hydrogen bonds with the backbone amides of N287 and N288 are broken, while the hydrogen bond to the N287 side chain is still possible. In this intermediate form the loop is more flexible than in the fully folded state, but the characteristic turns in the loop backbone are still largely present, and we observe refolding to the fully folded state in some of the trajectories. The transition to the fully unfolded conformation occurs when one or more of the backbone torsion angles in the long-loop rotate and the hydrogen bond between the side chains of D308 and N287 breaks. This transition is irreversible on the timescale of our simulations.
To corroborate our visual analysis of the simulation end points, we determined the time of the unfolding event by four additional criteria: the mean between last fully folded frame and first fully unfolded frame determined by visual inspection, the Cα-RMSD of the long-loop residues exceeds 0.2 nm, and breaking of the hydrogen bonds between the D308 carboxyl group or the backbone amide-hydrogen of N287 and N288. All four criteria confirm the first analysis (Fig. 10C).
If E285 is protonated, a hydrogen bond between the protonated carboxyl group of E285 and the unprotonated carboxyl group of D308 stabilizes a partially folded loop structure, such that for some criteria we observe even fewer unfolding events than by the simple visual analysis for this system. We determined the half-life periods t1/2 of the folded states from the decay plots of the folded trajectories (see Supporting information). Independent of the criterion, the decay is fastest, when D308 is protonated. In particular, unfolding is over twice as fast if D308 is protonated than if only H294 is protonated (t1/2 = 218 versus 93 ns, Fig. 10D). Some of the decays deviate from a single-exponential decay, hinting at a more complex underlying unfolding mechanism.
Since the folded conformation binds Ca2+ much more strongly than the unfolded conformation (Fig. 8), the equilibrium between folded and unfolded long-loop is critical for the overall Ca2+ affinity. Thus, the protonation of D308 has a 2-fold effect: First, it destabilizes the Ca2+ in the binding pocket. Second, after the Ca2+ has left the binding pocket, it destabilizes the folded loop conformation and thereby reduces the likelihood of Ca2+ rebinding.
The second pH sensor
In the ITC experiments the H294A mutant exhibits a pH sensitivity of ΔG = 3.2 kJ/mol, even though the pH sensor H294 is missing (17). This suggests that langerin has a second pH sensor. To convince ourselves that this residual pH sensitivity is indeed due to a second pH sensor, we checked whether K257 forms another potentially pH-sensitive hydrogen bond in the H294A mutant that could replace the pH-sensitive K257–H294 hydrogen bond and explain the residual pH sensitivity. In our simulations of the H294A mutant, K257 does not form any highly populated hydrogen bond. With 13% population the hydrogen bond between the side chain of K257 and the main-chain carbonyl group of E293 is the most frequently formed hydrogen bond. However, in wildtype langerin it is formed with the same frequency. All other hydrogen bonds of K257 are populated with less than 5%. Thus, the experimentally determined pH sensitivity in the H294A mutant does indeed indicate that wildtype langerin has a second pH sensor.
There are two possible mechanisms to explain the residual pH sensitivity. First, langerin could have a second allosteric pH sensor that, similar to H294, is activated by protonation from the surrounding solvent prior to the dissociation of Ca2+. Second, the carboxyl groups of the Ca2+-coordinating residues E285, E293, and D308 could form a dyad with an effective pKa that makes it sensitive to a pH change from 7 to 6. That is, after initial dissociation of Ca2+, one of the coordinating residues (Fig. 2D) is protonated and the protonated state is stabilized as a hydrogen bond to an unprotonated carboxyl group (56). We first discuss the possibility of a second allosteric pH sensor before investigating whether a dyad is possible.
H229 is the only other histidine residue in langerin. It is solvent exposed and will indeed be protonated when the pH changes from 7 to 6. However, H229 is located far away from the Ca2+-binding site, which makes an allosteric influence on the Ca2+-binding affinity unlikely (Fig. 1). This is further corroborated by the previously published mutual information analysis of the allosteric network in langerin and by chemical shift perturbation experiments (17). In the extended simulation data set used for this study, protonation of H229 has a local effect on the lower protein region including the α1 helix, but these conformational shifts are well separated from the Ca2+-binding site. We therefore exclude H229 as a potential pH sensor.
Other candidates for allosteric pH sensors are aspartic and glutamic acids, whose pKa (in water at 25 °C 4.15 for E and 3.71 for D) (27) can be shifted by several units by the local environment in the protein, such that their carboxyl groups could become sensitive to a pH change from 7 to 6 (57). Apart from the Ca2+-coordinating residues E285, E293, and D308, langerin has nine aspartic or glutamic acids. Using PROPKA 3.1 (58, 59), we calculated the distribution of the pKa values for these residues in holo-langerin in the neutral and the H294-protonated state, as well as for apo-langerin in the neutral and the H294-protonated state. The distributions are based on 10,000 to 30,000 structures extracted from the simulations of the corresponding systems and are reported along with the mean and the standard deviation in the Supporting information. The mean pKa value of all tested residues is below 5.0, and none of the distributions reaches into the critical region between pH 6 and 7, indicating that none of them acts as pH sensor. We therefore conclude that the residual pH sensitivity in langerin is not generated by a second allosteric pH sensor.
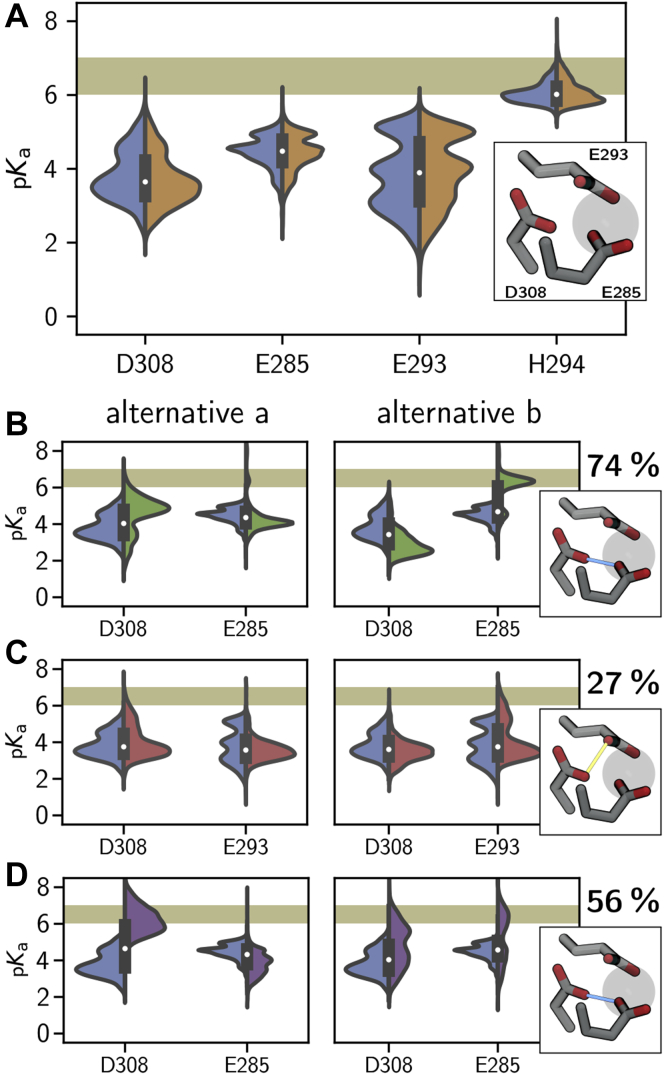
PROPKA 3.1 can detect the coupling between two carboxyl groups that are in close vicinity. It returns two alternative pKa values. In alternative a, one carboxyl group is protonated first and stabilized by the second (unprotonated) carboxyl group, in alternative b the situation is reversed. Figure 11A shows the pKa distribution of the Ca2+-coordinating residues E285, E293, and D308 as well as the pKa distribution of H294 for apo-langerin in the neutral and the H294-protonated state. No coupling between E285, E293, and D308 was detected by PROPKA 3.1. Their mean pKa value is below 5.0, and none of the distributions reaches into the critical region between pH 6 and 7. By contrast, the mean pKa values of H294 are about 6 in the neutral and the H294 protonated states, and the pKa distributions have a large overlap with the critical region between pH 6 and 7. Thus, from these simulations one would conclude that langerin does not have a protonatable dyad in the Ca2+-binding pocket and that only H294 is sensitive to a pH change from 7 to 6.
Figure 11.
Calculation ofpKavalueswith PROPKA 3.1.A, pKa distributions for the neutral (blue) and the H294 protonated (orange) apo-systems. B, distributions for residues involved in coupling, neutral versus E285 protonated (green), C, neutral versus E293 protonated (red), and D, neutral versus D308 protonated (purple). Alternative distributions due to the coupling left and right. Percentages of coupling frames are placed over the binding site illustrations.
However, in the neutral and the H294-protonated states, the carboxyl group of the Ca2+-coordinating residues are negatively charged and repel each other, making structures in which the two carboxyl groups are close enough to potentially stabilize a protonation unlikely. We therefore also calculated the pKa distribution for the following protonation states of apo-langerin: H294 and E285 protonated (Fig. 11B), H294 and E293 protonated (Fig. 11C), and H294 and D308 protonated (Fig. 11D). For these protonation states, substantial coupling between the Ca2+-coordinating residues is detected. D308 and E285 couple in 74% of all structure if E285 is protonated and in 56% of all structures if D308 is protonated. When E293 is protonated, E293 and D308 couple in 27% of all structures.
These couplings give rise to a strong shift of the pKa distributions compared with neutral apo-langerin. We report the distributions of both pKa estimates, which should be interpreted as limiting cases of the true distribution. If D308 is protonated, the pKa distributions of D308 for both limiting cases reach well into the critical region between pH 6 and 7, and for alternative a we obtain a mean pKa value in coupling frames of 6.4 ± 0.7 (Fig. 11D). If E285 is protonated, the coupling to D308 in alternative b yields a mean pKa value of 6.2 ± 0.6 for E285, and the corresponding distribution of all frames is almost centered on the critical region between pH 6 and 7 (Fig. 11B). The effect is not as strong, if E293 is protonated (Fig. 11B). For alternative a the pKa distribution of D308 reaches slightly into the region between pH 6 and 7, and for alternative b the pKa distribution of E293 reaches into this region. However, the corresponding pKa values, 5.2 ± 0.7 and 5.5 ± 0.7, are clearly lower than those for the coupling between D308 and E285.
These results show that, in the absence of Ca2+, D308 and E285 can form a protonated dyad with an effective pKa that is likely high enough to sense a pH change from 6 to 7. We therefore believe that the second pH sensor that is active in the H294A mutant is the dyad between D308 and E285. In wildtype langerin the pH sensor H294 and this dyad would amplify each other: the K257–D308 hydrogen bond increases the probability that D308 is protonated, and, after Ca2+ has escaped, the protonated D308 is stabilized by the D308–E285 dyad. Constant-pH simulations (60, 61, 62) or mixed quantum mechanics/molecular mechanics simulations (63, 64) could be used to verify whether D308 and E285 indeed form a dyad and constitute the second pH sensor.
Note that the conformational fluctuations in the Ca2+-binding pocket give rise to large fluctuations in the instantaneous pKa value (Fig. 11) with some distributions covering more than six pKa units. Thus, knowing the underlying conformational distribution is essential for a reliable estimate of the overall pKa value.
Comparison with other C-type lectins
To gain insight into whether the proposed mechanism for the pH sensitivity is found in other C-type lectins, we compared the sequences of human langerin with mouse langerin and with human variants of 15 related C-type lectins (Fig. S31). All 16 proteins exhibit the typical C-type-lectin fold, as evidenced by crystal structures (Fig. S32). The residues D308 and E285, which form the proposed second pH sensor, are highly conserved. However, one should be careful to interpret this as evidence for a conserved second pH sensor, because these residues are also essential for the coordination of Ca2+ and might be conserved for this reason.
The H294–K257 motif, the primary pH sensor in langerin, is not conserved in our sequence alignment. Thus, the proposed mechanism for the pH sensitivity of the Ca2+ affinity via H294 protonation does not seem to be the most widespread mechanism to sense a change in the environment in C-type lectins. But the sequence alignment points to possible other mechanisms for sensing a change in the environment.
The selectines P-, E-, and LSECtin share the lysine K257 with langerin in the same position. In addition, the preceding threonine T256 in langerin is replaced by an arginine in these three proteins, while H294 is replaced by an aspartic acid. Note that, in LSECtin Ca2+ affinity increases if the pH decreases (26). It is, however, unclear whether this reversed pH sensitivity is brought about by the change of the H294–K257 motif. Other lysine residues in the short-loop in comparable positions as K257 in langerin can also be found in the macrophage C-type lectin, lung surfactant protein (SP), CD23a, Endo180, and the macrophage mannose receptor.
H294 only appears in human and mouse langerin, and is replaced by aspartic acid in most of the other C-type lectins. Instead, we find a Ca2+ in the position where langerin has the H294–K257 hydrogen bond in 6 of 15 lectins in our analysis (ASGPR, MBP, DCSIGN, DCSIGNR, SP, SR). This Ca2+ would be partially solvent exposed even when a large entity (such as a pathogen) is bound to the C-type lectin (Fig. S32). One therefore might speculate that these lectins do not sense a change in pH but rather a change in Ca2+ concentration.
Several C-type lectins have histidines in other positions close to the Ca2+-binding site, which might act as pH sensors via a different mechanism. As already mentioned, ASGPR has a histidine residue that is close to the Ca2+ in the primary Ca2+-binding site and thereby acts as pH sensor. Furthermore, dectin-2 and the macrophage mannose receptor have a histidine residue as a neighbor to a Ca2+-coordinating residue, and Endo180 and the macrophage C-type lectin have histidines at the beginning of the long-loop. Whether these histidines act as pH sensors can be tested by mutating the histidine residue and measuring the pH sensitivity of the Ca2+ affinity and of the carbohydrate affinity. Once a residue is confirmed as a pH sensor, the approach presented in this contribution can be used to propose a molecular mechanism for the pH sensitivity.
Conclusion
We have described the consequences of a H294 protonation in langerin and its implications for its biological function as an endocytic pattern recognition receptor. When langerin enters the acidic environment of an endosome, it releases its Ca2+ cofactor and subsequently its pathogenic cargo, triggered by a moderate change in pH. The Ca2+-binding site is blocked from direct solvent access by the pathogen, and additionally, the Ca2+-coordinating residues have low protonation probabilities in the presence of calcium. Instead, H294 acts as an accessible site, sensing already a change in pH from 7 to 6 (17).
In this contribution, we have uncovered a mechanism in which protonation of H294 perturbs the hydrogen-bonded network of the surrounding residues and alters the conformational ensemble of langerin. A new conformation becomes accessible, in which the protonated K257 side chain forms a hydrogen bond with the Ca2+-coordinating D308, thereby moving a positive charge into the vicinity of the Ca2+-binding site. This alone can facilitate the Ca2+ release as shown by the reduction in the required force to pull out the ion from its binding site in our steered-MD experiments.
The close availability of K257 as a proton source next to the Ca2+-binding site possibly results in a proton transfer to the side chain of D308. At least it has been shown in a theoretical model that the neutral form of a lysine–aspartate pair can be favored over the salt bridge, if the dielectric constant of the medium is low as it can be the case in the environment of a protein (42). Thus, protonation of the initial pH sensor H294 likely triggers a cascade of events that ensures the unbinding of Ca2+: K257 transfers a proton to D308, protonation of D308 competes drastically with Ca2+ binding and, after Ca2+ is expelled, the protonation of D308 is stabilized by a dyad with E285. Protonation of D308 additionally accelerates the unfolding of the long-loop, preventing Ca2+ from rebinding.
For langerin’s role as endocytic pattern recognition receptor a fast and irreversible Ca2+ release is essential. On the cell surface, Ca2+ needs to be tightly bound such that the receptor is continuously ready to bind to pathogens. Yet, after endocytosis langerin is probably recycled within minutes (13, 65). This leaves little time for the release of the pathogen, which must be preceded by the unbinding of Ca2+. The mechanism that we proposed is an elegant solution to these contradicting requirements: the Ca2+-unbinding rate is increased by the K257–D308 hydrogen bond, and after the initial Ca2+ release, a transfer of the proton from K257 to D308 triggers a transition to a conformation to which Ca2+ cannot rebind.
Note that, although our results show that the K257–D308 interaction decreases the stability of Ca2+ in the binding pocket and that the protonation of D308 triggers the long-loop unfolding, the transfer of a proton from K257 to D308 is currently an assumption. More work is needed to study the equilibrium between the initial and the end states of the proton transfer. Computationally, this could be tackled by mixed quantum mechanics/molecular mechanics calculations (63, 64), free-energy calculations with classical force fields, or by constant pH simulations (60, 61, 62).
Another concern is that the point charge Ca2+ model might not capture the energetics of Ca2+ binding accurately enough, because the point charge model does not enforce coordination and neglects polarization effects. In our study, we tried to minimize the influence of these force-field effects by analyzing the differences between two protonation states. However, more elaborate Ca2+ models such as reparametrized point-charge models (66, 67), multisite models (68), or polarizable models (69) are available and should be used, for example, for the computation of state-specific Ca2+ binding free energies.
Our close atomistic inspection of langerin and its conformational shift upon protonation gives insight into how pH sensitivity can be incorporated in biological systems. What seemed like a general conformational shift upon protonation in Figure 3 could be focused to a specific rearrangement of a side chain (K257) to transport the information from the primary pH sensor (H294) to the allosterically regulated site (Ca2+-binding site). Even though the H294–K257 motif is not typical for C-type lectins, many of these proteins exhibit a highly specific pH sensitivity and have potential pH sensors in the vicinity of the primary Ca2+-binding site. Our approach can serve as a road map to elucidate the mechanism of pH sensitivity in these systems.
Experimental procedures
Molecular dynamics simulations
We used the software package GROMACS (70, 71, 72, 73, 74, 75, 76) in setup and production to simulate the considered systems in the NPT-ensemble (1 bar, 300 K) with AMBER99SB-ILDN force-field parameters (77) and the TIP3P water model (78). Prior to production, starting structures were put into a sufficiently large simulation box, solvated, neutralized, and equilibrated for several hundred picoseconds. For further details refer to the Supporting information.
Protein expression and purification
All standard chemicals and buffers used within this work were purchased from Sigma-Aldrich or Carl Roth if not indicated otherwise.
Human langerin CRD WT and all mutants (amino acids 193–328) were cloned from a codon-optimized langerin gene for bacterial expression (GenScript) into a pET-28a vector (GenScript) with His-tag, T7 promoter, and Kanamycin resistance. Insoluble expression was performed in E. coli BL21 (Thermo Fisher Scientific) in LB medium or in isotope-labeled M9 medium at 37 °C. Protein expression was induced by adding 1 mM IPTG. Bacteria were harvested 3 to 4 h after induction by centrifugation at 4000g for 30 min. Cell pellets were lysed in lysis buffer (50 mM Tris, 150 mM NaCl, 10 mM MgCl2, 0.1% Tween-20, pH 8) with 1 mg ml−1 lysozyme and 100 μg ml−1 DNase I (Applichem) for at least 3 h at RT. Inclusion bodies were washed twice with 20 to 30 ml lysis buffer and twice with water to remove soluble proteins. Inclusion bodies were denatured in 20 ml of denaturation buffer (6 M guanidinium hydrochloride in 100 mM Tris, pH 8) with 0.01% β-mercaptoethanol for at least 1 h at 37 °C by shaking or overnight at 4 °C by rotating. After centrifuging (15,000g, 90 min, 4 °C), the supernatant was slowly diluted 1:10 with langerin refolding buffer (0.4 M L-arginine in 50 mM Tris, 20 mM NaCl, 0.8 mM KCl, pH 7.5) with 1 mM reduced glutathione (GSH) and 0.1 mM oxidized glutathione (GSSG) while stirring at 4 °C for at least 24 h. The refolded protein solution was dialyzed against 2 l TBS buffer (50 mM Tris, 150 mM NaCl, 5 mM CaCl2) and subsequently centrifuged to remove precipitated protein (15,000g, 90 min, 4 °C). The supernatant was purified via Ni-NTA agarose affinity chromatography and the elution fractions were pooled and dialyzed against MES (25 mM MES, 40 mM NaCl, pH 6) or HBS (25 mM Hepes, 150 mM NaCl, pH 7) buffer. Precipitated protein was removed by centrifugation (15,000g, 90 min, 4 °C), and the supernatant was used for experiments. Note that this procedure varies slightly from the one in our previous paper (17).
ITC measurements
ITC experiments were performed using a MicroCal iTC200 (Malvern Instruments) using either chelex-filtered HBS (25 mM Hepes, 150 mM NaCl, pH 7) or low-salt MES buffer (25 mM MES, 40 mM NaCl, pH 6). The titrant was dissolved in the same buffer as was used for dialysis of the protein sample. Using the iTC200, the titrant CaCl2 (15 mM stock) was added in defined steps of 1 to 2.5 μl to 80 μl protein solution at 298 K while stirring at 750 rpm. The differential heat of each injection was measured and plotted against the molar ratio. The data were fitted to a one-set of sites binding model assuming a Hill coefficient of 1. Owing to the low c-values of the measurements (c < 5), the enthalpy could not be determined reliably. See also Figs. S29 and S30.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. All remaining data are contained within the article and its supporting information. The software used for common-nearest-neighbor clustering and core-set Markov-state model estimation is publicly available on GitHub (https://github.com/janjoswig/CommonNNClustering).
Supporting information
This article contains supporting information (16, 36, 38, 58, 59, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90).
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
Funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy – EXC 2008 – 390540038 – UniSysCat. The authors thank the North-German Supercomputing Alliance (HLRN), the Paderborn Center for Parallel Computing PC2, and the ZEDAT of the FU Berlin for computing time. Also funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) through CRC 765, and DFG RA1944/6-1. We thank the Max Planck Society for support.
Author contributions
J.-O. J. performed the computer experiments, except for the simulations of the long-loop unfolding, analyzed and interpreted the data, made all figures, and drafted and revised the manuscript. J. A. performed the computer experiments for the long-loop unfolding and analyzed the data. H. Z. performed the laboratory experiments and analyzed the data. C. R. interpreted the data of the laboratory experiments and drafted and revised the corresponding part of the manuscript. B. G. K. designed the study, interpreted the data, and drafted and revised the manuscript.
Biography

Jan-Oliver Joswig is a doctoral student at Freie Universität Berlin in the Molecular Dynamics Group led by Prof. Dr Bettina Keller. In his research he focuses on the application of molecular simulations to biomolecular systems with complex dynamics. In addition, he develops software solutions for advanced analyses methods, e.g., density-based clustering for the construction of kinetic models. GitHub: https://github.com/janjoswig.
Edited by Roger Colbran
Supporting information
References
- 1.Valladeau J., Duvert-Frances V., Pin J.J., Dezutter- Dambuyant C., Vincent C., Massacrier C., Vincent J., Yoneda K., Banchereau J., Caux C., Davoust J., Saeland S. The monoclonal antibody DCGM4 recognizes Langerin, a protein specific of Langerhans cells, and is rapidly internalized from the cell surface. Eur. J. Immunol. 1999;29:2695–2704. doi: 10.1002/(SICI)1521-4141(199909)29:09<2695::AID-IMMU2695>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 2.Valladeau J., Ravel O., Dezutter-Dambuyant C., Moore K., Kleijmeer M., Liu Y., Duvert-Frances V., Vincent C., Schmitt D., Davoust J., Caux C., Lebecque S., Saeland S. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of birbeck granules. Immunity. 2000;12:71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- 3.Zelensky A.N., Gready J.E. Comparative analysis of structural properties of the C-type- lectin-like domain (CTLD) Proteins. 2003;52:466–477. doi: 10.1002/prot.10626. [DOI] [PubMed] [Google Scholar]
- 4.Zelensky A.N., Gready J.E. The C-type lectin-like domain superfamily. FEBS J. 2005;272:6179–6217. doi: 10.1111/j.1742-4658.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- 5.Ng W.C., Londrigan S.L., Nasr N., Cunningham A.L., Turville S., Brooks A.G., Reading P.C. The C-type lectin Langerin functions as a receptor for attachment and infectious entry of influenza A virus. J. Virol. 2016;90:206–221. doi: 10.1128/JVI.01447-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Vlist M., deWitte L., de Vries R.D., Litjens M., de Jong M.A.W.P., Fluitsma D., de Swart R.L., Geijtenbeek T.B.H. Human Langerhans cells capture measles virus through Langerin and present viral antigens to CD4+ T cells but are incapable of cross-presentation. Eur. J. Immunol. 2011;41:2619–2631. doi: 10.1002/eji.201041305. [DOI] [PubMed] [Google Scholar]
- 7.de Witte L., Nabatov A., Pion M., Fluitsma D., de Jong M.A.W.P., de Gruijl T., Piguet V., van Kooyk Y., Geijtenbeek T.B.H. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat. Med. 2007;13:367–371. doi: 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- 8.de Jong M.A., Vriend L.E., Theelen B., Taylor M.E., Fluitsma D., Boekhout T., Geijtenbeek T.B. C-type lectin Langerin is a β-glucan receptor on human Langerhans cells that recognizes opportunistic and pathogenic fungi. Mol. Immunol. 2010;47:1216–1225. doi: 10.1016/j.molimm.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunger R.E., Sieling P.A., Ochoa M.T., Sugaya M., Burdick A.E., Rea T.H., Brennan P.J., Belisle J.T., Blauvelt A., Porcelli S.A., Modlin R.L. Langerhans cells utilize CD1a and Langerin to efficiently present nonpeptide antigens to T cells. J. Clin. Invest. 2004;113:701–708. doi: 10.1172/JCI19655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Dalen R., Fuchsberger F.F., Rademacher C., van Strijp J.A., van Sorge N.M. A common genetic variation in langerin (CD207) compromises cellular uptake of Staphylococcus aureus. J. Innate Immun. 2020;12:191–200. doi: 10.1159/000500547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribeiro C.M.S., Sarrami-Forooshani R., Setiawan L.C., Zijlstra-Willems E.M., van Hamme J.L., Tigchelaar W., van der Wel N.N., Kootstra N.A., Gringhuis S.I., Geijtenbeek T.B.H. Receptor usage dictates HIV-1 restriction by human TRIM5α in dendritic cell subsets. Nature. 2016;540:448–452. doi: 10.1038/nature20567. [DOI] [PubMed] [Google Scholar]
- 12.Sorkin A., von Zastrow M. Signal transduction and endocytosis: Close encounters of many kinds. Nat. Rev. Mol. Cell Biol. 2002;3:600–614. doi: 10.1038/nrm883. [DOI] [PubMed] [Google Scholar]
- 13.Cote R., Lynn Eggink L., Kenneth Hoober J. CLEC receptors, endocytosis and calcium signaling. AIMS Allergy Immunol. 2017;1:207–231. [Google Scholar]
- 14.Onizuka T., Shimizu H., Moriwaki Y., Nakano T., Kanai S., Shimada I., Takahashi H. NMR study of ligand release from asialoglycoprotein receptor under solution conditions in early endosomes. FEBS J. 2012;279:2645–2656. doi: 10.1111/j.1742-4658.2012.08643.x. [DOI] [PubMed] [Google Scholar]
- 15.Gerasimenko J.V., Tepikin A.V., Petersen O.H., Gerasimenko O.V. Calcium uptake via endocytosis with rapid release from acidifying endosomes. Curr. Biol. 1998;8:1335–1338. doi: 10.1016/s0960-9822(07)00565-9. [DOI] [PubMed] [Google Scholar]
- 16.Feinberg H., Powlesland A.S., Taylor M.E., Weis W.I. Trimeric structure of langerin. J. Biol. Chem. 2010;285:13285–13293. doi: 10.1074/jbc.M109.086058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanske J., Aleksic S., Ballaschk M., Jurk M., Shanina E., Beerbaum M., Schmieder P., Keller B.G., Rademacher C. Intradomain allosteric network modulates calcium affinity of the C-type lectin receptor langerin. J. Am. Chem. Soc. 2016;138:12176–12186. doi: 10.1021/jacs.6b05458. [DOI] [PubMed] [Google Scholar]
- 18.Stambach N.S., Taylor M.E. Characterization of carbohydrate recognition by langerin, a C-type lectin of Langerhans cells. Glycobiology. 2003;13:401–410. doi: 10.1093/glycob/cwg045. [DOI] [PubMed] [Google Scholar]
- 19.Loeb J.A., Drickamer K. Conformational changes in the chicken receptor for endocytosis of glycoproteins. J. Biol. Chem. 1988;263:9752–9760. [PubMed] [Google Scholar]
- 20.Wragg S., Drickamer K. Identification of amino acid residues that determine pH dependence of ligand binding to the asialoglycoprotein receptor during endocytosis. J. Biol. Chem. 1999;274:35400–35406. doi: 10.1074/jbc.274.50.35400. [DOI] [PubMed] [Google Scholar]
- 21.Mullin N.P., Hall K.T., Taylor M.E. Characterization of ligand binding to a carbohydraterecognition domain of the macrophage mannose receptor. J. Biol. Chem. 1994;269:28405–28413. [PubMed] [Google Scholar]
- 22.Guo Y., Feinberg H., Conroy E., Mitchell D.A., Alvarez R., Blixt O., Taylor M.E., Weis W.I., Drickamer K. Structural basis for distinct ligand-binding and targeting properties of the receptors DC-SIGN and DC-SIGNR. Nat. Struct. Mol. Biol. 2004;11:591–598. doi: 10.1038/nsmb784. [DOI] [PubMed] [Google Scholar]
- 23.Tabarani G., Thépaut M., Stroebel D., Ebei C., Vivès C., Vachette P., Durand D., Fieschi F. DC-SIGN neck domain is a pH-sensor controlling oligomerization. SAXS and hydrodynamic studies of extracellular domain. J. Biol. Chem. 2009;284:21229–21240. doi: 10.1074/jbc.M109.021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Probert F., Mitchell D.A., Dixon A.M. NMR evidence for oligosaccharide release from the dendritic-cell specific intercellular adhesion molecule 3-grabbing non-integrin-related (CLEC4M) carbohydrate recognition domain at low pH. FEBS J. 2014;281:3739–3750. doi: 10.1111/febs.12899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell D.A., Fadden A.J., Drickamer K. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. Subunit organisation and binding to multivalent ligands. J. Biol. Chem. 2001;276:28939–28945. doi: 10.1074/jbc.M104565200. [DOI] [PubMed] [Google Scholar]
- 26.Powlesland A.S., Fisch T., Taylor M.E., Smith D.F., Tissot B., Dell A., Pöhlmann S., Drickamer K. A novel mechanism for LSECtin binding to Ebola virus surface glycoprotein through truncated glycans. J. Biol. Chem. 2008;283:593–602. doi: 10.1074/jbc.M706292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lide R.D., editor. CRC Handbook of Chemistry and Physics. 87th Ed. CRC Press; West Palm Beach, FL: 2006. [Google Scholar]
- 28.Hyland L.J., Tomaszek T.A., Meek T.D. Human immunodeficiency virus-1 protease. 2. Use of pH rate studies and solvent kinetic isotope effects to elucidate details of chemical mechanism. Biochemistry. 1991;30:8454–8463. doi: 10.1021/bi00098a024. [DOI] [PubMed] [Google Scholar]
- 29.Torbeev V.Y., Kent S.B.H. Ionization state of the catalytic dyad asp25/250 in the HIV-1 protease: NMR studies of site-specifically 13C labelled HIV-1 protease prepared by total chemical synthesis. Org. Biomol. Chem. 2012;10:5887. doi: 10.1039/c2ob25569c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toulokhonova L., Metzler W.J., Witmer M.R., Copeland R.A., Marcinkeviciene J. Kinetic studies on β-site amyloid precursor proteincleaving enzyme (BACE) J. Biol. Chem. 2003;278:4582–4589. doi: 10.1074/jbc.M210471200. [DOI] [PubMed] [Google Scholar]
- 31.Huang Y., Yue Z., Tsai C.-C., Henderson J.A., Shen J. Predicting catalytic proton donors and nucleophiles in enzymes: How adding dynamics helps elucidate the structure–function relationships. J. Phys. Chem. Lett. 2018;9:1179–1184. doi: 10.1021/acs.jpclett.8b00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamazaki T., Nicholson L.K., Torchia D.A., Wingfield P., Stahl S.J., Kaufman J.D., Eyermann C.J., Hodge N.C., Lam P.Y.S., Ru Y., Jadhav P.K., Chang C.H., Weber P.C. NMR and X-ray evidence that the HIV protease catalytic aspartyl groups are protonated in the complex formed by the protease and a non-peptide cyclic urea-based inhibitor. J. Am. Chem. Soc. 1994;116:10791–10792. [Google Scholar]
- 33.Keller B.G., Rademacher C. Allostery in C-type lectins. Curr. Opin. Struct. Biol. 2020;62:31–38. doi: 10.1016/j.sbi.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Drickamer K. Engineering galactose-binding activity into a C-type mannose-binding protein. Nature. 1992;360:183–186. doi: 10.1038/360183a0. [DOI] [PubMed] [Google Scholar]
- 35.Drickamer K., Taylor M.E. Recent insights into structures and functions of C-type lectins in the immune system. Curr. Opin. Struct. Biol. 2015;34:26–34. doi: 10.1016/j.sbi.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li L., Li C., Zhang Z., Alexov E. On the dielectric “constant” of proteins: Smooth dielectric function for macromolecular modeling and its implementation in DelPhi. J. Chem. Theory Comput. 2013;9:2126–2136. doi: 10.1021/ct400065j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jolliffe I.T. 2nd Ed. Springer; New York, NY: 2002. Principal Component Analysis. [Google Scholar]
- 38.Scherer M.K., Trendelkamp-Schroer B., Paul F., Pérez-Hernández G., Hoffmann M., Plattner N., Wehmeyer C., Prinz J.-H., Noé F. PyEMMA 2: A software package for estimation, validation, and analysis of Markov models. J. Chem. Theory Comput. 2015;11:5525–5542. doi: 10.1021/acs.jctc.5b00743. [DOI] [PubMed] [Google Scholar]
- 39.Keller B.G., Daura X., van Gunsteren W.F. Comparing geometric and kinetic cluster algorithms for molecular simulation data. J. Chem. Phys. 2010;132 doi: 10.1063/1.3301140. [DOI] [PubMed] [Google Scholar]
- 40.Lemke O., Keller B.G. Density-based cluster algorithms for the identification of core sets. J. Chem. Phys. 2016;145:164104. doi: 10.1063/1.4965440. [DOI] [PubMed] [Google Scholar]
- 41.Lemke O., Keller B.G. Common nearest neighbor clustering – a benchmark. Algorithms. 2018;11:19. [Google Scholar]
- 42.Nagy P.I., Erhardt P.W. Theoretical studies of salt-bridge formation by amino acid side chains in low and medium polarity environments. J. Phys. Chem. B. 2010;114:16436–16442. doi: 10.1021/jp103313s. [DOI] [PubMed] [Google Scholar]
- 43.Schütte C., Noé F., Lu J., Sarich M., Vanden- Eijnden E. Markov state models based on milestoning. J. Chem. Phys. 2011;134:204105. doi: 10.1063/1.3590108. [DOI] [PubMed] [Google Scholar]
- 44.Prinz J.-H., Wu H., Sarich M., Keller B., Senne M., Held M., Chodera J.D., Schütte C., Noé F. Markov models of molecular kinetics: Generation and validation. J. Chem. Phys. 2011;134:1–23. doi: 10.1063/1.3565032. [DOI] [PubMed] [Google Scholar]
- 45.Prinz J.-H., Keller B., Noé F. Probing molecular kinetics with Markov models: Metastable states, transition pathways and spectroscopic observables. Phys. Chem. Chem. Phys. 2011;13:16912. doi: 10.1039/c1cp21258c. [DOI] [PubMed] [Google Scholar]
- 46.Schwantes C.R., Pande V.S. Improvements in Markov state model construction reveal many non-native interactions in the folding of NTL9. J. Chem. Theory Comput. 2013;9:2000–2009. doi: 10.1021/ct300878a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pérez-Hernández G., Paul F., Giorgino T., De Fabritiis G., Noé F. Identification of slow molecular order parameters for Markov model construction. J. Chem. Phys. 2013;139:15102. doi: 10.1063/1.4811489. [DOI] [PubMed] [Google Scholar]
- 48.Izrailev S., Stepaniants S., Isralewitz B., Kosztin D., Lu H., Molnar F., Wriggers W., Schulten K. In: Computational Molecular Dynamics: Challenges, Methods, Ideas. Deuflhard P., Hermans J., Leimkuhler B., Mark A.E., Reich S., Skeel R.D., editors. Springer-Verlag; Berlin: 1998. pp. 39–65. [Google Scholar]
- 49.Dudko O.K., Hummer G., Szabo A. Theory, analysis, and interpretation of single-molecule force spectroscopy experiments. Proc. Natl. Acad. Sci. U. S. A. 2008;105:15755–15760. doi: 10.1073/pnas.0806085105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rico F., Russek A., González L., Grubmüller H., Scheuring S. Heterogeneous and ratedependent streptavidin–biotin unbinding revealed by high-speed force spectroscopy and atomistic simulations. Proc. Natl. Acad. Sci. U. S. A. 2019;116:6594–6601. doi: 10.1073/pnas.1816909116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng F., Shen J., Luo X., Jiang H., Chen K. Steered molecular dynamics simulations on the “tail helix latch” hypothesis in the gelsolin activation process. Biophys. J. 2002;83:753–762. doi: 10.1016/S0006-3495(02)75206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guzmán D.L., Roland J.T., Keer H., Kong Y.P., Ritz T., Yee A., Guan Z. Using steered molecular dynamics simulations and single-molecule force spectroscopy to guide the rational design of biomimetic modular polymeric materials. Polymer. 2008;49:3892–3901. doi: 10.1016/j.polymer.2008.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nielbo S., Thomsen J.K., Graversen J.H., Jensen P.H., Etzerodt M., Poulsen F.M., Thøgersen H.C. Structure of the plasminogen kringle 4 binding calcium-free form of the C-type lectin-like domain of tetranectin. Biochemistry. 2004;43:8636–8643. doi: 10.1021/bi049570s. [DOI] [PubMed] [Google Scholar]
- 54.Poget S.F., Freund S.M.V.V., Howard M.J., Bycroft M. The ligand-binding loops in the tunicate C-type lectin TC14 are rigid. Biochemistry. 2001;40:10966–10972. doi: 10.1021/bi002698z. [DOI] [PubMed] [Google Scholar]
- 55.Ng K.K.S., Park-Snyder S., Weis W.I. Ca2+-dependent structural changes in C-type mannosebinding proteins. Biochemistry. 1998;37:17965–17976. doi: 10.1021/bi981972a. [DOI] [PubMed] [Google Scholar]
- 56.Kim M.O., Blachly P.G., McCammon J.A. Conformational dynamics and binding free energies of inhibitors of BACE-1: From the perspective of protonation equilibria. PLoS Comput. Biol. 2015;11:1–28. doi: 10.1371/journal.pcbi.1004341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pace C.N., Grimsley G.R., Scholtz J.M. Protein ionizable groups: pK values and their contribution to protein stability and solubility. J. Biol. Chem. 2009;284:13285–13289. doi: 10.1074/jbc.R800080200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Søndergaard C.R., Olsson M.H.M., Rostkowski M., Jensen J.H. Improved treatment of ligands and coupling effects in empirical calculation and rationalization of pKa values. J. Chem. Theory Comput. 2011;7:2284–2295. doi: 10.1021/ct200133y. [DOI] [PubMed] [Google Scholar]
- 59.Olsson M.H.M., Søndergaard C.R., Rostkowski M., Jensen J.H. PROPKA3: Consistent treatment of internal and surface residues in empirical pKa predictions. J. Chem. Theory Comput. 2011;7:525–537. doi: 10.1021/ct100578z. [DOI] [PubMed] [Google Scholar]
- 60.Khandogin J., Brooks C.L. Constant pH molecular dynamics with proton tautomerism. Biophys. J. 2005;89:141–157. doi: 10.1529/biophysj.105.061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee J., Miller B.T., Damjanovic A., Brooks B.R. Enhancing constant-pH simulation in explicit solvent with a two-dimensional replica exchange method. J. Chem. Theory Comput. 2015;11:2560–2574. doi: 10.1021/ct501101f. [DOI] [PubMed] [Google Scholar]
- 62.Radak B.K., Chipot C., Suh D., Jo S., Jiang W., Phillips J.C., Schulten K., Roux B. Constant-pH molecular dynamics simulations for large biomolecular systems. J. Chem. Theory Comput. 2017;13:5933–5944. doi: 10.1021/acs.jctc.7b00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paasche A., Schirmeister T., Engels B. Benchmark study for the cysteine–histidine proton transfer reaction in a protein environment: Gas phase, COSMO, QM/MM approaches. J. Chem. Theory Comput. 2013;9:1765–1777. doi: 10.1021/ct301082y. [DOI] [PubMed] [Google Scholar]
- 64.Duster A.W., Lin H. Tracking proton transfer through titratable amino acid side chains in adaptive QM/MM simulations. J. Chem. Theory Comput. 2019;15:5794–5809. doi: 10.1021/acs.jctc.9b00649. [DOI] [PubMed] [Google Scholar]
- 65.Jonker C.T.H., Deo C., Zager P.J., Tkachuk A.N., Weinstein A.M., Rodriguez-Boulan E., Lavis L.D., Schreiner R. Accurate measurement of fast endocytic recycling kinetics in real time. J. Cell Sci. 2020;133 doi: 10.1242/jcs.231225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoo J., Wilson J., Aksimentiev A. Improved model of hydrated calcium ion for molecular dynamics simulations using classical biomolecular force fields. Biopolymers. 2016;105:752–763. doi: 10.1002/bip.22868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Timr S., Kadlec J., Srb P., Ollila O.H.S., Jungwirth P. Calcium sensing by recoverin: Effect of protein conformation on ion affinity. J. Phys. Chem. Lett. 2018;9:1613–1619. doi: 10.1021/acs.jpclett.8b00495. [DOI] [PubMed] [Google Scholar]
- 68.Saxena A., Sept D. Multisite ion models that improve coordination and free energy calculations in molecular dynamics simulations. J. Chem. Theory Comput. 2013;9:3538–3542. doi: 10.1021/ct400177g. [DOI] [PubMed] [Google Scholar]
- 69.Jing Z., Liu C., Cheng S.Y., Qi R., Walker B.D., Piquemal J.-P., Ren P. Polarizable force fields for biomolecular simulations: Recent advances and applications. Annu. Rev. Biophys. 2019;48:371–394. doi: 10.1146/annurev-biophys-070317-033349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berendsen H.J.C., van der Spoel D., vanDrunen R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 1995;91:43–56. [Google Scholar]
- 71.Lindahl E., Hess B., van der Spoel D. GROMACS 3.0: A package for molecular simulation and trajectory analysis. J. Mol. Model. 2001;7:306–317. [Google Scholar]
- 72.van der Spoel D., Lindahl E., Hess B., Groenhof G., Mark A.E., Berendsen H.J. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005;26:1701–1718. doi: 10.1002/jcc.20291. [DOI] [PubMed] [Google Scholar]
- 73.Hess B., Kutzner C., van der Spoel D., Lindahl E. GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 2008;4:435–447. doi: 10.1021/ct700301q. [DOI] [PubMed] [Google Scholar]
- 74.Pronk S., Páll S., Schulz R., Larsson P., Bjelkmar P., Apostolov R., Shirts M.R., Smith J.C., Kasson P.M., van der Spoel D., Hess B., Lindahl E. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics. 2013;29:845–854. doi: 10.1093/bioinformatics/btt055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Páll S., Abraham M.J., Kutzner C., Hess B., Lindahl E. Tackling Exascale software challenges in molecular dynamics simulations with GROMACS. In: Markidis S., Laure E., editors. Solving Software Challenges for Exascale. EASC 2014. Lecture Notes in Computer Science, vol 8759. Springer; Cham: 2015. [Google Scholar]
- 76.Abraham M.J., Murtola T., Schulz R., Páll S., Smith J.C., Hess B., Lindahl E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1-2:19–25. [Google Scholar]
- 77.Lindorff-Larsen K., Piana S., Palmo K., Maragakis P., Klepeis J.L., Dror R.O., Shaw D.E. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins. 2010;78:1950–1958. doi: 10.1002/prot.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jorgensen W.L., Chandrasekhar J., Madura J.D., Impey R.W., Klein M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983;79:926–935. [Google Scholar]
- 79.Feinberg H., Taylor M.E., Razi N., McBride R., Knirel Y.A., Graham S.A., Drickamer K., Weis W.I. Structural basis for langerin recognition of diverse pathogen and mammalian glycans through a single binding site. J. Mol. Biol. 2011;405:1027–1039. doi: 10.1016/j.jmb.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bussi G., Donadio D., Parrinello M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007;126 doi: 10.1063/1.2408420. [DOI] [PubMed] [Google Scholar]
- 81.Parrinello M., Rahman A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phy. 1981;52:7182–7190. [Google Scholar]
- 82.Hess B. P-LINCS: A parallel linear constraint solver for molecular simulation. J. Chem. Theory Comput. 2008;4:116–122. doi: 10.1021/ct700200b. [DOI] [PubMed] [Google Scholar]
- 83.van Gunsteren W.F., Berendsen H.J.C. A leap-frog algorithm for stochastic dynamics. Mol. Simulat. 1988;1:173–185. [Google Scholar]
- 84.Essmann U., Perera L., Berkowitz M.L., Darden T., Lee H., Pedersen L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995;103:8577–8593. [Google Scholar]
- 85.Blomberg F., Maurer W., Rüterjans H. Nuclear magnetic resonance investigation of 15N-labeled histidine in aqueous solution. J. Am. Chem. Soc. 1977;99:8149–8159. doi: 10.1021/ja00467a005. [DOI] [PubMed] [Google Scholar]
- 86.Hass M.A.S., Hansen D.F., Christensen H.E.M., Led J.J., Kay L.E. Characterization of conformational exchange of a histidine side chain: Protonation, rotamerization, and tautomerization of His61 in plastocyanin from Anabaena variabilis. J. Am. Chem. Soc. 2008;130:8460–8470. doi: 10.1021/ja801330h. [DOI] [PubMed] [Google Scholar]
- 87.Hansen A.L., Kay L.E. Measurement of histidine pKa values and tautomer populations in invisible protein states. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E1705–E1712. doi: 10.1073/pnas.1400577111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Humphrey W., Dalke A., Schulten K. VMD: Visual molecular dynamics. J. Mol. Graphics. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 89.Berendsen H.J.C., Postma J.P.M., van Gunsteren W.F., DiNola A., Haak J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984;81:3684–3690. [Google Scholar]
- 90.Dogan T., MacDougall A., Saidi R., Poggioli D., Bateman A., O’Donovan C., Martin M.J. UniProt-DAAC: domain architecture alignment and classification, a new method for automatic functional annotation in UniProtKB. Bioinformatics. 2016;32:2264–2271. doi: 10.1093/bioinformatics/btw114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. All remaining data are contained within the article and its supporting information. The software used for common-nearest-neighbor clustering and core-set Markov-state model estimation is publicly available on GitHub (https://github.com/janjoswig/CommonNNClustering).