Highlights
-
•
First study investigating ICA-based structural covariance in patient with OCD.
-
•
Results show significantly altered structural covariance patterns in patients with OCD.
-
•
Common characteristics associated with the disorder may lead to a disorder-specific grey matter pattern organization.
Keywords: OCD, Grey matter, ICA, Structural covariance, Duration of illness
Abstract
Background
Changes in grey matter volume have frequently been reported in patients with obsessive-compulsive disorder (OCD). Most studies performed whole brain or region-of-interest based analyses whereas grey matter volume based on structural covariance networks has barely been investigated up to now. Therefore, the present study investigated grey matter volume within structural covariance networks in a sample of 228 participants (n = 117 OCD patients, n = 111 healthy controls).
Methods
First, an independent component analysis (ICA) was performed on all subjects’ preprocessed T1 images to derive covariance-dependent morphometric networks. Then, grey matter volume from each of the ICA-derived morphometric networks was extracted and compared between the groups. In addition, we performed logistic regressions and receiver operating characteristic (ROC) analyses to investigate whether network-related grey matter volume could serve as a characteristic that allows to differentiate patients from healthy volunteers. Moreover, we assessed grey matter pattern organization by correlating grey matter volume in all networks across all participants. Finally, we explored a potential association between grey matter volume or whole-brain grey matter pattern organization and clinical characteristics in terms of symptom severity and duration of illness.
Results
There were only subtle group differences in network-related grey matter volume. Network-related grey matter volume had moreover a very poor discrimination performance. We found, however, significant group differences with regard to grey matter pattern organization. When correlating grey matter volume in all networks across all participants, patients showed a significantly higher homogeneity across all networks and a significantly lower heterogeneity, as assessed by the coefficient of variation across all networks as well as in several single networks. There was no association with clinical characteristics.
Conclusion
The findings of the present study suggest that the pathological mechanisms of OCD reduce interindividual grey matter variability. We assume that common characteristics associated with the disorder may lead to a more uniform, disorder-specific morphometry.
1. Introduction
There is increasing evidence showing that obsessive-compulsive disorder is associated with structural brain alterations in specific regions or networks. These alterations manifest in both the white matter and grey matter of the brain (Koch et al., 2014, Piras et al., 2013). Specifically, alterations are observed in grey matter volume, its thickness, surface area as well as its gyrification in specific regions (Boedhoe et al., 2017, Reess et al., 2018a, Rotge et al., 2010, Rus et al., 2017a, Rus et al., 2017b). Association of these alterations with underlying pathogenesis as well as clinical characteristics, such as symptom severity or duration of ilness, are still a matter of debate since some of these studies showed a close association with clinical characteristics while others did not find any correlations (Nakamae et al., 2012, Piras et al., 2015, Reess et al., 2018a, Rus et al., 2017a, Zhang et al., 2021).
These previous findings are mainly based on studies using whole-brain or ROI-based methods, whereas structural alterations in covariance-derived morphometric networks have been investigated in OCD by only a few studies so far (Reess et al., 2018b, Subirà et al., 2016, Yun et al., 2020, Yun et al., 2015). The majority of these studies used graph theory for the investigation of structural covariance such as a large meta-analysis by Yun et al. (2020) which showed that patients with OCD have reduced parameters of brain structural covariance networks of the cortical surface area, cortical thickness, subcortical volume measures compared to healthy controls. Another approach explores structural covariance analysis based on the structural similarity between cortical areas or networks. Thereby, an independent-component analysis (ICA) on the grey matter maps of multiple individuals is performed, to identify population-derived morphometric networks that share a common variance across subjects and are thus assumed to be related or connected to each other. Instead of performing a whole-brain analysis or using a-priori defined, atlas-based regions this approach is purely data-driven and therefore especially useful as a basis for prediction or classification. Hence, the method has the advantage that it is less dependent on the specific choice of an anatomical atlas or a specific a-priori hypothesis thus yielding less biased results than, for instance, classical ROI-based methods. Networks derived using this approach have been found to be altered in several neurodegenerative diseases (Coppen et al., 2016, Hafkemeijer et al., 2016, Lee et al., 2018, Zhou et al., 2020) as well as in patients with depression (Watanabe et al., 2020) and schizophrenia (Xu et al., 2009).
This procedure complies with the underlying assumption that brain regions which increase or decrease in volume at the same rate over the course of years exhibit strong structural similarity and covariance across individuals (Alexander-Bloch et al., 2013a, Alexander-Bloch et al., 2013b). This volumetric increase or decrease over time might be the consequence of disease-related processes or, more generally, the result of use-dependent neuroplasticity. Thus, use-dependent synchronous firing of several neurons within a region or network can cause synaptogenesis between these neurons leading to an increase in connectivity and grey matter volume (Katz and Shatz, 1996). Our own findings of an increased grey matter thickness in regions of the motor circuitry in OCD patients suffering mainly from motor rituals (Wagner et al., 2019) can be assumed to constitute the result of such use- or experience-dependent coordinated growth. In this fashion, alterations in structural covariance in regions and networks implicated in the disorder in general may also be strongly linked to use- or experience-dependent coordinated growth (Subirà et al., 2016).
In sum, grey matter volume in OCD might be changed within specific covariance networks as a result of disorder-related alterations in regional growth. In the latter case, a close association with illness onset or duration would likewise have to be expected considering that experience and development over the course of the years is assumed to play a major role in this regard. Against this background, the aim of the current study was to investigate grey matter volume and homogeneity of grey matter patterns within structural covariance networks in a large sample of OCD patients and healthy control participants. In addition, we aimed to investigate the underlying mechanisms by examining a potential association with illness duration as well as exploring whether these networks and their characteristics can serve as specific disorder attributes, allowing for a clear differentiation between patients with OCD and healthy people.
2. Material and methods
2.1. Participants
The study comprised a total of 228 participants (111 controls, 117 patients with OCD). 28 patients were recruited and scanned at the University Hospital for Psychiatry and Psychotherapy, Jena, Germany. The remaining 89 patients were recruited from the Windach Institute and Hospital of Neurobehavioral Research and Therapy (WINTR), an institution specialized in the treatment of OCD, and scanned at the Klinikum rechts der Isar, Technische Universität München, Germany. All patients were in-house patients diagnosed with OCD as their primary diagnosis by an experienced psychiatrist according to DSM-5 criteria. At the time of the study, n = 43 patients were drug-naïve or medication free for at least 3 weeks. The Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) was used to assess clinical severity of obsessive-compulsive symptoms. 111 age and gender-matched healthy subjects, recruited by advertisements, were scanned at the Klinikum rechts der Isar, TUM, Germany. Exclusion criteria for all subjects were a history of clinically important head injuries, seizures or neurological diseases. There were no significant differences between healthy controls and OCD patients regarding age (two-sample t-test, two-tailed; t(226) = 0.03, p = 0.97) or gender (Chi-Square test; χ2(1) = 3.29, p = 0.07). For demographical and clinical sample characteristics, see Table 1.
Table 1.
Demographic and clinical data.
| Characteristic | OCD (N = 117) | Controls (N = 111) | Group difference |
|---|---|---|---|
| Mean (SD) | Mean (SD) | p-value | |
| Sex, male:female | 43:74 | 54:57 | χ 2 = 3.29, n.s. |
| Age | 32.16 (10.73) | 32.20 (10.17) | t = 0.03, n.s. |
| Medication, yes/no * | 72/44 | – | – |
| SSRI: 53 SNRI: 9 neuroleptic: 3 TCA: 4 tetracyclic: 1 Amphetamine: 3 NDRI: 1 analgetic: 1 |
|||
| Duration of illness * | 14.78 (10.75) | – | – |
| Age at onset * | 17.46 (7.81) | – | – |
| Comorbidity, yes/no * depression: 28 anxiety disorders: 10 personality disorder: 3 PTSD: 1 bulimia: 1 pain disorder: 1 ADHD: 1 |
37/79 | ||
| Y-BOCS total Obsessions Compulsions |
21.82 (5.49) 11.04 (3.05) 10.78 (3.61) |
– | – |
* Note: Missing data in one patient.
Multiple comorbid diagnosis as well as different medication types can be present in a single patient.
Abbreviations: OCD, obsessive-compulsive disorder; Y-BOCS, Yale-Brown Obsessive-Compulsive Scale, SSRI, selective serotonin reuptake inhibitor; SNRI, selective serotonin-norepinephrine reuptake inhibitor; TCA, tricyclic antidepressant; NDRI, norepinephrine-dopamine reuptake inhibitor; PTSD, post-traumatic stress disorder; ADHD, attention deficit hyperactivity disorder.
The study was approved by the local Ethics Committee of the Klinikum rechts der Isar, München and the Ethics Committee of the University Hospital Jena. The study protocol was in compliance with the Declaration of Helsinki. Data have been made publicly available via the Open Science Framework (https://osf.io/92rve).
2.2. Image acquisition
The T1-weighted MPRAGE sequence at the Munich site was conducted on a 3 T Philips Ingenia (Philips Healthcare, Best, The Netherlands) using a 12-channel (SENSE) head coil (170 slices, sagittal orientation, 240x240 matrix, 1 mm isotropic resolution, TR = 9 ms, TE = 4 ms, flip angle = 8°) and a 32-channel (SENSE) head coil (230 slices, sagittal orientation, 368 × 340 matrix, 0.7 × 0.7 × 0.7 mm resolution, TR = 11 ms, TE = 5.1 ms, flip angle = 8°). Structural imaging at the Jena site was conducted on a 3 T Siemens MAGNETOM (Siemens Medical Solutions, Erlangen, Germany) using a 12-channel receive-only head matrix coil. It consisted of a T1-weighted MPRAGE sequence with the following parameters: 192 slices, sagittal orientation, 256x256 matrix, 1 mm isotropic resolution, TR = 2300 ms, TE = 3.03 ms, flip angle = 9°.
2.3. Image preprocessing
T1-weighted structural images were segmented into grey matter, white matter, and CSF using Statistical Parametric Mapping (SPM12, http://www.fil.ion.ucl.ac.uk/spm/software/spm12/), running on MATLAB version 2018b. Grey matter images went through Diffeomorphic Anatomical Registration through Exponentiated Lie Algebra toolbox (DARTEL) (Ashburner, 2007) which creates a sample-specific template representative of all subjects by iterative alignment of all images. Subsequently, the template underwent non-linear registration with modulation for linear and non-linear deformations to the MNI-ICBM152 template. Each participant’s grey matter map was then registered to the group template and smoothed with an 8 mm3 isotropic Gaussian kernel.
2.4. Independent component analysis
ICA was performed on the grey matter maps of all individuals to derive population-based networks of grey matter covariance. With this aim, all individually modulated and smoothed grey matter maps were concatenated to create a 4D file, which served as the basis for the ICA. To ensure that only grey matter voxels were retained for the ICA, an absolute grey matter threshold of 0.1 was applied to all images. ICA was performed using the Multivariate Exploratory Linear Optimized Decomposition into Independent Components (MELODIC) method (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/MELODIC) as implemented in the FSL analysis package (Jenkinson et al., 2012) version 6.0. To derive common data-driven components based on patients and healthy control subjects, the ICA was performed on all subjects (n = 228) thus identifying common spatial components based on the covariation of grey matter patterns across all participants. In line with previous work which employed similar methods we chose 30 components (Pichet Binette et al., 2020, Zeighami et al., 2015) which allows the investigation of a relatively fine-grained organization and represents one of the most frequent choices in resting state ICA analyses. For exploratory reasons and to investigate the stability of the results, we additionally performed all analyses with an ICA based on 20 components. Results are reported in the Supplement S1. To eliminate spurious results each of the 30 components or 30 morphometric networks was thresholded at z = 3.5 and binarized (Beckmann et al., 2009, Pichet Binette et al., 2020). Finally, for each participant grey matter volume was extracted from each of the 30 morphometric networks.
3. Statistical analyses
3.1. Grey matter volume
To investigate group differences in grey matter volume across brain networks, we used repeated-measures ANCOVA with grey matter volume in the 30 networks as within-subjects factor and group as between-subjects factor. Given a significant positive correlation between grey matter volume and total intracranial volume (TIV) in both groups (patients: Pearson’s r = 0.79, p < 0.001; controls: Pearson’s r = 0.67, p < 0.001) and a significant negative correlation between grey matter volume and age (patients: Pearson’s r = −0.46, p < 0.001; controls: Pearson’s r = −0.44, p < 0.001), in addition to scanner site age and TIV were entered as covariates in the repeated-measures ANCOVA. All follow-up analyses were performed with the grey matter values corrected for the influence of age, TIV and scanner site. To investigate the classification performance of the morphometric networks, we performed binary logistic regression using the Matlab function fitglm. It classified OCD patients versus healthy controls with the average grey matter volume in each of the 30 networks as input. We then performed receiver operating characteristic (ROC) analyses and assessed the area under the curve (AUC) to evaluate the classification performance of each network.
3.2. Whole-brain grey matter pattern
To investigate potential differences in grey matter pattern organization between the groups, we assessed grey matter pattern similarity (i.e., homogeneity) by correlating the grey matter volume in the 30 morphometric networks of each individual to the grey matter volume in the 30 brain networks of every other subject.
Hence, this parameter indicates the similarity or correlation of the whole-brain network profile from one subject with the whole-brain network profile of all other subjects (i.e., it illustrates the homogeneity of the group with regard to the whole-brain grey matter pattern).
This resulted in a 111 × 111 matrix of whole-brain grey matter pattern between all healthy controls and a 117 × 117 matrix of whole-brain grey matter pattern between all patients. To investigate whether groups differed in the variability of these grey matter patterns, we used the Levene’s Test for the comparison of variances.
3.3. Heterogeneity of grey matter volumes
Finally, we investigated potential differences in intra-network variability of grey matter volume between the groups by calculating the coefficient of variation (i.e., standard deviation divided by mean of grey matter volume) in each of the 30 networks. Thus, this parameter indicates the similarity or variability of the grey matter volume from each network between the subjects as well as across all 30 networks. We used the modified signed-likelihood ratio (MSLR) test from the R software package cvequality (https://cran.r-project.org/web/packages/cvequality/index.html) version 0.1.3 (Marwick, 2019) with 1000 simulations to test for significant differences in the coefficients of variation of grey matter volume between groups.
3.4. Correlations with clinical scores
Finally, a potential association between clinically relevant information (Y-BOCS, duration of illness) and grey matter volume as well of grey matter pattern variability were assessed by Pearson correlations. The correlation with duration of illness was performed both with and without correcting for age and TIV. Results were considered significant at p < 0.008 (i.e., p = 0.05 divided by 6 correlations).
4. Results
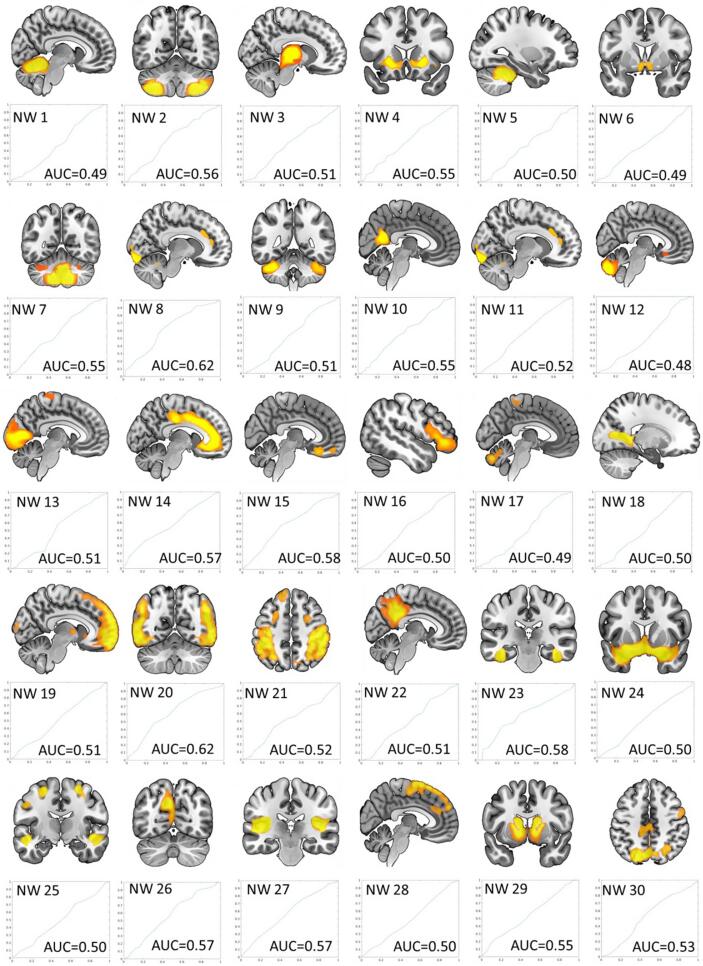
The 30 morphometric networks are shown in Fig. 1 and their anatomical description as determined by the probability maps implemented in the JuBrain Anatomy toolbox (Eickhoff et al., 2005) can be found in Supplement S2. The majority of morphometric networks showed clearly defined anatomical regions, such as the cerebellum, basal ganglia, cingulate cortex or the thalamus. Most networks also displayed bilateral distribution. The networks with lower component numbers were more focused.
Fig. 1.
ICA-derived morphometric networks (NW) with component numbers ascending from top left to bottom right and ROC curves with AUC values for each network. The x- and y-axes of the ROC curves contain values ranging from 0 to 1 with the y-axis showing the true positive rate (i.e., sensitivity) and the x-axis illustrating the false positive rate (i.e., 1-specificity).
4.1. Grey matter volume differences between groups
Results of the repeated-measures ANCOVA, with grey matter volume of the 30 networks as the within-subject factor, group as the between-subject factor and age, TIV and scanner site as covariates, showed a significant main effect of group (F(1, 223) = 4.71, p < 0.03) indicating a significantly smaller grey matter volume in patients compared to controls. Moreover, there was a significant main effect of network-related grey matter volume (F(8,1725) = 5.93, p < 0.001) illustrating significant differences in grey matter volume between the networks.
Moreover, there was a significant interaction between network-related grey matter volume and sequence (F(8, 1725) = 7.99, p = 0.001) as well as a significant interaction between network-related grey matter volume and group (F(8, 1725) = 2.04, p = 0.04). Post-hoc tests indicated significant group differences for network 8, 20 and 27 comprising mainly the amygdala, hippocampus, planum temporale, temporal pole and occipital regions.
With regard to the covariates, there was a significant interaction between grey matter volume and age (F(8, 1725) = 17.61, p < 0.001) as well as a significant interaction between grey matter volume and TIV (F(8, 1725) = 7.63, p < 0.001). All between-subject effects were Greenhouse-Geisser corrected due to a significant result in the Mauchly sphericity test.
The AUCs from the ROC analyses, representing the overall performance of each population-derived morphometric network to classify patients versus controls, showed poor classification performance. This demonstrated that the network-related grey matter volume is not a suitable parameter to differentiate the groups (i.e., all AUC’s ranged inbetween 0.49 and 0.62, see ROC curves in Fig. 1).
4.2. Whole-brain grey matter pattern differences between groups
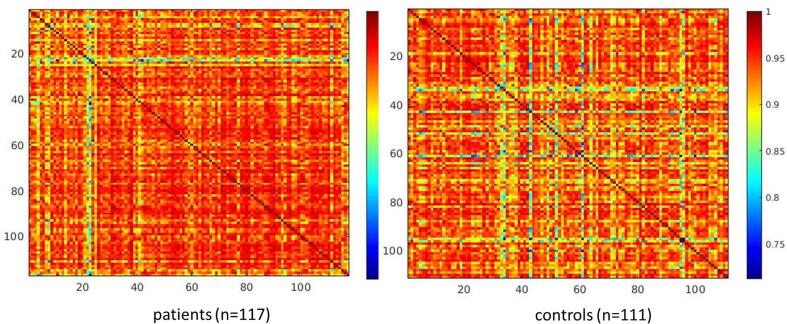
To investigate potential differences in whole-brain grey matter pattern between groups we assessed grey matter pattern similarity (i.e., homogeneity) by correlating the grey matter volume in the 30 morphometric networks of each individual with the grey matter volume in the 30 brain networks of every other subject. These correlations indicate how similar one’s whole-brain organization is with every other individual. Fig. 2 shows the grey matter pattern similarity matrix for each group.
Fig. 2.
Correlation matrix for each group showing the correlation between grey matter volume in the 30 morphometric networks between all individuals. Colour-coded Pearson’s r values indicate how one’s whole-brain organization is similar to every other individual.
The results illustrate a visibly lower variability in the patient group. Comparing the variances between the groups using Levene’s test corroborated the visual impression and revealed a significant result indicating a significantly higher homogeneity in the patient group (F(1,226) = 16.56, p < 0.001).
4.3. Differences in heterogeneity of grey matter volumes between groups
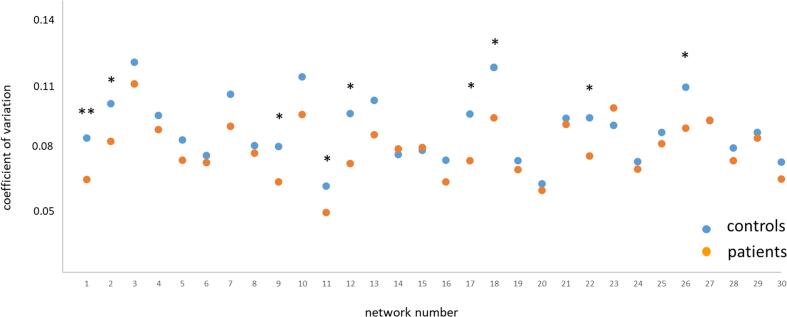
The modified signed-likelihood ratio (MSLR) test to assess significant differences in the coefficients of variation of grey matter volume (see Fig. 3) showed a main effect of group on coefficient of variation across all 30 networks (p = 0.02), as well as on network 2 (p = 0.038), network 9 (p = 0.011), network 11 (p = 0.009), network 12 (p = 0.004), network 17 (p = 0.005), network 18 (p = 0.014), network 22 (p = 0.021) and network 26 (p = 0.029) at an uncorrected level and on network 1 (p = 0.0004) at a corrected level (i.e., corrected for the 30 network yielding a p-value of p = 0.002).
Fig. 3.
Network-specific heterogeneity as assessed by the coefficient of variation for both groups. Group differences were investigated using the modified signed-likelihood ratio (MSLR) test; * significant at p < 0.05 uncorrected, ** significant at p < 0.05 corrected for multiple comparisons (i.e., 30 networks).
All differences were based on a decreased coefficient of variation (i.e., heterogeneity) in patients relative to healthy controls. Thus, these results revealed a significantly lower heterogeneity in patients both across all networks as well as in several single networks, confirming the results of the overall grey matter pattern analysis.
4.4. No association with clinical scores
The correlation between Y-BOCS total and grey matter volume across all networks as well as grey matter pattern variability yielded no significant results (r = −0.09, p = 0.33; r = −0.04, p = 0.71, respectively). The correlation between duration of illness and grey matter volume across all networks (uncorrected for TIV and age) showed a highly significant correlation (r = −0.37, p < 0.00005) which was no longer significant after correcting for age and TIV (r = 0.06, p = 0.56). The correlation with grey matter pattern variability showed a non-significant result on the corrected level (r = 0.21, p = 0.03) and was also not significant after correcting for age and TIV (r = 0.04, p = 0.68).
5. Discussion
5.1. Grey matter volume
The current study investigated grey matter volume within population-derived structural covariance networks in a large sample of OCD patients and healthy participants. We found a lower grey matter volume in patients compared to healthy controls across all networks. Although specific regions, such as the pallidum, have been found to exhibit an increased grey matter volume in patients compared to control participants (Boedhoe et al., 2018), most previous studies employing voxel- or surface-based methods reported a reduced grey matter volume of cortical regions (Boedhoe et al., 2018, Rotge et al., 2010). Present findings of an overall decreased network-related grey matter volume in patients are in line with previous studies, albeit it has to be kept in mind that our results are based on population-based networks (i.e., networks derived from the volume maps of both patients and healthy controls). This most probably explains why the classification performance of the single covariance networks turned out to be very poor and implies that the volume differences (i.e., in terms of neuronal density and architecture) between groups within these common networks are very subtle. Thus, present results differ from previous findings in other patient populations, such as Alzheimer’s disease (Pichet Binette et al., 2020) where – not surprisingly - most structural covariance networks showed a relatively high classification performance. Hence, present findings suggest gross volumetric alterations are not present within covariance-based morphometric networks in OCD.
5.2. Grey matter pattern
When analysing grey matter pattern similarity (i.e., homogeneity) in the 30 morphometric networks we found a significantly lower variability in the patient group (Fig. 2). This finding was moreover supported by a significantly decreased heterogeneity in the patient group across all networks (i.e., an overall decreased coefficient of variation) as well as a significantly decreased heterogeneity within several specific networks containing predominantly frontal and cerebellar regions. Thus, the study’s findings give rise to the impression of OCD as an “equalizing” process which, to some degree, seems to reduce “healthy” or “normal” interindividual morphometric heterogeneity. As previously mentioned, it is assumed that brain regions that increase or decrease in volume at the same rate over the course of years demonstrate strong structural covariance across individuals from the same “population”, such as patients suffering from OCD (Alexander-Bloch et al., 2013a). Moreover, it is well-known that the grey matter structure of the human brain undergoes strong alterations during childhood and adolescence (Raznahan et al., 2011, Shaw et al., 2008). Given that in many cases OCD symptoms first manifest during adolescence or early adulthood (Brakoulias et al., 2017), alterations in structural covariance due to clinical symptoms during this sensitive neurodevelopmental period are plausible and might to some degree explain the increased uniformity or homogeneity in structural covariance in the patient group. Our own recent work investigating structural covariance in OCD based on alterations in gyrification likewise pointed to changes time-locked to a specific neurodevelopmental period (Reess et al., 2018b). However, the ontogenetic development of cortical gyrification is only partially comparable to the developmental process of grey matter volume (Armstrong et al., 1995).
Another relevant mechanism of structural covariance is use-dependent neuroplasticity. Synchronous neuronal firing in combination with synaptogenesis between these neurons (Bi and Poo, 1999, Katz and Shatz, 1996) is the basis of use-dependent coordinated growth and explains findings on grey matter volume increases in association with intensive learning, training or long-term experience (Koch et al., 2016, Kühn et al., 2014, Niemann et al., 2014, Wu et al., 2020). This mechanism should also be taken into consideration when interpreting grey matter volume changes in OCD such as those mentioned previously in patients suffering from motor-related compulsion (Wagner et al., 2019). Although other studies could not detect any direct association with specific symptoms, we believe that common characteristics of OCD such as increased fear responses, impaired inhibition or intolerance of uncertainty could drive the increased homogeneity in structural covariance. It should be taken into consideration, however, that without doubt influencing factors not assessed in this study, such as life-time medication exposure, therapeutic treatment or other environmental aspects, also had a significant impact on use-dependent neuroplasticity.
Moreover, our finding of a decreased inter-network heterogeneity (i.e., increased homogeneity in structural covariance across several networks) within the patient group can most probably be traced back to this mechanism, as well. Here, experience-dependent changes in grey matter structure due to symptomatology and common disorder characteristics mentioned before which, in its whole complexity, are known to involve a variety of core regions independent from individual symptom profile or symptom severity, may well explain an increased uniformity (or decreased heterogeneity) of structural covariance in the patient group. The lacking association with symptom severity corroborates this assumption and indicates that the alterations in structural covariance might represent a rather stable marker of the disorder that is independent from the current clinical status. Future studies, however, should investigate the influence of additional factors such as life-time medication or therapeutic treatment in more detail.
One final influencing factor should not go unnoticed when talking about structural covariance. Several studies show that common genetic predispositions explains a large percentage of the structural covariance in healthy people (Schmitt et al., 2008, Schmitt et al., 2010). More specifically, it has been demonstrated that 5-HTTLPR polymorphism, which is also being discussed to influence the susceptibility to OCD (Sinopoli et al., 2017), has an impact on structural covariance between the amygdala and the anterior cingulate (Pezawas et al., 2005). In addition, the covariance between homologous contralateral regions has been reported to have predominantly strong genetic underpinnings (Alexander-Bloch et al., 2013a, Schmitt et al., 2009). The fact that our finding of an increased homogeneity of structural covariance is based on predominantly homologous, bilateral networks, might point to the assumption that common genetic underpinnings related to OCD and to the development of structural covariance could have also played a role in the development of a more uniform, disease-specific structural covariance. This calls for classifying patients and controls according to their genetic profile when assessing their structural covariance.
Due to a lack of genetic information and other data (such as, for instance, anxiety, depression, IQ or socio-economic status) as well as the cross-sectional design of the present study it is, however, difficult to reliably determine which factors have significantly contributed to the increased uniformity or homogeneity of structural covariance that we encountered in our OCD patient sample.
To conclude, the present study investigating grey matter volume of population-derived morphometric networks in patients with OCD showed that – despite an overall difference in network-related grey matter volume – there were only subtle group differences in grey matter volume of population-derived networks. Nonetheless, there were distinctions with regard to the similarity of these networks. Our findings suggest that these single covariance networks can not serve as disease-related characteristics or predictors allowing for a morphometric differentiation between patients with OCD and healthy individuals. Moreover, we found significant differences in the heterogeneity across networks as well as differences in network-specific heterogeneity. On a speculative note, the pathological mechanisms of OCD might leave a morphometric imprint by reducing the healthy interindividual morphometric variability.
Due to the cross-sectional design of the study as well as the lack of additional data on potential influencing factors (such as, e.g., socio-economic status) a significant impact of other parameters cannot be ruled out. Thus, there could be influencing factors characterizing the OCD group that do not constitute a consequence of the disorder and might make the patient group more similar from the start.
Nevertheless, our results clearly speak against profound and extended morphometric alterations in OCD but rather imply discreet processes seemingly leading to a more pronounced morphometric similarity within the patient population.
6. Role of the funding source
This study was supported by German Research Foundation (DFG) grants to KK (DFG KO 3744/7-1). The funding agencies had no influence on the design and conduct of the study including collection, management, analysis and interpretation of the data, as well as preparation, review or approval of the manuscript. The authors reported no biomedical financial interests or potential conflicts of interest.
CRediT authorship contribution statement
Kathrin Koch: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Writing - original draft. Daniela Rodriguez Manrique: Writing - original draft. Oana Georgiana Rus-Oswald: Data curation, Investigation, Writing - original draft. Deniz A. Gürsel: Data curation, Investigation. Götz Berberich: Conceptualization, Investigation. Miriam Kunz: Formal analysis, Methodology. Claus Zimmer: Conceptualization, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102727.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Alexander-Bloch A., Giedd J.N., Bullmore E. Imaging structural co-variance between human brain regions. Nat. Rev. Neurosci. 2013;14:322–336. doi: 10.1038/nrn3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander-Bloch A., Raznahan A., Bullmore E., Giedd J. The convergence of maturational change and structural covariance in human cortical networks. J. Neurosci. 2013;33:2889–2899. doi: 10.1523/JNEUROSCI.3554-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong E., Schleicher A., Omran H., Curtis M., Zilles K. The ontogeny of human gyrification. Cereb. Cortex. 1995;5:56–63. doi: 10.1093/cercor/5.1.56. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., Mackay C.E., Filippini N., Smith S.M. Group comparison of resting-state FMRI data using multi-subject ICA and dual regression. Neuroimage. 2009;47:S148. [Google Scholar]
- Bi G.-Q., Poo M.-M. Distributed synaptic modification in neural networks induced by patterned stimulation. Nature. 1999;401:792–796. doi: 10.1038/44573. [DOI] [PubMed] [Google Scholar]
- Boedhoe P.S., Schmaal L., Abe Y., Alonso P., Ameis S.H., Anticevic A., Arnold P.D., Batistuzzo M.C., Benedetti F., Beucke J.C. Cortical abnormalities associated with pediatric and adult obsessive-compulsive disorder: findings from the ENIGMA Obsessive-Compulsive Disorder Working Group. Am. J. Psychiatry. 2018;175:453–462. doi: 10.1176/appi.ajp.2017.17050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boedhoe P.S., Schmaal L., Abe Y., Ameis S.H., Arnold P.D., Batistuzzo M.C., Benedetti F., Beucke J.C., Bollettini I., Bose A. Distinct subcortical volume alterations in pediatric and adult OCD: a worldwide meta-and mega-analysis. Am. J. Psychiatry. 2017;174:60–69. doi: 10.1176/appi.ajp.2016.16020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakoulias V., Starcevic V., Belloch A., Brown C., Ferrao Y.A., Fontenelle L.F., Lochner C., Marazziti D., Matsunaga H., Miguel E.C. Comorbidity, age of onset and suicidality in obsessive–compulsive disorder (OCD): an international collaboration. Compr. Psychiatry. 2017;76:79–86. doi: 10.1016/j.comppsych.2017.04.002. [DOI] [PubMed] [Google Scholar]
- Coppen E.M., van der Grond J., Hafkemeijer A., Rombouts S.A., Roos R.A. Early grey matter changes in structural covariance networks in Huntington's disease. NeuroImage. 2016;12:806–814. doi: 10.1016/j.nicl.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S.B., Stephan K.E., Mohlberg H., Grefkes C., Fink G.R., Amunts K., Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Hafkemeijer A., Möller C., Dopper E.G., Jiskoot L.C., van den Berg-Huysmans A.A., van Swieten J.C., van der Flier W.M., Vrenken H., Pijnenburg Y.A., Barkhof F. Differences in structural covariance brain networks between behavioral variant frontotemporal dementia and Alzheimer's disease. Hum. Brain Mapp. 2016;37:978–988. doi: 10.1002/hbm.23081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E., Woolrich M.W., Smith S.M. Fsl. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Katz L.C., Shatz C.J. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Koch K., Reess T.J., Rus O.G., Zimmer C. Extensive learning is associated with gray matter changes in the right hippocampus. NeuroImage. 2016;125:627–632. doi: 10.1016/j.neuroimage.2015.10.056. [DOI] [PubMed] [Google Scholar]
- Koch K., Reeß T.J., Rus O.G., Zimmer C., Zaudig M. Diffusion tensor imaging (DTI) studies in patients with obsessive-compulsive disorder (OCD): a review. J. Psychiatr. Res. 2014;54:26–35. doi: 10.1016/j.jpsychires.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Kühn S., Gleich T., Lorenz R.C., Lindenberger U., Gallinat J. Playing Super Mario induces structural brain plasticity: gray matter changes resulting from training with a commercial video game. Mol. Psychiatry. 2014;19:265–271. doi: 10.1038/mp.2013.120. [DOI] [PubMed] [Google Scholar]
- Lee P.-L., Chou K.-H., Lu C.-H., Chen H.-L., Tsai N.-W., Hsu A.-L., Chen M.-H., Lin W.-C., Lin C.-P. Extraction of large-scale structural covariance networks from grey matter volume for Parkinson’s disease classification. Eur. Radiol. 2018;28:3296–3305. doi: 10.1007/s00330-018-5342-1. [DOI] [PubMed] [Google Scholar]
- Marwick, K.K., 2019. cvequality: Tests for the Equality of Coefficients of Variation from Multiple Groups. R software package version 0.1.3. Retrieved from https://github.com/benmarwick/cvequality, on 07/01/2019.
- Nakamae T., Narumoto J., Sakai Y., Nishida S., Yamada K., Kubota M., Miyata J., Fukui K. Reduced cortical thickness in non-medicated patients with obsessive-compulsive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2012;37:90–95. doi: 10.1016/j.pnpbp.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Niemann C., Godde B., Voelcker-Rehage C. Not only cardiovascular, but also coordinative exercise increases hippocampal volume in older adults. Front. Aging Neurosci. 2014;6:170. doi: 10.3389/fnagi.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L., Meyer-Lindenberg A., Drabant E.M., Verchinski B.A., Munoz K.E., Kolachana B.S., Egan M.F., Mattay V.S., Hariri A.R., Weinberger D.R. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat. Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Pichet Binette A., Gonneaud J., Vogel J.W., La Joie R., Rosa-Neto P., Collins D.L., Poirier J., Breitner J.C., Villeneuve S., Vachon-Presseau E. Morphometric network differences in ageing versus Alzheimer’s disease dementia. Brain. 2020;143:635–649. doi: 10.1093/brain/awz414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piras F., Piras F., Caltagirone C., Spalletta G. Brain circuitries of obsessive compulsive disorder: a systematic review and meta-analysis of diffusion tensor imaging studies. Neurosci. Biobehav. Rev. 2013;37:2856–2877. doi: 10.1016/j.neubiorev.2013.10.008. [DOI] [PubMed] [Google Scholar]
- Piras F., Piras F., Chiapponi C., Girardi P., Caltagirone C., Spalletta G. Widespread structural brain changes in OCD: a systematic review of voxel-based morphometry studies. Cortex. 2015;62:89–108. doi: 10.1016/j.cortex.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Raznahan A., Shaw P., Lalonde F., Stockman M., Wallace G.L., Greenstein D., Clasen L., Gogtay N., Giedd J.N. How does your cortex grow? J. Neurosci. 2011;31:7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reess T.J., Rus O.G., Gürsel D.A., Schmitz-Koep B., Wagner G., Berberich G., Koch K. Association between hippocampus volume and symptom profiles in obsessive–compulsive disorder. NeuroImage. 2018;17:474–480. doi: 10.1016/j.nicl.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reess T.J., Rus O.G., Gürsel D.A., Schmitz-Koep B., Wagner G., Berberich G., Koch K. Network-based decoupling of local gyrification in obsessive-compulsive disorder. Hum. Brain Mapp. 2018;39:3216–3226. doi: 10.1002/hbm.24071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotge J.-Y., Langbour N., Guehl D., Bioulac B., Jaafari N., Allard M., Aouizerate B., Burbaud P. Gray matter alterations in obsessive–compulsive disorder: an anatomic likelihood estimation meta-analysis. Neuropsychopharmacology. 2010;35:686–691. doi: 10.1038/npp.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rus O., Reess T., Wagner G., Zaudig M., Zimmer C., Koch K. Hypogyrification in obsessive-compulsive disorder. Psychol. Med. 2017;47:1053. doi: 10.1017/S0033291716003202. [DOI] [PubMed] [Google Scholar]
- Rus O.G., Reess T.J., Wagner G., Zaudig M., Zimmer C., Koch K. Structural alterations in patients with obsessive–compulsive disorder: a surface-based analysis of cortical volume, surface area and thickness. J. Psychiatry Neurosci. 2017 doi: 10.1503/jpn.170030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt J., Lenroot R., Wallace G., Ordaz S., Taylor K., Kabani N., Greenstein D., Lerch J., Kendler K., Neale M. Identification of genetically mediated cortical networks: a multivariate study of pediatric twins and siblings. Cereb. Cortex. 2008;18:1737–1747. doi: 10.1093/cercor/bhm211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt J.E., Lenroot R.K., Ordaz S.E., Wallace G.L., Lerch J.P., Evans A.C., Prom E.C., Kendler K.S., Neale M.C., Giedd J.N. Variance decomposition of MRI-based covariance maps using genetically informative samples and structural equation modeling. Neuroimage. 2009;47:56–64. doi: 10.1016/j.neuroimage.2008.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt J.E., Wallace G.L., Lenroot R.K., Ordaz S.E., Greenstein D., Clasen L., Kendler K.S., Neale M.C., Giedd J.N. A twin study of intracerebral volumetric relationships. Behav. Genet. 2010;40:114–124. doi: 10.1007/s10519-010-9332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Kabani N.J., Lerch J.P., Eckstrand K., Lenroot R., Gogtay N., Greenstein D., Clasen L., Evans A., Rapoport J.L. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinopoli V.M., Burton C.L., Kronenberg S., Arnold P.D. A review of the role of serotonin system genes in obsessive-compulsive disorder. Neurosci. Biobehav. Rev. 2017;80:372–381. doi: 10.1016/j.neubiorev.2017.05.029. [DOI] [PubMed] [Google Scholar]
- Subirà M., Cano M., De Wit S.J., Alonso P., Cardoner N., Hoexter M.Q., Kwon J.S., Nakamae T., Lochner C., Sato J.R. Structural covariance of neostriatal and limbic regions in patients with obsessive–compulsive disorder. J. Psychiatry Neurosci. 2016;41:115. doi: 10.1503/jpn.150012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G., Köhler S., Peikert G., de la Cruz F., Reess T.J., Rus O.G., Schultz C.C., Koch K., Bär K.-J. Checking and washing rituals are reflected in altered cortical thickness in obsessive-compulsive disorder. Cortex. 2019;117:147–156. doi: 10.1016/j.cortex.2019.03.012. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Kakeda S., Katsuki A., Ueda I., Ikenouchi A., Yoshimura R., Korogi Y. Whole-brain structural covariance network abnormality in first-episode and drug-naïve major depressive disorder. Psychiatry Res.: Neuroimag. 2020;300 doi: 10.1016/j.pscychresns.2020.111083. [DOI] [PubMed] [Google Scholar]
- Wu, H., Yan, H., Yang, Y., Xu, M., Shi, Y., Zeng, W., Li, J., Zhang, J., Chang, C., Wang, N., 2020. Occupational neuroplasticity in the human brain: a critical review and meta-analysis of neuroimaging studies. Front. Human Neurosci. 14. [DOI] [PMC free article] [PubMed]
- Xu L., Groth K.M., Pearlson G., Schretlen D.J., Calhoun V.D. Source-based morphometry: the use of independent component analysis to identify gray matter differences with application to schizophrenia. Hum. Brain Mapp. 2009;30:711–724. doi: 10.1002/hbm.20540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun J.-Y., Boedhoe P.S., Vriend C., Jahanshad N., Abe Y., Ameis S.H., Anticevic A., Arnold P.D., Batistuzzo M.C., Benedetti F. Brain structural covariance networks in obsessive-compulsive disorder: a graph analysis from the ENIGMA Consortium. Brain. 2020;143:684–700. doi: 10.1093/brain/awaa001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun J.-Y., Jang J.H., Kim S.N., Jung W.H., Kwon J.S. Neural correlates of response to pharmacotherapy in obsessive-compulsive disorder: individualized cortical morphology-based structural covariance. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2015;63:126–133. doi: 10.1016/j.pnpbp.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Zeighami Y., Ulla M., Iturria-Medina Y., Dadar M., Zhang Y., Larcher K.-M.-H., Fonov V., Evans A.C., Collins D.L., Dagher A. Network structure of brain atrophy in de novo Parkinson's disease. Elife. 2015;4 doi: 10.7554/eLife.08440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Chye Y., Braganza L., Fontenelle L.F., Harrison B.J., Parkes L., Sabaroedin K., Maleki S., Yücel M., Suo C. Severity related neuroanatomical alteration across symptom dimensions in obsessive-compulsive disorder. J. Affect. Disorders Rep. 2021;100129 [Google Scholar]
- Zhou C., Gao T., Guo T., Wu J., Guan X., Zhou W., Huang P., Xuan M., Gu Q., Xu X. Structural covariance network disruption and functional compensation in Parkinson’s disease. Front. Aging Neurosci. 2020;12:199. doi: 10.3389/fnagi.2020.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.