Abstract
Background
After the advent of new treatment options for advanced hepatocellular carcinoma (HCC), the identification of prognostic factors is crucial for the selection of the most appropriate therapy for each patient.
Patients and methods
With the aim to fill this gap, we applied recursive partitioning analysis (RPA) to a cohort of 404 patients treated with lenvatinib.
Results
The application of RPA resulted in a classification based on five variables that originated a new prognostic score, the lenvatinib prognostic index (LEP) index, identifying three groups: low risk [patients with prognostic nutritional index (PNI) >43.3 and previous trans-arterial chemoembolization (TACE)]; medium risk [patients with PNI >43.3 but without previous TACE and patients with PNI <43.3, albumin-bilirubin (ALBI) grade 1 and Barcelona Clinic Liver Cancer stage B (BCLC-B)]; high risk [patients with PNI <43.3 and ALBI grade 2 and patients with PNI <43.3, albumin-bilirubin (ALBI) grade 1 and Barcelona Clinic Liver Cancer stage C (BCLC-C)]. Median overall survival was 29.8 months [95% confidence interval (CI) 22.8-29.8 months] in low risk patients (n = 128), 17.0 months (95% CI 15.0-24.0 months) in medium risk (n = 162) and 8.9 months (95% CI 8.0-10.7 months) in high risk (n = 114); low risk hazard ratio (HR) 1 (reference group), medium risk HR 1.95 (95% CI 1.38-2.74), high risk HR 4.84 (95% CI 3.16-7.43); P < 0.0001. The LEP index was validated in a cohort of 127 Italian patients treated with lenvatinib. While the same classification did not show a prognostic value in a cohort of 311 patients treated with sorafenib, we also show a possible predictive role in favor of lenvatinib in the low risk group.
Conclusions
LEP index is a promising, easy-to-use tool that may be used to stratify patients undergoing systemic treatment of advanced HCC.
Key words: hepatocellular carcinoma, lenvatinib prognostic index, recursive partitioning analysis
Highlights
-
•
This study shows a new prognostic index (LEP index) for patients undergoing systemic therapy for hepatocellular carcinoma.
-
•
LEP index is an easy-to-use tool, based on clinical and laboratory features, that identifies three risk groups.
-
•
LEP index may be used to stratify hepatocellular carcinoma patients in order to select the most appropriate treatment.
Introduction
Liver cancer is the fourth leading cause of cancer death in the world; 75%-85% of cases are represented by hepatocellular carcinoma (HCC).1 Although the majority of cases arise during surveillance in cirrhosis, a non-malignant condition,2 only a part of HCC (~40%) can undergo local treatment with radical aim (e.g. surgical resection, radiofrequency ablation),3 and most cases are only amenable to systemic therapy.
Sorafenib, a tyrosine kinase inhibitor (TKI), has been the first drug to show a survival benefit in advanced HCC;4 then lenvatinib, another TKI, showed non-inferiority in survival when compared with sorafenib in the first-line setting.5 While TKIs target, although not specifically, the vascular endothelial growth factor (VEGF) pathway, the advent of immunotherapy allowed us to exploit a new field in HCC therapy. Nowadays, the most promising treatment strategies in first-line HCC are represented by combination regimens with an immune checkpoint inhibitor and a VEGF-targeted agent: atezolizumab plus bevacizumab resulted in a survival advantage compared with sorafenib in a phase III trial,6 and promising results also come from the combination of pembrolizumab and lenvatinib in a phase Ib study.7
After the advent of new therapeutic options, with combination regimens and different toxicity profiles, the identification of prognostic factors is crucial to identify patients with different a priori probabilities of survival in order to assign every patient the most appropriate treatment (e.g. TKI in patients with higher probability of long survival, combination or clinical trial in patients with estimated poor prognosis).
In this context, many possible prognostic factors have been identified in HCC patients treated with sorafenib, such as the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), albumin-bilirubin (ALBI) score, prognostic nutritional index (PNI) and systemic immune-inflammation index (SII).8, 9, 10, 11, 12 While these indices address relevant features that reflect the HCC microenvironment or patients' metabolic imbalance, they all analyze a specific aspect, neglecting other potentially relevant factors, e.g. previous treatments. On the other hand, only a few studies explored possible prognostic factors in patients treated with lenvatinib.13, 14, 15
To fill this gap, the aim of the present study was to evaluate a new prognostic index through a statistical method for analysis of multiple variables, named recursive partitioning analysis (RPA), that creates a regression-tree-based on prognostic value.16
Patients and methods
The study population derived from prospectively collected data of patients treated with lenvatinib as first-line for Barcelona Clinic Liver Cancer (BCLC) stage B or C HCC, deemed not eligible for first treatment or for re-treatment with surgical or loco-regional therapies. The training cohort included Eastern populations from Japan between March 2018 and June 2020. The validation cohort included a Western population from Italy between September 2019 and February 2021. Eligible patients had HCC diagnosis confirmed histologically or confirmed clinically in accordance with international guidelines. None of them received previous systemic therapy. Common inclusion criteria for the use of lenvatinib were applied.
In order to explore the potential application of the lenvatinib prognostic (LEP) index to a population treated with sorafenib, we enrolled a cohort of patients treated with sorafenib. This cohort included a Western population from Italy, and those derived from prospectively collected data in the same setting as the lenvatinib cohort, enrolled in our previous studies.8,17, 18, 19 Then, the classification based on the five variables identified in the lenvatinib cohort was applied to this cohort. Finally, outcomes of the two therapies were compared in the three risk classes, and an interaction test was carried out. Data have been presented by means of forest plot. Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100190, shows the flow diagram of the study.
Lenvatinib was administered as described in the REFLECT trial;5 thus, patients received 12 mg if baseline body weight was ≥60 kg or 8 mg if baseline body weight was <60 kg; lenvatinib was given once daily orally. Sorafenib was administered as in common clinical practice, and all patients in the sorafenib group received a starting dose of 400 mg orally twice daily.4 Patients continued lenvatinib or sorafenib if they had clinical benefit as judged by the physician in charge or until unacceptable toxicity. Treatment interruptions and dose reductions were allowed to manage adverse events.
The present study was approved by the ethics committee at each center, complied with the provisions of the Good Clinical Practice guidelines and the Declaration of Helsinki and local laws and fulfilled the Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data.
Statistical analysis
Information on clinical features and hematologic blood tests carried out at baseline (the day before the start of treatment) was collected.
X-tile 3.6.1 software (Yale University, New Haven, CT) was used to determine the cut-off value for baseline levels.
We used survival tree regression to define risk groups in the test cohort after dichotomizing each variable. Starting with the full test cohort, we carried out univariate Cox proportional hazards regression for each predictor variable. Our criteria to define groups at each level included the variable with the highest hazard ratio (HR), a P value < 0.05. We then dichotomized the test cohort using the selected predictor variable and repeated the univariate Cox models within each group. We recursively repeated this process within each new group until no variable met the criteria for selection. After defining initial groups or ‘nodes’, HRs for each node were calculated relative to the lowest-risk node. Kaplan–Meier methods were used to estimate survival for each node, and an overall log-rank test was calculated for the model.
MedCalc package (MedCalc® version 16.8.4) was used for statistical analysis.
Results
Eastern training cohort treated with lenvatinib
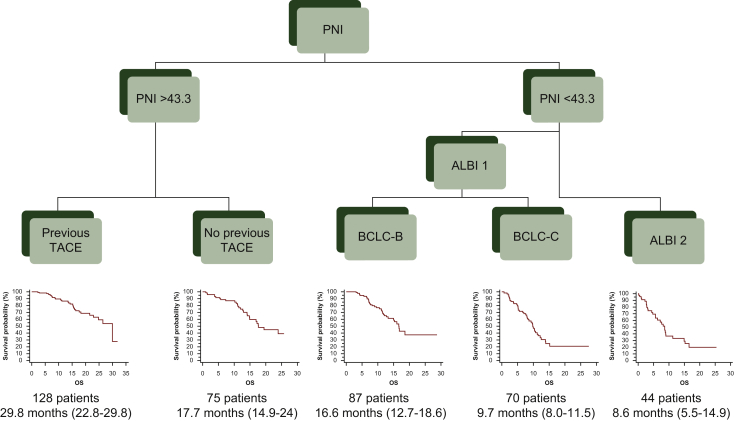
RPA analysis was carried out on 404 Japanese patients treated with lenvatinib; their main characteristics are summarized in Table 1. Univariate results indicate a survival difference according to alpha-fetoprotein (<400 versus >400), ALBI grade (1 versus 2), aspartate aminotransferase (<31 versus >31), BCLC (B versus C), Child–Pugh (A versus B), NLR (<2.7 versus >2.7) and PNI (<43.3 versus >43.3). The first node split by PNI (Figure 1) indicates that the survival difference between patients with PNI <43.3 versus PNI >43.3 is greater than the difference between any other patient subset. The same process has been recursively applied to the resulting subpopulations, giving rise to the partitioning tree depicted in Figure 1. Among the 201 patients with PNI <43.3, the most significant split was by ALBI grade, between patients with ALBI grade 1 (n = 157) and patients with ALBI grade 2 (n = 44). Among the 157 patients with PNI <43.3 and ALBI grade 1, the final split was determined between patients with BCLC-B (n = 87) and patients with BCLC-C (n = 70). Among the 203 patients with PNI >43.3, the most significant and final split was between patients with or without previous trans-arterial chemoembolization (TACE; n = 128 and n = 75, respectively).
Table 1.
Patient baseline characteristics
| Lenvatinib Eastern population % |
Lenvatinib Italian population % |
Sorafenib % |
P value between the two cohorts of lenvatinib | P value between lenvatinib Eastern population and sorafenib | |
|---|---|---|---|---|---|
| Age, years | |||||
| <70 | 34.4 | 51.2 | 59.8 | 0.0008 | <0.00001 |
| >70 | 65.6 | 48.8 | 40.3 | ||
| Sex | |||||
| Male | 78.0 | 75.6 | 88.1 | 0.62 | 0.0004 |
| Female | 22.0 | 24.4 | 11.9 | ||
| BCLC stage | |||||
| B | 58.2 | 22.0 | 25.1 | <0.000001 | <0.000001 |
| C | 41.8 | 78.0 | 74.9 | ||
| Etiology | |||||
| HCV | 43.3 | 48.0 | 51.4 | ||
| HBV | 14.6 | 15.0 | 20.6 | 0.07 | 0.0004 |
| Others | 42.1 | 37.0 | 28.0 | ||
| Performance status | |||||
| 0 | 86.6 | 86.6 | 61.4 | 1.00 | <0.000001 |
| 1 | 13.4 | 13.4 | 38.6 | ||
| Portal vein thrombosis | |||||
| No | 85.6 | 72.4 | 61.1 | 0.001 | <0.000001 |
| Yes | 14.4 | 27.6 | 38.9 | ||
| Child–Pugh score | |||||
| A | 88.6 | 94.5 | 92.6 | ||
| B | 11.4 | 5.5 | 7.4 | 0.06 | 0.07 |
| AFP | |||||
| <400 | 74.7 | 57.6 | 68.5 | 0.0003 | 0.06 |
| >400 | 25.3 | 42.4 | 31.5 | ||
| TACE | |||||
| Yes | 65.8 | 41.7 | 49.8 | 0.00002 | 0.00001 |
| No | 34.2 | 58.3 | 50.2 | ||
| ALBI | |||||
| 1 | 89.1 | 91.9 | 93.6 | 0.40 | 0.04 |
| 2 | 10.9 | 8.1 | 6.4 | ||
| PNI | |||||
| <43.3 | 49.7 | 33.1 | 86.5 | 0.001 | <0.000001 |
| >43.3 | 50.3 | 66.9 | 13.5 |
The positive results were in bold.
AFP, α-fetoprotein; ALBI, albumin-bilirubin score; BCLC, Barcelona Clinic Liver Cancer; HBV, hepatitis B virus; HCV, hepatitis C virus; PNI, prognostic nutritional index; TACE, trans-arterial chemoembolization.
Figure 1.
Classification of patients treated with lenvatinib according to recursive partitioning analysis (RPA).
RPA identifies three risk groups: low risk [patients with prognostic nutritional index (PNI) >43.3 and with previous trans-arterial chemoembolization (TACE); n = 128]; medium risk [patients with PNI >43.3 but without previous TACE and patients with PNI <43.3, ALBI grade 1 and Barcelona Clinic Liver Cancer stage B (BCLC-B); n = 162]; high risk [patients with PNI <43.3 and albumin-bilirubin (ALBI) grade 2 and patients with PNI <43.3, ALBI grade 1 and Barcelona Clinic Liver Cancer stage C (BCLC-C); n = 114].
According to the RPA tree, we have identified three groups of patients with different outcomes in terms of overall survival (OS). The first class, renamed ‘low risk’, includes patients with PNI >43.3 and with previous TACE. The second class, renamed ‘medium risk’, includes patients with PNI >43.3 but without previous TACE and patients with PNI <43.3, ALBI grade 1 and BCLC-B. Finally, the third class, renamed ‘high risk’, includes patients with PNI <43.3, ALBI grade 2 and patients with PNI <43.3, ALBI grade 1 and BCLC-C.
We have named our new score the lenvatinib prognostic index ‘LEP index’. We have created a tool for easy calculation of the score based on the five variables identified (albumin, bilirubin, lymphocytes, BCLC stage, previous TACE). The calculator is available online (https://casadeigardini.wixsite.com/lepindex) and gives, as a result, the patient’s risk class (low, medium or high risk).
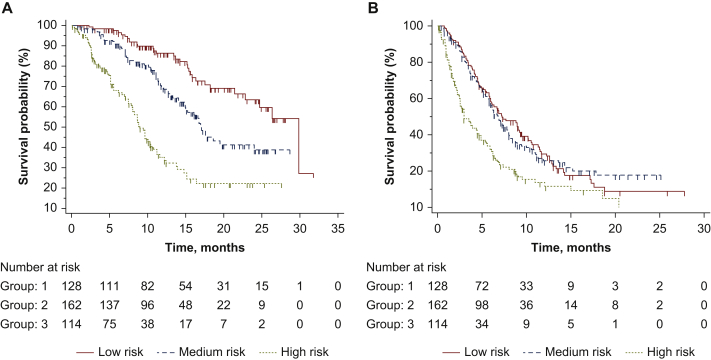
Median OS was 29.8 months [95% confidence interval (CI) 22.8-29.8 months] in patients with low risk (n = 128), 17.0 months (95% CI 15.0-24.0 months) in medium risk (n = 162) and 8.9 months (95% CI 8.0-10.7 months) in high risk (n = 114); low risk hazard ratio (HR) 1 (reference group), medium risk HR 1.95 (95% CI 1.38-2.74), high risk HR 4.84 (95% CI 3.16-7.43); P < 0.0001 (Figure 2A). Receiver operating characteristic (ROC) curve analysis showed an area under the curve (AUC) of 0.73 (95% CI 0.67-0.79; P < 0.0001).
Figure 2.
Survival analysis of patients treated with lenvatinib based on risk groups identified with recursive partitioning analysis (RPA).
(A) Median overall survival (OS) was 29.8 months [95% confidence interval (CI) 22.8-29.8 months] in the low risk group, 17.0 months (95% CI 15.0-24.0 months) in medium risk and 8.9 months (95% CI 8.0-10.7 months) in high risk; low risk hazard ratio (HR) 1, medium risk HR 1.95 (95% CI 1.38-2.74), high risk HR 4.84 (95% CI 3.16-7.43); P < 0.0001. (B) Median progression-free survival (PFS) was 7.2 months (95% CI 6.0-9.4 months) in the low risk group, 6.9 months (95% CI 5.9-7.9 months) in medium and 3.0 months (95% CI 2.5-4.7 months) in high risk; low risk HR 1, medium risk HR 1.00 (95% CI 0.78-1.30), high risk HR 2.04 (95% CI 1.46-2.84); P < 0.0001.
Median progression-free survival (PFS) was 7.2 months (95% CI 6.0-9.4 months) in patients with low risk, 6.9 months (95% CI 5.9-7.9 months) in medium and 3.0 months (95% CI 2.5-4.7 months) in high risk; low risk HR 1 (reference group), medium risk HR 1.00 (95% CI 0.78-1.30), high risk HR 2.04 (95% CI 1.46-2.84); P < 0.0001(Figure 2B).
The three groups of patients had a different percentage of progressive disease (PD) at the first computed tomography (CT) response assessment (low risk 15.9%; medium risk 11.6%; high risk 43.1%; P < 0.0001). No difference in PD at first response assessment was found between patients with low and medium risk (P = 0.36).
In the three groups of patients, different profiles of toxicity have been reported, in particular in terms of hand-foot skin reaction (low risk 33.6%; medium risk 19.7%; high risk 17.5%, P = 0.0046), hypertension (low risk 51.5%; medium risk 37.0%; high risk 23.6%, P < 0.0001) and proteinuria (low risk 39.8%; medium risk 35.2%; high risk 21.9%, P = 0.0091) (Table 2).
Table 2.
Adverse events reported in patients treated with lenvatinib
| Low risk n | Medium risk n | High risk n | P value | |
|---|---|---|---|---|
| HSFR | ||||
| Yes | 43 (33.6) | 32 (19.7) | 20 (17.5) | 0.0046 |
| No | 85 | 130 | 94 | |
| Diarrhea | ||||
| Yes | 37 | 47 | 21 | 0.0939 |
| No | 91 | 115 | 93 | |
| Hypertension | ||||
| Yes | 66 (51.5) | 60 (37.0) | 27 (23.6) | <0.0001 |
| No | 62 | 102 | 87 | |
| Fatigue | ||||
| Yes | 49 | 68 | 39 | 0.4251 |
| No | 79 | 94 | 75 | |
| Decreased appetite | ||||
| Yes | 45 | 68 | 40 | 0.3798 |
| No | 83 | 94 | 74 | |
| Proteinuria | ||||
| Yes | 51 (39.8) | 57 (35.2) | 25 (21.9) | 0.0091 |
| No | 77 | 105 | 89 | |
| Hypothyroidism | ||||
| Yes | 56 | 64 | 38 | 0.2509 |
| No | 72 | 98 | 76 | |
| Other toxicity | ||||
| Yes | 79 | 95 | 55 | 0.0871 |
| No | 49 | 67 | 59 |
The positive results were in bold.
HSFR, hand-foot skin reaction.
Western validation cohort treated with lenvatinib
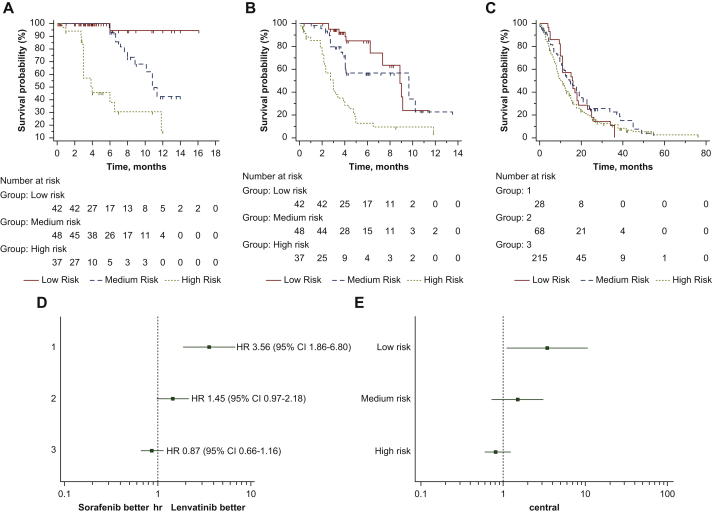
The classification identified with RPA has been applied to a cohort of 127 Italian patients treated with lenvatinib, whose characteristics are summarized in Table 1. Median OS was not reached in low risk patients (n = 42), 10.8 months (95% CI 8.8-11.3 months) in medium risk (n = 48) and 3.9 months (95% CI 3.0-11.8 months) in high risk (n = 37); low risk HR 1 (reference group), medium risk HR 9.35 (95% CI 4.31-20.27), high risk HR 41.54 (95% CI 14.46-119.36); P < 0.0001 (Figure 3A). Median PFS 8.8 months (95% CI 7.3-9.1 months) in low risk patients, 9.7 months (95% CI 4.0-10.2 months) in medium risk and 3.0 months (95% CI 2.2-3.9 months) in high risk; low risk HR 1 (reference group), medium risk HR 1.51 (95% CI 0.87-2.60), high risk HR 4.85 (95% CI 2.43-9.65); P < 0.0001 (Figure 3B).
Figure 3.
Application of the lenvatinib prognostic (LEP) index to lenvatinib validation cohort and to patients treated with sorafenib and comparison of the two treatments in the three risk groups identified with recursive partitioning analysis RPA.
(A) Overall survival analysis of Italian patients treated with lenvatinib based on risk groups identified with RPA. (B) Progression-free analysis of Italian patients treated with lenvatinib based on risk groups identified with RPA. (C) Survival analysis of patients treated with sorafenib based on risk groups identified with RPA. (D) Forest plot comparing sorafenib with lenvatinib training cohort (Eastern population). (E) Forest plot comparing sorafenib with lenvatinib validation cohort (Italian population).
The three groups of patients had a different percentage of PD at the first CT response assessment (low risk 14.3%; medium risk 20.8%; high risk 51.3%; P = 0.005). No difference in PD at first response assessment was found between patients with low and medium risk (P = 0.42).
Patients treated with sorafenib
The classification identified with RPA has been applied to a cohort of 311 Italian patients treated with sorafenib, whose characteristics are summarized in Table 1. Median OS was 15.8 months (95% CI 11.0-36.0 months) in low risk patients (n = 28), 14.8 months (95% CI 10.9-19.0 months) in medium risk (n = 68) and 10.5 months (95% CI 8.6-36.3 months) in high risk (n = 215); low risk HR 1 (reference group), medium risk HR 0.81 (95% CI 0.53-1.27), high risk HR 1.13 (95% CI 0.76-1.67); P = 0.1177 (Figure 3C).
Forest plot of OS highlighted the efficacy of lenvatinib over sorafenib in patients with low risk from the Eastern population (Figure 3D). This was confirmed when we compared lenvatinib versus sorafenib in the Italian cohort (Figure 3E). Interaction tests for both analyses highlighted a positive predictive role of low risk class in patients treated with lenvatinib (P < 0.0001).
Discussion
The application of RPA to a cohort of HCC patients treated with lenvatinib has originated a new prognostic index in this population: the LEP index. This has highlighted a very clear difference in OS in the three groups identified (low, medium and high risk: 29.8, 17.0 and 8.9 months, respectively). Notably, the low risk population (128 out of 404 patients analyzed, 31.7%) outperforms by far the lenvatinib arm (29.8 versus 13.6 months) of the phase III trial.5 Furthermore, the low risk population showed a longer OS than the atezolizumab + bevacizumab arm in the IMbrave 150 trial (19.2 months),20 conversely the medium risk population showed a similar OS. Although this is an indirect comparison, it may suggest that different risk classes are worthy of different treatments, and lenvatinib may be suitable in first-line treatment of HCC even after the advent of combination therapies (e.g. lenvatinib may be used in patients with low risk, or in medium risk with contraindications to the combination therapy, such as history of autoimmune disease or concerns for major bleeding, while the combination would be preferred in patients with high risk). In this context, the LEP index may be a tool to allocate patients the most appropriate treatment. However, further investigation is warranted, with direct comparative studies considering different treatment allocation based on risk stratification.
The LEP index was validated in a Western population treated with lenvatinib. It should be noted that Eastern and Western populations have different characteristics (Table 1), and this point improves the prognostic impact of our index.
On the other hand, evaluation of PFS and response rate highlighted a poor outcome in the high risk group but did not show any differences between low and medium risk groups (both in Eastern and Western populations). Data in these two categories were consistent with other reports, showing that TACE itself has an effect on survival,21 but it does not affect response rate and PFS in patients that subsequently received lenvatinib.22 These data raise attention on the optimal integration of TACE and lenvatinib, even in early stages of HCC.23,24
PNI, although with a slightly different cut-off (>40), has already been shown to be a good prognostic indicator in HCC patients treated with lenvatinib,15 whereas in our study, the definition of low risk includes both high PNI (>43.3) and having received TACE; therefore, one might speculate that, in order to achieve maximum benefit, patients should receive lenvatinib as soon as they become refractory to TACE. Previous studies have already reported a beneficial effect of lenvatinib as an early treatment in TACE-refractory disease, a setting where lenvatinib has shown better performance in PFS than sorafenib.23,25 Data confirmed recently by our group, where lenvatinib provided longer survival than sorafenib in patients previously submitted to TACE.26 Furthermore, some data are emerging about the role of lenvatinib even in an earlier setting, i.e. before TACE;27 indeed, systemic treatment may be preferred when intermediate-stage HCC is deemed TACE-unsuitable, i.e. when TACE will likely be ineffective or result in deterioration of hepatic function.28 Another promising strategy in intermediate HCC may be the alternation of lenvatinib and trans-arterial therapy upon the appearance of new nodules.24
A very interesting point in our study is the finding of different toxicity profiles, i.e. patients in the longer survival group reported adverse effects (specifically, hand-foot skin reaction, hypertension and proteinuria) more frequently than in the lower survival population; this is consistent with other reports that correlate the onset of adverse events during lenvatinib with a better outcome.29,30 These findings once again raise awareness about the management of adverse effects, especially taking into account the importance of maintaining a high relative dose intensity (≥70%-75%) in the first 4-8 weeks of treatment with lenvatinib in order to achieve a better outcome, both in response and survival.31, 32, 33
Different from lenvatinib, the LEP index had no prognostic significance in patients treated with sorafenib. It should be noted though that the interaction test and forest plot highlighted the positive predictive role in favor of lenvatinib in patients classified as low risk. This data reinforces the use of lenvatinib over sorafenib in early stage, especially in patients refractory to TACE.23,25 Although it should be confirmed in a prospective study, this data discloses a new scenario for systemic treatment in patients with hepatocellular carcinoma. Indeed, lenvatinib and sorafenib show some differences in their mechanism of action:34 although they both are TKIs that act mainly on the VEGF pathway, they have significant off-target activity on molecules such as platelet-derived growth factor receptor α (PDGFR-α), c-KIT and rearranged during transfection,4,5 where they show a somewhat different spectrum.35 This is underscored by the different toxicity profiles5 and by the non-overlapping clinical activity, as also shown in thyroid cancer.36
The LEP index has some relevant advantages: it is easily calculated, it is based on variables (albumin, bilirubin, lymphocytes, BCLC stage, previous TACE yes/no) that are commonly assessed in patients with HCC (thus not requiring additional examinations) and clearly identifies three groups with sharp differences in patients treated with lenvatinib, with a considerable sample size (n = 404). Furthermore, and potentially more important, the comprehensive nature of the LEP index should be underscored. Many of the existing indices point to a specific aspect of HCC: some are mainly focused on the activation of an immune-inflammatory system such as the NLR, PLR, SII or C-reactive protein,37, 38, 39 while others mainly assess the metabolic imbalance associated with the disease such as the Child–Pugh score, ALBI or PNI.40, 41, 42 All these indices disregard other relevant features of the disease. Conversely, the LEP index takes into account a higher number of variables (five), that include multiple clinical and laboratory features of HCC, i.e. activation of the immune-inflammatory system (lymphocytes), liver function and metabolic status (albumin, bilirubin), disease stage (BCLC) and previous treatments (TACE yes/no). Consequently, the LEP index is, to our knowledge, the most comprehensive prognostic factor in advanced HCC treated with lenvatinib.
Our study has some limitations: first of all, its retrospective nature and for this reason we did not have some important data of our patients (e.g. dose intensity and treatment duration); second, while with lenvatinib, the three groups identified were comparable in number (training cohort: n = 128 in low risk, n = 162 in medium risk; n = 114 in high risk; validation cohort: n = 42, n = 48, n = 37 in low, medium and high risk, respectively), the application of the same criteria to the sorafenib cohort resulted in three groups with an imbalance in number (n = 28 in low risk; n = 68 in medium risk; n = 215 in high risk); in particular, the very small sample size of the low risk group could not be fully representative. We cannot exclude that this imbalance in groups may reflect a real difference in the two populations; on the other hand, Western populations treated with sorafenib or lenvatinib had similar basal characteristics, thus strengthening the prognostic impact of our index and allowing its extension to a Western population treated with lenvatinib.
In conclusion, the new index identified in our study, named the LEP index, might represent a promising and easy-to-use tool to estimate the prognosis of HCC patients treated with lenvatinib, and strengthens the preliminary data for the use of lenvatinib over sorafenib in the early stage of the disease.
Ethics approval and consent to participate
All patients provided written informed consent before the enrolment in the study. The study was approved by ethics committee at each center and complied with the provisions of the Good Clinical Practice guidelines and the Declaration of Helsinki and local laws.
Acknowledgements
The authors thank Shinichiro Nakamura, Kojiro Michitaka, Norio Itokawa, Tomomi Okubo, Taeang Arai, Yoichi Hiasa, Masashi Hirooka, Yohei Koizumi, Kunihiko Tsuji, Toru Ishikawa, Michitaka Imai, Koichi Takaguchi, Akemi Tsutsui, Takuya Nagano, Kazuhiro Nouso, Kazuya Kariyama, Ei Itobayashi, Kouji Joko, Hironori Ochi, Satoshi Yasuda, Shinya Fukunishi, Hideko Ohama and Noritomo Shimada for their kind collaboration.
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
Data sharing
Data are available upon reasonable request.
Supplementary data
Flow diagram of the study.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 3.Llovet J.M., Zucman-Rossi J., Pikarsky E. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- 4.Llovet J.M., Ricci S., Mazzaferro V. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 5.Kudo M., Finn R.S., Qin S. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 6.Finn R.S., Qin S., Ikeda M. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 7.Finn R.S., Ikeda M., Zhu A.X. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38(26):2960–2970. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casadei-Gardini A., Dadduzio V., Rovesti G. Utility of neutrophil-to-lymphocyte ratio to identify long-term survivors among HCC patients treated with sorafenib. Medicine (Baltimore) 2020;99(22):e19958. doi: 10.1097/MD.0000000000019958. [DOI] [PubMed] [Google Scholar]
- 9.Liu L., Gong Y., Zhang Q., Cai P., Feng L. Prognostic roles of blood inflammatory markers in hepatocellular carcinoma patients taking sorafenib. A systematic review and meta-analysis. Front Oncol. 2020;9:1557. doi: 10.3389/fonc.2019.01557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong B.-Y., Yan Z.-P., Sun J.-H. Prognostic performance of albumin–bilirubin grade with artificial intelligence for hepatocellular carcinoma treated with transarterial chemoembolization combined with sorafenib. Front Oncol. 2020;10:525461. doi: 10.3389/fonc.2020.525461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caputo F., Dadduzio V., Tovoli F. The role of PNI to predict survival in advanced hepatocellular carcinoma treated with sorafenib. PLoS One. 2020;15(5):e0232449. doi: 10.1371/journal.pone.0232449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conroy G., Salleron J., Belle A. The prognostic value of inflammation-based scores in advanced hepatocellular carcinoma patients prior to treatment with sorafenib. Oncotarget. 2017;8(56):95853–95864. doi: 10.18632/oncotarget.21401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiraoka A., Kumada T., Atsukawa M. Prognostic factor of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions—multicenter analysis. Cancer Med. 2019;8(8):3719–3728. doi: 10.1002/cam4.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiraoka A., Kumada T., Kariyama K. Clinical importance of muscle volume in lenvatinib treatment for hepatocellular carcinoma: analysis adjusted with inverse probability weighting. J Gastroenterol Hepatol. 2020 doi: 10.1111/jgh.15336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiraoka A., Kumada T., Tada T. Nutritional index as prognostic indicator in patients receiving lenvatinib treatment for unresectable hepatocellular carcinoma. Oncology. 2020;98(5):295–302. doi: 10.1159/000506293. [DOI] [PubMed] [Google Scholar]
- 16.Gaspar L., Scott C., Rotman M. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 17.Rovesti G., Orsi G., Kalliopi A. Impact of baseline characteristics on the overall survival of HCC patients treated with sorafenib: ten years of experience. Gastrointest Tumors. 2019;6(3-4):92–107. doi: 10.1159/000502714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casadei-Gardini A., Marisi G., Dadduzio V. Association of NOS3 and ANGPT2 gene polymorphisms with survival in patients with hepatocellular carcinoma receiving sorafenib: results of the multicenter prospective INNOVATE study. Clin Cancer Res. 2020 Sep;26(17):4485–4493. doi: 10.1158/1078-0432.CCR-19-3897. [DOI] [PubMed] [Google Scholar]
- 19.Orsi G., Tovoli F., Dadduzio V. Prognostic role of blood eosinophil count in patients with sorafenib-treated hepatocellular carcinoma. Target Oncol. 2020;15(6):773–785. doi: 10.1007/s11523-020-00757-3. [DOI] [PubMed] [Google Scholar]
- 20.Finn R.S., Qin S., Ikeda M. IMbrave150: updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC) Gastrointestinal Cancers Symposium. 2021;39(suppl 3):267. [Google Scholar]
- 21.Kong J.Y., Li S.M., Fan H.Y., Zhang L., Zhao H.J., Li S.M. Transarterial chemoembolization extends long-term survival in patients with unresectable hepatocellular carcinoma. Medicine (Baltimore) 2018;97(33):e11872. doi: 10.1097/MD.0000000000011872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang D.X., Yang X., Lin J.Z. Efficacy and safety of lenvatinib for patients with advanced hepatocellular carcinoma: a retrospective, real-world study conducted in China. World J Gastroenterol. 2020;26(30):4465–4478. doi: 10.3748/wjg.v26.i30.4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimose S., Kawaguchi T., Tanaka M. Lenvatinib prolongs the progression-free survival time of patients with intermediate-stage hepatocellular carcinoma refractory to transarterial chemoembolization: a multicenter cohort study using data mining analysis. Oncol Lett. 2020;20(3):2257–2265. doi: 10.3892/ol.2020.11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimose S., Iwamoto H., Tanaka M. Alternating lenvatinib and trans-arterial therapy prolongs overall survival in patients with inter-mediate stage hepato cellular carcinoma: a propensity score matching study. Cancers (Basel) 2021;13(1):160. doi: 10.3390/cancers13010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J., Sung P.S., Yang H. A real-world comparative analysis of lenvatinib and sorafenib as a salvage therapy for transarterial treatments in unresectable HCC. J Clin Med. 2020;9(12):4121. doi: 10.3390/jcm9124121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casadei-Gardini A., Scartozzi M., Tada T. Lenvatinib versus sorafenib in first-line treatment of unresectable hepatocellular carcinoma: an inverse probability of treatment weighting analysis. Liver Int. 2021;41:1389–1397. doi: 10.1111/liv.14817. [DOI] [PubMed] [Google Scholar]
- 27.Kudo M., Ueshima K., Chan S. Lenvatinib as an initial treatment in patients with intermediate-stage hepatocellular carcinoma beyond up-to-seven criteria and Child–Pugh a liver function: a proof-of-concept study. Cancers (Basel) 2019;11(8):1084. doi: 10.3390/cancers11081084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kudo M., Han K.H., Ye S.L. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: Asia-Pacific Primary Liver Cancer Expert Consensus statements. Liver Cancer. 2020;9(3):245–260. doi: 10.1159/000507370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sung M.W., Finn R.S., Qin S. Association between overall survival and adverse events with lenvatinib treatment in patients with hepatocellular carcinoma (REFLECT) J Clin Oncol. 2019;37(suppl 4):317. [Google Scholar]
- 30.Shomura M., Okabe H., Sato E. Hypothyroidism is a predictive factor for better clinical outcomes in patients with advanced hepatocellular carcinoma undergoing lenvatinib therapy. Cancers (Basel) 2020;12(11):1–14. doi: 10.3390/cancers12113078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi A., Moriguchi M., Seko Y. Impact of relative dose intensity of early-phase lenvatinib treatment on therapeutic response in hepatocellular carcinoma. Anticancer Res. 2019;39(9):5149–5156. doi: 10.21873/anticanres.13710. [DOI] [PubMed] [Google Scholar]
- 32.Kirino S., Tsuchiya K., Kurosaki M. Relative dose intensity over the first four weeks of lenvatinib therapy is a factor of favorable response and overall survival in patients with unresectable hepatocellular carcinoma. PLoS One. 2020;15(4):e0231828. doi: 10.1371/journal.pone.0231828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohki T., Sato K., Kondo M. Relationship between outcomes and relative dose intensity of lenvatinib treatment in patients with advanced hepatocellular carcinoma. Liver Res. 2020;4(4):199–205. [Google Scholar]
- 34.Kim S.Y., Kim S.M., Chang H. Safety of tyrosine kinase inhibitors in patients with differentiated thyroid cancer: real-world use of lenvatinib and sorafenib in Korea. Front Endocrinol (Lausanne) 2019;10:384. doi: 10.3389/fendo.2019.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarcognato S., García-Lezana T., Villanueva A. Mechanisms of action of drugs effective in hepatocellular carcinoma. Clin Liver Dis. 2019;14(2):62–65. doi: 10.1002/cld.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takinami M., Yokota T. Rechallenge with lenvatinib after refractoriness to initial lenvatinib followed by sorafenib in a patient with metastatic papillary thyroid carcinoma. Case Rep Oncol. 2020;13(2):522–527. doi: 10.1159/000507344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh B.S., Jang J.W., Kwon J.H. Prognostic value of C-reactive protein and neutrophil-to-lymphocyte ratio in patients with hepatocellular carcinoma. BMC Cancer. 2013;13:78. doi: 10.1186/1471-2407-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin W.F., Zhong M.F., Zhang Y.R. Prognostic role of platelet-to-lymphocyte ratio in hepatocellular carcinoma with different BCLC stages: a systematic review and meta-analysis. Gastroenterol Res Pract. 2018;2018:5670949. doi: 10.1155/2018/5670949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang B., Huang Y., Lin T. Prognostic impact of elevated pre-treatment systemic immune-inflammation index (SII) in hepatocellular carcinoma: a meta-analysis. Medicine (United States) 2020;99(1):e18571. doi: 10.1097/MD.0000000000018571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng Y., Wei Q., He Y. ALBI versus Child–Pugh in predicting outcome of patients with HCC: a systematic review. Expert Rev Gastroenterol Hepatol. 2020;14(5):383–400. doi: 10.1080/17474124.2020.1748010. [DOI] [PubMed] [Google Scholar]
- 41.Tanemura A., Mizuno S., Hayasaki A. Onodera's prognostic nutritional index is a strong prognostic indicator for patients with hepatocellular carcinoma after initial hepatectomy, especially patients with preserved liver function. BMC Surg. 2020;20(1):261. doi: 10.1186/s12893-020-00917-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hatanaka T., Naganuma A., Shibasaki M. The role of the albumin-bilirubin score for predicting the outcomes in Japanese patients with advanced hepatocellular carcinoma treated with ramucirumab: a real-world study. Oncology. 2021;99:203–214. doi: 10.1159/000511734. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow diagram of the study.