Abstract
Mitochondrial dysfunction has been proposed as one of the pathobiological underpinnings in Parkinson's disease. Environmental stressors, such as paraquat, induce mitochondrial dysfunction and promote reactive oxygen species production. Targeting oxidative stress pathways could prevent mitochondrial dysfunction and thereby halt the neurodegeneration in Parkinson's disease. Since curcumin is touted as an antioxidant and neuroprotective agent, the aim of this study was to investigate if curcumin is a suitable therapy to target mitochondrial dysfunction in Parkinson's disease using a paraquat-toxicity induced model in fibroblasts from LRRK2-mutation positive Parkinson's disease individuals and healthy controls. The fibroblasts were exposed to five treatment groups, (i) untreated, (ii) curcumin only, (iii) paraquat only, (iv) pre-curcumin group: with curcumin for 2hr followed by paraquat for 24hr and (v) post-curcumin group: with paraquat for 24hr followed by curcumin for 2hr. Mitochondrial function was determined by measuring three parameters of mitochondrial respiration (maximal respiration, ATP-associated respiration, and spare respiratory capacity) using the Seahorse XFe96 Extracellular Flux Analyzer. As expected, paraquat effectively disrupted mitochondrial function for all parameters. Pre-curcumin treatment improved maximal and ATP-associated respiration whereas, post-curcumin treatment had no effect. These findings indicate that curcumin may be most beneficial as a pre-treatment before toxin exposure, which has implications for its therapeutic use. These promising findings warrant future studies testing different curcumin dosages, exposure times and curcumin formulations in larger sample sizes of Parkinson's disease and control participants.
Keywords: Turmeric, Mitochondrial function, Paraquat, Oxidative stress
Abbreviations: ATP, Adenosine Triphosphate; DMEM, Dulbecco's Modified Eagle Medium; DMSO, Dimethyl Sulfoxide; FCCP, Carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone; LRRK2, Leucine Rich Repeat Kinase 2; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; OCR, oxygen consumption rate; PD, Parkinson's disease
Highlights
-
•
Paraquat reduced respiration in Parkinson's disease and control fibroblasts.
-
•
Curcumin, an antioxidant, improved mitochondrial respiration, as a pre-treatment.
-
•
Post-treatment with curcumin did not improve mitochondrial respiration.
1. Introduction
Parkinson's disease (PD) is a neurodegenerative disorder with extensive neuronal cell loss mainly in the substantia nigra, however, the underlying cause is still unclear. Mitochondrial dysfunction has been proposed as one of the possible pathobiological underpinnings in PD [1]. Brain samples of deceased PD patients and animal PD models showed reduced activity of mitochondrial complex I and impaired mitochondrial function [2,3]. Also, the PD-causing LRRK2 genetic mutations, G2019S and R1441C, have been shown to play a role in the disproportionate degradation of mitochondria, via mitophagy [4]. LRRK2 mutations are one of the most common causes of familial PD worldwide, with an estimated prevalence of three familial PD cases per 100 000 individuals [5]. Furthermore, LRRK2 mutations are also observed in non-familial, sporadic forms of PD which highlights the importance of utilizing LRRK2 mutations to elucidate disease mechanisms for all types of PD. Various environmental stressors have also been linked to increased risk of PD. The well-known environmental stressor, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), leads to acute parkinsonian syndrome and is known to disrupt mitochondrial complex I [6]. The herbicide paraquat, chemically similar to MPTP, has also caused PD-related symptoms and neurodegeneration in animal and cellular models [7]. These environmental stressors are known to induce mitochondrial dysfunction and promote production of reactive oxygen species [8], implying a relationship between mitochondrial impairment and oxidative stress. This relationship could be exploited by targeting oxidative stress to prevent mitochondrial dysfunction and thereby halting or slowing neurodegeneration in PD.
The polyphenol curcumin has been touted as an antioxidant and anti-inflammatory agent [9]. A previous study by our group, using a siRNA-mediated knock down PINK1 model of PD, showed that curcumin protected against mitochondrial dysfunction and cell death [10]. The antioxidant and cellular-protective effects of curcumin highlights it as a suitable therapeutic candidate to target the mitochondrial dysfunction observed in PD pathology. Therefore, the aim of this exploratory study was to investigate the effect of curcumin on mitochondrial dysfunction in a cellular model of PD, specifically within dermal fibroblasts derived from LRRK2-mutation positive PD and healthy control participants.
2. Materials and methods
2.1. Participant recruitment and cell culture
Ethical clearance was obtained from the Health Research Ethics Committee of Stellenbosch University, Cape Town, South Africa (2002/C059). Skin samples were obtained from the upper arm, and dermal fibroblasts derived, from three LRRK2-mutation positive PD participants and six healthy control participants from Tygerberg Academic Hospital and surrounding areas in Cape Town, South Africa (Table 1). LRRK2 mutation status was confirmed using Sanger sequencing for the heterozygous G2019S (N = 1) [11] and R1441C (N = 2) mutations (unpublished). Fibroblasts were cultured in DMEM growth media containing 10% foetal bovine serum and 1% penicillin-streptomycin (1000U/ml).
Table 1.
Demographic information for the Parkinson's disease and control participants investigated in this study.
| Genetic Mutation | Disease Status | Population Group | Sex | Age at Biopsy (yrs) | Age at Onset (yrs) |
|---|---|---|---|---|---|
| LRRK2 R1441C | PD case 1 | MA | M | 72 | 62 |
| LRRK2 G2019S | PD case 2 | White | F | 75 | 42 |
| LRRK2 R1441C | PD case 3 | MA | F | 70 | 61 |
| Wild type | Control 1 | MA | F | 67 | |
| Wild type | Control 2 | Afrikaner | M | 75 | |
| Wild type | Control 3 | MA | F | 67 | |
| Wild type | Control 4 | White | F | 61 | |
| Wild type | Control 5 | MA | M | 77 | |
| Wild type | Control 6 | MA | M | 67 |
Age at biopsy refers to the age when a skin biopsy was performed to obtain the dermal fibroblasts from the PD and control participants. Age at onset refers to the age when PD was first diagnosed in the PD participant, by the neurologist. Afrikaner population group represents individuals mainly from Dutch, German and French ancestral origins. F: Female, LRRK2: Leucine Rich Repeat Kinase 2, M: Male, MA: Mixed Ancestry (representing an admixture of African, European and Asian ancestries unique to South Africa), PD: Parkinson's disease, White: Individuals of European ancestry, yrs: Years.
2.2. Curcumin and paraquat treatments
Curcumin treatment was investigated in the presence and absence of the herbicide, paraquat, which is commonly used as a PD toxicity model. Initially, curcumin and paraquat dosage curves were performed to identify optimal concentrations for the study. The concentrations and incubation times for curcumin and paraquat were determined using the cell viability, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The MTT reagent (10 mg/ml) was added to cells, seeded at 7000 per well, and incubated for 3hr at 37 °C and 5% CO2. Absorbance, and by inference, cell viability, was determined at 570 nm using a spectrophotometer (BioTek Instruments Inc., USA). The final curcumin concentration (1 μM) used in the treatment incubations was determined as the optimal concentration with no decrease in viability, whereas the final paraquat concentration (14 mM) was determined where at least 50% viability was lost. Five treatment groups were selected, namely: (i) untreated (contained DMSO solvent), (ii) 1 μM curcumin for 2hr, (iii) 14 mM paraquat for 24hr, (iv) pre-curcumin with 1 μM curcumin for 2hr followed by 14 mM paraquat for 24hr and (v) post-curcumin with 14 mM paraquat for 24hr followed by 1 μM curcumin for 2hr. These five treatments groups were used to determine the effect of curcumin on a paraquat-exposed model of PD under these various conditions.
2.3. Mitochondrial oxygen consumption
All fibroblasts were exposed to the five treatment groups, and thereafter mitochondrial respiration experiments were performed using the Seahorse XFe96 Extracellular Flux Analyzer (Seahorse Bioscience, USA). The analyzer determines respiration by measuring the rate cells are consuming oxygen, or the oxygen consumption rate (OCR), effectively reporting on substrate-driven respiration of the mitochondrial electron transfer system (ETS). Briefly, cells were seeded at 12 000 cells per well and divided into the five treatment groups in a 96-well XF Culture Plate. There were six replicates per treatment group for each participant. In total, three experiments were performed across three separate 96-well plates with one case and two controls included on each plate. After treatment, media was replaced with XF assay media (1 mM pyruvate, 10 mM glucose, 2 mM l-Glutamine, pH 7.4) and incubated for 1hr in a non-CO2 incubator. The OCR was measured across four conditions: i) basal, ii) after the addition of a complex V inhibitor, oligomycin (1 μM); iii) after the addition of FCCP (Carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone, an uncoupling agent and an indication of electron transfer system capacity, 0.8 μM); and iv) after the addition of complex I and complex III inhibitors, rotenone (1 μM) and antimycin A (1 μM), respectively. The respiration measures (OCR, pmol/min/arbitrary cell number) were normalized using the CyQuant Cell Proliferation Assay Kit (Thermo Fisher Scientific, USA) and analyzed using version 2.6 of the Seahorse Wave software. This assay uses these four compounds (oligomycin, FCCP, rotenone and antimycin A) to sequentially disrupt the ETS to assess key aspects of mitochondrial respiration, including basal respiration, ATP production, proton leak, maximal respiration, spare respiratory capacity and non-mitochondrial respiration. Basal respiration is the oxygen consumed to produce the energy required for basal cellular function, which results from ATP production and proton leak in the mitochondria. ATP production is the respiration that is dedicated to synthesizing ATP and is measured when oligomycin inhibits ATP synthase in the ETS. Proton leak is the difference between ATP production and basal respiration. Maximal respiration is the maximal oxygen consumed by the ETS and occurs when FCCP disrupts the proton gradient and mitochondrial membrane potential, allowing free flow of electrons and unregulated production of energy (limited only by ADP levels). Spare respiratory capacity is the difference between basal and maximal respiration. Non-mitochondrial respiration is the (baseline) oxygen consumed by enzymes outside of the mitochondria, and is measured using rotenone and antimycin A which inhibit complexes I and III, respectively, and ultimately shuts down the ETS and energy production from the mitochondria. The parameters of mitochondrial respiration included in this analysis were maximal respiration, ATP production and spare respiratory capacity. In terms of biological importance, maximal respiration depicts the cell's ability to meet a physiological energy demand above that of basal conditions and reflects the overall metabolic activity of the mitochondria. ATP production reflects the capacity of the cell to synthesize ATP, the major source of energy, to meet its physiological energy demands. Finally, spare respiratory capacity indicates the cellular ‘fitness’ or ability of cells to rapidly respond to changes in energy demands. An increase or no difference, compared to the untreated group, in these respiration parameters indicated an optimal mitochondrial metabolism, i.e. oxidative phosphorylation, and mitochondrial health. However, decreased respiration indicated a dysfunction in mitochondrial metabolism and in turn, compromised cellular health.
2.4. Statistical analyses
A total of nine participants were included in the statistical analyses (PD, n = 3; Controls, n = 6). This PD to control ratio of 1:2 was selected as using more controls ensured that findings are not confounded by normal cellular function in healthy individuals, as well as being based on previous studies on patient-derived fibroblasts [12]. Graphical representation and descriptive summary analysis were performed using GraphPad Prism 5 software (GraphPad Software, Inc.) and STATA statistical software, release 16 [13]. The mean mitochondrial respiration values were reported across the five treatment groups for PD cases and controls to compare whether (i) curcumin was “optimal for normal cell function” (vs. untreated) (ii) paraquat was toxic (vs. untreated), and (iii) curcumin pre- and post-treatments (vs. paraquat only) protected against paraquat-induced toxicity.
3. Results
3.1. Only pre- and not post-curcumin had an effect on respiration
Due to the limited sample size, we report the findings as descriptive data for the effect of curcumin treatment on respiration for each PD case compared to its two matched controls (Table 2, Fig. 1).
Table 2.
Mitochondrial respiration values (mean) across the curcumin treatment groups for LRRK2-mutation positive Parkinson's disease and unaffected control participants.
| Respiration parameter | Treatment group | PD case 1 |
Control 1 |
Control 2 |
PD case 2 |
Control 3 |
Control 4 |
PD case 3 |
Control 5 |
Control 6 |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | ||
| Maximal respiration | Untreated | 2,81 | 3,64 | 1,56 | 3,47 | 2,05 | 3,11 | 0,78 | 1,20 | 1,32 |
| Curcumin | 3,00 | 3,10 | 1,96 | 2,63 | 2,41 | 2,57 | 0,85 | 1,80 | 2,11 | |
| Paraquat | 0,44 | 0,22 | 0,12 | 0,29 | 0,26 | 0,66 | 0,24 | 0,09 | 0,67 | |
| Pre-curcumin | 1,10 | 0,60 | 0,65 | 0,96 | 0,65 | 1,26 | 0,11 | 0,17 | 0,15 | |
| Post-curcumin | 0,56 | 0,07 | 0,14 | 0,14 | 0,20 | 0,55 | 0,08 | 0,32 | 0,86 | |
| ATP-associated respiration | Untreated | 0,99 | 1,23 | 0,53 | 1,63 | 1,24 | 2,02 | 0,24 | 0,53 | 0,67 |
| Curcumin | 0,81 | 0,98 | 0,47 | 1,10 | 1,06 | 0,91 | 0,16 | 0,51 | 0,46 | |
| Paraquat | 0,24 | 0,04 | 0,11 | 0,21 | 0,11 | 0,40 | 0,00 | 0,02 | 0,42 | |
| Pre-curcumin | 0,86 | 0,32 | 0,42 | 0,79 | 0,43 | 0,81 | 0,09 | 0,15 | 0,18 | |
| Post-curcumin | 0,39 | 0,10 | 0,08 | 0,11 | 0,19 | 0,33 | 0,10 | 0,08 | 0,64 | |
| Spare-respiratory capacity-associated respiration | Untreated | 1,69 | 2,20 | 0,54 | 1,81 | 0,66 | 0,96 | 0,45 | 0,49 | 0,26 |
| Curcumin | 2,10 | 2,01 | 0,95 | 1,44 | 1,28 | 1,56 | 0,67 | 0,93 | 1,29 | |
| Paraquat | 0,00 | 0,00 | 0,00 | 0,00 | 0,00 | 0,00 | 0,11 | 0,16 | 0,00 | |
| Pre-curcumina | 0,00 | 0,00 | 0,00 | 0,00 | 0,00 | 0,00 | 0,00 | 0,00 | 0,00 | |
| Post-curcumina | 0,00 | 0,00 | 0,00 | 0,00 | 0,00 | 0,00 | 0,00 | 0,23 | 0,00 |
Respiration data (oxygen consumption rates, pmol/min/AU) were represented as mean values for the five treatment groups between each of the LRRK2-mutant PD and unaffected control participants. PD case 1 was compared to Controls 1 and 2; PD case 2 was compared to Controls 3 and 4; PD case 3 was compared to Controls 5 and 6. aFor spare respiratory capacity, the zero values for means of pre- and post-curcumin groups were possibly due to excess cellular energy depleted by the stressors, paraquat and FCCP. ATP: Adenosine Triphosphate, FCCP: Carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone, PD: Parkinson's disease.
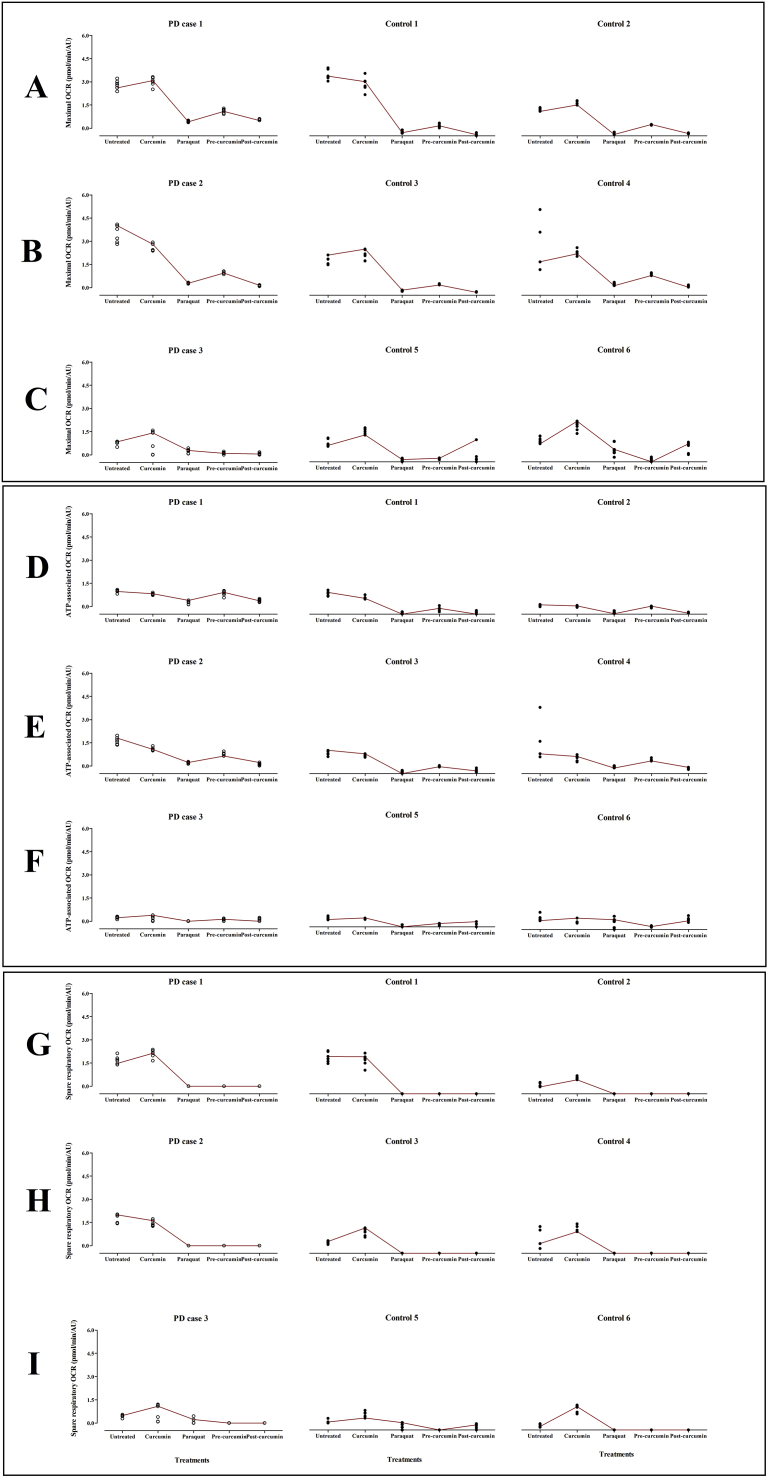
Fig. 1.
Scatterplot profile of mitochondrial respiration parameters (pmol/min/AU) across five treatment groups in fibroblast cells derived from Parkinson's disease and control participants. The three respiration parameters evaluated were maximal (A–C), ATP-associated (D–F) and spare respiratory (G–I) respiration. The raw respiration values (4–6 technical replicates) are indicated by open circles for PD cases and closed circles for controls. The respiration parameters were compared between a mutation-positive PD case and their matched controls (PD case 1 with controls 1 and 2; PD case 2 with controls 3 and 4; PD case 3 with controls 5 and 6). ATP: Adenosine Triphosphate, OCR: oxygen consumption rate, PD: Parkinson's disease.
3.2. PD case 1 and controls
For PD case 1 and its controls, the curcumin only treatment group, when compared to the untreated group, showed no change for maximal (Fig. 1: Row A) and ATP-associated respiration (Fig. 1: Row D), (Table 2). Curcumin slightly increased spare respiratory respiration in this PD case (Fig. 1: Row G). Paraquat reduced respiration, compared to the untreated, for maximal (Fig. 1: Row A), and ATP-associated respiration (Fig. 1: Row D). The pre-curcumin treatment group showed increased respiration, compared to paraquat only, for maximal respiration (Fig. 1: Row A). However, no change was noted for ATP-associated respiration (Fig. 1: Row D). Post-curcumin treatment did not alter any of the three respiration measures, when compared to the paraquat only group. For spare respiratory respiration, zero values were obtained for the paraquat, pre-curcumin and post-curcumin treatment groups (Fig. 1: Row G).
3.3. PD case 2 and controls
For PD case 2 and its controls, the curcumin only treatment group did not differ from the untreated group, for maximal (Fig. 1: Row B) and ATP-associated respiration in PD case 2 and controls (Fig. 1: Row E), (Table 2). Curcumin slightly increased spare respiratory respiration in the controls but not for the PD case (Fig. 1: Row H). Paraquat reduced maximal (Fig. 1: Row B) and ATP-associated respiration (Fig. 1: Row E). The pre-curcumin treatment, compared to paraquat, increased maximal (Fig. 1: Row B) and ATP-associated respiration (Fig. 1: Row E). However, the post-curcumin treatment showed no change in maximal (Fig. 1: Row B) and ATP-associated respiration (Fig. 1: Row E), when compared to paraquat. Similarly to PD case 1, zero values for spare respiratory respiration were also obtained for the paraquat, pre-curcumin and post-curcumin groups (Fig. 1: Row H).
3.4. PD case 3 and controls
For PD case 3 and its controls, the curcumin only treatment group, when compared to untreated, increased maximal (Fig. 1: Row C) and spare respiratory respiration (Fig. 1: Row I), (Table 2). However, no change was observed for ATP-associated respiration (Fig. 1: Row F). The paraquat only group reduced respiration for all three parameters (Fig. 1: Rows C, F and I). The pre-curcumin treatment, compared to paraquat, had no effect on respiration for all three parameters. Similarly, the post-curcumin treatment did not differ from paraquat for all three respiration parameters.
4. Discussion
In this exploratory study, we investigated the treatment effect of curcumin in a paraquat-induced toxicity model of PD, in fibroblasts from LRRK2-mutation positive PD and control participants. Mitochondrial function was assessed in the form of mitochondrial respiration parameters, specifically maximal respiration, ATP production and spare respiratory capacity. In this study, a descriptive summary of the data on the PD and control participants was reported (Fig. 1; Table 2).
When examining the individual treatment groups, paraquat consistently reduced mitochondrial respiration for all three parameters. These findings confirm that paraquat toxicity may disrupt mitochondrial metabolic activity by preventing the cell from meeting its energy demands and in turn compromising cellular health. This supports the previous literature on paraquat effectively inducing mitochondrial damage [14], and therefore provides further support for paraquat as a model of mitochondrial dysfunction. In the absence of paraquat, curcumin showed no change in maximal respiration and ATP-associated respiration but improved spare respiratory capacity.
Interestingly, we found that in the presence of paraquat, the timing of the insult (before or after curcumin treatment) had different effects on the ability of curcumin to protect against mitochondrial dysfunction. Pre-treating the cells with curcumin, before paraquat treatment, improved maximal respiration and ATP-associated respiration. However, this pre-curcumin treatment showed no effect on spare respiratory capacity. In contrast, the post-treatment of curcumin, after paraquat treatment, did not improve mitochondrial respiration across the three parameters. It is possible that the mitochondrial and cellular damage was too extensive as a result of the paraquat toxicity and therefore could not be rescued by curcumin. The exact mechanism of action for paraquat on mitochondrial function is still unclear with conflicting evidence for its role as a mitochondrial complex I uncoupler [7]. Paraquat is thought to interfere with mitochondrial redox cycling [15] and this mechanism may be irreversible leading to cell death and thereby limiting the protective effect of curcumin in the post-treatment experiments, in our study. Furthermore, since many studies only perform a pre-curcumin treatment, we are unable to compare our findings to other published studies, and therefore further studies to corroborate our observations are warranted.
A limitation of the present study is the small sample size (n = 9) which constrains the statistical power. Therefore, the findings reported here should be interpreted with caution and is limited in the inferences that can be made to a larger population. Recruitment of additional LRRK2-mutation positive PD patients for this study was challenging within the South African context as these mutations seem to be relatively uncommon within this population with a frequency of 1.2%, as indicated by our previous study [11]. Another limitation of our study was that for spare respiratory capacity, the extensive metabolic damage recorded respiration values as zero, for treatments including the paraquat toxin, thereby indicating that the excess cellular energy was depleted (Fig. 1: Rows G – I, Table 2). It is possible that the cells were stressed beyond their residual energy capacity due to paraquat, in addition to the electron transfer system uncoupler FCCP. Strengths of this exploratory study include that this work was done on an ex-vivo model from the unique South African admixed population from sub-Saharan Africa. Also, to our knowledge, it is the first study to investigate the effect of curcumin on mitochondrial respiration in a human ex-vivo model of PD.
In conclusion, our study showed that using curcumin as a pre-treatment had potential protective effects, which has implications for its therapeutic usage. Although speculative, it appears likely that curcumin would be most beneficial before PD symptoms or excessive cellular damage has occurred and therefore may be useful as a dietary supplement before the onset of disease. These promising findings warrant further studies in larger sample sizes of PD and control participants and in carriers of other PD-causing mutations. Future studies should also investigate the effect of different curcumin dosages, treatment exposure times and formulations of curcumin (e.g. nanocurcumin) in patient-derived cellular models and healthy controls. More research into nutraceuticals, such as curcumin, are needed as a potential therapeutic agent that could be used in combination with existing/conventional drug therapies to prevent or slow the progression of neurodegeneration in PD and related disorders.
Conflicts of interest
No conflicts of interest to declare.
Acknowledgements
This work is based on the research supported wholly/in part by the National Research Foundation of South Africa, NRF, (Grant Numbers: 106052, 96072, 120719), the South African Medical Research Council (Self-Initiated Research Grant), and Stellenbosch University. SA is supported by the NRF and the Faculty of Medicine and Health Sciences, Stellenbosch University.
We would like to acknowledge the study participants for their involvement in this study, and the clinical team (neurologist Prof J. Carr and nurse D. Acker) for recruiting the participants and obtaining skin biopsies. We also acknowledge the support of the DST-NRF Centre of Excellence for Biomedical Tuberculosis Research, South African Medical Research Council Centre for Tuberculosis Research, Division of Molecular Biology and Human Genetics, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa.
Data accessibility statement
The raw data can be made available for research purposes upon request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Larsen S.B., Hanss Z., Krüger R. The genetic architecture of mitochondrial dysfunction in Parkinson's disease. Cell Tissue Res. 2018;373:21–37. doi: 10.1007/s00441-017-2768-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arun S., Liu L., Donmez G. Mitochondrial biology and neurological diseases. Curr. Neuropharmacol. 2017;54:2752–2762. doi: 10.2174/1570159x13666150703154541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schapira A., Gu M., Taanman J., Tabrizi S., Seaton T., Cleeter M., Cooper J. Mitochondria in the etiology and pathogenesis of Parkinson's disease. Ann. Neurol. 1998;44:S89–S98. doi: 10.1002/ana.410440714. [DOI] [PubMed] [Google Scholar]
- 4.3rd Cherra S., Steer E., Gusdon A., Kiselyov K., Chu C. Mutant LRRK2 elicits calcium imbalance and depletion of dendritic mitochondria in neurons. Am. J. Pathol. 2013;182:474–484. doi: 10.1016/j.ajpath.2012.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Healy D.G., Falchi M., O'Sullivan S.S., Bonifati V., Durr A., Bressman S., Brice A., Aasly J., Zabetian C.P., Goldwurm S., Ferreira J.J., Tolosa E., Kay D.M., Klein C., Williams D.R., Marras C., Lang A.E., Wszolek Z.K., Berciano J., Schapira A.H., Lynch T., Bhatia K.P., Gasser T., Lees A.J., Wood N.W. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson's disease: a case-control study. Lancet Neurol. 2008;7:583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Przedborski S., Jackson-Lewis V. Mechanisms of MPTP toxicity. Mov. Disord. 1998;13:35–38. doi: 10.15586/codonpublications.parkinsonsdisease.2018.ch5. [DOI] [PubMed] [Google Scholar]
- 7.Berry C., La Vecchia C., Nicotera P. Paraquat and Parkinson's disease. Cell Death Differ. 2010;17:1115–1125. doi: 10.1038/cdd.2009.217. [DOI] [PubMed] [Google Scholar]
- 8.Dias V., Junn E., Mouradian M. The role of oxidative stress in Parkinson's disease. J Park. Dis. 2013;3:461–491. doi: 10.3233/JPD-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 10.van der Merwe C., van Dyk H.C., Engelbrecht L., van der Westhuizen F.H., Kinnear C., Loos B., Bardien S. Curcumin rescues a PINK1 knock down SH-SY5Y cellular model of Parkinson's disease from mitochondrial dysfunction and cell death. Mol. Neurobiol. 2017;54:2752–2762. doi: 10.1007/s12035-016-9843-0. [DOI] [PubMed] [Google Scholar]
- 11.du Toit N., van Coller R., Anderson D.G., Carr J., Bardien S. Frequency of the LRRK2 G2019S mutation in South African patients with Parkinson's disease. Neurogenetics. 2019;20:215–218. doi: 10.1007/s10048-019-00588-z. [DOI] [PubMed] [Google Scholar]
- 12.Smith G.A., Jansson J., Rocha E.M., Osborn T., Hallett P.J., Isacson O. Fibroblast biomarkers of sporadic Parkinson's disease and LRRK2 kinase inhibition. Mol. Neurobiol. 2016;53:5161–5177. doi: 10.1007/s12035-015-9435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.StataCorp Stata. Statistical software. Release. 2019;16 [Google Scholar]
- 14.Chen Y.W., Yang Y.T., Hung D.Z., Su C.C., Chen K.L. Paraquat induces lung alveolar epithelial cell apoptosis via Nrf-2-regulated mitochondrial dysfunction and ER stress. Arch. Toxicol. 2012;86:1547–1558. doi: 10.1007/s00204-012-0873-8. [DOI] [PubMed] [Google Scholar]
- 15.Bonneh-Barkay D., Reaney S.H., Langston W.J., Di Monte D.A. Redox cycling of the herbicide paraquat in microglial cultures. Mol. Brain Res. 2005;134:52–56. doi: 10.1016/j.molbrainres.2004.11.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data can be made available for research purposes upon request.