Abstract
Background
Prostate cancer (PCa) progression depends on androgen receptor activity. Cholesterol is required for biosynthesis of all steroid hormones, including androgens. Impact of cholesterol-lowering statins on androgens is unknown. We explored atorvastatin influence on serum and prostatic tissue steroidomic profiles (SP) to expose novel pathways for limiting androgen concentration in men with PCa.
Methods
This is a pre-planned post hoc analysis of ESTO-1 pilot randomised, double-blinded, clinical trial. Statin naïve men, scheduled for radical prostatectomy due to localised PCa, were randomised 1:1 to use daily 80 mg of atorvastatin or placebo before the surgery for a median of 28 days. Participants were recruited and treated at the Pirkanmaa Hospital District, Tampere, Finland. 108 of the 158 recruited men were included in the analysis based on sample availability for hormone profiling. Serum and prostatic tissue steroid profiles were determined using liquid chromatography mass spectrometry. Wilcoxon rank sum test and bootstrap confidence intervals (CI) were used to analyse the difference between placebo and atorvastatin arms.
Findings
Most serum and prostatic steroids, including testosterone and dihydrotestosterone, were not associated with atorvastatin use. However, atorvastatin use induced serum SP changes in 11-ketoandrostenedione (placebo 960pM, atorvastatin 617.5pM, p-value <0.0001, median difference -342.5; 95% CI -505.23 – -188.98). In the prostatic tissue, atorvastatin was associated with plausible downshift in 11- ketodihydrotestosterone (placebo 25.0pM in 100 mg tissue/1 mL saline, atorvastatin 18.5pM in 100 mg tissue/1 mL saline, p-value 0.027, median difference -6.53; 95% CI -12.8 – -0.29); however, this association diminished after adjusting for multiple testing. No serious harms were reported.
Interpretation
Atorvastatin was associated with adrenal androgen downshift in the serum and possibly in the prostate. The finding warrants further investigation whether atorvastatin could improve androgen deprivation therapy efficacy.
Funding
Funded by grants from the Finnish Cultural Foundation, Finnish Cancer Society, Academy of Finland, and the Expert Responsibility Area of the Tampere University Hospital.
Clinicaltrials.gov identifier
Keywords: Prostate cancer, Serum adrenal androgens, Prostatic tissue adrenal androgens, Statins, Clinical trial
Research in Context.
Evidence before this study
Prostate cancer cellular proliferation is driven by androgen steroids. Cholesterol, which is the precursor for all steroids, lowering statin use has been associated with improved prostate cancer survival, but the mechanism is still unclear. Moreover, statin use has been associated with improved efficacy of androgen deprivation therapy (a common therapy in advanced prostate cancer management) than androgen therapy alone.
Added value of this study
This clinical trial investigated the influence of atorvastatin to serum and prostatic tissue steroids. Atorvastatin lowers serum adrenosterone concentration significantly, which is produced in the adrenal. Adrenosterone itself is not a potent androgen but is metabolised into ketotestosterone and ketodihydrotestosterone which both are potent androgens, similar to the common androgens testosterone and dihydrotestosterone. Moreover, atorvastatin was associated with lower ketodihydrotestosterone concentration in prostatic tissue, compared to placebo; however, this association was not robust when adjusted for multiple comparison.
Implications of all the available evidence
Implications of all the available evidence: These findings suggest that the underlying biological mechanism between improved prostate cancer survival and atorvastatin use may be partly mediated by adrenal androgens. Moreover, it may partly explain the association between atorvastatin use and improved efficacy of androgen deprivation therapy. The finding warrants for further investigation and confirmation of these results.
Alt-text: Unlabelled box
Introduction
In 2018 the global prostate cancer (PCa) death toll was 358,000 and new diagnosed cases reached 1.2 million [1], making PCa the second most common cancer in men, superseded only by lung cancer [1,2]. Roughly 15–20% of the diagnosed PCa cases are high-risk, potentially fatal PCa cancers [3,4]. PCa cells are known to exploit increased androgen hormone intake to support cellular proliferation. This is facilitated by androgen receptors (AR) [5]. Treatment of advanced PCa includes systemic androgen deprivation therapy (ADT), leading to castrate testosterone level due to inhibition of testicular androgen production [6].
Cholesterol is a precursor for steroid hormones including androgens, the drivers of PCa progression [7]. Statins are a commonly used class of cholesterol-lowering drugs which lower blood cholesterol level by inhibiting cholesterol-producing mevalonate pathway in the liver [8]. However, mevalonate pathway is active also in the prostate [9]. In ESTO1 clinical trial atorvastatin induced lipidomic changes in the serum and was associated with prostatic tissue lipidome compared to placebo [10]. It is unknown whether this is also reflected in steroid hormone production. Statin use has been associated with improved PCa survival compared to non-users [11]. Moreover, combination of statin use, and ADT have been observed to limit PCa progression longer than ADT alone [12]. Therefore, statin use may be beneficial in PCa treatments, especially in the context of ADT.
Concentrations of main AR agonists testosterone (T) and dihydrotestosterone (DHT) have not been observed to decrease in the serum after statin use in epidemiological studies [13,14], while post hoc analyses of clinical trials suggest a slight decrease in T [15]. Moreover, to our knowledge, the impact of statins on intraprostatic steroid hormone profiles, including T and DHT concentrations, has not been studied before. It is unclear whether statin use could affect hormone profiles similarly in the serum and prostatic tissue. This is a pre-planned post hoc analysis of ESTO1 clinical trial which was a piloting hypothesis generating study. As a first of a kind study, compelling power calculation based on existing research was impossible to calculate in ESTO1. Although the main analysis result of ESTO1 was negative, some subgroup analyses were positive. In this pre-planned post hoc analysis, we comprehensively explore the impact of atorvastatin on the serum and on the prostatic tissue steroid hormone profiles, with the a priori hypothesis that atorvastatin changes steroid hormone levels compared to placebo in both.
Methods
Prospective randomised, double-blind, placebo-controlled, clinical trial ESTO-1 was started in 2012. The participants were recruited at urology outpatient clinic of the Pirkanmaa Hospital District, Tampere, Finland between 2012 and 2016. The study is registered at EudraCT (22/05/2012, registration number: 2011–005,438–20) and clinicaltrials.gov (01/04/2013, identifier: NCT01821404).
Ethical statement
The study was performed in accordance with Declaration of Helsinki and good clinical practice. An informed consent was obtained from all the participants in this study. ESTO-1 was approved by the ethics committee of the Pirkanmaa Hospital District (decision number ETL R03230).
Sample size and randomization
Due to this being first-in-man randomized study comparing atorvastatin in placebo amongst PCa patients the sample size calculations were based on Ki-76 differences observed in cell culture models after statin treatment. In the ESTO1, the target sample size of 160 men was based on statistical power 0.80 (α=0.05) to detect 12% difference in prostatic tissue Ki-67 levels and 13% difference in serum prostate specific antigen (PSA), with assumed 10% dropout rate [16] (Supplementary file 1). This pilot post hoc analysis of atorvastatin affecting serum and tissue steroidomic profile is exploratory in nature; therefore, formal sample size for these outcomes were not assessed. Randomization was done by hospital pharmacy of Tampere University Hospital which produced the blinded study drug capsules. All participants, study physicians, pathologists, and researchers evaluating the outcomes remained blinded to the allocation until all data had been collected. After data collection was completed, allocation concealment was removed by matching the patient research IDs with randomization list obtained from the hospital pharmacy.
Participants
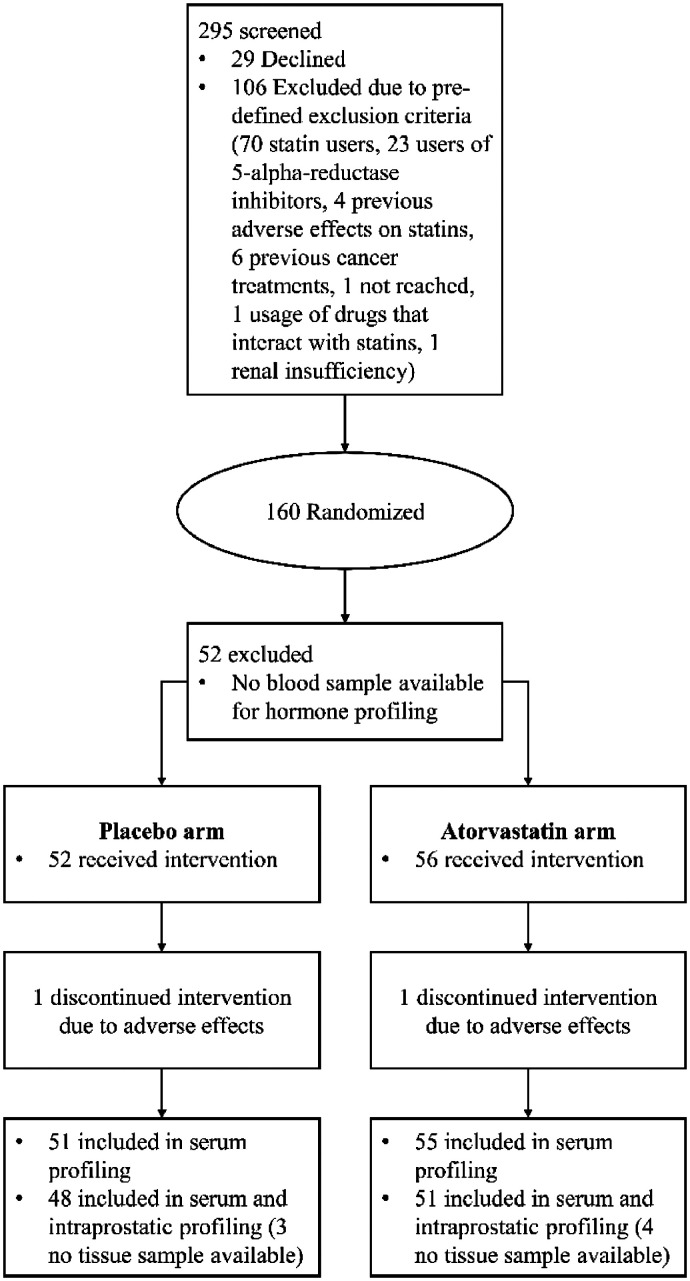
ESTO-1 recruited 160 statin-naive Finnish men diagnosed with PCa who were scheduled for radical prostatectomy for localised PCa. Participants were randomised 1:1 to use either 80 mg atorvastatin or placebo daily [16]. In total, 158 men completed the study. The 108 men who had blood sample available before and after the intervention for steroidomic assessment were included into pre-planned post hoc analysis of steroid hormone profile changes induced by statin use. Out of the 108 men, 99 had prostate tissue sample available for tissue steroid profile assessment. Fig. 1 displays the participant and randomisation scheme as a flowchart (Fig. 1). ESTO1 clinical trial was a pilot hypothesis generating study therefore compelling power calculation for the sample size was impossible to calculate based on existing research.
Fig. 1.
Flowchart of the patient recruitment, randomization, and allocation scheme.
Study design and setting
Participants were randomised with 1:1 ratio to use daily 80 mg dose of atorvastatin or placebo from recruitment until radical prostatectomy. The median intervention time was 28 (IQR 14.5) days. No minimum exposure time was set as the ethics committee of the Pirkanmaa Hospital District decreed that study participation must not delay cancer treatment. Allocation concealment was ensured by randomising and blinding the equal looking drug capsules at manufacturing site and containing them in equal looking boxes. Each box was assigned with a rolling ID number from 1 to 160 before distributing the drugs to the patients. Intervention compliance was monitored by counting of left-over drug capsules at the time of prostatectomy showing overall compliance of 96% [16]. No one in the placebo arm reported post-randomisation statin use when queried. An unblinded interim analysis was done once 60 patients were recruited and statistical significance (p < 0.05) of the difference was tested but not achieved; the clinical trial would have been terminated otherwise. The trial ended once all the participants were analysed, as planned. Due to exploratory pilot nature of the study no bias adjustments nor adjustment on confidence level in terms of data accumulation, i.e., unblinding, or any other, concerning the interim analysis were made. This is a pre-planned post hoc analysis of ESTO1 clinical trial and no changes to design or methods or the trial were done for this analysis. Operational bias was eliminated by blinding of a study allocation both for the physicians taking care of patients and researchers who evaluated the study outcomes. No adaptation decisions to study protocol or analysis were made during the trial. Full trial protocol is available as Supplementary file 1.
Serum and prostatic tissue steroidomic profile assessment
Serum and prostatic steroid profiles were quantitated with validated liquid chromatography tandem mass spectrometry (LC-MS/MS) method as described earlier [17]. In short, 50µL serum or 150µL tissue homogenate (15 mg tissue/150µL saline) were spiked with isotope-labelled steroids as internal standards; next, the steroids were extracted with toluene and derivatised with hydroxylamine prior to simultaneous LC-MS/MS analysis of all the steroids using Agilent 1290 UHPLC and Agilent 6495 QQQ. All serum steroid concentrations are continuous variables within the measurable (technical) range and the unit is pM. Some prostatic steroids are outside the measurable range showing min or max values for all the participants; therefore, these steroids are not included in the analysis as the variance is zero. The unit for prostatic steroid concentration is pM in homogenate (100 mg tissue / 1 mL saline).
Statistical analyses
For confirmatory analysis we used Wilcoxon rank sum test since the underlying distribution is non-normal. Type 1 error was controlled by adjusting the p-values for multiple hypothesis testing using Benjamini-Hochberg method (false discovery rate). The locations were estimated using median and scatter by using interquartile range (IQR), reported as interquartiles. Median differences in serum / prostatic steroid concentrations (atorvastatin – placebo) were calculated and 95% bootstrap confidence intervals (CI) for the median differences were estimated using 1000 bootstrap samples with replacement.
Exploratory analysis for internal validation was conducted by using random forest classification (RFC). RFC is a non-parametric supervised learning method based on classification and regression tree (CART) [18]. The RFC method is well established and studied although sparsely used in clinical trials. In RFC, multiple CARTs are grown by using bootstrap sample of the original sample , where i is the i:th bootstrap sample, n is the sample size, and p is the number of features (steroids in our case). At each splitting node √p features are randomly selected from the p features and are used one-by-one to minimise Gini impurity function; the one minimising the Gini impurity is used to split the sample into two sub samples. The trees are either allowed to fully grow or by defining minimum node size. Typically, 100 to 500 trees are grown in one random forest model. RFC classification performance is estimated by calculating so-called out-of-bag (O—O-B) error. In short, when a tree is grown by using bootstrap sample, the observations that were not in that bootstrap sample are propagated through the decision tree, i.e., predicted; an observation is misclassified if it ends into any of the wrong terminal nodes and is correctly classified if it ends into correct terminal node. Finally, the trees are averaged by a majority vote; each tree where the observation was not in the bootstrap sample casts one class vote for that observation. The interpretation for the O—O-B error is roughly such that: 50% represents a model as good as a coin-flip, 40 – 49% the model is slightly better than a coin-flip, 20 – 30% the model is good, 10 – 20% the model is excellent. Moreover, RFC can also be used in an unsupervised fashion by calculating so-called proximity matrix. Proximity is defined as: if two observations share a terminal node in a tree, their proximity is 1, and zero otherwise. The proximities are accumulated over all trees in the model. Essentially, the proximity is a distance measure between two points, like Euclidian distance is a distance measure. The proximities can be used for 2-dimensional cluster visualisation by applying multidimensional scaling (very similar to principal component transformation) to the proximity matrix [19].
RFC can capture non-linear and complex relationships due to the nature of decision trees. In RFC, a single tree can be over-fitted which is countered by taking the average over all bootstrapped trees. When signal-to-noise ratio is poor, RFC can perform poorly since the probability that a signal feature gets selected in a split gets lower as the number of noise features increase.
We used separate RFC models for serum steroidomic profile before and after, and intraprostatic tissue steroidomic profile after. All models were set to grow 500 trees and to use √p (rounded into nearest integer value) features at each tree branching where p is the total number of classifiers. To counter the random forest Monte Carlo error, inherent to RFC, an estimate for the classification error rate and Monte Carlo confidence intervals were obtained by repeating each RFC model 1000 times, followed by calculating the median, and finding the upper and lower 95% confidence intervals using the percentile method [20]. Moreover, we applied backward feature selection when needed to counter the poor signal-to-noise ratio (all models are reported for transparency). The O—O-B error rates were calculated for each model. In addition, proximity plots were generated to visualise the classification performance and in-class similarity of the study arms of each RFC model.
Wilcoxon rank sum test and RFC have different mathematical assumptions, therefore if the results from these two modelling strategies are similar, it would make a stronger case for the results than either method alone.
All statistical analyses were conducted using R (version 4.0.4). Random forest was implemented with R package ‘randomForest’ (version 4.6–14).
Role of funding source
Finnish Cultural Foundation, Finnish Cancer Society, Academy of Finland, and the Expert Responsibility Area of the Tampere University Hospital provided only financial support and did not interfere nor participate with the study in any other fashion.
Results
The key background and clinical factors are divided rather equally between the randomised study arms. The atorvastatin arm includes more smokers compared to the placebo arm. Distribution of background and key clinical characteristics are shown in Table 1. There were only 4 CTCAE 4.0 grade 2 adverse reactions, all in the atorvastatin arm. Grade 1 adverse reaction were distributed similarly between the study arms. They were not associated with any of the outcomes. Baseline serum steroid concentrations are shown in Supplementary file 2, Table 1. Background characteristics table of the full ESTO1 clinical trial population is displayed in Supplementary file 2, Table 2.
Table 1.
Patient characteristic, tumour characteristic, and background variable distribution table. For continuous variables, median and interquartiles (IQ) are reported. For categorical variables, number and percentage of patients are reported. 1Proportion of study drug doses used from the target amount given the duration of intervention. 2One man from both study arms suspended the study early.
| Continuous, median (IQ) | Placebo (n = 52) | Atorvastatin (n = 56) |
|---|---|---|
| Age at recruitment, years | 64.5 (58–68) | 64.5 (59–68) |
| Intervention time, days | 27 (20.5–36) | 28 (22.5–35) |
| BMI, kg/m2 | 26.4 (24.6 – 28.7) | 26.1 (24.4 – 29.2) |
| PSA, ng/mL | 7.6 (5.8–10) | 8.4 (5.7–12) |
| %Used / Target capsules1 | 97.00 (89.7–100) | 97.64 (90–100) |
| Categorical, n (%) | ||
| Smoking | ||
| - Non smoker | 43 (84.3) | 42 (75.0) |
| - Regular smoker | 5 (9.8) | 11 (19.6) |
| - Occasional smoker | 3 (5.9) | 2 (3.6) |
| - Previous smoker | 0 (0) | 1 (1.8) |
| Pathological Gleason grade | ||
| - 5 | 1 (2.0) | 0 (0) |
| - 6 | 9 (17.7) | 12 (21.4) |
| - 7 | 35 (68.6) | 40 (71.4) |
| - 8 | 3 (5.9) | 1 (1.8) |
| - 9 | 3 (5.9) | 3 (5.4) |
| Pathological T-stage | ||
| - N/A | 1 (1.9) | 0 (0) |
| - T2a – T2c | 28 (53.8) | 30 (53.6) |
| - T3a, or higher | 23 (44.2) | 26 (46.4) |
| Diabetes | ||
| - No | 45 (88.2) | 52 (92.9) |
| - Yes | 6 (11.8) | 4 (7.1) |
| Hypertension | ||
| - No | 33 (64.7) | 35 (62.5) |
| - Yes | 18 (35.3) | 21 (37.5) |
| Completed study2 | ||
| - Yes | 51 (98.1) | 55 (98.2) |
| - No | 1 (1.9) | 1 (1.8) |
| Sex | ||
| - Male | 52 | 56 |
| Ethnicity | ||
| - Finnish | 52 | 56 |
Table 2.
Median (interquartiles), Wilcoxon rank-sum test p-value, median difference (atorvastatin – placebo), and 95% bootstrap confidence intervals for median difference. The concentration units are pM for serum and pM in homogenate (100 mg tissue/1 mL saline) for prostatic tissue steroid hormone profile. Cortisol and 11KA4 levels have decreased after atorvastatin intervention in the atorvastatin arm (n = 56) compared to placebo (n = 52), and the difference is statistically significant. After adjusting the Wilcoxon rank-sum test p-values for multiple comparison, the adjusted p-value for the 11KA4 difference between the study arms is 0.001. For prostatic tissue hormone profile, the 11KDHT, Estrone, and DHEA are significantly differing between the study arms (atorvastatin n = 51, placebo n = 48). After adjusting the p-values for multiple comparison all p-values inflate above significance level 0.05. In the serum, the median difference of 11KA4 is significantly differing between the study arms (−342.5, 95% bootstrap CI-505.23 −188.98). In the prostatic tissue, the median difference of 11KDHT is significantly differing between the study arms (−6.53, 95% bootstrap CI −12.80 −0.29).
| Serum hormone | Placebo, median (Q1 – Q3), n = 52 | Atorvastatin, median (Q1 – Q3), n = 56 | p-value | Median diff. | 95% bootstrap CI for median difference. | |||
|---|---|---|---|---|---|---|---|---|
| Cortisol | 333,333.5 (267,289.25–420,965.5) | 291,364.5 (234,340–358,946.75) | 0.035 | −41,969 | −86,983.9 | 8754.69 | ||
| Cortisone | 61,173 (53,556–67,695) | 57,485 (50,435.75–68,475.25) | 0.202 | −3688 | −9014.5 | 1254.43 | ||
| 11KA4 | 960 (715.75–1254.25) | 617.5 (506.5–825) | <0.0001 | −342.5 | −505.23 | −188.98 | ||
| 11KT | 1275.5 (826.25–1852.25) | 1214.5 (829–1546.5) | 0.324 | −61 | −456.66 | 264.04 | ||
| 11OHT | 567 (444.75–800) | 573.5 (444–728.25) | 0.515 | 6.5 | −173 | 133.64 | ||
| 11bOH A4 | 6043 (4073–8225.75) | 5292.5 (4098–6489.5) | 0.187 | −750.5 | −2033 | 360.08 | ||
| Estrone | 142.5 (109–189.5) | 129 (104–160) | 0.108 | −13.6 | −44.03 | 15.01 | ||
| 11DOX | 849 (547.5–1372) | 903 (563.25–1185.75) | 0.963 | −221.33 | 260.03 | |||
| 17OH Pregne | 2815 (1928.25–4232.75) | 2121.5 (1567–3472.25) | 0.053 | −693.5 | −1656.03 | 168.71 | ||
| DHEA | 9862.5 (6098.75–11,945.25) | 7479 (5098.25–10,040.75) | 0.079 | −2383 | −4388 | 326 | ||
| Androstenedione | 2794.5 (1971.5–3702.5) | 2598.5 (1949.25–3487) | 0.322 | −196 | −620.09 | 219.01 | ||
| Testo | 15,058.5 (12,481.75–20,652.25) | 15,613 (11,116.5–20,581.25) | 0.742 | 554.5 | −2877.69 | 2981 | ||
| DHT | 1252.5 (923–1648.25) | 1248.5 (815.25–1720.5) | 0.717 | −4 | −344.01 | 363 | ||
| Androstanedione | 212.5 (134.5–259) | 166 (114.75–231.75) | 0.066 | −46.5 | −83.013 | 20.5 | ||
| 17OH Proge | 2430 (1579–3889.25) | 2076.5 (1627–2738) | 0.19 | −353.5 | −982.13 | 358 | ||
| Pregnenolone | 2232.5 (1514.75–2852.75) | 1746 (1240–2689.75) | 0.062 | −486.5 | −863.08 | 38.2 | ||
| Progesterone | 168 (127.25–287.25) | 163.5 (112.75–204) | 0.116 | −4.5 | −47.5 | 28 | ||
| Prostatic tissue hormone | Placebo, median (Q1 – Q3), n = 48 | Atorvastatin, median (Q1 – Q3), n = 51 | p-value | Median diff. | 95% bootstrap CI for median difference | |||
| Cortisol | 1218.775 (603.0525–2476.285) | 1605 (791.42–2561.97) | 0.204 | 386.23 | −182.19 | 868.96 | ||
| Cortisone | 225 (118.1375–437.7025) | 280.25 (168.3–493.73) | 0.196 | 55.25 | −38.11 | 191.60 | ||
| 11KDHT | 25.015 (19.1–34.3125) | 18.49 (13.1–26.82) | 0.027 | −6.53 | −12.80 | −0.29 | ||
| Estrone | 10.62 (5.7525–13.7075) | 12.08 (9.17–18.69) | 0.044 | 1.46 | −0.88 | 5.43 | ||
| 17OH Pregne | 66.065 (49.155–106.5875) | 68.77 (47.56–112.175) | 0.703 | 2.71 | −14.54 | 27.12 | ||
| DHEA | 2243.835 (1151.7075–3240.335) | 3123.46 (1772.325–5043.845) | 0.037 | 879.63 | −20.65 | 1622.79 | ||
| Androstenedione | 11.325 (7.22–16.565) | 11.08 (7.635–15.275) | 0.966 | −0.245 | −3.28 | 3.06 | ||
| Testo | 10.08 (8.075–19.6925) | 13.37 (7.72–31.145) | 0.171 | 3.29 | −0.29 | 8.24 | ||
| DHT | 824.325 (677.6175–1050.8925) | 864.02 (684.585–1047.76) | 0.958 | 39.70 | −137.01 | 151.21 | ||
| AndrostAnedione | 77.55 (59.02–106.965) | 78 (58.585–113.94) | 0.853 | 0.45 | −21.57 | 17.12 | ||
| Pregnenolone | 394.76 (268.285–548.795) | 444.5 (273.615–699.67) | 0.350 | 49.74 | −50.00 | 160.87 | ||
Analysing the serum steroid hormone change by location and scatter, the 11-ketoandrostenedione (11KA4) and Cortisol levels have decreased by 35.6% and 12.5% in the atorvastatin arm, respectively. The 11KA4 difference between the study arms is statistically significant (Wilcoxon rank-sum test p-value <0.0001, median difference −324.5, 95% bootstrap CI −505.23 −188.98) (Table 2). Adjusting the p-values for multiple comparison by Benjamini-Hochberg method, 11KA4 difference between the treatment arms retain the statistical significance (Wilcoxon rank-sum test p-value 0.001). Cortisol level difference between the study arms is statistically significant (Wilcoxon rank-sum test p-value 0.035), but top and bottom 95% bootstrap confidence interval have different signs (median difference −41,969, 95% bootstrap CI −86,983.9 – 8754.69) (Table 2). Moreover, Cortisol levels are changing naturally during the day by circadian rhythm thus the difference might also be due to patient sampling time which was not standardised in this trial. This might have induced non-differential misclassification of cortisol values. 17-OH-pregnenolone, Pregnenolone, DHEA, and Androstenedione display slightly lower concentrations in the atorvastatin arm with borderline statistically significant difference by Wilcoxon rank-sum test (Table 2), which is lost after controlling for false discoveries. No changes in common androgens T or DHT were observed by Wilcoxon rank-sum test (Table 2). Boxplots showing the serum steroid concentration distributions by study arm are shown in Supplementary file 2, Figs. 3 to 36.
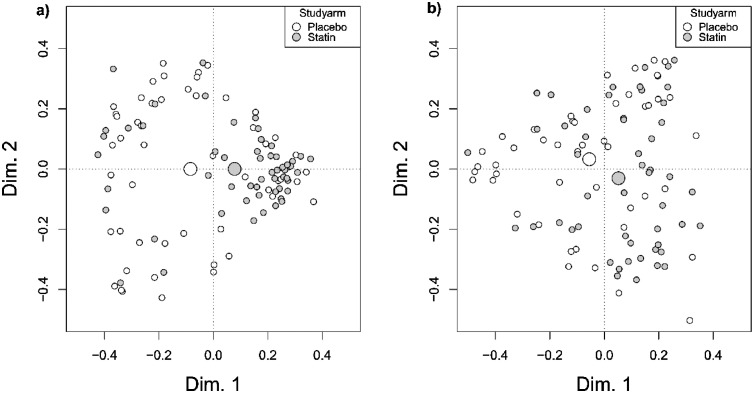
Fig. 3.
Random forest proximity plots for the serum and prostatic tissue hormone profiles. Grey dots represent men who received atorvastatin intervention and white dots represent patients who received placebo. The large grey and white dots are the mean centroids of atorvastatin and placebo arms, respectively. More densely clustered patients demonstrate similar within-group hormone profiles. Fig. 3a) serum profile after intervention shows densely clustered atorvastatin (n = 56) users indicating similarity in their hormone profiles whereas placebo (n = 52) users are randomly scattered indicating that no harmonic changes occurred in placebo arm. Fig. 3b) prostatic tissue profile after intervention does not show as clear clustering amongst atorvastatin (n = 51) users compared to serum hormone profiles, and no clustering of placebo arm (n = 48). The mean centroids are separated which indicates overall difference between the study arms.
For prostatic tissue hormone profile, the 11-ketodihydrotestosterone (11KDHT) concentration are lower by median 26% amongst atorvastatin users compared to placebo and the difference was statistically significant (Wilcoxon rank-sum test p-value 0.027, median difference −6.53, 95% bootstrap CI −12.8 −0.29) (Table 2). On the contrary, Estrone and DHEA concentrations are higher in the atorvastatin arm by median 13.7% and median 39%, respectively, compared to placebo, and the difference is statistically significant (Wilcoxon rank-sum test p-value 0.044 and 0.037 for Estrone and DHEA respectively) (Table 2). After adjusting for multiple comparisons by Benjamini-Hochberg method, differences in prostatic steroid concentrations are no longer statistically significant with confidence level 0.05. Therefore, the association between atorvastatin use and prostatic tissue steroidomic profile is not strong by Wilcoxon rank sum test. Other prostatic steroid hormone concentrations, including DHT and T, are clearly indifferent between the study arms (Table 2). Boxplots showing the prostatic tissue steroid concentration distributions by study arm are shown in Supplementary file 2, figures 37 to 47.
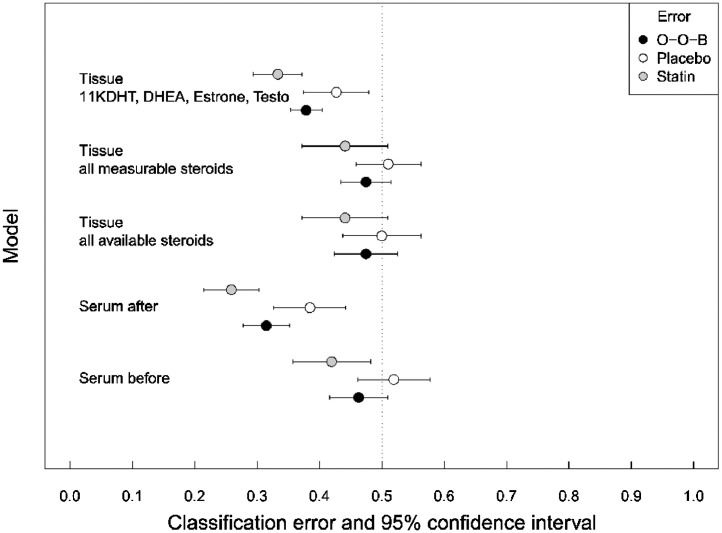
In the secondary analysis, the RFC model median classification error using the serum steroidome before the intervention is 46.30% (95% CI 41.67 – 50.93) reflecting no difference between the study arms. For serum steroidome after the intervention, the median classification error is markedly lower 31.48% (27.78–35.19) indicating a systematic change. Moreover, the atorvastatin arm class-specific median classification error is lower (25.89% (95% CI 21.43–30.36)) than the median classification error of the placebo arm (38.46% (95% CI 32.69–44.23)), which indicates a harmonising impact of atorvastatin use. This indicates systematic impact of atorvastatin on serum steroidomic hormone profile.
Using all available prostatic tissue steroid hormones, the median classification error is 47.47% (95% CI 42.42–52.53) suggesting no overall difference between the study arms in the prostatic tissue steroidome. Limiting the analysis into measurable steroid hormones, the median classification error is still relatively high at 47.47% (95% CI 43.43–51.52). In random forest, when most of the features are invariant between the classes, i.e., non-classifying (or noise), the probability that only noisy features are selected at each tree branch splitting node is high whereas the probability that a class separating feature gets selected is low.
To counter the weak signal, we used backward feature selection and selected only the features that had significant impact on the Gini impurity measure in the first RFC model including all available steroids. The variable importance plot is shown in Supplementary file 2, Fig. 1. Testosterone (T), Dehydroepiandrosterone (DHEA), Estrone, and 11KHDT fulfilled this criterion, thus they were chosen as classifiers in a separate analysis. This model yielded low median classification error 37.88 (95% CI 35.35 – 40.40) suggesting that these steroid hormones are differing between the study arms. Moreover, the class-specific median classification error for atorvastatin arm is 33.33% (29.41–37.25). This is low enough to indicate that atorvastatin use is associated with systematic harmonic pattern in the prostatic tissue steroidomic hormone profile amongst atorvastatin users. The median classification error and class-specific classification error for all models are displayed on Fig. 2. Moreover, the RFC and Wilcoxon rank sum modelling strategies agree, since RFC finds T, DHEA, Estrone, and 11KHDT the most-important classifiers; these same variables also display the smallest p-values in the Wilcoxon rank sum test.
Fig. 2.
Out-of-bag classification error (black points) and 95% confidence intervals (bars) for random forest classification models as a forest plot. Grey and white points are classification errors for atorvastatin and placebo arm, respectively; the bars are 95% confidence intervals. The confidence interval is for the Monte Carlo error. The vertical dotted line represents the 50% classification error, i.e., as-good-as-coin-flip; Out-of-bag classification error below 50% can be considered better model than random. Serum steroidomic hormone profile after the intervention classifies the treatment arms well. In the prostatic tissue, reduced model, with 11KDHT, DHEA, Estrone, and Testosterone as classifiers, classified the treatment arms with moderately low prediction error, whereas using all features failed in the classification task. For the serum, the sample sizes are n = 52 placebo and n = 56 atorvastatin. For the tissue, the sample sizes are n = 48 placebo and n = 51 atorvastatin.
After the intervention, serum steroid hormones in the atorvastatin arm are densely clustered in the random forest proximity plot reflecting systematic changes whereas placebo arm remains randomly scattered (Fig. 3a). The systematic differences between the atorvastatin and placebo arm steroidomic profile are not as pronounced in the prostate as suggested by the random forest proximity plot using Testo, DHEA, Estrone, and 11KHDT as classifiers; the atorvastatin arm is clearly less clustered (Fig. 3b) compared to the serum (Fig. 3a). At baseline, serum steroidomic profile shows random distribution pattern in both study arms (Supplementary file 2, Fig. 2).
Additional Pearson correlation analysis between serum (before and after), prostatic tissue (before and after), and PSA change are shown in Supplementary file 2 as correlation matrix heatmaps (Figure 50a placebo, Figure 50b atorvastatin, Figure 51 correlation coefficient difference atorvastatin – placebo).
Discussion
In this first-in-man pilot study, high-dose atorvastatin use induced clear changes in serum adrenal androgens, and most prominently in 11KA4. Atorvastatin use was also associated with prostatic tissue 11KDHT concentration. To our knowledge, this is the first time that atorvastatin has been observed to lower adrenal androgens compared to placebo in vivo clinical trial. Remarkably, the steroidomic profile differences, compared to placebo, differed between the serum and prostatic tissue. This suggests that intraprostatic and serum steroidomic profile milieus are dissimilar and possibly under differing regulation in men with PCa [21].
Ketosteroids are mainly secreted by the adrenal gland. In the serum, 11KA4 concentration was median 35.6% lower amongst atorvastatin users compared to placebo. 11KA4 metabolises into remarkable and potent androgen 11-ketotestosterone (11KT) [22]. Moreover, 11KA4 > 11KT conversion is mediated by steroidogenic enzyme, aldoketoreductase 1C3 (AKR1C3) [22] which is over-expressed in castration-resistant PCa [23]. The clearly lowered 11KA4 concentration in the treatment arm suggests that atorvastatin can modify androgen supply in the serum but not by limiting DHT and T.
In the prostatic tissue, 11KDHT was lower by median 25% in the atorvastatin arm, compared to placebo. 11KDHT is a remarkable androgen, nearly as potent as DHT, and can induce upregulation of the hallmark PCa markers KLK3 and TMPRESS2 in vitro. This clearly underlines the significance of lowered 11KDHT in the context of PCa.
We suggest that atorvastatin use modulates adrenal steroidogenesis by simply reducing available cholesterol, the precursor for all steroids. The benefit of statin use in PCa survival has been observed but the mechanism is unclear. According to our study, it is plausible that the benefit is partly mediated by lowered adrenal androgen concentration.
ADT and statin use has been associated with increased ADT efficacy [24,25]. ADT is usually implemented with GNRH agonists or antagonists which limits T production in the testes by nearly 100% whereas adrenal gland androgen production is limited only by 20 – 30% [26]. Therefore, we suggest that the synergism between statin use and ADT is partly facilitated by the decreased adrenal androgens. Moreover, similar juxtaposition scheme could apply to abiraterone and statin anti-PCa effect, which have also been previously suggested to have additive benefits on PCa survival, compared to either drug alone [27]. Abiraterone inhibits CYP17A1 which catalyses androgen biosynthesis from pregnenolone and progesterone [28]. Statins on the other hand inhibit cholesterol production thereby limiting pregnenolone and progesterone production in the first place. Therefore, statin and abiraterone are more likely to be synergistic rather than competing agents.
Statin use has been linked with decreased risk of aggressive PCa [29]. In the ESTO-1 clinical trial, we observed PSA decline amongst men who had high-grade PCa (Gleason score 4 + 3 or third Gleason grade 5) and used atorvastatin, compared to high-grade PCa in placebo arm [16]. PSA (KLK3) is an AR regulated gene, hence the atorvastatin impact on PSA could be partly mediated by limited local androgen supply causing lower gene reading rate of AR in the prostate. Therefore, decreased PSA levels due to atorvastatin may reflect lowered androgen stimulus in the atorvastatin arm.
Prostate cancer cells are known to exploit lipid metabolism pathways and increased fatty acid intake for cell proliferation [30,31]. Previously, we have demonstrated that atorvastatin induces significant changes in serum lipidomic profile and moderately associates with prostatic tissue lipidomic profile [10]. Therefore, we suggest that the anti-PCa mechanism of atorvastatin is multimodal, involving modified steroidome and lipidome. These modifications take place both in the serum and locally in the prostate tissue.
The major strength of this study stems from the RCT study design which mitigates confounding by known and unknown background variables. Moreover, the steroidomic hormone profiles in the serum and the prostate were determined from the same patients. To our knowledge, this is the first time such comprehensive steroidome characterisation has been done in a well-defined clinical trial population. Although the background and clinical features were roughly equally distributed between the study arms, the sample size is relatively small making the sample prone to random effects and bias. However, the random effects were countered by selecting appropriate statistical methods. Moreover, the atorvastatin intervention time was relatively short (median 28 days) – statins are typically used for years – we cannot say whether longer exposure time would expose weaker or stronger changes in steroid hormones. Our sample consisted of Caucasian men only, therefore generalisability of these results to men of other ethnicities is uncertain.
Conclusions
In this first-in-man pilot study, we demonstrate for the first time that atorvastatin lowers adrenal androgen concentration in the serum in men with prostate cancer. The association between atorvastatin use and adrenal androgens were weak but not diminished in the prostatic tissue. The findings suggest that one of the anti-PCa mechanisms of atorvastatin is mediated by lowered adrenal androgen concentration. Atorvastatin may provide a novel well-tolerated way to enhance ADT against PCa with simultaneous cardiological benefits. Clinical efficacy of atorvastatin especially in combination with ADT requires further evaluation.
Contributors
Study concept and design: Raittinen, Murtola, Syvälä, Tammela.
Acquisition of data: Murtola, Auriola, Häkkinen.
Analysis and interpretation of data: Raittinen, Murtola, Syvälä, Häkkinen, Auriola, Ilmonen.
Drafting of the manuscript: Raittinen.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Raittinen, Murtola, Ilmonen.
Obtaining funding: Murtola, Tammela.
Administrative, technical, or material support: Tammela.
Supervision: Tammela.
All authors have read and approved the final version of this manuscript. The consistency of the data has been validated by Raittinen, Murtola, Häkkinen, and Auriola
Declaration of Competing Interest
Financial disclosures: Teemu J. Murtola certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (e.g., employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following:
Dr. Murtola reports grants from Finnish Cultural Foundation, Finnish Cancer Society, Academy of Finland, and Expert Responsibility Area of the Tampere University Hospital during the conduct of the study; personal fees from Astellas and Janssen, and other from Astellas and Bayer, outside the submitted work.
Dr. Tammela reports grants from Expert Responsibility Area of the Tampere University Hospital, during the conduct of the study; personal fees from Astellas, Bayer, and Roche, outside the submitted work.
Other authors have nothing to disclose.
Acknowledgments
Acknowledgements
The study was financially supported by Finnish Cultural Foundation, Finnish Cancer Society, Academy of Finland, and the Expert Responsibility Area of the Tampere University Hospital. UEF metabolomics laboratory is supported by Biocenter Finland and Biocenter Kuopio.
Data sharing statement
The data is available from the corresponding author upon reasonable request.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2021.103432.
Contributor Information
Paavo V.H. Raittinen, Email: paavo.raittinen@aalto.fi.
Heimo Syvälä, Email: heimo.syvala@tuni.fi.
Teuvo L.J. Tammela, Email: teuvo.tammela@tuni.fi.
Merja R. Häkkinen, Email: merja.hakkinen@uef.fi.
Pauliina Ilmonen, Email: pauliina.ilmonen@aalto.fi.
Seppo Auriola, Email: seppo.auriola@uef.fi.
Teemu J. Murtola, Email: teemu.murtola@tuni.fi.
Appendix. Supplementary materials
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Culp M.B., Soerjomataram I., Efstathiou J.A., Bray F., Jemal A. Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol. 2020;77(1):38–52. doi: 10.1016/j.eururo.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Mahal B.A., Butler S., Franco I., Spratt D.E., Rebbeck T.R., D’Amico A.V. Use of active surveillance or watchful waiting for low-risk prostate cancer and management trends across risk groups in the United States, 2010-2015. JAMA. 2019;321(7):704–706. doi: 10.1001/jama.2018.19941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang A.J., Autio K.A., Roach I.II.M., Scher H.I. High-risk prostate cancer—Classification and therapy. Nature Rev Clin Oncol. 2014;11(6):308. doi: 10.1038/nrclinonc.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heinlein C.A., Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25(2):276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 6.Heidenreich A., Bastian P.J., Bellmunt J., Bolla M., Joniau S., van der Kwast T. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65(2):467–479. doi: 10.1016/j.eururo.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Berg J.M., Tymoczko J.L., Stryer L. 5th ed. Vol. 38. WH Freeman; New York: 2006. p. 76. (Biochemistry). [Google Scholar]
- 8.Stancu C., Sima A. Statins: mechanism of action and effects. J Cell Mol Med. 2001;5(4):378–387. doi: 10.1111/j.1582-4934.2001.tb00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murtola T.J., Syvälä H., Pennanen P., Bläuer M., Solakivi T., Ylikomi T. The importance of LDL and cholesterol metabolism for prostate epithelial cell growth. PLoS ONE. 2012;7(6):e39445. doi: 10.1371/journal.pone.0039445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raittinen P., Niemistö K., Pennanen E., Syvälä H., Auriola S., Riikonen J. Circulatory and prostatic tissue lipidomic profiles shifts after high-dose atorvastatin use in men with prostate cancer. Sci Rep. 2020;10(1):1–10. doi: 10.1038/s41598-020-68868-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joentausta R.M., Rannikko A., Murtola T.J. Prostate cancer survival among statin users after prostatectomy in a Finnish nationwide cohort. Prostate. 2019;79(6):583–591. doi: 10.1002/pros.23768. [DOI] [PubMed] [Google Scholar]
- 12.Gutt R., Tonlaar N., Kunnavakkam R., Karrison T., Weichselbaum R.R., Liauw S.L. Statin use and risk of prostate cancer recurrence in men treated with radiation therapy. J Clin Oncol. 2010;28(16):2653–2659. doi: 10.1200/JCO.2009.27.3003. [DOI] [PubMed] [Google Scholar]
- 13.Hall S.A., Page S.T., Travison T.G., Montgomery R.B., Link C.L., McKinlay J.B. Do statins affect androgen levels in men? Results from the Boston area community health survey. Cancer Epidemiol Prevent Biomarker. 2007;16(8):1587–1594. doi: 10.1158/1055-9965.EPI-07-0306. [DOI] [PubMed] [Google Scholar]
- 14.Mondul A.M., Selvin E., Rohrmann S., Menke A., Feinleib M., Kanarek N. Association of serum cholesterol and cholesterol-lowering drug use with serum sex steroid hormones in men in NHANES III. Cancer Causes Control. 2010;21(10):1575–1583. doi: 10.1007/s10552-010-9586-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schooling C.M., Yeung S.L.A., Freeman G., Cowling B.J. The effect of statins on testosterone in men and women, a systematic review and meta-analysis of randomized controlled trials. BMC Med. 2013;11(1):57. doi: 10.1186/1741-7015-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murtola T.J., Syvälä H., Tolonen T., Helminen M., Riikonen J., Koskimäki J. Atorvastatin versus placebo for prostate cancer before radical prostatectomy—A randomized, double-blind, placebo-controlled clinical trial. Eur Urol. 2018;74(6):697–701. doi: 10.1016/j.eururo.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 17.Häkkinen M.R., Murtola T., Voutilainen R., Poutanen M., Linnanen T., Koskivuori J. Simultaneous analysis by LC–MS/MS of 22 ketosteroids with hydroxylamine derivatization and underivatized estradiol from human plasma, serum and prostate tissue. J Pharm Biomed Anal. 2019;164:642–652. doi: 10.1016/j.jpba.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 18.Breiman L. Random forests. Mach Learn. 2001;45(1):5–32. [Google Scholar]
- 19.Hastie T., Tibshirani R., Friedman J. Springer Science & Business Media; 2009. The elements of statistical learning: data mining, inference, and prediction. [Google Scholar]
- 20.Diciccio T.J., Romano J.P. A review of bootstrap confidence intervals. J R Stat Soc: Ser B (Methodological) 1988;50(3):338–354. [Google Scholar]
- 21.Page S.T., Lin D.W., Mostaghel E.A., Hess D.L., True L.D., Amory J.K. Persistent intraprostatic androgen concentrations after medical castration in healthy men. J Clin Endocrinol Metabol. 2006;91(10):3850–3856. doi: 10.1210/jc.2006-0968. [DOI] [PubMed] [Google Scholar]
- 22.Pretorius E., Arlt W., Storbeck K. A new dawn for androgens: novel lessons from 11-oxygenated C19 steroids. Mol Cell Endocrinol. 2017;441:76–85. doi: 10.1016/j.mce.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Yepuru M., Wu Z., Kulkarni A., Yin F., Barrett C.M., Kim J. Steroidogenic enzyme AKR1C3 is a novel androgen receptor-selective coactivator that promotes prostate cancer growth. Clin Cancer Res. 2013;19(20):5613–5625. doi: 10.1158/1078-0432.CCR-13-1151. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton R.J., Ding K., Crook J.M., O'Callaghan C.J., Higano C.S., Dearnaley D.P. The association between statin use and outcomes in patients initiating androgen deprivation therapy. Eur Urol. 2020 doi: 10.1016/j.eururo.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 25.Harshman L.C., Wang X., Nakabayashi M., Xie W., Valenca L., Werner L. Statin use at the time of initiation of androgen deprivation therapy and time to progression in patients with hormone-sensitive prostate cancer. JAMA Oncol. 2015;1(4):495–504. doi: 10.1001/jamaoncol.2015.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishii M., Nomura M., Sekine Y., Koike H., Matsui H., Shibata Y. Luteinizing Hormone (LH)–Releasing hormone agonist reduces serum adrenal androgen levels in prostate cancer patients: implications for the effect of LH on the adrenal glands. J Androl. 2012;33(6):1233–1238. doi: 10.2164/jandrol.112.016493. [DOI] [PubMed] [Google Scholar]
- 27.Harshman L.C., Werner L., Tripathi A., Wang X., Maughan B.L., Antonarakis E.S. The impact of statin use on the efficacy of abiraterone acetate in patients with castration-resistant prostate cancer. Prostate. 2017;77(13):1303–1311. doi: 10.1002/pros.23390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeVore N.M., Scott E.E. Structures of cytochrome P450 17A1 with prostate cancer drugs abiraterone and TOK-001. Nature. 2012;482(7383):116–119. doi: 10.1038/nature10743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Platz E.A., Leitzmann M.F., Visvanathan K., Rimm E.B., Stampfer M.J., Willett W.C. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006;98(24):1819–1825. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- 30.Koundouros N., Poulogiannis G. Reprogramming of fatty acid metabolism in cancer. Br J Cancer. 2019:1–19. doi: 10.1038/s41416-019-0650-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu X., Daniels G., Lee P., Monaco M.E. Lipid metabolism in prostate cancer. Am J Clin Exp Urol. 2014;2(2):111. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.