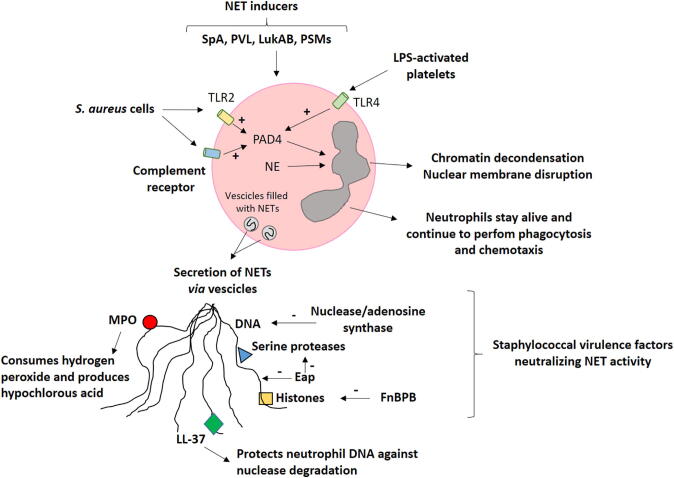
Graphical abstract
Keywords: Neutrophil extracellular traps, Staphylococcus aureus, Virulence factors
Abstract
Neutrophil extracellular traps (NETs) are considered part of the innate human immune system because they are involved in host defense during bacterial infections. NETs are formed by activated neutrophils and consist of a DNA backbone combined with proteins with different biological functions. The activity of NETs can be significantly reduced by a Staphylococcus aureus DNase, which degrades the DNA backbone and enables the liberation of bacteria from NETs, and by Eap, a secreted protein which binds and aggregates linearized DNA, suppressing the formation of NETs. Furthermore, the pathogen can resist NET-mediated killing by expressing the surface protein FnBPB, which neutralizes the bactericidal activity of histones. Finally, the anti-staphylococcal activity of NETs is counteracted and blocked by S. aureus biofilm. Staphylococcal cells and several virulence factors such as protein A and phenol-soluble modulins can also elicit the formation of NETs which in turn can cause tissue injury, enhancing bacterial performance in host colonization. The identification of additional virulence factors involved in NET formation/neutralization could provide the basis for therapeutic interventions against this formidable pathogen.
1. Introduction
The main mechanisms used by neutrophils to clear pathogens include a) phagocytosis, b) release of antimicrobial peptides, c) production of antimicrobial reactive oxygen and nitrogen species, and d) production of neutrophil extracellular traps (NETs) [1]. NETs are fragile structures composed of a branching network of extracellular DNA filaments decorated with cytoskeletal proteins and proteases mainly involved in host defense during bacterial, fungal and viral infections [2]. NETs not only contribute to pathogen elimination but in parallel can cause damage to bystander cells. In sepsis, a systemic acute infective disease with high morbidity and mortality, NETs may promote patient survival [3], although as the disease progresses, they may accumulate in organs and cause detrimental tissue damage [4], [5]. Platelets and NETs cooperate to promote intravascular coagulation during sepsis in mice [6] and platelet-neutrophil interactions induce the release of intravascular NETs that in turn ensnare bacteria from the bloodstream and cause liver damage [7]. Moreover, as revealed by intravital imaging, during a bloodstream infection with methicillin-resistant S. aureus, neutrophils infiltrate the liver and release NETs into the vasculature. The NETs remain anchored to the vascular wall via von Willebrand factor and produce profound hepatic injury [8] (Fig. 1).
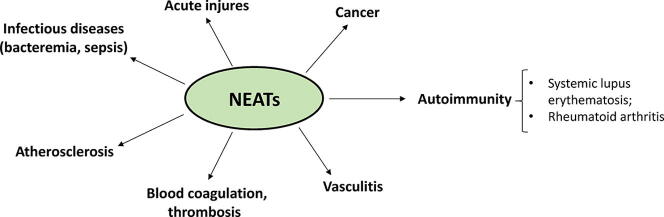
Fig. 1.
Schematic representation of NET involvement in infectious and noninfectious diseases. NET formation induced by staphylococcal bacteremia and sepsis can cause tissue damage and enhance bacterial permeation of deeper structures in the body. NETosis also has a role in noninfectious disease such as autoimmunity (systemic lupus erythematosus and rheumatoid arthritis), atherosclerosis, vasculitis, and cancer.
Importantly, NET formation can also occur in noninfectious chronic inflammation such as autoimmunity (for example, systemic lupus erythematosus and rheumatoid arthritis) [9], vasculitis [10], [11], cancer [12], [13], and atherosclerosis [14], [15]. Moreover, NETs play an important role in thrombosis [16] and induce platelet activation, coagulation, and thrombus formation [17], [18] (Fig. 1). Hence, due to the combination of various proteases, cytotoxic proteins, and enzymes bound to the NETs, the NETs can be a double-edged sword.
In the face of this potentially harmful arsenal of weapons associated with NETs, microorganisms are endowed with a variety of virulence factors that effectively contrast the action of these host structures.
Staphylococcus aureus is the etiological agent of diseases ranging from mild infections to severe diseases such as sepsis, pneumonia, endocarditis, and medical device-associated infections [19]. Among the variety of virulence factors to combat host defenses [20], [21], S. aureus has evolved pathogenetic activities to escape NETs or induce NET formation to facilitate bacterial performance in the colonized host (Fig. 2). In this mini-review, we discuss the mechanisms of NET induction and formation by S. aureus, as well as the S. aureus virulence factors and the role they may have in counteracting the bactericidal activities of NETs.
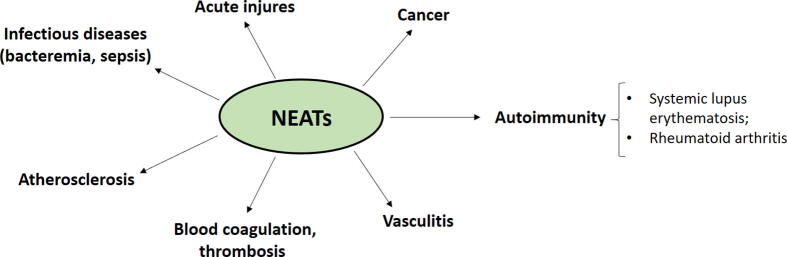
Fig. 2.
Representation of the S. aureus surface and secreted virulence factors. S. aureus is endowed with a number of surface-expressed and secreted virulence factors. The most known surface proteins are the cell wall-anchored proteins that promote adhesion to extracellular matrix proteins and are involved in biofilm formation. Secreted virulence factors include toxins, autolysins, and modulators of the complement pathways.
2. NET formation
NET formation or NETosis can occur by two mechanisms:
2.1. Lytic or suicidal mechanism
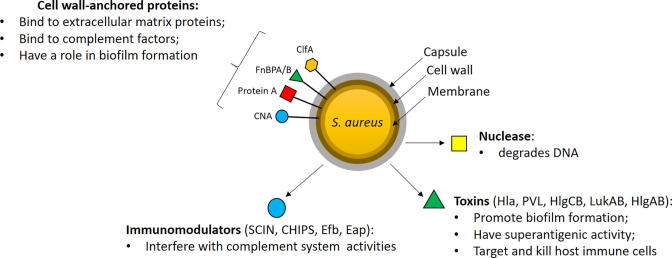
Stimulation of neutrophils with phorbol myristate acetate (PMA), autoantibodies, and cholesterol crystals results in the activation of NADPH oxidase, via PKC and RAF-MEK-ERK signaling pathways and the consequent generation of reactive oxygen species (ROS). This activates peptidyl arginine deiminase (PAD4), which induces the conversion of arginine to citrulline (citrullination) in histones. Through citrullination, PAD4 modulates the conversion of positively charged arginine side chains into uncharged side chains of histones causing chromatin decondensation. The hydrogen peroxide is in turn consumed by MPO to produce hypochlorous acid and other oxidants and the generation of oxidants liberates neutrophil elastase from azurophilic granules, allowing it to translocate to the nucleus where it promotes the further unfolding of chromatin and nuclear membrane disruption. After nuclear membrane disintegration, chromatin is released into the cytosol where it associates with granular and cytosolic proteins [22], [23]. NE also cleaves gasdermin D (GSDMD) in the cytosol to its active form (GSDMD-NT), which forms pores in the plasma membrane and granular membranes. Finally, NETs are released into the extracellular space and neutrophils die [24] (Fig. 3).
Fig. 3.
Lytic NET formation. Different stimuli activate neutrophils via the activation of NADPH oxidase and induce consequent ROS formation. Then, PAD4 is activated and citrullinates histones in the nucleus, causing chromatin decondensation. At same time, myeloperoxidase and elastase translocate from cytosol to the nucleus where they contribute to further unfolding of chromatin. Elastase also activates gasdermin D, which forms pores in the nuclear and plasma membranes. Consequently, chromatin is released in the cytosol and mixed with cytosolic proteins forming NETs. After the secretion of NETs, neutrophils die.
2.2. Nonlytic or vital NET release mechanism
Nonlytic NET formation is a rapid process induced by the recognition of stimuli through complement receptors and does not depend on NADPH activation. Vital NET formation is induced by S. aureus through both complement receptors and TLR-2 ligands, or by Escherichia coli directly via TLR-4 or indirectly via TLR-4 activated platelets. As reported for lytic NET formation, during nonlytic NET formation PAD4 and elastase are activated and translocate to the nucleus where they promote chromatin decondensation. The unfolded chromatin is released into the cytosol, becomes decorated with cytosolic proteins and is finally expelled via vesicular export into the extracellular environment [25]. Importantly, after the release of NETs, this pathway preserves the integrity of neutrophils’ plasma membranes and the anucleated neutrophils named cytoplasts remain alive and retain the capacity to migrate and phagocytose [26], [27] (Fig. 4).
Fig. 4.
Nonlytic NET formation promoted by S. aureus and S. aureus virulence factors. S. aureus cells activate complement receptor or TLR2 on neutrophils. Afterwards, PAD4 is activated and along with elastase translocates to the nucleus where they induce chromatin decondensation. Chromatin decorated with cytosolic proteins is expelled via vesicles without plasma membrane disruption. Loss of the nucleus does not compromise processes such as phagocytosis, chemotaxis and release of cytotoxic molecules. Paradoxically, S. aureus virulence factors such as nuclease, Eap, and FnBPB can interact with specific components of NETs and block their anti-bacterial activity.
3. NET composition, structure, and bactericidal function
The composition of NETs is critical for their pathological impact. Due to its structure and charge, DNA represents the structural unit around which the other components of NETs are assembled. The DNA backbone in NETs is coated with at least 20–30 different proteins including nuclear proteins (histones), granule proteins (NE (neutrophyl elastase), MPO (myeloperoxidase), lactoferrin, cathepsin G, proteinase-3) and cytosolic proteins (S100 calcium-binding proteins A8, A9, A12, as well as actin, α-actinin and calprotectin), which are attached to DNA by electrostatic forces [28], [29], [30].
The mechanisms by which NETs contrast and eventually kill microbial invaders remain controversial. Menegazzi et al showed that when captured by NETs, microorganisms such as S. aureus and Candida albicans are trapped but not killed by these structures [31]. On the other hand, several reports state that NETs efficiently kill bacteria. According to Halverson et al, DNA possesses a rapid bactericidal activity due to its ability to sequester surface bound cations, disrupt membrane integrity and lyse bacterial cells [32]. The cationic antimicrobial peptides such as cathelicidin LL-37 can protect neutrophil-derived DNA from bacterial nuclease degradation [33]. The antimicrobial function of NETs has also been attributed to NET-bound proteins including histones [34], [35], the zinc-chelating protein calprotectin [30], and the granular serine protease cathepsin G [36]. Moreover, MPO associated with NETs exhibits a bactericidal activity needed to kill the pathogen in the presence of hydrogen peroxide [37]. The molecular details by which the different components of NETs interfere with the viability of the microorganisms remain to be elucidated.
4. S. aureus and its arsenal of virulence factors
The explanation for the high pathogenetic potential of S. aureus lies in the ability of the bacterium to express a large number of virulence factors and the capacity to colonize and infect host tissues and organs. The virulence factors are mostly surface-associated or secreted proteinaceous products. A significant number of cell wall-anchored (CWA) proteins act as receptors for extracellular matrix components (fibronectin, fibrinogen, collagen) and play additional roles in biofilm formation [38]. Biofilms are multicellular microbial communities formed on either biological or inorganic surfaces that are encased within a self-produced matrix. Fibronectin-binding proteins FnBPA and FnBPB and clumping factor A (ClfA) are the most relevant adhesins that bind to fibrinogen. Furthermore, as a result of their fibronectin-binding activity, FnBPA and FnBPB mediate S. aureus colonization of fibronectin rich-tissues and fibronectin-mediated host cell invasion. Protein A (SpA), another important CWA protein, binds to the Fc domain of IgG in an incorrect orientation which results in the protection of staphylococci from opsonophagocytosis and killing. SpA also binds to the Fab region of surface IgM located on B lymphocytes, triggering the proliferation and apoptotic collapse of adaptive immune responses [39], [40], [41] (Fig. 2).
S. aureus expresses more than 60 surface-exposed lipoproteins (Lpp), which are involved in a number of metabolic processes such as nutrient uptake (ion, sugar, and amino acids and oligopeptide transporters), enzymes and foldases [42].
S. aureus has also evolved a series of secreted proteins/peptides that functionally interfere with complement C3 and C5 convertase activities and reduce the chemotactic activity of neutrophils. For example, the extracellular adherence protein Eap specifically inhibits both the classical and lectin pathways, disrupting the formation of C4bC2 proconvertase. This results in the inhibition of C3b formation and consequent reduction of S. aureus phagocytosis and killing by neutrophils. Moreover, S. aureus secretes a peptide named CHIPS (Chemotaxis Inhibitory Protein of S. aureus) that binds to formyl peptides and C5a receptors and this reduces neutrophil activation and migration to the site of infection [21]. To further contribute to pathogenesis, S. aureus secretes a vast group of cytotoxins, among them Hla, leukocidins and phenol-soluble modulins (PSMs). After binding to the host receptor ADAM-10, the Hla monomer oligomerizes to form heptameric pores in host cell membranes, causing the lysis and death of many cell types. Leukocidins are composed of two monomers, termed S- and F-subunits. The S-subunit recognizes a specific receptor on the plasma membrane and then dimerizes with the F-subunit. This is followed by oligomerization of three additional dimers to form a complete octameric pore in the plasma membrane of leukocytes. PSMs are cytotoxins belonging to a family of amphipathic peptides (20–25 amino acid residues) that have a variety of roles in S. aureus pathogenesis such as cell lysis, immune modulation and biofilm formation [43] (Fig. 2).
5. S. aureus induces NET formation by specific, secreted virulence factors
Nonlytic NET formation is caused primarily by intact cells of S. aureus (Fig. 2). NET formation is also promoted by released staphylococcal products. PVL (Panton-Valentine Leukocidin), but not the structurally similar leukotoxin gamma hemolysin CB (HlgCB), was reported as the dominant inducer of lytic NET formation [44], [25]. There have been recent insights concerning the mechanism of PVL-promoted NET formation [25]. The mechanism starts with PVL binding to its specific receptor, which in turn leads to its endocytosis by neutrophils. In parallel, cytosolic intracellular Ca2+ is rapidly mobilized from endoplasmic reticulum and a direct interaction of PVL with mitochondrial membranes occurs. The increase of intracellular calcium triggers the activation of small conductance potassium channels, SK and PVL binding to neutrophil mitochondria induces an increase of reactive oxygen species (ROS) and triggers the enzymatic activity of MPO. Finally, PVL promotes PAD4 activation and the consequent formation of citrullinated histone 3.
Importantly, PVL is also a potent cytotoxic factor and can induce rapid death in human and rabbit neutrophils [44]. We can hypothesize that the differential effects of the PVL action in the studies reported above (cell lysis and NETosis in one case and cell death in the second) may be explained, in part, by the experimental conditions used. Specifically, under a critical threshold of PVL concentration, NET formation and release might favorably prepare the host to resist infection.
Aside from PVL, other staphylococcal molecules contribute to NET formation. For example, leukotoxin LukGH (also named LukAB) promotes the release of NETs which, in turn, ensnare but do not kill S. aureus cells. It has been proposed that the ability of LukAB to promote the formation of NETs contributes to the inflammatory response and host defense against S. aureus infection [45]. Notably, LukAB released by staphylococcal biofilm contributes to the neutralization of NETs (see below) [46].
Moreover, phenol-soluble modulin α (PSMα) induces rapid formation of NETs through a ROS-independent pathway [47]. Finally, staphylococcal SpA has been shown to be involved in NET formation [48]. NET formation by S. aureus is facilitated by the cathelicidin LL37 released by epithelial cells and phagocytes upon infection [49] (Fig. 4).
It must be kept in mind that the biological activity of these staphylococcal virulence factors has been determined in vitro and in environmental conditions of pH and ionic strength that may differ from in vivo operational conditions. Moreover, it is not known whether the expression of each of the above factors is expressed in bloodstream and host tissues in sufficient amounts to induce NET formation. Therefore, to validate the supposed role of these NET inducers, more studies with animal models and the use of specific deletion mutants will be needed.
6. NET neutralization by S. Aureus
6.1. Degradation of NETs by staphylococcal nuclease
Several Gram-positive bacteria including S. aureus express 5′-nucleotidases (5′-NTs), enzymes that catalyze the hydrolysis of nucleoside monophosphates to produce nucleosides and phosphate [50]. In a study performed to examine the potential role of S. aureus nuclease in NET degradation and virulence in a murine respiratory tract infection model, Berends et al showed that an isogenic deficient-nuclease mutant lacked the ability to degrade NETs compared with the parental strain and consequently appeared to be more susceptible to extracellular killing by activated neutrophils. On the contrary, nuclease expression by S. aureus enhanced the escape of bacteria from NETs in an in vivo mouse model of S. aureus respiratory tract injection [51]. In a more detailed study Thammavongsa and colleagues found that S. aureus escapes host defenses by converting DNA in NETs to deoxyadenosine (dAdo) through the concerted action of two enzymes, nuclease and adenosine synthase (AdsA). dAdo in turn kills macrophages, preventing their infiltration into S. aureus-induced abscesses and thereby reducing their antimicrobial action [52]. Moreover, data produced by Herzog et al demonstrates that high nuclease activity of S. aureus isolates correlates with long-term persistence and survival within the airways of cystic fibrosis patients due to the protection against NET-mediated killing [53] (Fig. 4). In summary, S. aureus nuclease exhibits a critical role in NET degradation in vivo. However, due to the presence of a number of DNase inhibitors in serum (for example, C1q of the complement system), it remains to be determined whether S. aureus nuclease keeps its activity intact in body fluids.
6.2. Eap protein and its effect on the bactericidal activity of NETs
The Extracellular Adherence Protein Eap blocks NETs formation and activities. By using atomic force microscopy, evidence has been provided that Eap can bind and aggregate linearized DNA. Consistent with this, Eap interferes with the formation of NETs, suggesting that it may protect bacteria from being trapped by structures such as microthrombi (Fig. 1) [54]. Notably, Eap and its homologues Ehp1 and Ehp2 potently inhibit the neutrophil serine proteases (NSPs) elastase (NE), proteinase 3, and cathepsin G [55]. Thus, Eap proteins could potentially block the enzymatic activities of NET proteases. However, considering that the NET-bound NSP could be inactivated by high concentrations of NSP inhibitors such as alpha1-proteinase inhibitor in serum [56], it is unclear whether the serine-protease inhibitory activity of Eap proteins plays an effective role in blocking the anti-bacterial activity of NETs (Fig. 4).
6.3. FnBPB confers resistance to bactericidal activity of NETs
As reported by several authors, histones are expressed and extruded in NETs in abundant amounts, estimated at 2.5 μg/106 neutrophils, such that histones comprise more than two thirds of the total protein content within the NET structure. In a recent study it was discovered that fibronectin-binding protein B (FnBPB) is the main histone receptor and that histone H3 displays the highest affinity. Importantly, an FnBPB-deletion mutant bound less H3 and was more susceptible to histone bactericidal activity, whereas a mutant overexpressing FnBPB bound more H3 and was more resistant to killing by histone. This information raised the question whether inhibition by FnBPB of histone-mediated bacterial killing is biologically significant. As a matter of fact, in a bactericidal assay promoted by NETs it was shown that FnBPB protected staphylococci from killing by NETs, demonstrating that FnBPB-mediated resistance is important when histones are present in a biological relevant milieu [57] (Fig. 4). The histone-neutralizing activity of FnBPB is reminiscent of the behavior of the M1 protein, a classical Streptococcus pyogenes surface virulence factor, which also protects bacteria against released extracellular histones in NETs [58] (Fig. 4).
6.4. S. aureus biofilm induces NETosis and blocks their anti-microbial activity
When they encounter neutrophils, S. aureus biofilms release the leukocidins PVL and HlgAB and produce NETs and cytoplasts. The generated anuclear neutrophils, although still capable of permeating the biofilm structure and phagocytosing bacteria, were not effective at clearing the biofilms. Likewise, the induced NETs were not sufficient for clearing S. aureus biofilms. The inefficiency of these structures is attributed to the leukocidin LukAB, a toxin which promotes S. aureus survival during phagocytosis [46]. The persistence of biofilm bacteria trapped in NETs is also facilitated by S. aureus nuclease-mediated degradation of DNA in NETs, resulting in the dispersal of bacteria and persistence of the chronic infection [59].
7. Conclusions
Virulence factors of S. aureus and the role they play in the formation and destruction of NETs were examined here. Up to now, at least three staphylococcal factors have been identified which interfere with the anti-microbial activity of NETs: a) a nuclease that degrades DNA in NETs; b) the Eap protein that forms complexes with linear DNA and, possibly, stabilizes the DNA structure and protects the molecule from enzymatic attack; 3) FnBPB, a staphylococcal surface protein that neutralizes the antimicrobial activity of histones (Fig. 2). Due to the complexity of the structure of NETs, other staphylococcal factors may target constituents of NETs. Scl-1, a streptococcal collagen-like protein in M1T1 group A Streptococcus, interferes with MPO activity and mediates bacterial survival in NETs [60]. Additionally, the M1 protein allows the survival of S. pyogenes in phagocytic extracellular traps through LL-37 inhibition [61]. Thus, we speculate that unidentified surface or secreted factors of S. aureus can further neutralize the function of enzymes, antimicrobial peptides, or ion chelating agents of NETs.
S. aureus cells can also induce the formation of NETs (Fig. 2) [44]. Specific S. aureus factors such as SpA [62], PVL [25], LukAB [45] and PSMα [49] directly elicit NET formation. The induction of NETosis is promoted by the alpha enolase and pneumolysin from Streptococcus pneumoniae [49], [63], by gingipains of Porphyromonas gingivalis [64], and by hydrogen peroxide produced by Streptococcus sanguinis [65]. Therefore, NET formation induced by bacterial species is a common event. How can we solve the apparent paradox that virulence factors produced to protect bacteria from the host defense mechanisms also promote the formation of anti-bacterial structures? As reported above, NETs may either induce bacterial killing or mediate tissue damage both during acute and chronic inflammation [9], [66], [67]. Thus, it is possible that under specific pathological circumstances, S. aureus (and other bacterial species) benefit more from inducing the formation of NETs and using these structures to damage host tissues than from blocking the antibacterial activity of NETs. This strategy could allow bacteria to have better access to metabolic resources, favor the colonization of deeper tissues and definitively ensure safer and optimal survival in the host.
A better understanding of the biochemical details of these alternative strategies and a clearer definition of the role played by new staphylococcal factors in NET formation and destruction in vivo is key for the development of novel therapeutic approaches to control and combat this formidable pathogen.
Author statement
P.S. and G.P. conceptualized and wrote the manuscript. P.S. and G.P. supervised the overall conceptualization and draft writing. The authors have read and agreed to the published version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We wish to thank Prof. Timothy J. Foster for the fruitful discussions.
References
- 1.Nathan C. Neutrophils and immunity: challenges and opportunities. Review Nat Rev Immunol. 2006;6(3):173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 2.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018;18(2):134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 3.Park S.Y., Shrestha S., Youn Y.-J., Kim J.-K., Kim S.-Y., Kim H.J. Autophagy Primes Neutrophils for Neutrophil Extracellular Trap Formation during Sepsis. Am J Respir Crit Care Med. 2017;196(5):577–589. doi: 10.1164/rccm.201603-0596OC. [DOI] [PubMed] [Google Scholar]
- 4.Saffarzadeh M., Juenemann C., Queisser M.A., Lochnit G., Barreto G., Galuska S.P. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS ONE. 2012;7(2):e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber C. Liver: Neutrophil extracellular traps mediate bacterial liver damage. Nat Rev Gastroenterol Hepatol. 2015;12:251. doi: 10.1038/nrgastro.2015.60. [DOI] [PubMed] [Google Scholar]
- 6.McDonald B., Davis R.P., Kim S.J., Tse M., Esmon C.T. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood. 2017;129(10):1357–1367. doi: 10.1182/blood-2016-09-741298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald B., Urrutia R., Yipp B., Jenne C., Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe. 2012;12(3):324–333. doi: 10.1016/j.chom.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Kolaczkowska E., Jenne C.N., Surewaard B.G.J., Thanabalasuriar A., Lee W.-Y., Sanz M.-J. Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nat Commun. 2015;6(1) doi: 10.1038/ncomms7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jorch S.K., Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat Med. 2017;23(3):279–287. doi: 10.1038/nm.4294. [DOI] [PubMed] [Google Scholar]
- 10.Kessenbrock K., Krumbholz M., Schönermarck U., Back W., Gross W.L., Werb Z. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15(6):623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Söderberg D., Kurz T., Motamedi A., Hellmark T., Eriksson P., Segelmark M. Increased levels of neutrophil extracellular trap remnants in the circulation of patients with small vessel vasculitis, but an inverse correlation to anti-neutrophil cytoplasmic antibodies during remission. Rheumatology (Oxford) 2015;54(11):2085–2094. doi: 10.1093/rheumatology/kev217. [DOI] [PubMed] [Google Scholar]
- 12.Breitbach C.J., De Silva N.S., Falls T.J., Aladl U., Evgin L., Paterson J. Targeting tumor vasculature with an oncolytic virus. Mol Ther. 2011;19(5):886–894. doi: 10.1038/mt.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cedervall J., Zhang Y., Huang H., Zhang L., Femel J., Dimberg A. Neutrophil Extracellular Traps Accumulate in Peripheral Blood Vessels and Compromise Organ Function in Tumor-Bearing Animals. Cancer Res. 2015;75(13):2653–2662. doi: 10.1158/0008-5472.CAN-14-3299. [DOI] [PubMed] [Google Scholar]
- 14.Swirski F.K., Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339(6116):161–166. doi: 10.1126/science:1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nahrendorf M., Swirski F.K. Neutrophil-macrophage communication in inflammation and atherosclerosis. Science. 2015;349(6245):237–238. doi: 10.1126/science:aac7801. [DOI] [PubMed] [Google Scholar]
- 16.Martinod K., Wagner D.D. Thrombosis: tangled up in NETs. Blood. 2014;123:2768–2776. doi: 10.1182/blood-2013-10-463646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs T.A., Brill A., Duerschmied D., Schatzberg D., Monestier M., Myers D.D. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107(36):15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kambas K., Mitroulis I., Ritis K. The emerging role of neutrophils in thrombosis-the journey of TF through NETs. Front Immunol. 2012;3:385. doi: 10.3389/fimmu.2012.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowy F.D. Staphylococcus aureus infections. N Engl J Med. 1998;339(8):520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 20.Foster T.J. The MSCRAMM Family of Cell-Wall-Anchored Surface Proteins of Gram-Positive Cocci. Trends Microbiol. 2019;27(11):927–941. doi: 10.1016/j.tim.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 21.de Jong N.W.M., van Kessel K.P.M., van Strijp J.A.G., Fischetti V.A., Novick R.P., Ferretti J.J. Immune Evasion by Staphylococcus aureus. Microbiol Spectr. 2019;7(2) doi: 10.1128/microbiolspec.GPP3-0061-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenny E.F., Herzig A., Krüger R., Muth A., Mondal S. Diverse stimuli engage different neutrophil extracellular trap pathways. Elife. 2017;6 doi: 10.7554/eLife.24437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sollberger G., Tilley D.O., Zychlinsky A. Neutrophil Extracellular Traps: The Biology of Chromatin Externalization. Dev Cell. 2018;44(5):542–553. doi: 10.1016/j.devcel.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Sollberger G., Choidas A., Burn G.L., Habenberger P., Di Lucrezia R., Kordes S. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci Immunol. 2018;3(26):eaar6689. doi: 10.1126/sciimmunol.aar6689. [DOI] [PubMed] [Google Scholar]
- 25.Pilsczek F.H., Salina D., Poon K.K.H., Fahey C., Yipp B.G., Sibley C.D. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol. 2010;185(12):7413–7425. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 26.Yipp B.G., Petri B., Salina D., Jenne C.N., Scott B.N.V., Zbytnuik L.D. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med. 2012;18(9):1386–1393. doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yipp B.G., Kubes P. NETosis: how vital is it? Blood. 2013;122:2784–2794. doi: 10.1182/blood-2013-04-457671. [DOI] [PubMed] [Google Scholar]
- 28.Chapman E.A., Lyon M., Simpson D., Mason D., Beynon R.J. Caught in a Trap? Proteomic Analysis of Neutrophil Extracellular Traps in Rheumatoid Arthritis and Systemic Lupus Erythematosus. Front Immunol. 2019;10:423. doi: 10.3389/fimmu.2019.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petretto A., Bruschi M., Pratesi F., Croia C., Candiano G. Neutrophil extracellular traps (NET) induced by different stimuli: A comparative proteomic analysis. PLoS One. 2019;14 doi: 10.1371/journal.pone.0218946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urban C.F., Ermert D., Schmid M., Abu-Abed U., Goosmann C. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menegazzi R., Decleva E., Dri P. Killing by neutrophil extracellular traps: fact or folklore? Blood. 2012;119:1214–1216. doi: 10.1182/blood-2011-07-364604. [DOI] [PubMed] [Google Scholar]
- 32.Halverson T.W.R., Wilton M., Poon K.K.H., Petri B., Lewenza S., Weiss D. DNA is an antimicrobial component of neutrophil extracellular traps. PLoS Pathog. 2015;11(1):e1004593. doi: 10.1182/blood-2011-07-364604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neumann A., Völlger L., Berends E.T.M., Molhoek E.M., Stapels D.A.C., Midon M. Novel role of the antimicrobial peptide LL-37 in the protection of neutrophil extracellular traps against degradation by bacterial nucleases. J Innate Immun. 2014;6(6):860–868. doi: 10.1159/000363699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richards R.C., O'Neil D.B., Thibault P., Ewart K.V. Histone H1: an antimicrobial protein of Atlantic salmon (Salmo salar) Biochem Biophys Res Commun. 2001;284(3):549–555. doi: 10.1006/bbrc.2001.5020. [DOI] [PubMed] [Google Scholar]
- 35.Parseghian M.H., Luhrs K.A. Beyond the walls of the nucleus: the role of histones in cellular signaling and innate immunity. Biochem Cell Biol. 2006;84:589–604. doi: 10.1139/o06-082. [DOI] [PubMed] [Google Scholar]
- 36.Lappann M., Danhof S., Guenther F., Olivares-Florez S., Mordhorst I.L., Vogel U. In vitro resistance mechanisms of Neisseria meningitidis against neutrophil extracellular traps. Mol Microbiol. 2013;89(3):433–449. doi: 10.1111/mmi.2013.89.issue-310.1111/mmi.12288. [DOI] [PubMed] [Google Scholar]
- 37.Parker H., Albrett A.M., Kettle A.J., Winterbourn C.C. Myeloperoxidase associated with neutrophil extracellular traps is active and mediates bacterial killing in the presence of hydrogen peroxide. J Leukoc Biol. 2012;91(3):369–376. doi: 10.1189/jlb.0711387. [DOI] [PubMed] [Google Scholar]
- 38.Speziale P., Pietrocola G., Foster T.J., Geoghegan J.A. Protein-based biofilm matrices in Staphylococci. Front Cell Infect Microbiol. 2014;4:171. doi: 10.3389/fcimb.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foster T.J., Geoghegan J.A., Ganesh V.K., Höök M. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol. 2014;12:49–62. doi: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foster T.J., Fischetti V.A., Novick R.P., Ferretti J.J., Portnoy D.A., Braunstein M. Surface Proteins of Staphylococcus aureus. Microbiol Spectr. 2019;7(4) doi: 10.1128/microbiolspec.GPP3-0046-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Speziale P., Pietrocola G. The Multivalent Role of Fibronectin-Binding Proteins A and B (FnBPA and FnBPB) of Staphylococcus aureus in Host Infections. Front Microbiol. 2020;11:2054. doi: 10.3389/fmicb.2020.02054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen M.T., Matsuo M., Niemann S., Herrmann M., Götz F. Lipoproteins in Gram-Positive Bacteria: Abundance, Function. Fitness Front Microbiol. 2020;11 doi: 10.3389/fmicb.2020.582582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tam K., Torres V.J. Staphylococcus aureus secreted toxins and extracellular enzymes. Microbiol Spectr. 2019;7 doi: 10.1128/microbiolspec.GPP3-0039-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mazzoleni V., Zimmermann‐Meisse G., Smirnova A., Tarassov I., Prévost G. Staphylococcus aureus Panton-Valentine Leukocidin triggers an alternative NETosis process targeting mitochondria. FASEB J. 2021;35(2) doi: 10.1096/fsb2.v35.210.1096/fj.201902981R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Löffler B., Hussain M., Grundmeier M., Brück M., Holzinger D., Varga G. Staphylococcus aureus panton-valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 2010;6(1):e1000715. doi: 10.1371/journal.ppat.1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malachowa N., Kobayashi S.D., Freedman B., Dorward D.W., DeLeo F.R. Staphylococcus aureus leukotoxin GH promotes formation of neutrophil extracellular traps. J Immunol. 2013;191(12):6022–6029. doi: 10.4049/jimmunol.1301821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhattacharya M., Berends E.T.M., Chan R., Schwab E., Roy S., Sen C.K. Staphylococcus aureus biofilms release leukocidins to elicit extracellular trap formation and evade neutrophil-mediated killing. Proc Natl Acad Sci U S A. 2018;115(28):7416–7421. doi: 10.1073/pnas.1721949115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Björnsdottir H., Dahlstrand Rudin A., Klose F.P., Elmwall J., Welin A., Stylianou M. Phenol-Soluble Modulin α Peptide Toxins from Aggressive Staphylococcus aureus Induce Rapid Formation of Neutrophil Extracellular Traps through a Reactive Oxygen Species-Independent Pathway. Front Immunol. 2017;8 doi: 10.3389/fimmu.2017.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoppenbrouwers T., Sultan A.R., Abraham T.E., Lemmens-den Toom N.A., Hansenová Maňásková S., van Cappellen W.A. Staphylococcal Protein A Is a Key Factor in Neutrophil Extracellular Traps Formation. Front Immunol. 2018;9 doi: 10.3389/fimmu.2018.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neumann A., Berends E.T., Nerlich A., Molhoek E.M., Gallo R.L. The antimicrobial peptide LL-37 facilitates the formation of neutrophil extracellular traps. Biochem J. 2014;464:3–11. doi: 10.1042/BJ20140778. [DOI] [PubMed] [Google Scholar]
- 51.Soh K.Y., Loh J.M.S., Proft T. Cell wall-anchored 5'-nucleotidases in Gram-positive cocci. Mol Microbiol. 2020;113(4):691–698. doi: 10.1111/mmi.v113.410.1111/mmi.14442. [DOI] [PubMed] [Google Scholar]
- 52.Berends E.T.M., Horswill A.R., Haste N.M., Monestier M., Nizet V., von Köckritz-Blickwede M. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. J Innate Immun. 2010;2(6):576–586. doi: 10.1159/000319909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thammavongsa V., Missiakas D.M., Schneewind O. Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science. 2013;342(6160):863–866. doi: 10.1126/science:1242255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herzog S., Dach F., de Buhr N., Niemann S., Schlagowski J., Chaves-Moreno D. High Nuclease Activity of Long Persisting Staphylococcus aureus Isolates Within the Airways of Cystic Fibrosis Patients Protects Against NET-Mediated Killing. Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.0255210.3389/fimmu.2019.02552.s001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eisenbeis J., Saffarzadeh M., Peisker H., Jung P., Thewes N., Preissner K.T. The Staphylococcus aureus Extracellular Adherence Protein Eap Is a DNA Binding Protein Capable of Blocking Neutrophil Extracellular Trap Formation. Front Cell Infect Microbiol. 2018;8 doi: 10.3389/fcimb.2018.0023510.3389/fcimb.2018.00235.s001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stapels D.A.C., Ramyar K.X., Bischoff M., von Kockritz-Blickwede M., Milder F.J., Ruyken M. Staphylococcus aureus secretes a unique class of neutrophil serine protease inhibitors. Proc Natl Acad Sci U S A. 2014;111(36):13187–13192. doi: 10.1073/pnas.1407616111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silverman G.A., Bird P.I., Carrell R.W., Church F.C., Coughlin P.B., Gettins P.G.W. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem. 2001;276(36):33293–33296. doi: 10.1074/jbc.R100016200. [DOI] [PubMed] [Google Scholar]
- 58.Pietrocola G., Nobile G., Alfeo M.J., Foster T.J., Geoghegan J.A., De Filippis V. Fibronectin-binding protein B (FnBPB) from Staphylococcus aureus protects against the antimicrobial activity of histones. J Biol Chem. 2019;294(10):3588–3602. doi: 10.1074/jbc.RA118.005707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Döhrmann S., LaRock C.N., Anderson E.L., Cole J.N., Ryali B., Stewart C. Group A Streptococcal M1 Protein Provides Resistance against the Antimicrobial Activity of Histones. Sci Rep. 2017;7(1) doi: 10.1038/srep43039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhattacharya M., Berends E.T.M., Zheng X., Hill P.J., Chan R., Torres V.J. Leukocidins and the Nuclease Nuc Prevent Neutrophil-Mediated Killing of Staphylococcus aureus Biofilms. Infect Immun. 2020;88(10) doi: 10.1128/IAI.00372-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Döhrmann S., Anik S., Olson J., Anderson E.L., Etesami N., No H. Role for streptococcal collagen-like protein 1 in M1T1 group A Streptococcus resistance to neutrophil extracellular traps. Infect Immun. 2014;82(10):4011–4020. doi: 10.1128/IAI.01921-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lauth X., von Köckritz-Blickwede M., McNamara C.W., Myskowski S., Zinkernagel A.S., Beall B. M1 protein allows Group A streptococcal survival in phagocyte extracellular traps through cathelicidin inhibition. J Innate Immun. 2009;1(3):202–214. doi: 10.1159/000203645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mori Y., Yamaguchi M., Terao Y., Hamada S., Ooshima T., Kawabata S. α-Enolase of Streptococcus pneumoniae induces formation of neutrophil extracellular traps. J Biol Chem. 2012;287(13):10472–10481. doi: 10.1074/jbc.M111.280321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bryzek D., Ciaston I., Dobosz E., Gasiorek A., Makarska A., Sarna M. Triggering NETosis via protease-activated receptor (PAR)-2 signaling as a mechanism of hijacking neutrophils function for pathogen benefits. PLoS Pathog. 2019;15(5):e1007773. doi: 10.1371/journal.ppat.1007773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sumioka R., Nakata M., Okahashi N., Li Y., Wada S. Streptococcus sanguinis induces neutrophil cell death by production of hydrogen peroxide. PLoS One. 2017;12 doi: 10.1371/journal.pone.0172223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Castanheira F.V.S., Kubes P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood. 2019;133:2178–2185. doi: 10.1182/blood-2018-11-844530. [DOI] [PubMed] [Google Scholar]
- 67.Mutua V., Gershwin L.J. A Review of Neutrophil Extracellular Traps (NETs) in Disease: Potential Anti-NETs Therapeutics. Clin Rev Allergy Immunol. 2020:1–18. doi: 10.1007/s12016-020-08804-7. [DOI] [PMC free article] [PubMed] [Google Scholar]