Highlights
-
•
Epileptologists can accurately review single-channel EEG data.
-
•
All seizures were visible in single-channel EEG from at least one Epilog location.
-
•
Epileptologists review of Epilog EEG is more accurate than personal seizure diaries.
Keywords: Seizure Counting, Wearables, Remote Monitoring, Telehealth, Seizure Reporting
Abstract
Objective
Recording seizures using personal seizure diaries can be challenging during everyday life and many seizures are missed or mis-reported. People living with epilepsy could benefit by having a more accurate and objective wearable EEG system for counting seizures that can be used outside of the hospital. The objective of this study was to (1) determine which seizure types can be electrographically recorded from the scalp below the hairline, (2) determine epileptologists’ ability to identify electrographic seizures from single-channels extracted from full-montage wired-EEG, and (3) determine epileptologists’ ability to identify electrographic seizures from Epilog, a wireless single-channel EEG sensor.
Methods
Epilog sensors were worn concurrently during epilepsy monitoring unit (EMU) monitoring. During standard-of-care review, epileptologists were asked if the electrographic portion of the seizure was visible on single channels of wired electrodes at locations proximal to Epilog sensors, and if focal-onset, which electrode was closest to the focus. From these locations, single channels of EEG extracted from wired full-montage EEG and the proximal Epilog sensor were presented to 3 blinded epileptologists along with markers for when known seizures occurred (taken from the standard-of-care review). Control segments at inter-ictal times were included as control. The epileptologists were asked whether a seizure event was visible in the single channel EEG record at or near the marker.
Results
A total of 75 seizures were recorded from 22 of 40 adults that wore Epilog during their visit to the EMU. Epileptologists were able to visualize known seizure activity on at least one of the wired electrodes proximal to Epilog sensors for all seizure events. Epileptologists accurately identified seizures in 71% of Epilog recordings and 84% of single-channel wired recordings and were 92% accurate identifying seizures with Epilog when those seizures ended in a clinical convulsion compared to those that did not (>55%).
Conclusions
Epileptologists are able to visualize seizure activity on single-channels of EEG at locations where Epilog sensors are easily placed on the scalp below hairline. Manual review of seizure annotations can be done quickly and accurately (>70% TP and >98% PPV) on single-channel EEG data. Reviewing single-channel EEG is more accurate than what has been reported in the literature on self-reporting seizures in seizure diaries, the current standard of care for seizure counting outside of the EMU.
Significance
Wearable EEG will be important for seizure monitoring outside of the hospital. Epileptologists can accurately identify seizures in single-channel EEG, better than patient self-reporting in diaries based on the literature. Automated or semi-automated seizure detection on single channels of EEG could be used in the future to objectively count seizures to complement the standard of care outside of the EMU without the overt burden upon epileptologist review.
1. Introduction
Epilepsy affects 1% of the population or ∼70 million people world-wide. 30–40% of people with epilepsy continue to have seizures despite having tried multiple anti-seizure medications. Seizures are difficult to manage and are an immense financial burden to the person with epilepsy, their family, and society. Uncontrolled seizures drastically reduce quality of life (Schuele and Lüders, 2018, Luoni et al., 2011). Accurate seizure counting is important to optimize treatment for individual patients and is critical for analysis of clinical therapy trials. The current standard for personal seizure counting is paper/digital seizure diaries recorded by the person with epilepsy or their caregivers. But this is often an inaccurate practice which misses up to 55% of all seizures (Blum et al., 1996, Hoppe et al., 2007, Poochikian-Sarkissian et al., 2009). Reasons for errors include: seizures in sleep, seizures that involve memory related brain structures, seizures in people with cognitive impairments that impact their ability to communicate, and subclinical seizures. To optimize treatment options following initial diagnosis, health care providers would ideally obtain a record of their patients’ seizures by obtaining high-quality, long-term EEG outside of the hospital epilepsy monitoring unit (EMU). Current clinical ambulatory EEG systems, consisting of 19+ wired channels applied in the ‘International 10–20 system’, are prohibitively expensive for the purpose of counting seizures at home, are time consuming to set up, and extremely inconvenient for patients.
Recent efforts to improve seizure counting at home using non-EEG methods (beyond diaries) have been demonstrated with indirect biomarkers. The Empatica Embrace® system uses electrodermal and accelerometry readings from the wrist (Poh et al., 2012, Gutierrez et al., 2018) and Brain Sentinel SPEAC® uses electromyogram recorded from the biceps muscle (Halford et al., 2017, Beniczky et al., 2018). These non-EEG devices have gained market clearance through FDA, but only for convulsive generalized tonic-clonic seizures. EEG is the only clinically-recognized method for detection of all seizure types. However, social stigma and ease-of-use are key acceptance factors for everyday use by many people with epilepsy (Patel et al., 2016) and current ambulatory EEG systems are bulky and are not designed for use in public. Implanted subgaleal EEG offers one solution to long-term EEG recording and UNEEG Medical has recently received CE-labeling for use of their 2-channel system in Europe (Duun-Henriksen et al., 2020). While subgaleal EEG has been shown to have similar temporal and frequency characteristics as simultaneously-recorded scalp EEG, it is expensive and invasive (Weisdorf et al., 2018). Long-term seizure counting outside of the EMU is lacking as there remains no suitable non-invasive EEG system that can provide epileptologists with a reliable long-term quantitative report of a patient’s electrographic seizure activity (Elger and Hoppe 2018).
To address this need, Epitel, Inc., has developed EpilogTM, a miniature, wireless, wearable EEG sensor capable of recording EEG throughout a person’s daily life (Fig. 1). Epilog is designed to be physically small and lightweight (27 × 27 × 5.8 mm, 6.6 g), allowing a user unrestricted mobility. The Epilog sensor records a single channel of EEG through a differential electrode pair spaced 18 mm center-to-center similar to high-density EEG (Freeman et al., 2003) and has been shown to have signal quality comparable to current commercial EEG system recordings (Frankel et al., in preparation). The placement of the sensor is individualized, based on data including seizure semiology, imaging, and EEG from an initial seizure diagnosis where epileptologist guidance provides optimal sensor placement given four scalp locations (Fig. 1, Supplementary Fig. S1). We hypothesize that a ‘well-placed’, Epilog EEG sensor has great potential to be suitable for counting seizures outside of the EMU. We sought to answer two critical questions: (1) Are seizures are visible in single channels of scalp EEG below the hairline?, and (2) How accurately can epileptologists review single channels of EEG to detect electrographic seizure activity, from both standard wired-EEG recordings and Epilog sensor recordings?
Fig. 1.
Epilog sensors. The Epilog miniature wearable EEG sensor uses one-piece disposable “stickers”, that are both the adhesive and conductive hydrogel that serve as the interface between Epilog and the scalp when used below hairline.
2. Methods
Epilog EEG was recorded alongside the gold-standard of 19-channel, full-montage, video-EEG (herein referred to as “wired-EEG”) in adults during EMU stays at the University of Colorado Anschutz Medical Center. The patients’ video-EEG included a full array of 19 wired electrodes in a standard International 10–20 configuration, T1, T2, and eye leads.
2.1. General methods
All protocols were approved the by Institutional Review Board of the University of Colorado. Adults entering the EMU for long-term EEG evaluation were called prior to their appointment to discuss the study objectives. Each subject was consented in the EMU. Epilog sensors were placed by the trained study coordinator after the full-montage wired-EEG electrodes were placed by an EEG technician. Each patient wore four Epilog sensors, placed at scalp locations below the hairline, on the forehead and behind each ear, using an adhesive sticker with embedded conductive hydrogel (see Supplementary Fig. S1 for proximity to 10–20 locations). The IRB approval allowed for up to seven days of continuous EEG recording. The adhesive sticker can be worn continuously for up to 7 days in a normal EMU environment and required no daily maintenance from the patient or medical staff. Routine video-EEG review and associated seizure identification was part of the standard patient care. Seizure onset times were taken directly from epileptologists’ clinical video-EEG reports. Only patients whose clinical video-EEG reports included at least one visible electrographic seizure were included in this analysis.
2.2. EEG recordings
Epilog records a single channel of EEG through gold electrodes ∅6 mm, spaced 18 mm center-to-center and data is extracted from the sensor’s onboard memory into the European Data Format Plus file type (EDF+). Epilog data were recorded at 10-bit, 512 Hz with an amplifier passband of 0.8–92 Hz. The full-scale signal amplitude was ±175 µV. While the Epilog sensor is designed for a rechargeable battery that lasts 24–30 h, the sensor was modified for this study to use a primary battery that supported continuous EEG recording for 7 days without replacement or recharging. Video-EEG in the EMU was recorded with standard clinical equipment and settings (Supplementary Table S1).
2.3. Workflow
The study protocol involved a study coordinator, a clinical data management specialist, and a statistician to provide streamlined data collection and analysis. First, the study coordinator, working with the on-service epileptologist, pre-contacted all patients, consented all patients upon arrival, placed each Epilog sensor (using training material provided by Epitel) after the EEG Technician had placed wired-EEG electrodes, and managed the reporting and data retrieval. The Epilog and wired-EEG were time-synced using a sequence of “taps” on both the Epilog sensor and the Fp1 wired-EEG electrode. After the recording session, the clinical data management specialist uploaded de-identified patient data (wired-EEG and Epilog) to an HIPAA-compliant database.
2.4. Seizure event determination and single-channel visibility
Seizure event onset times were determined from the clinical video-EEG evaluation report as part of the standard patient care. During the evaluation, the epileptologist was asked the following question for each seizure event, “Is the electrographic portion of the seizure visible on the individual single-channel wired-EEG at location X”, where X is a 10–20 wired electrode location proximal to the four possible Epilog scalp locations (F7, F8, T5, and T6). This was asked of all four locations regardless of whether the seizure was of focal or generalized onset. Additionally, if the seizure was of focal onset, the epileptologist was asked for the wired-EEG electrode location in the 10–20 system nearest to the seizure onset focus.
2.5. Single-channel seizure detection
This part of the study design was “open-label, non-inferiority”. A minimum sample size of 61 seizure epochs and 137 non-seizure epochs were required to achieve 90% power with 90% specificity and confidence intervals of 0.805 – 0.959 given non-inferiority (Δ) specificity of 0.80 with one-sided alpha of 0.025 in an exact binomial test (events detected or undetected for each condition [Epilog vs wired-EEG]). Likewise, the same minimum number of seizure and non-seizure epochs were required to achieve 90% power with a sensitivity of 95% and confidence intervals of 0.861 – 0.990 and non-inferiority (Δ) sensitivity of 0.81 with one-sided alpha of 0.025 in the exact binomial test. Three epileptologists were recruited for this analysis to provide a consensus decision.
For each patient, the epileptologists were provided with two single-channel EDF+ files of EEG, one for Epilog and one for the wired-EEG electrode extracted from the full montage video-EEG. They were also provided with the patient’s dominant seizure type and location of seizure focus (if focal onset) from the long-term video-EEG report. For generalized onset seizures, the Epilog sensor and proximal single-channel wired-EEG electrode were randomly selected. For focal onset seizures, the single-channel wired-EEG electrode and nearest Epilog sensor were chosen based on the seizure onset focus determined in the single-channel visibility discussed above.
A random subset of 31 seizure events were extracted from the seizures recorded based on the electrographic seizure times determined during standard-of-care video-EEG review, for a total of 62 recorded seizures (31 from each of time-synced Epilog and wired-EEG, 62 total to meet the statistical power detailed above without overburdening epileptologist reviewers). An additional 83 non-ictal events were randomly extracted, spread across each patient’s recording session as 2.7× the number of ictal events that occurred. The 31 ictal and 83 non-ictal events were added to the Epilog and wired-EEG single-channel EDF+ files of EEG as annotation markers, where each marker was 1 s in length. The epileptologists were then tasked with scrolling through the continuous single-channel EDF+ file for each patient and labeling each annotation within the file as containing either ictal (seizure) or inter-ictal (non-seizure) activity, and providing how confident they were of their decision (0 = no confidence, 1 = moderate confidence, 2 = complete confidence). Reviewers were blinded to the patient identity and whether the single-channel EEG record came from an Epilog sensor or extracted from the wired-EEG. Standardization and scaling based on background noise z-scores was done to both Epilog and single-channel wired-EEG recordings to account for amplitude differences between recording systems and across patients. Inter-rater reliability was measured with Cohen’s Kappa for pair-wise reviewers and Fleiss’ Kappa for group reliability.
3. Results
A total of 40 adult subjects (ages 18–64) were enrolled in the study of which 22 (55%) had at least one seizure in the EMU (mean 4, median 4). In total, 75 seizures were recorded and classified according to International League Against Epilepsy (ILAE) seizure type in the EMU (Table 1). An average of 2.45 days (58.8 h) of Epilog EEG were recorded per patient. An example of a focal onset seizure with altered awareness recorded with Epilog can be seen in Fig. 2.
Table 1.
Distribution of seizure types recorded simultaneously with Epilog and classified according to the International League Against Epilepsy system alongside video-EEG in the EMU.
| ILAE | Seizure Type | Count |
|---|---|---|
| IA1 | Focal onset, aware, w/ motor | 4 |
| IA2-4 | Focal onset, aware, w/o motor | 8 |
| IB | Focal onset, impaired awareness | 36 |
| IC | Focal onset to bilateral tonic-clonic | 20 |
| IIA | Generalized absence | 5 |
| IIB-E | Generalized tonic-clonic | 2 |
| Total | 75 |
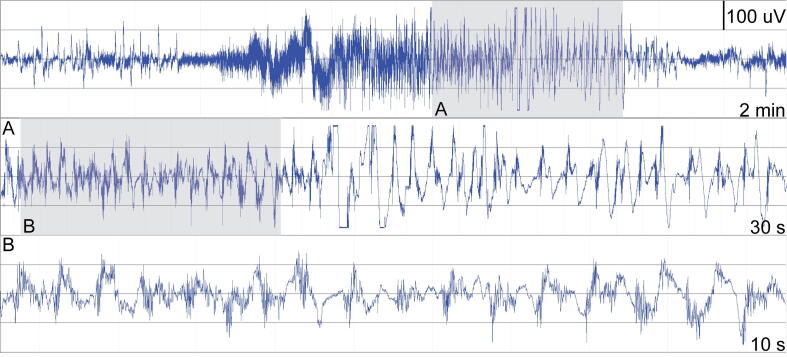
Fig. 2.
Epilog sensor seizure recording. Seizure recording from Epitel’s single-channel Epilog during a focal-onset seizure with altered awareness, as measured on the left-forehead. Top panel is a 2-minute display. Middle panel is a 30-second enlargement of the gray area marked by A in the top panel during the latter half of the seizure. Bottom panel is a 10-second enlargement of the gray area marked by B in the middle panel during latter half of seizure.
3.1. Single-channel visibility
The visibility of each electrographic seizure on a single-channel wired-EEG electrode proximal to Epilog locations is shown in Supplementary Table S2. In general, the majority of all seizures were visible at each individual location (≥79%). There was at least one single-channel location where the seizure activity was visible for every seizure event (the “Any 1” column). Generalized onset seizures were visible at all locations for all seizures. Focal onset seizures were more difficult to visualize across all single channel locations, especially those without a motor component. More seizures were visible on both the F8 and T6 locations for focal onset seizures with impaired awareness (>80% for F8/T6 vs 69% for F7/T5). Similarly, more seizures were visible on both the F7 and T5 locations for focal onset evolving to bilateral tonic-clonic (≥90% for F7/T5 vs 75% for F8/T6).
3.2. Single-channel seizure detection
Epileptologists blindly reviewed single-channel EEG, from both wired-EEG and Epilog sensors, using annotation markers denoting suspected seizure activity. Results are shown in Table 2, Table 3, Table 4 and Supplementary Table S3.
Table 2.
Three epileptologists independently reviewed identical sets of data to obtain consensus. Data in each table are the percent of epileptologists’ consensus responses in each category of true positive (TP), false negative (FN), false positive (FP), and true negatives (TN). Sz = seizure. EMU Report = seizures identified by the gold-standard of clinical video-EEG reporting. Single Channel Response = the consensus response of 2 of 3 epileptologists.
| Single-Channel Response |
Single-Channel Response |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Wired | Sz | No Sz | Epilog | Sz | No Sz | ||||
| EMU | Sz | 84 TP | 16 FN | n = 31 | EMU | Sz | 71 TP | 29 FN | n = 31 |
| Report | No Sz | 2 FP | 98 TN | n = 83 | Report | No Sz | 0 FP | 100 TN | n = 83 |
Table 3.
Seizure-type specific analysis for electrographically focal vs. electrographically generalized seizure events. In this case, electrographically-generalized seizures include focal onset evolving to bilateral tonic-clonic seizures. Percentages are given for true positive (TP) classification of ictal events alongside epileptologist confidence for both correct (TP) and incorrect (False Negative, FN) classification.
| Confidence |
Confidence |
|||||
|---|---|---|---|---|---|---|
| Focal | TP | FN | Generalized | TP | FN | |
| Wired | 88% TP | 1.67 | 1.58 | 80% TP | 1.82 | 1.58 |
| Epilog | 56% TP | 1.44 | 1.06 | 87% TP | 1.69 | 1.00 |
Table 4.
Distribution of Epilog sensor placement by seizure type during single-channel seizure detection. The number of seizure events correctly identified out of the total events for each location and seizure type is shown along with the average true positive confidence and false negative confidence in brackets as [Conf TP, Conf FN].
| Epilog Location |
||||||
|---|---|---|---|---|---|---|
| Sz Type | LF | RF | LE | RE | Total Correct | n |
| All | 13/17 [1.5, 1.1] | 5/6 [1.8, 1.3] | 2/4 [1.5, 1.5] | 2/4 [1.3, 0.7] | 71% | 31 |
| Focal onset, aware, w/motor | 1/2 [1.7, 1.3] | 1/1 [1.7, n/a] | 67% | 3 | ||
| Focal onset, aware, w/o motor | 1/1 [1.7, n/a] | 100% | 1 | |||
| Focal onset, impaired awareness | 4/5 [1.3, 1.0] | 0/1 [n/a, 1.3] | 1/3 [1.3, 1.5] | 1/3 [1.3, 0.7] | 55% | 12 |
| Focal onset to bilateral tonic-clonic | 3/3 [1.7, n/a] | 4/4 [1.8, n/a] | 1/1 [1.7, n/a] | 1/1 [1.3, n/a] | 100% | 9 |
| Generalized absence | 3/5 [1.4, 1.0] | 60% | 5 | |||
| Generalized tonic-clonic | 1/1 [2.0, n/a] | 100% | 1 | |||
Single-Channel Detection (Table 2). Epileptologists were able to classify seizure activity correctly in 84% (26 out of 31) of the single-channel wired-EEG events and 71% (22/31) of the Epilog events, and non-seizure activity correctly in 98% (81/83) of the single-channel wired-EEG events and 100% (83/83) of the Epilog events. Incorrectly identifying seizure activity as non-ictal occurred in 16% (5/31) of the single-channel wired-EEG events and 29% (9/31) in the Epilog events, and incorrectly identifying non-seizure activity as ictal occurred in 2% (2/83) of the single-channel wired-EEG events and 0% (0/83) of the Epilog events. The positive predictive value (PPV) was 92% for single-channel wired-EEG events and 100% for Epilog events. A chi-squared contingency analysis provided p = 0.32 between the single-channel wired-EEG and Epilog classifications.
Single-Channel Confidence. Epileptologists were most confident in their decisions when correctly identifying activity (1.90 out of 2 for single-channel wired-EEG and 1.91/2 for Epilog for non-ictal activity; 1.72/2 for single-channel wired-EEG and 1.59/2 for Epilog for ictal activity). They were less confident when incorrectly identifying activity (1.60/2 for single-channel wired-EEG and 1.11/2 for Epilog for falsely identifying ictal activity as non-ictal; 1.33/2 for single-channel wired-EEG and N/A for Epilog for falsely identifying non-ictal activity as ictal).
Electrographic Seizure-Type Specific Analysis and Inter-Rater Reliability. Epileptologists were better at correctly identifying electrographically-focal (Focal) versus electrographically-generalized (Generalized, includes focal-onset with progression to generalized convulsions) seizures in the single-channel wired-EEG (88% vs 80%), yet were better at correctly identifying Generalized vs Focal seizures in the Epilog EEG (87% vs 56%) as shown in Table 3. The distribution of Epilog placement during the seizures analyzed is shown in Table 4. In all scenarios, epileptologists were more confident about their decision when they were correct. The inter-rater reliability (Supplementary Table S3) was consistent between all reviewers and across the group (≥0.79 for all values). The inter-rater reliability was greater when the group was reviewing the single-channel wired-EEG data versus the Epilog data (0.91 vs 0.81 group kappa).
4. Discussion
We sought to answer the question as to how accurately epileptologists could review single channels of EEG below the hairline to detect electrographic seizure activity. This was performed with single channels extracted from clinical, full-montage video-EEG as well as with Epilog, a self-contained, single-channel EEG sensor developed by Epitel.
4.1. Electrographic seizure activity is visible on single channels of EEG below the hairline
The results show that seizure activity can be visualized from single channels of EEG at scalp locations below the hairline where Epilog sensors can be placed (Supplementary Table S2). Across all seizure types, approximately 80% of all seizures were visible at each of the four possible Epilog locations (left/right forehead and left/right behind-the-ear), and there was at least one location where the electrographic seizure was visible for every seizure noted in the clinical video-EEG review. The electrographic seizure activity was visible at all locations for the generalized onset seizure types (Generalized absence and Generalized tonic-clonic), which would be expected given their generalized nature. There were differences in which locations some of the focal onset seizures were visible, which is to be expected. Focal onset seizures have localized electrographic activity and would not be expected to be evenly distributed in this patient population with regards to left/right hemisphere or frontal/temporal lobe focus.
These results provide high confidence that a “well-placed” single-channel Epilog EEG sensor would record the majority of a person’s electrographic seizures. The initial intended use of Epilog sensors would be after an initial diagnosis of epilepsy. An epileptologist would prescribe the sensor and direct the patient on where best to place it based on known seizure-onset characteristics.
4.2. Epileptologists can distinguish between ictal and non-ictal activity in blinded single-channel EEG
The results show that epileptologists are able to identify seizure and non-seizure activity in single channels of EEG (Table 2, Table 3, Table 4) at much higher accuracy than results reported in the literature on self-reporting in seizure diaries. Reviewer consensus was 70% correct in identifying true seizure activity, while over 98% correct in identifying non-seizure activity. Because epileptologists often use multi-channel montages alongside video recordings to detect seizures, it is not surprising that they are not as accurate with single-channel EEG identification. While the reviewers were better at identifying seizures with the single-channel data extracted from the wired-EEG, there was no significant difference than with Epilog EEG (p = 0.32). Ultimately, these data suggest that single-channel EEG review may be better than average individual performance self-reporting seizures in seizure diaries.
Reviewers were more accurate in determining true seizure events in Epilog EEG when the seizures were electrographically generalized or progressed from a focal-onset to a generalized tonic-clonic convulsion (Table 3) where they were 87% accurate versus 56% accurate for electrographically focal seizures which did not evolve to a tonic-clonic convulsion. Epileptologists were nearly 100% accurate (12/13) identifying seizures with Epilog when those seizures ended in a clinical convulsion (Table 4) including focal-onset with motor, focal-onset evolving to bilateral tonic-clonic, and generalized tonic-clonic seizures. Epileptologists were least accurate in identifying seizures that did not end in a convulsion, including focal-onset with impaired awareness (55%) followed by generalized absence seizures (60%).
The missed seizure events varied across patients, locations, and time of day. The two missed absence events in the Epilog review were from the same patient and both occurred between 4p and 9p. The missed focal-onset seizure events in the Epilog review were all from different patients. Two of the focal-onset events that were missed in the Epilog review occurred between 6p and 10p and the rest occurred between 2a and 8a. Two of the generalized events that were missed from the wired single-channel review were from the same patient and both events occurred between 2a and 7a. All of the other missed wired single-channel events occurred between 2a and 11a and were varied across patients and both temporal and frontal locations.
One potential reason for the difference in Epilog vs wired single-channel detection could be attributed to the scalp location of each recording. For single-channels of wired-EEG, the “best scalp location” for recording that seizure activity was determined by epileptologists from the full wired montage, only after all recordings occurred. Epilog, however, is constrained to four scalp locations nearest to these best locations (when available) because of the adhesive sticker with embedded conductive hydrogel that does not currently work through hair. The spacing between the wired electrode dipoles is much larger than Epilog which uses a single, fixed bipolar electrode pair. Short electrode spacing can drastically affect the spatial dipole observed in the temporal domain, most similar to the bipolar derivation of high-density EEG montages. Furthermore, this short electrode spacing may be subject to in-phase cancellation of electrographic activity. All of these confounds may have been a contributing factor to the ability and confidence in distinguishing seizures from Epilog EEG.
Additional possibilities for difficulty in discerning events could be due to signal quality concerns including saturation and/or artifacts. While Epilog mitigates wired-system issues such as long-wire movement artifacts, other issues such as muscle artifacts may influence results. While not part of this study, future research will investigate if issues like saturation and/or artifacts influence a reviewer’s ability to discern events and whether EEG artifact rejection strategies specific to single-channel data (Dhindsa, 2017) can minimize or mitigate these issues.
4.3. Towards semi-automated and automated analysis
In this study, up to 4.5 days of Epilog EEG were recorded per patient. Manually reviewing even this limited amount of data with standard-of-care techniques would be exorbitantly time consuming and prohibitively expensive outside of the clinic. Such “seizure counting” does not currently fit within the workflow of the management of epilepsy outside of seizure diaries. Semi-automated methods could be used to reduce days of single-channel EEG down to few discrete, auto-detected events that an epileptologist could quickly scan within the full EEG record. Use of single-channel EEG alongside traditional seizure diaries could help epileptologists understand how many seizures their patients are aware of and can identify with greater accuracy than self-reporting, though a head-to-head comparison would need to be performed. Such information could provide a more accurate, objective measure of how many seizures a patient is having that an epileptologist could use to provide personalized care in the management of a seizure disorder.
Automated seizure detection systems with market clearance through FDA for use in the EMU have high positive predictive value (>80%) with modest false positive rates (<5 per day) (Wilson et al., 2004, Kelly et al., 2010), highlighting that no automated seizure detection system is perfect. Yet, they have been successfully used in the EMU to manage workflow and reduce the time to review the EEG record (Scheuer and Wilson, 2004). Ultimately, automated seizure detection on single channels of EEG could likely help epileptologists objectively count seizures outside of the EMU (Kjaer et al., 2018, Gu et al., 2017).
4.4. Multi-sensor systems
While this study focused on single-channel EEG review, results could likely be improved by having patients wear multiple Epilog sensors. This would provide increased scalp coverage and ensure seizure events are captured by the sensors, which would be most important for those with multi-focal onset epilepsy. Indeed, there was at least one single-channel location where the seizure activity was visible for every seizure event (Supplementary Table S2). Future studies will assess multi-channel and low channel-count montages, and report on more intricate differences among the systems, including blinded review of full-montage EEG for better comparisons. Multi-channel systems would be expected to provide better results for clinician review and automated analysis, but patient adherence and acceptability will be more important for long-term wearability in daily-life. The simplicity and ease of a discreet single sensor like Epilog may be the best option to meet the long-term needs of both the patient and epileptologist.
4.5. Conclusions
Epileptologists are able to visualize seizure activity on single-channels of EEG at locations where Epilog sensors are easily placed below hairline. Manual review of seizure annotations can be done quickly and accurately (>70% TP and >98% PPV) on single-channel EEG data. Reviewing single-channel EEG is expected to be more accurate than self-reporting seizures in seizure diaries, the current standard of care for seizure counting outside of the EMU. Automated or semi-automated seizure detection on single channels of EEG could be used in the future to aid epileptologists in objectively counting seizures outside of the EMU.
Acknowledgments
Acknowledgements
Funding provided by the State of Utah Economic Development Council, the Epilepsy Foundation, and NIH SBIR NS100235. We greatly appreciate the help of biostatistician Stefan Sillau, PhD (University of Colorado).
Author statements
The corresponding author, Mitchell Frankel, has full access to all data and the right to publish such data. All authors participated in a meaningful way in the preparation of the manuscript.
Disclosure of competing interest
MAF and MJL have financial interests in Epitel, Inc. None of the authors from University of Colorado Anschutz Medical Center have any conflict of interest to disclose.
Ethical publication statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cnp.2021.04.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.
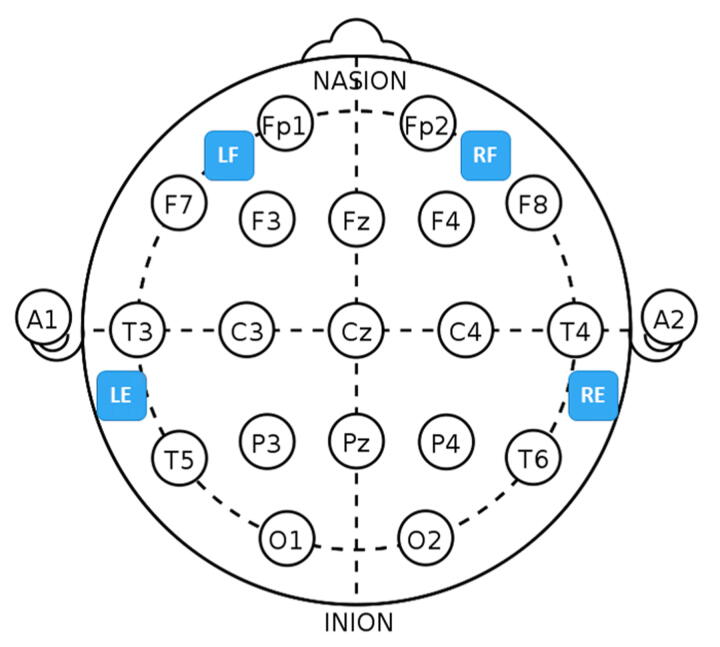
Epilog sensor placement relative to the international 10-20 system. For the forehead locations, LF is left-forehead closest to F7, RF is right-forehead closest to F8, and the Epilog sensors were placed as far from forehead/eye muscles as possible. For the behind-the-ear locations, LE is behind left ear closest to T5, and RE is behind right ear closest to T6, and the Epilog sensors were placed as high up as possible while still being below the hairline, making sure placement was not directly over the neck muscles.
References
- Beniczky S., Conradsen I., Wolf P. Detection of convulsive seizures using surface electromyography. Epilepsia. 2018;59(Suppl 1):23–29. doi: 10.1111/epi.14048. [DOI] [PubMed] [Google Scholar]
- Blum D.E., Eskola J., Bortz J.J., Fisher R.S. Patient awareness of seizures. Neurology. 1996;47(1):260–264. doi: 10.1212/wnl.47.1.260. [DOI] [PubMed] [Google Scholar]
- Dhindsa K. Filter-bank artifact rejection: high performance real-time single-channel artifact detection for EEG. Biomed Signal Process Control. 2017;38:224–235. [Google Scholar]
- Duun-Henriksen J., Baud M., Richardson M.P., Cook M., Kouvas G., Heasman J.M., Friedman D., Peltola J., Zibrandtsen I.C., Kjaer T.W. A new era in electroencephalographic monitoring? Subscalp devices for ultra–long-term recordings. Epilepsia. 2020;61(9):1805–1817. doi: 10.1111/epi.16630. [DOI] [PubMed] [Google Scholar]
- Elger C.E., Hoppe C. Diagnostic challenges in epilepsy: seizure under-reporting and seizure detection. Lancet Neurol. 2018;17(3):279–288. doi: 10.1016/S1474-4422(18)30038-3. [DOI] [PubMed] [Google Scholar]
- Freeman W.J., Holmes M.D., Burke B.C., Vanhatalo S. Spatial spectra of scalp EEG and EMG from awake humans. Clin. Neurophysiol. 2003;114(6):1053–1068. doi: 10.1016/s1388-2457(03)00045-2. [DOI] [PubMed] [Google Scholar]
- Gu Y., Cleeren E., Dan J., Claes K., Van Paesschen W., Van Huffel S. Comparison between scalp EEG and behind-the-ear EEG for development of a wearable seizure detection system for patients with focal epilepsy. Sensors (Basel) 2017;18(1):pii: E29. doi: 10.3390/s18010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez E.G., Crone N.E., Kang J.Y., Carmenate Y.I., Krauss G.L. Strategies for non-EEG seizure detection and timing for alerting and interventions with tonic-clonic seizures. Epilepsia. 2018;59(Suppl 1):36–41. doi: 10.1111/epi.14046. [DOI] [PubMed] [Google Scholar]
- Halford J.J., Sperling M.R., Nair D.R., Dlugos D.J., Tatum W.O., Harvey J. Detection of generalized tonic-clonic seizures using surface electromyographic monitoring. Epilepsia. 2017;58(11):1861–1869. doi: 10.1111/epi.13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe C., Poepel A., Elger C.E. Epilepsy: accuracy of patient seizure counts. Arch. Neurol. 2007;64(11):1595–1599. doi: 10.1001/archneur.64.11.1595. [DOI] [PubMed] [Google Scholar]
- Kelly K.M., Shiau D.S., Kern R.T., Chien J.H., Yang M.C., Yandora K.A. Assessment of a scalp EEG-based automated seizure detection system. Clin. Neurophysiol. 2010;121(11):1832–1843. doi: 10.1016/j.clinph.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer T.W., Sorensen H.B., Groenborg S., Pedersen C.R., Duun-Henriksen J. Detection of paroxysms in long-term, single-channel EEG-monitoring of patients with typical absence seizures. IEEE J. Trans. Eng. He. 2018;5:1–8. doi: 10.1109/JTEHM.2017.2649491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoni C., Bisulli F., Canevini M.P., De Sarro G., Fattore C., Galimberti C.A. Determinants of health-related quality of life in pharmacoresistant epilepsy: results from a large multicenter study of consecutively enrolled patients using validated quantitative assessments. Epilepsia. 2011;52(12):2181–2191. doi: 10.1111/j.1528-1167.2011.03325.x. [DOI] [PubMed] [Google Scholar]
- Patel A.D., Moss R., Rust S.W., Patterson J., Strouse R., Gedela S. Patient-centered design criteria for wearable seizure detection devices. Epilepsy Behav. 2016;64:116–121. doi: 10.1016/j.yebeh.2016.09.012. [DOI] [PubMed] [Google Scholar]
- Poh M.Z., Loddenkemper T., Reinsberger C., Swenson N.C., Goyal S., Sabtala M.C. Convulsive seizure detection using a wrist-worn electrodermal activity and accelerometry biosensor. Epilepsia. 2012;53(5):e93–e97. doi: 10.1111/j.1528-1167.2012.03444.x. [DOI] [PubMed] [Google Scholar]
- Poochikian-Sarkissian S., Tai P., del Campo M., Andrade D.M., Carlen P.L., Valiante T. Patient awareness of seizures as documented in the epilepsy monitoring unit. Can. J. Neurosci. Nurs. 2009;31(4):22–23. [PubMed] [Google Scholar]
- Scheuer M.L., Wilson S.B. Data analysis for continuous EEG monitoring in the ICU: seeing the forest and the trees. J. Clin. Neurophysiol. 2004;5:353–378. [PubMed] [Google Scholar]
- Schuele S.U., Lüders H.O. Intractable epilepsy: management and therapeutic alternatives. Lancet Neurol. 2018;7(6):514–524. doi: 10.1016/S1474-4422(08)70108-X. [DOI] [PubMed] [Google Scholar]
- Weisdorf S., Gangstad S.W., Duun-Henriksen J., Mosholt K.S.S., Kjær T.W. High similarity between EEG from subcutaneous and proximate scalp electrodes in patients with temporal lobe epilepsy. Neurophysiology. 2018;120(3):1451–1460. doi: 10.1152/jn.00320.2018. [DOI] [PubMed] [Google Scholar]
- Wilson S.B., Scheuer M.L., Emerson R.G., Gabor A.J. Seizure detection: evaluation of the Reveal algorithm. Clin. Neurophysiol. 2004;115(10):2280–2291. doi: 10.1016/j.clinph.2004.05.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.