Abstract
Stress is a fundamental biological response that can be associated with alterations in cognitive processes. Unhealthy dietary habits are proposed to modulate this effect, notably through their pro-inflammatory potential. This cross-sectional study aimed to evaluate the influence of an obesogenic dietary pattern with inflammatory potential on stress-induced cognitive alterations in healthy volunteers.
Fifty healthy adult participants were stratified into two diet groups: obesogenic vs. non-obesogenic, based on their self-reported consumption of fat, sugar, and salt, assessed by the French National Program for Nutrition and Health questionnaire and a food frequency questionnaire. Serum high-sensitive C-reactive protein (hsCRP) was measured as a marker of systemic inflammation using ELISA. Verbal memory and sustained attention were evaluated through the Verbal Recognition Memory (VRM) test and the Rapid Visual Information Processing (RVP) test respectively, from the Cambridge Neuropsychological Test Automated Battery. Assessments were performed before and after exposure to the psychological stressor Trier Social Stress Test (TSST). Stress response was evaluated by subjective stress perception, salivary cortisol, blood pressure, and heart rate.
Twenty-two participants (44%) presented an obesogenic diet. Systemic inflammation was significantly higher in the obesogenic diet group (p=0.005). The TSST induced a significant stress response, regardless of dietary habits (Time effect p < 0.001). In the whole sample, exposure to TSST was associated with cognitive changes in the form of impaired performance on the VRM test and overall improved RVP scores. However, the obesogenic diet group exhibited an increased total number of false alarms (Time x Diet: p=0.014) on the RVP test after TSST exposure as well as a greater impairment in immediate verbal recognition on the VRM test (Time x Diet: p=0.002). This effect was not associated with the inflammatory component of the obesogenic diet.
These results suggest that an obesogenic diet may sensitize healthy individuals to the detrimental effects of acute stress on cognitive performance.
Keywords: Obesogenic diet, Inflammation, Acute stress, Verbal memory, Sustained attention
Highlights
-
•
Obesogenic diet is associated with serum levels of high-sensitive C-reactive protein.
-
•
Obesogenic diet potentiates stress-induced cognitive impairments in healthy adults.
-
•
This potentiation effect is not accounted by high-sensitive C-reactive protein.
Abbreviations
- CANTAB®
Cambridge neuropsychological test automated battery
- CRO
contract research organization
- CRP
C-reactive protein
- DBP
diastolic blood pressure
- FET
Fisher's exact test
- FFQ
food frequency questionnaire
- GLM RM
general linear models for repeated measures
- HR
heart rate
- HS
high sensitive
- PNNS
French National Program for Nutrition and Health
- RVPA’
rapid visual information processing signal A'
- SBP
systolic blood pressure
- TSST
Trier social stress test
- VAS
visual analog scale
- VRM
verbal recognition memory
1. Introduction
Psychological stressors have a strong impact on mental and cognitive health (Lupien et al., 2018). Even after acute exposure, stress was shown to alter cognitive abilities, notably attention and episodic memory (Arnsten, 2015; Shields et al., 2017). However, this effect is not always consistent in the literature (Banks et al., 2014; Domes et al., 2004). Such heterogeneity is, in part, explained by the multiplicity of factors that are likely to modulate the relationship between stress and cognitive function, among which dietary habits appear as potent candidates (Adan et al., 2019).
Poor dietary habits, including western diets rich in fat, refined sugar, and salt, have been repeatedly associated with deleterious health outcomes. In addition to promote overweight and obesity, these diets can impact brain health and cognitive function (Attuquayefio and Stevenson, 2015; Spencer et al., 2017a). Accordingly, correlational and interventional clinical studies indicate that Western-style diets are associated with smaller left hippocampal volumes (Jacka et al., 2015), and worse cognitive performance (Francis and Stevenson, 2011), notably poorer verbal memory and attention (Attuquayefio et al., 2017; Edwards et al., 2011; Stevenson et al., 2020). Unbalanced diets were also found to increase the risk of long-term cognitive deficits and the development of age-related memory decline, independently of body weight, in preclinical and longitudinal epidemiological studies (Kesse-Guyot et al., 2011; Noble et al., 2021; Spencer et al., 2017b). The specific influence of unbalanced nutritional patterns on stress-induced cognitive alterations remains poorly investigated. Nevertheless, reports in overweight/obese subjects and animal models of diet-induced obesity show that cumulative exposure to stress potentiates obesity-related memory and attention impairments (Alzoubi et al., 2009; Lasikiewicz et al., 2013; Verdejo-Garcia et al., 2015). Altogether, these data suggest that obesogenic dietary profiles may increase the vulnerability to stress-induced cognitive disturbances, independently of body weight.
While different factors may contribute to the association of unhealthy dietary habits with stress-induced cognitive changes, there is converging evidence for the involvement of inflammatory processes. A large body of data substantiates the role of inflammation, both at peripheral and central levels, in cognitive impairment (Fourrier et al., 2019; Noble et al., 2010). Interestingly, the association of inflammation with cognitive function is also observed in the context of Western-type diets (Beilharz et al., 2014), which are known to harbor a high inflammatory potential, notably through their fat and sugar components and obesogenic characteristics (Christ et al., 2019). The immune and stress response systems are tightly interconnected and interact with each other, consistent with findings showing an increased stress response to an acute stressor in inflammatory conditions (Horowitz and Zunszain, 2015). Accordingly, it is thus possible that low-grade inflammation related to obesogenic dietary patterns can sensitize individuals to stronger stress-induced cognitive alterations.
This study aimed to evaluate the influence of an obesogenic dietary pattern with inflammatory potential on stress-induced cognitive alterations in healthy adult volunteers. In particular, we investigated if such an obesogenic pattern is associated with an increased vulnerability to cognitive alterations in an experimental model of acute stress, and if systemic inflammation is associated with this effect.
2. Subjects and methods
2.1. Study participants
Fifty healthy adult volunteers (aged 18–50 years) were recruited between March 2017 and February 2018 from an existing database available at a contract research organization (CRO, CEN Nutriment) based in Dijon, France. The database contains information from a large cohort of volunteers from the Dijon area, stratified on their dietary habits based on adherence to French dietary recommendations, assessed by an online questionnaire of the French National Program for Nutrition and Health (“Programme National Nutrition et Santé", PNNS).
Exclusion criteria were: body mass index (BMI) greater than 35 kg/m2; current diagnosis of severe or uncontrolled medical illness; traumatic or stressful event over the last six months; current anti-inflammatory, immune-modulator, or lipid-lowering treatment; recent (last six months) or current psychotropic treatment, intake of food supplements for anxiety, depression or insomnia or for improving/maintaining cognitive function; regular intake of corticosteroids; consumption of illegal psychotropic substances; elevated alcohol and/or cigarette consumption; recent (last three months) or current engagement in weight loss programs; and pregnancy.
Eligible participants were screened by phone to verify suitability for study inclusion.
All participants provided written informed consent after reading a complete description of the study. The study was approved by the Institutional Committee of Protection of Persons of the East Region I (registration number 2016/40).
2.2. Procedures
2.2.1. Study protocol
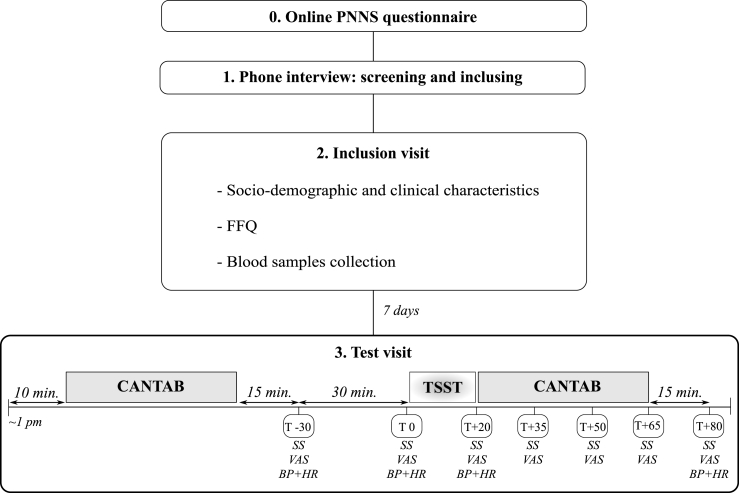
The study design is summarized in Fig. 1. Eligible participants were asked to come to the CRO for the inclusion visit, during which a brief interview was carried out, and fasting blood samples were collected by trained professionals. A new visit to the CRO was scheduled seven days later to assess stress-induced cognitive alterations (test visit). This 2-h visit took place in the early afternoon (from 1:00 p.m.) to reduce the effects of circadian variation on cortisol concentrations. Participants were instructed not to smoke or drink caffeinated beverages 2 h before and during the visit and to withdraw from eating or drinking 1 h prior to the scheduled time. Upon arrival, subjects rested for 10 min and then performed a battery of cognitive tests (first session, pre-stress). After completing the test battery and resting for 15 min, subjects provided the first saliva sample (T-30), reported their perceived stress level on a visual analog scale (VAS-stress), and measures of systolic/diastolic blood pressure (SBP and DBP, respectively) and heart rate (HR) were taken. These measurements were repeated 30 min after (T0), followed by exposure to the stress-induction paradigm. At the end of the test (T+20), stress responses were re-assessed, and the second (post-stress) cognitive evaluation was performed during which three saliva samples were collected and the VAS-stress was quantified every 15 min (T+35, T+50, and T+65).
Fig. 1.
Study protocol.
Participants were drawn from an existing database on a large cohort of volunteers with information available on adherence to the French dietary recommendations (based on answers on the PNNS questionnaire completed online) (point 0). Potentially eligible participants were screened by phone to verify suitability for study inclusion (point 1). Study participants (n = 50) were asked to come to the contract research organization for an inclusion visit that included the measurement of sociodemographics and clinical characteristics, the administration of the FFQ, and the collection of blood samples (point 2). A 2-h new visit (test visit) was scheduled seven days later, early in the afternoon (from 1:00 p.m.) (point 3). During this visit, participants were submitted to a battery of cognitive tests from the CANTAB®, before and after TSST exposure. The stress response elicited by the TSST was monitored through salivary free-cortisol, perceived stress level (VAS-stress), SBP, DBP, and HR. Saliva samples and VAS-stress were collected 30 min (T-30) and immediately before (T0, baseline) the TSST, immediately after (T+20), and at regular intervals of 15 min until the end of the visit (T+35, T+50, T+65, T+80). SBP, DBP, and HR were measured at T-30, T0, T+20, and T+80.
CANTAB®, Cambridge Neuropsychological Test Automated Battery; DBP, diastolic blood pressure; FFQ, Food Frequency Questionnaire; HR, heart rate; PNNS, French National Program for Nutrition and Health; SBP: systolic blood pressure; SS, saliva sample; TSST: Trier social stress test; VAS: visual analog scale.
The last saliva sample collection, VAS-stress, SBP, DBP, and HR assessments took place 15 min after the end of the cognitive tests battery (T+80). The cognitive assessment and stress-induction paradigm were performed in two different rooms.
2.2.2. Stress-induction paradigm: Trier Social Stress Test (TSST)
Acute stress was induced by the TSST, a validated stress protocol based on public speaking and evaluation designed to induce moderate psychosocial stress in a laboratory setting. This paradigm incorporates novelty, uncontrollability, and social-evaluative threat and was found to reliably elicit cortisol and other neuroendocrine responses (Dickerson and Kemeny, 2004). The TSST is fully described elsewhere (Kirschbaum et al., 1993). Briefly, it consists of a role-playing scenario where subjects have to outline their suitability for the “dream job” and complete an unexpected mental arithmetic task (serial subtraction) in front of a panel of two unresponsive committee members. A microphone and a video-camera supposedly recording were placed in the room. Participants were given 10 min to prepare their speech, 5 min to present it, and 5 min to perform the mental arithmetic task the fastest and more precise as possible.
2.3. Measurements
2.3.1. Socio-demographic, clinical, and lifestyle characteristics
Socio-demographics, clinical information (e.g., anthropometric data, medical history, current treatments), and smoking and caffeine consumption in the last 12 h were collected at study inclusion and at the test visit, respectively. BMI was calculated as the ratio of the weight (kg) to the square of height (m).
2.3.2. Dietary assessment
Dietary habits were assessed using the self-reported PNNS questionnaire in addition to a qualitative food frequency questionnaire (FFQ) (Fig. 1). The PNNS questionnaire assesses the adherence to the main points of the French nutritional recommendations, first defined in 2001 (Hercberg et al., 2008). The version used in this study was adapted from previous works (Buscail et al., 2018; Méjean et al., 2010) and comprises twelve questions. The first eight items refer to the frequency of intake of bread and cereals, starch, legumes, milk and dairy products, meat/poultry/eggs, fish and seafood, fruits, and vegetables. The remaining four items assess the consumption of added fat, sugary foods, salt, and water, self-evaluated as “insufficient”, “satisfactory”, “fairly high” or “very high”. The FFQ assesses the habitual consumption of broad food groups and beverages, as described elsewhere (Cougnard-Grégoire et al., 2016). In the present study, the assessed groups were focused on the use of dietary fats for cooking, dressing, or spreading, in four categories: “never”, “rarely”, “most of the times”, and “always”. Added fats included butter, cream, margarine, corn oil, peanut oil, sunflower oil, olive oil, mixed oil, colza oil, walnut oil, and soya oil.
2.3.3. Obesogenic diet
Obesogenic dietary pattern was defined a priori based on intake profile of relevant PNNS and FFQ items, including sugary foods, salt, and added fat. These food items are known to promote weight gain (Schulz et al., 2002; Tappy et al., 2018; Zhou et al., 2019) and for their inflammatory potential (Christ et al., 2019). Subjects were included in the obesogenic diet group when they reported being “high consumers” of at least two of the three food groups. For salt and sugary products, “high consumers” referred to those reporting a “fairly high” or “very high” intake of the respective items on the PNNS questionnaire. For added fat, “high consumers” were the ones who reported 1) a “fairly high” or “very high” intake of total added fat on the PNNS questionnaire or 2) to use cream or butter, and peanut, sunflower, or corn oil “most of the times” or “always” for spreading, cooking or dressing, on the FFQ questionnaire (Cougnard-Grégoire et al., 2016; Samieri et al., 2013). Conversely, participants who did not report this dietary pattern were included in the non-obesogenic diet group.
2.3.4. Cognitive performance
Cognitive performance was assessed using the Verbal Recognition Memory (VRM) and Rapid Visual Information Processing (RVP) tests of the CANTAB®, a validated battery of computer-based neuropsychological assessments (Sahakian and Owen, 1992).
The VRM test assesses verbal episodic memory and new learning. Participants were instructed to memorize a list of eighteen words appearing on the screen, one at a time. Afterward, they had to recall out loud the maximum number of words possible (free recall). Immediately after this stage, participants were asked to indicate if they recognized these words from a randomized sequence of thirty-six words, half belonging to the original list to be memorized (targets) and half new (distracters) (immediate recognition). This step was repeated 20 min after (delayed recognition). Altogether, the three stages lasted approximately 8 min. At each stage (immediate free recall and recognition, delayed recognition), the total number of words correctly recalled or recognized, i.e., all targets recognized and all distracters rejected, was used as the outcome measure. Parallel versions with different lists of words were used at each session to avoid learning effects.
The RVP test is a sustained attention/vigilance task with a small working memory component. In this task, digits ranging from 2 to 9 appeared on the screen, one at a time in a pseudo-random order, at a rate of 100 digits/minute for 4 min. Subjects were asked to press a response pad when they detected the target sequences: 2-4-6; 3-5-7; 4-6-8. Target sequences appeared 27 times. Performance on the RVP test was measured using the signal A' (RVPA’), i.e., signal detection measure of sensitivity to the target regardless of response tendency (ranging from 0/bad to 1/good), the mean latency for correct responses, the total number of hits (ranging from 0 to 27), and the total number of false alarms (ranging from 0 to 19).
2.3.5. Stress response
Subjective perception of stress was assessed by a VAS-stress, ranging from 1 (not stressed) to 10 (very stressed). SBP, DBP, HR measurements were taken as markers of sympathetic nervous system activity using an automatic sphygmomanometer. The hypothalamic-pituitary-adrenal (HPA) axis response to TSST was examined by salivary-free cortisol (Stahl and Dörner, 1982). Saliva samples were collected by passive drooling into Salivettes (saliva sampling devices, Sarstedt Ltd., London, England). Samples were frozen at −80 °C until analysis. High-sensitive (hs) salivary cortisol concentrations were determined by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's specifications (Salimetrics©, PA, USA). Assay sensitivity was 0.007 μg/dL, and the intra- and inter-assay coefficients of variation were ±4.6% and ±6.0%, respectively.
2.3.6. Inflammatory profile
Fasting blood samples were collected for the measurement of serum concentrations of high-sensitive C-reactive protein (hsCRP) as a marker of systemic inflammation. After 30–45 min at room temperature, samples were centrifuged (3200 rpm, 10 min at 4 °C) and stored at −80 °C until further analysis. Serum concentrations of hsCRP were determined by ELISA according to the manufacturer's specifications (CYT298, Millipore, Billerica, Massachusetts). Assays sensitivity and intra- and inter-assay variability were respectively 0.20 ng/mL, ± 4.6%, and ±6.0%.
2.4. Analytical strategy and statistical analysis
Raw values for salivary-free cortisol and serum hsCRP concentrations were log-transformed due to non-normal distribution, as assessed by the Shapiro-Wilk test. Of note, hsCRP was missing for one participant in the non-obesogenic diet group due to technical problems at blood collection. Data from subjects who did not fully complete cognitive tests were removed from the respective analyses. This was the case for one participant for the VRM test and two participants for the RVP test, all belonging to the non-obesogenic diet group.
Albeit parallel versions of the VRM test are supposed to be comparable in terms of frequency of word use, target words displayed at the first VRM session (pre-TSST) appeared to be overall more frequent than the ones in the second VRM session (post-TSST) (70.31 vs. 47.25), as per the online database “Lexique”, version 3.83 (New et al., 2007). Accordingly, the lists of words (targets and distracters), presented pre- and post-TSST, were uniformized for data analyses to provide them with a similar frequency and avoid any potential list effect. This uniformization was performed by excluding six words for which the frequency was not identical, with the final targets and distracters lists comprising twelve words each (mean frequency 23.23 and 23.65).
Dietary, socio-demographic, and clinical characteristics of the two groups were compared using Student's t-tests for continuous variables and Chi-squared test (χ2) or Fisher's exact test (FET) for categorical variables, when adequate. All further analyses were controlled for age, sex, BMI, treatments, and metabolic/endocrine comorbidities, due to their potential confounding effect. Moreover, given the potential effect of smoking and coffee consumption on the stress reactivity, analyses on TSST-induced stress response were further controlled for these two factors.
Circulating concentrations of hsCRP were compared between the two diet groups using analyses of covariance (ANCOVA). Mean differences in stress response, VRM and RVP scores, between the two diet groups before and after the TSST were analyzed using general linear models for repeated measures (GLMs RM), with “Time” as the repeated measure factor and “Diet” as the between-subjects variable. For stress response measures, T0, i.e., the assessment point immediately before TSST, was taken as the baseline time-point since it represents a more stable measure than the one at T-30 (Balodis et al., 2010). For all GLMs RM, the sphericity assumption was assessed by the Maulchy's test, and if violated, Greenhouse-Geisser corrections were applied. When appropriate, simple main effects and post-hoc comparisons using Bonferroni corrected test were performed. Delta performance on VRM and RVP tests was calculated when a significant “Time × Diet” interaction was observed, by subtracting scores of the post-stress test session to the ones of the pre-TSST test session. Delta scores were compared between the two diet groups with ANCOVA, controlling for all covariates mentioned earlier and pre-TSST (baseline) performance. The relationship between hsCRP serum concentrations and delta scores was estimated by multiple regression analyses.
All analyses were repeated after excluding the four participants with class I obesity in the obesogenic diet group to confirm that obesity did not drive the obtained results. Statistical analyses were performed with the Statistical Package for the Social Sciences version 25 (IBM SPSS Statistics for Windows; IBM Corp., Armonk, NY, USA). All probabilities were two-sided, with the degree of significance set at p < 0.05.
4. Results
4.1. Dietary characterization of obesogenic diet
As shown in Table 1, twenty-two participants (44%) presented an obesogenic dietary profile. As expected, participants in this group reported a significantly higher intake of sugar, salt, and fat, compared to those in the non-obesogenic diet group. The obesogenic dietary profile was also characterized by lower daily consumption of fruits and vegetables. No significant differences were observed for the remaining food groups assessed by the PNNS questionnaire.
Table 1.
Dietary characterization.
| Non-obesogenic diet group | Obesogenic diet group | Statistics | Effect size | p | |
|---|---|---|---|---|---|
| Sample size, n(%) | 28 (56) | 22 (44) | |||
| Obesogenic food groups | |||||
| High consumers of sugar, n(%) | 11 (42.3) | 15 (57.7) | χ2 = 4.1 | ϕ = 0.3 | 0.04 |
| High consumers of salt, n(%) | 4 (14.3) | 15 (68.2) | χ2 = 15.2 | ϕ = 0.6 | <0.001 |
| High consumers of fat, n(%) | 3 (10.7) | 20 (90.9) | χ2 = 31.9 | ϕ = 0.8 | <0.001 |
| Other food and beverage groupsa | |||||
| Meat, poultry and eggs, times/day (SD) | 0.6 (0.91) | 0.6 (0.91) | t = 0.4 | d = 0.1 | 0.71 |
| Dairy products, times/day (SD) | 1.6 (1.17) | 1.5 (1.44) | t = 0.2 | d = 0.1 | 0.85 |
| Fruits and Vegetables, times/day (SD) | 3.5 (1.62) | 2.4 (1.99) | t = 2.2 | d = 0.6 | 0.03 |
| Fish and seafood, times/week (SD) | 1.2 (0.99) | 1.3 (0.83) | t = −0.4 | d = −0.1 | 0.66 |
| Cereals, times/day (SD) | 2.7 (1.29) | 2.9 (1.63) | t = 0 | d = −0.1 | 0.63 |
| Water as main beverage, yes(%)b,c | 27 (96.4) | 17 (77.3) | χ2 = 4.3 | ϕ = 0.3 | 0.08 |
Continuous variables are presented as mean and standard deviation and compared using Student's t-test, whereas categorical variables are presented as n (%) and compared by chi-squared test or Fisher's exact test when 25% or more of the observations had an expected frequency of five or less. Effect sizes correspond to Cohen's D coefficient for continuous variables and to the Phi (ϕ) coefficient for categorical variables.
Extracted from the PNNS questionnaire.
Fisher's exact test was used to compare categorical variables.
Equivalent to reporting “drinking only or mostly water” in the PNNS questionnaire.
4.2. Clinical characteristics of study participants
As shown in Table 2, there was no significant difference between the two diet groups in terms of age, sex, weight, BMI, education level and occupational category. Consistent with dietary habits, four (18.2%) subjects in the obesogenic diet group presented a BMI ≥ 30 kg/m2, indicative of class I obesity. The prevalence of metabolic and endocrine abnormalities, i.e., mild hypertension, mild hepatopathy, and hypothyroidism, was comparable across the two diet groups. Use of any treatment was overall more frequent in the non-obesogenic diet group.
Table 2.
Socio-demographic and clinical characteristics of study participants.
| Non-obesogenic diet group | Obesogenic diet group | Statistics | Effect size | p | |
|---|---|---|---|---|---|
| Sample size, n(%) | 28 (56) | 22 (44) | |||
| Age, years (SD) | 38.8 (7.3) | 36.3 (8.5) | t = −1.1 | d = −0.3 | 0.27 |
| Females, n(%)a | 26 (92.9) | 17 (77.3) | χ2 = 2.5 | ϕ = 0.2 | 0.22 |
| Weight, kg (SD) | 66.3 (10.2) | 70.6 (15.2) | t = 1.2 | d = 0.3 | 0.24 |
| BMI, kg/m2(SD) | 23.8 (3.3) | 24.99 (5.0) | t = 1.0 | d = 0.3 | 0.32 |
| Normal weight, n(%)a | 18 (64.3) | 10 (45.5) | χ2 = 5.5 | V = 0.3 | 0.07 |
| Overweight, n(%) | 10 (35.7) | 8 (36.4) | |||
| Obese, n(%) | 0 (0) | 4 (18.2) | |||
| Metabolic and endocrine alterations, n yes(%)a | 2 (7.4) | 3 (13.6) | χ2 = 0.6 | ϕ = −0.1 | 0.64 |
| Hypertension, n(%)a | 0 (0) | 1 (4.5) | χ2 = 1.3 | ϕ = 0.2 | 0.44 |
| Hypercholesterolemia, n(%)a | 1 (3.6) | 0 (0) | χ2 = 0.8 | ϕ = −0.1 | 1.00 |
| Hepatopathy, n(%)a | 0 (0) | 1 (4.5) | χ2 = 1.3 | ϕ = 0.1 | 0.44 |
| Hypothyroidism, n(%)a | 1 (3.6) | 2 (9.1) | χ2 = 0.7 | ϕ = −0.1 | 0.58 |
| Treatments, n yes(%) | 18 (64.3) | 5 (22.7) | χ2 = 8.6 | ϕ = 0.4 | 0.003 |
| Hypothyroidism treatment, n(%)a | 1 (3.6) | 1 (4.5) | χ2 = 0 | ϕ = 0 | 1.00 |
| Contraception/hormonal replacement treatment, n(%)a,b | 13 (46.4) | 5 (22.7) | χ2 = 3.0 | ϕ = 0.2 | 0.08 |
| Proton pump inhibitor treatment, n(%)a | 1 (3.6) | 0 (0) | χ2 = 0.8 | ϕ = 0.1 | 1.00 |
| Antihistaminic treatment, n(%)a | 2 (7.1) | 0 (0) | χ2 = 1.6 | ϕ = 0.2 | 0.50 |
| Education levelc | |||||
| Up to high school, n(%) | 8 (28.6) | 4 (18.2) | χ2 = 0.6 | ϕ = −0.1 | 0.45 |
| Undergraduate or graduate degree, n(%) | 19 (67.9) | 16 (72.7) | |||
| Occupational categorya,c | |||||
| Unemployed or without activity, n(%) | 2 (7.4) | 2 (10.0) | |||
| Self-employed/artisan, n(%) | 1 (3.7) | 1 (5.0) | |||
| High qualified workers, n(%) | 2 (7.4) | 4 (20.0) | χ2 = 2.4 | V = 0.2 | 0.7 |
| Technicians/associate professionals, n(%) | 16 (59.3) | 8 (40.0) | |||
| Employees, n(%) | 6 (22.2) | 5 (25) | |||
Continuous variables are presented as mean and standard deviation and compared using Student's t-test, whereas categorical variables are presented as n (%) and compared by chi-squared test or Fisher's exact test when 25% or more of the observations had an expected frequency of five or less. Effect sizes correspond to Cohen's D coefficient for continuous variables and to the Phi (ϕ) or Cramer's V coefficients for categorical variables. BMI, body mass index.
Fisher's exact test was used to compare categorical variables.
Analysis performed only in women.
Information was missing for three participants.
4.3. Inflammatory profile
Differences in serum concentrations of hsCRP were assessed by ANCOVA. As shown in Fig. 2, subjects in the obesogenic diet group exhibited significantly higher circulating concentrations of hsCRP than those in the non-obesogenic diet group [F (1,42) = 8.8, p = 0.005, η2 = 0.1], in line with the inflammatory potential of obesogenic dietary patterns. This result was independent of obesity since it remained significant after excluding from the analyses the four participants with class I obesity (Table S1).
Fig. 2.
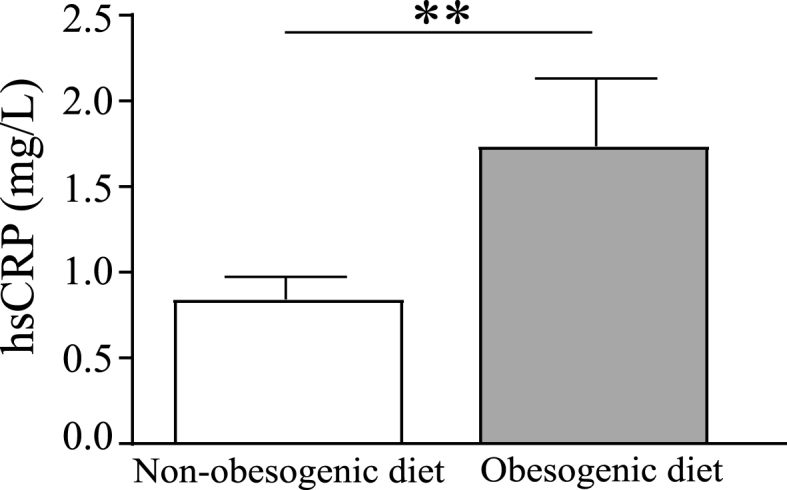
Serum concentrations of hsCRP in the non-obesogenic diet group (n = 27, white bar) compared to the obesogenic group (n = 22, grey bar). Statistical analysis was performed on log-transformed data using ANCOVA controlling for age, sex, BMI, current treatments, and presence of metabolic/endocrine comorbidities. Data are expressed as unadjusted means (+SEM) of raw data for readability purposes, **p < 0.01. hsCRP: high-sensitive C-reactive protein.
4.4. TSST-induced stress response
Stress response elicited by TSST was assessed using GLMs RM within the whole sample and between the two diet groups, controlling for age, sex, BMI, current treatments, presence of metabolic/endocrine comorbidities, and nicotine/caffeine consumption in the 12 h preceding the test.
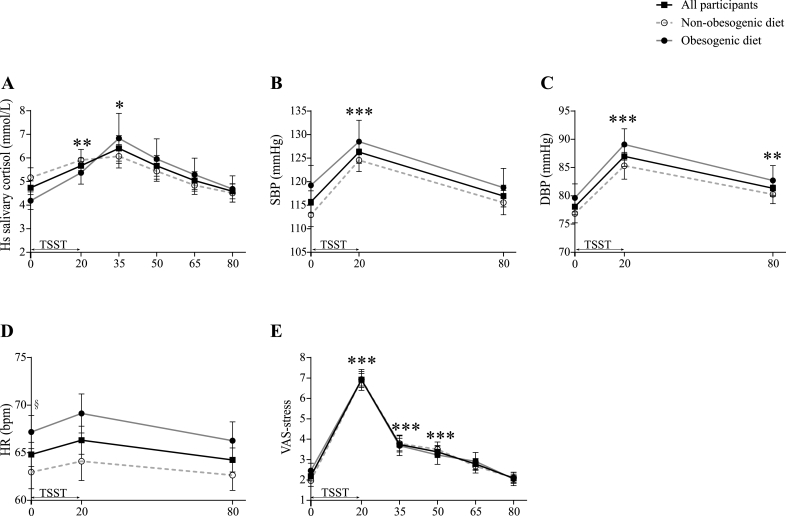
As shown in Fig. 3A, high sensitive salivary cortisol concentrations were increased in all study participants after TSST, with a peak at T+35, returning to baseline at T+80 [Time effect: F(2.1, 90.6) = 11.8, p < 0.001, η2 = 0.2 (T0 vs. T+35: p = 0.022; T0 vs. T+80, p=1.00)]. No significant differences were found between the two diet groups with respect to TSST-induced cortisol response.
Fig. 3.
Stress response in the whole population under study (n = 50, solid black lines, filled square) and in the two diet groups (non-obesogenic diet: n = 28, dashed grey lines with open circle; obesogenic diet: n = 22, solid grey lines with filled circle).
TSST-induced stress response was assessed by measuring A) salivary high-sensitive cortisol concentrations, B–C) blood pressure, D) heart rate, and E) VAS-stress. Panels A–C and E: TSST induced a stress response in the whole sample, regardless of dietary habits. Panel D: No significant change was measured in heart rate following TSST. Statistical analyses were performed on log-transformed data for salivary cortisol and on non-transformed data for the remaining measures, using GLMs RM, followed by post-hoc comparisons using the corrected Bonferroni test, when adequate. All analyses were controlled for age, sex, BMI, current treatments, presence of metabolic/endocrine comorbidities, and nicotine/caffeine consumption in the 12 h preceding the test. Data are expressed as unadjusted means (±SEM) of raw data for readability purposes. * represent the differences in the whole sample vs. T0. *p < 0.05; **p < 0.01; ***p < 0.001. § represents the differences between the obesogenic and non-obesogenic diet groups at T0. §p < 0.05. DBP, diastolic blood pressure; HR, heart rate; hs, high-sensitive; SBP, systolic blood pressure; VAS, visual analog scale.
TSST-induced changes in SBP and DBP were also observed (Fig. 3B and C), with significant increases at T+20, regardless of dietary classification [Time effect: F(1.7, 73.2) = 31.1, p < 0.001, η2 = 0.4 for SBP; F(1.8,78.2) = 29.9, p < 0.001, η2 = 0.4 for DBP; T0 vs. T+20, p < 0.001 for both measures]. In contrast to BP measures, HR was not significantly altered by TSST exposure (Fig. 3D). Of note, subjects in the obesogenic diet group presented higher baseline HR values than those in the non-obesogenic diet group [Diet effect: F(1,42) = 4.3, p=0.044; η2 = 0.1; obesogenic vs. non-obesogenic diet groups at T0, p=0.049].
Finally, exposure to TSST produced an equally marked psychological response, assessed by the VAS-stress, in the two diet groups (Fig. 3E) [Time effect: F(3.1, 132.0) = 114.7, p < 0.001, η2 = 0.7]. Accordingly, participants from both groups reported feeling significantly more stressed immediately after the TSST (T0 vs. T+20, p < 0.001), time point after which the VAS-stress scores decreased gradually and returned to baseline at T+80 (T0 vs. T+80, p=1.00). Interestingly, stress response was not affected by obesity, as an identical response pattern was observed after excluding from analyses subjects with BMI ≥30 kg/m2 (Table S2).
4.5. Relationship between diet and TSST-induced cognitive alterations
The influence of dietary habits on TSST-induced cognitive alterations was assessed using GLMs RM controlled for age, sex, BMI, current treatments, and presence of metabolic/endocrine comorbidities.
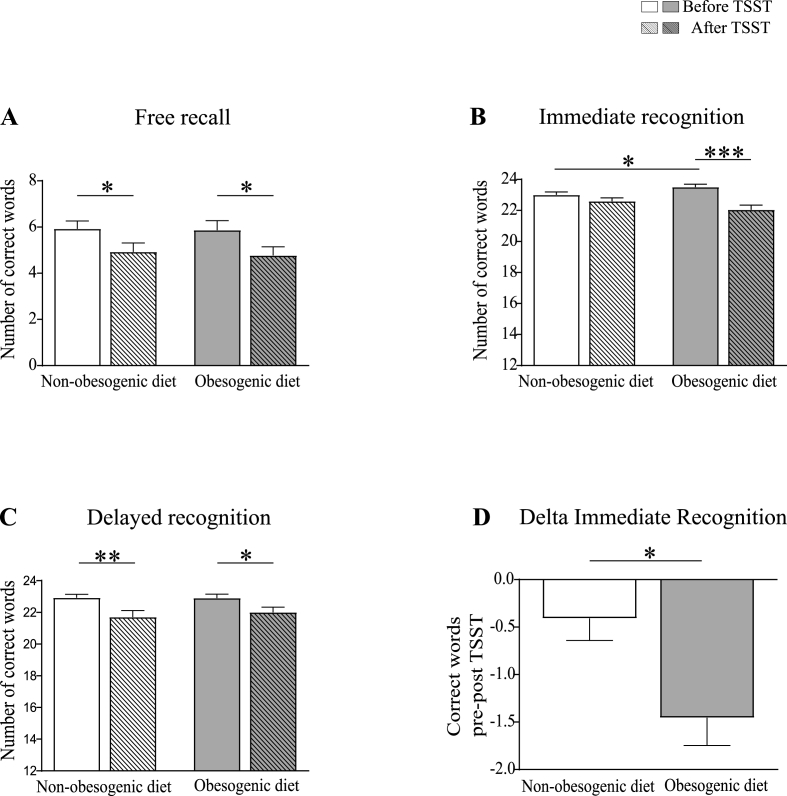
Exposure to TSST was associated with an overall impaired performance on the VRM test [Time effect: F(1,42) = 10.4, p=0.002, η2 = 0.2 for free recall; F(1,42) = 30.0, p < 0.001, η2 = 0.4 for immediate recognition; F(1,42) = 21.0, p < 0.001, η2 = 0.3 for delayed recognition] (Fig. 4A–C). For the free recall and delayed recognition tasks, this impairment was not affected by diet, with both groups showing a similar decrease in the number of correct words following TSST (Fig. 4A and C). On the immediate recognition task, a significant Time × Diet interaction was observed, with only subjects from the obesogenic diet group exhibiting a significant reduction in the total number of words correctly recognized after TSST compared to baseline [Time × Diet: F(1,42) = 11.0, p=0.002, η2 = 0.2; obesogenic diet group: before vs. after TSST p < 0.001] (Fig. 4B). Albeit performance before the TSST was greater in subjects presenting an obesogenic diet than in subjects presenting a non-obesogenic diet (p=0.018), this difference did not influence the worsening effect of obesogenic diet on TSST-induced memory impairment. Indeed, the magnitude of the change in performance at the immediate recognition stage of the VRM (delta post-pre TSST) remained significantly higher in the obesogenic diet group after controlling for pre-stress scores (F(1,41) = 6.3; p=0.016, η2 = 0.1) (Fig. 4D).
Fig. 4.
Total number of words correctly recalled or recognized on the VRM test before (solid bars) and after (striped bars) TSST exposure, in the non-obesogenic (white bars; n = 27), and obesogenic (grey bars; n = 22) diet groups. Panels A-C: Exposure to TSST was associated with overall impaired performance. Panels A and C: Diet did not influence free recall and delayed recognition performance. Panels B and D: Only participants in the obesogenic diet group exhibited a significant reduction in the total number of words correctly recognized/rejected after TSST compared to baseline, in the immediate recognition stage of the test. This result is confirmed by the comparison of the delta performance (post vs. pre-TSST) between the two diet groups. Statistical analyses were performed using GLMs RM, followed by post-hoc comparisons using the corrected Bonferroni test when adequate (panels A–C) and ANCOVA (panel D). Analyses were controlled for age, sex, BMI, current treatments, and presence of metabolic/endocrine comorbidities (panels A–D) and pre-TSST performance (panel D). Data are expressed as unadjusted means (+SEM); *p < 0.05; **p < 0.01; ***p < 0.001.
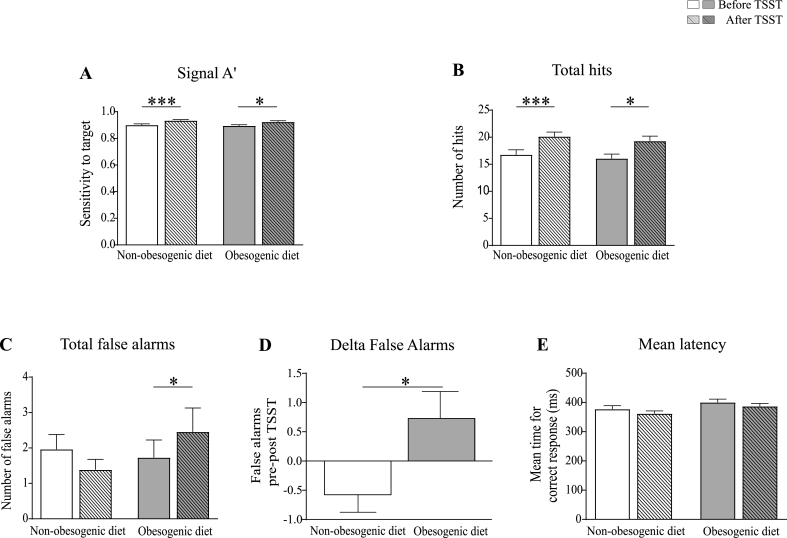
In contrast to VRM, performance on the RVP test was overall improved on both groups after exposure to the TSST, as reflected in increased RVPA’ (sensitivity to the target sequences, regardless of response tendency) and number of total hits (Fig. 5A and B) [Time effect: F(1,41) = 20.9, p < 0.001, η2 = 0.3 for RVPA’; F(1,41) = 21.8, p < 0.001, η2 = 0.4 for total hits]. However, subjects from the obesogenic diet group exhibited a higher number of false alarms after TSST compared to baseline (Fig. 5C) [Time × Diet effect: F(1,41) = 6.6, p=0.014, η2 = 0.1; obesogenic diet group: before vs. after TSST p < 0.05]. In contrast, subjects from the non-obesogenic diet group tended to show a reduced number of false alarms after TSST compared to baseline, as reflected in the delta performance for false alarms between the two diet groups (Fig. 5D) (F(1,40) = 6.3; p=0.017, η2 = 0.1). No effect of TSST was measured on the mean latency for correct response in both groups (Fig. 5E).
Fig. 5.
Performance on the RVP test, before (solid bars) and after (striped bars) TSST exposure, in the non-obesogenic (white bars; n = 26), and obesogenic (grey bars; n = 22) diet groups. Panels A-B: The signal RVPA′ and the number of total hits were increased in both diet groups after TSST. Panels C and D: The number of total false alarms was increased after TSST exposure only in the obesogenic diet group. This result is confirmed by the comparison of the delta performance (post vs. pre-TSST) between the two diet groups. Panel E: Mean latency for correct response was not affected by TSST in both diet groups. Statistical analyses were performed using GLMs RM, followed by post-hoc comparisons using the corrected Bonferroni test when adequate (panels A–C and E) and ANCOVA (panel D). Aanalyses were controlled for age, sex, BMI, current treatments, and presence of metabolic/endocrine comorbidities (panels A–C and E) and pre-TSST performance (panel D). Data are expressed as unadjusted means (+SEM); *p < 0.05; ***p < 0.001.
Similar results were obtained after excluding class I obese participants (Tables S3 and S4).
4.6. Association of inflammation with diet-related TSST-induced cognitive alterations
Multiple regression analyses were performed to investigate if serum concentrations of hsCRP were associated with the cognitive alterations measured in the obesogenic diet group following TSST. As shown in Table 3, the reduced scores of immediate recognition at the VRM test and the increased number of false alarms on the RVP were not significantly associated with hsCRP concentrations in the whole population and in subjects from the obesogenic diet group. Nevertheless, a statistical trend between performance in immediate recognition and hsCRP levels was measured in the whole sample.
Table 3.
Association of serum hsCRP concentrations with diet-related TSST-induced cognitive changes.
| Delta immediate recognition VRM | Delta total false alarms RVP | |||||||
|---|---|---|---|---|---|---|---|---|
| β | F | R2 | p | β | F | R2 | p | |
| Whole population | ||||||||
| Log hsCRP | −0.30 | 1.85 | 0.10 | 0.06 | 0.04 | 0.91 | −0.01 | 0.81 |
| Obesogenic diet group | ||||||||
| Log hsCRP | 0.11 | 1.19 | 0.05 | 0.69 | 0.12 | 1.33 | 0.09 | 0.68 |
Multiple regression analyses controlled for age, sex, BMI, current treatment use, and metabolic/endocrine comorbidities. Whole population: n = 48 for delta immediate recognition on the VRM test, and n = 47 for delta total false alarms on the RVP test. Obesogenic diet group: n = 22 on both tests. hsCRP, high sensitive C-reactive protein; RVP, rapid visual information processing; VRM, verbal recognition memory.
Similar results were obtained after excluding class I obese participants (Table S5).
5. Discussion
There is increasing emphasis on the sensitizing role of unhealthy dietary habits in the development of cognitive alterations. While most research has focused on aging contexts, the present study aimed to evaluate the influence of dietary habits with obesogenic and inflammatory potentials on stress-induced cognitive alterations in healthy adult volunteers. We showed that healthy adults with obesogenic dietary habits exhibit higher systemic inflammation (heightened hsCRP concentrations) and an increased sensitivity to TSST-induced alterations in verbal memory and sustained attention. Moreover, our results suggest that diet-related systemic inflammation did not significantly account for stress-induced changes in cognitive performance in subjects from the obesogenic diet group.
Forty-four percent of the study sample reported having obesogenic dietary habits, defined by high intake of at least two unhealthy food groups: fat, sugar, and salt. Interestingly, the daily intake of fruits and vegetables was also decreased in this group. Previous studies have identified similar obesogenic dietary patterns in representative cohorts of the French adult population. However, the prevalence of this dietary pattern was lower than the one found in our study (Gazan et al., 2016). This difference may rely on the fact that subjects from the present study were recruited on the basis of their adherence (or not) to the French dietary recommendations. Consistent with previous reports on the inflammatory potential of unhealthy diets (Griep et al., 2013; Shivappa et al., 2018), individuals in the obesogenic diet group exhibited significantly higher serum hsCRP concentrations than the non-obesogenic diet group. Importantly, this difference was independent of BMI and remained significant after excluding participants with class I obesity from the analysis.
While chronic low-grade inflammation represents a fundamental characteristic of obesity, it is also well recognized that diets rich in fat, sugar, and/or salt can, per se, trigger inflammatory responses through different mechanisms (Christ et al., 2019). Among these, the gut microbiota has been identified as a potential modulator of inflammation (Christ et al., 2019). Unhealthy diets, mainly those rich in saturated fat, have been related to altered microbiota composition, disrupted intestinal permeability, and increased endotoxemia, that may all contribute to chronic low-grade inflammation (Sandhu et al., 2017). However, clinical evidence integrating dietary habits, gut microbiota composition, and systemic inflammation is still scarce, especially in healthy subjects (Chun Kong et al., 2014), and further studies are needed to unravel the mechanisms by which unhealthy diet promotes inflammation.
To test the hypothesis that obesogenic diet sensitizes healthy individuals to stress-induced cognitive alterations, we used the TSST, a gold-standard acute laboratory stressor, as an experimental model of acute stress, and the CANTAB®, a standardized and validated test battery for the assessment of cognitive performance. Our results confirmed that the TSST induced a significant stress response, measured by salivary cortisol levels, blood pressure, and subjective stress perception, in the whole sample regardless of dietary habits. Moreover, exposure to TSST was associated with decreased performance on the VRM test of CANTAB® battery, together with overall improved RVP scores. Importantly, a Time × Diet interaction was measured for the immediate recognition stage of the VRM and the total number of false alarms on the RVP, with reduced performance being observed only in the obesogenic diet group. In particular, subjects in the obesogenic diet group exhibited a significant reduction in the total number of words correctly recognized after TSST (VRM) and an increased number of false alarms (RVP) compared to baseline. These alterations, notably as they relate to episodic verbal memory, are consistent with previous reports documenting associations between high-fat/high-sugar diet and reduced verbal memory performance in healthy human participants (Attuquayefio et al., 2016).
Although acute stressors have been reported to impair verbal memory and sustained attention (Olver et al., 2015; Stawski et al., 2008), inconsistencies are found in the literature, with some studies reporting improved performance (Kamp et al., 2019; Schwabe et al., 2013) and others no changes (Banks et al., 2014; Domes et al., 2004; Luethi et al., 2009). Differences in study design (e.g., time between stress onset and cognitive evaluation) and sample features (e.g., sex and BMI) may explain such heterogeneity of results between studies (Shields et al., 2017). Interestingly, stress-induced alterations in verbal memory and sustained attention (increased false alarms) in the obesogenic diet group were not related to obesity status, suggesting that dietary habits, per se, may modulate the relationship between stress and cognitive performance. In support of this notion, a healthy diet enriched in omega-3 polyunsaturated fatty acids and vitamin A was found to normalize the memory deficits induced by a chronic stress in rats (Provensi et al., 2019).
The influence of the obesogenic dietary profile on immediate recognition (VRM) and total false alarms (RVP) post-TSST, but not on the other cognitive outcomes, suggest that these cognitive processes present a specific sensitivity to dietary factors. In line with this, clinical studies in non-stress conditions have shown that macro and micronutrients do not equally affect each of the VRM domains (Bensalem et al., 2019; Gibson et al., 2013). The three domains of verbal episodic memory in the VRM test – free recall, immediate and delayed recognition – differ in terms of memory processes, task complexity and neuroanatomical substrates (Gagnon and Wagner, 2016; Shields et al., 2017). Similarly, the components of the RVP test – total hits, total false alarms, mean latency - rely on different cognitive functions, involve different levels of cognitive demand, and reflect distinct neuropsychological processes. It is possible that tasks involving higher cognitive demand/attentional resources or relying on specific neuroanatomical networks are more prone to be modulated by diet. In addition, the increase in false alarms measured post-TSST in subjects with obesogenic dietary habits may reflect a higher sensitivity to stress-induced attentional bias or impulsive behavior. Further clinical studies, with measures of perceived task-related difficulty and psychological processes, as well as preclinical research with neuroanatomical investigations, are needed to address this issue. Surprisingly, the obesogenic diet group performed better than the non-obesogenic diet group on immediate recognition pre-TSST. The reliability of this finding needs to be confirmed in larger clinical samples, using a more systematic and detailed assessment of dietary intake.
In contrast to what was expected, alterations in the immediate recognition stage of the VRM and the increased number of false alarms on the RVP test were not associated with serum levels of hsCRP. This observation is not consistent with a large body of literature proposing inflammation as a mediator of diet-induced cognitive alterations (Kanoski and Davidson, 2011; Spencer et al., 2017a). There are several possible explanations for this discrepancy. First, hsCRP may not be the most relevant or specific marker of diet-related systemic inflammation. Second, it is possible that the magnitude of the increase in hsCRP in relation to obesogenic diet is not sufficient to affect cognition. Third, given that TSST may exacerbate inflammatory processes (Prather et al., 2008), it is possible that obesogenic diet leads to a heightened TSST-induced inflammatory response, consistent with the inflammatory priming effect of high-fat diets in rodents exposed to stress (Sobesky et al., 2014). This effect is likely to modulate diet-related TSST-induced cognitive alterations. Since blood collection was performed seven days before the tests, this priming effect could not be investigated.
The present study bears some limitations that should be taken into consideration when interpreting the results. First, the small sample size may have contributed to reduce the power to detect significant associations between the two diet groups. Accordingly, further studies in larger samples are required to confirm the findings. Second, the dietary information used in this study was derived from qualitative questionnaires with questions on broad food groups. Thus no/limited information was available on the portion size, sources of added fat, sugary and salty foods, or nutritional composition. Although the present dietary characterization was in line with the literature in terms of obesogenic and inflammatory potential, more detailed dietary and nutritional information is needed to refine the profile of obesogenic food “high consumers”, who are at risk for stress-induced cognitive alterations. The type of assessment of food intake at inclusion and the small size of the clinical samples impeded the constitution of a “strict” non-obesogenic food group comprising subjects being simultaneously “low consumers” of fat, sugar, and salt. However, a “strict” obesogenic/non-obesogenic diet stratification (i.e., high versus low consumers of all the obesogenic food groups) would increase the robustness of the results. Third, while participants were asked to refrain from eating and drinking 1 h before the test visit, pre-TSST glycemia levels could not be measured, precluding the assessment of their influence on stress response and cognitive performance. Finally, the lack of a control “non-stressed” group did not allow us to determine the respective effect of dietary pattern and acute stress on study outcomes. Albeit an extensive analysis of the relationship between diet and stress reactivity was beyond the scope of this study, future epidemiological studies evaluating the influence of regular dietary habits on stress reactivity would be highly relevant.
In conclusion, findings from the present study suggest that an obesogenic diet, defined by a “high intake” of at least two of the three obesogenic food groups (fat, sugar, and salt), may sensitize healthy individuals to stress-induced alterations in verbal memory (reduced immediate recognition) and sustained attention (increased total false alarms). While the underlying mechanisms still need to be determined, these findings open new avenues for future research. A better understanding/characterization of these effects on representative samples is highly relevant to define nutritional strategies for the prevention of stress-related cognitive deficits, in modern societies burdened by Western-type diets and psychosocial stress.
Sources of support
This work was supported by funds from the JPI HDHL Nutrition and Cognitive Function (AMBROSIAC, French National Research Agency, ANR-15-HDHL-0001-03, LC; 15/JPHDHL/3270 JFC), JPI HDHL Biomarkers for Nutrition and Health (HEALTHMARK, French National Research Agency, ANR-16-HDHL-0003-03, LC; 16/ERAHDHL/3362 JFC), the National Research Institute for Agriculture, Food and Environment (INRAE), and LabEX Brain PhD extension grant (EB 660 NEURO/7200R GP/7200R-1 BRAIN, ID).
CRediT authorship contribution statement
Inês Delgado: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – original draft, Visualization. Sandra Dexpert: Investigation, Data curation. Julie Sauvant: Formal analysis. John F. Cryan: Conceptualization, Methodology, Writing – review & editing, Project administration, Funding acquisition. Lucile Capuron: Conceptualization, Methodology, Writing – original draft, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
None.
Acknowledgments
The authors thank F. Herpin and the CRO CEN Nutriment staff for recruiting the participants and conducting study visits.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2021.100353.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Adan R.A.H., Beek E.M. Van Der, Buitelaar J.K., Cryan J.F., Hebebrand J., Higgs S., Schellekens H., Dickson S.L. Nutritional psychiatry : towards improving mental health by what you eat. Eur. Neuropsychopharmacol. 2019;29:1321–1332. doi: 10.1016/j.euroneuro.2019.10.011. [DOI] [PubMed] [Google Scholar]
- Alzoubi K.H., Abdul-Razzak K.K., Khabour O.F., Al-Tuweiq G.M., Alzubi M.A., Alkadhi K.A. Adverse effect of combination of chronic psychosocial stress and high fat diet on hippocampus-dependent memory in rats. Behav. Brain Res. 2009;204:117–123. doi: 10.1016/j.bbr.2009.05.025. [DOI] [PubMed] [Google Scholar]
- Arnsten A.F.T. Stress weakens prefrontal networks: molecular insults to higher cognition. Nat. Neurosci. 2015;18:1376–1385. doi: 10.1038/nn.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attuquayefio T., Stevenson R.J. A systematic review of longer-term dietary interventions on human cognitive function: emerging patterns and future directions. Appetite. 2015;95:554–570. doi: 10.1016/j.appet.2015.08.023. [DOI] [PubMed] [Google Scholar]
- Attuquayefio T., Stevenson R.J., Boakes R.A., Oaten M.J., Yeomans M.R., Mahmut M., Francis H.M. A high-fat high-sugar diet predicts poorer hippocampal-related memory and a reduced ability to suppress wanting under satiety. J Exp Psychol Anim Learn Cogn. 2016;42:415–428. doi: 10.1037/xan0000118. [DOI] [PubMed] [Google Scholar]
- Attuquayefio T., Stevenson R.J., Oaten M.J., Francis H.M. A four-day Western-style dietary intervention causes reductions in hippocampal-dependent learning and memory and interoceptive sensitivity. PloS One. 2017;12:1–21. doi: 10.1371/journal.pone.0172645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodis I.M., Wynne-Edwards K.E., Olmstead M.C. The other side of the curve: examining the relationship between pre-stressor physiological responses and stress reactivity. Psychoneuroendocrinology. 2010;35:1363–1373. doi: 10.1016/j.psyneuen.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Banks J.B., Tartar J.L., Welhaf M.S. Where's the impairment: an examination of factors that impact sustained attention following a stressor. Cognit. Emot. 2014;28:856–866. doi: 10.1080/02699931.2013.857643. [DOI] [PubMed] [Google Scholar]
- Beilharz J.E., Maniam J., Morris M.J. Short exposure to a diet rich in both fat and sugar or sugar alone impairs place, but not object recognition memory in rats. Brain Behav. Immun. 2014;37:134–141. doi: 10.1016/j.bbi.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Bensalem J., Dudonné S., Etchamendy N., Pellay H., Amadieu C., Gaudout D., Dubreuil S., Paradis M.-E., Pomerleau S., Capuron L., Hundon C., Layé S., Desjardins Y., Pallet V. Polyphenols from grape and blueberry improve episodic memory in healthy elderly with lower level of memory performance: a bicentric double-blind, randomized, placebo-controlled clinical study. Journals Gerontol. Ser. A. 2019;74:996–1007. doi: 10.1093/gerona/gly166. [DOI] [PubMed] [Google Scholar]
- Buscail C., Margat A., Petit S., Gendreau J., Daval P., Lombrail P., Hercberg S., Latino-Martel P., Maurice A., Julia C. Fruits and vegetables at home (FLAM): a randomized controlled trial of the impact of fruits and vegetables vouchers in children from low-income families in an urban district of France. BMC Publ. Health. 2018;18:1065. doi: 10.1186/s12889-018-5908-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ A., Lauterbach M., Latz E. Western diet and the immune system: an inflammatory connection. Immunity. 2019;51:794–811. doi: 10.1016/j.immuni.2019.09.020. [DOI] [PubMed] [Google Scholar]
- Chun Kong L., Holmes B.A., Cotillard A., Habi-Rachedi F., Brazeilles R., Gougis S., Gausserè N., Cani P.D., Fellahi S., Bastard J.-P., Kennedy S.P., Doré J., Ehrlich S.D., Zucker J.-D., Rizkalla S.W., Clément K. Dietary patterns differently associate with inflammation and gut microbiota in overweight and obese subjects. PloS One. 2014;9 doi: 10.1371/journal.pone.0109434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cougnard-Grégoire A., Merle B.M.J., Korobelnik J.-F., Rougier M.-B., Delyfer M.-N., Le Goff M., Samieri C., Dartigues J.-F., Delcourt C. Olive oil consumption and age-related macular degeneration: the alienor study. PloS One. 2016;11 doi: 10.1371/journal.pone.0160240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson S.S., Kemeny M.E. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Domes G., Heinrichs M., Rimmele U., Reichwald U., Hautzinger M. Acute stress impairs recognition for positive words - association with stress-induced cortisol secretion. Stress. 2004;7:173–181. doi: 10.1080/10253890412331273213. [DOI] [PubMed] [Google Scholar]
- Edwards L.M., Murray A.J., Holloway C.J., Carter E.E., Kemp G.J., Codreanu I., Brooker H., Tyler D.J., Robbins P.A., Clarke K. Short‐term consumption of a high‐fat diet impairs whole‐body efficiency and cognitive function in sedentary men. Faseb. J. 2011;25:1088–1096. doi: 10.1096/fj.10-171983. [DOI] [PubMed] [Google Scholar]
- Fourrier C., Singhal G., Baune B.T. Neuroinflammation and cognition across psychiatric conditions. CNS Spectr. 2019;24:4–15. doi: 10.1017/S1092852918001499. [DOI] [PubMed] [Google Scholar]
- Francis H.M., Stevenson R.J. Higher reported saturated fat and refined sugar intake is associated with reduced hippocampal-dependent memory and sensitivity to interoceptive signals. Behav. Neurosci. 2011;125:943–955. doi: 10.1037/a0025998. [DOI] [PubMed] [Google Scholar]
- Gagnon S.A., Wagner A.D. Acute stress and episodic memory retrieval: neurobiological mechanisms and behavioral consequences. Ann. N. Y. Acad. Sci. 2016;1369:55–75. doi: 10.1111/nyas.12996. [DOI] [PubMed] [Google Scholar]
- Gazan R., Béchaux C., Crépet A., Sirot V., Drouillet-Pinard P., Dubuisson C., Havard S. Dietary patterns in the French adult population: a study from the second French national cross-sectional dietary survey (INCA2) (2006-2007) Br. J. Nutr. 2016;116:300–315. doi: 10.1017/S0007114516001549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson E.L., Barr S., Jeanes Y.M. Habitual fat intake predicts memory function in younger women. Front. Hum. Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griep L.M.O., Wang H., Chan Q. Empirically derived dietary patterns, diet quality scores, and markers of inflammation and endothelial dysfunction. Curr Nutr Rep. 2013;2:97–104. doi: 10.1007/s13668-013-0045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercberg S., Chat-Yung S., Chauliac M. The French national nutrition and health program: 2001-2006-2010. Int J Public Heal. 2008;53:68–77. doi: 10.1007/s00038-008-7016-2. [DOI] [PubMed] [Google Scholar]
- Horowitz M.A., Zunszain P.A. Neuroimmune and neuroendocrine abnormalities in depression: two sides of the same coin. Ann. N.Y. Acad. Sci. ISSN. 2015;1351:68–79. doi: 10.1111/nyas.12781. [DOI] [PubMed] [Google Scholar]
- Jacka F.N., Cherbuin N., Anstey K.J., Sachdev P., Butterworth P. Western diet is associated with a smaller hippocampus: a longitudinal investigation. BMC Med. 2015;13:1–8. doi: 10.1186/s12916-015-0461-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp S.-M., Endemann R., Domes G., Mecklinger A. Effects of acute psychosocial stress on the neural correlates of episodic encoding: item versus associative memory. Neurobiol. Learn. Mem. 2019;157:128–138. doi: 10.1016/j.nlm.2018.12.006. [DOI] [PubMed] [Google Scholar]
- Kanoski S.E., Davidson T.L. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol. Behav. 2011;103:59–68. doi: 10.1016/j.physbeh.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesse-Guyot E., Amieva H., Castetbon K., Henegar A., Ferry M., Jeandel C., Hercberg S., Galan P., SU.VI.MAX 2 research Group Adherence to nutritional recommendations and subsequent cognitive performance: findings from the prospective Supplementation with Antioxidant Vitamins and Minerals 2 (SU.VI.MAX 2) study. Am. J. Clin. Nutr. 2011;93:200–210. doi: 10.3945/ajcn.2010.29761. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C., Pirke K.M., Hellhammer D.H. The “Trier social stress test” - a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Lasikiewicz N., Hendrickx H., Talbot D., Dye L. Exploring stress-induced cognitive impairment in middle aged, centrally obese adults. Stress. 2013;16:44–53. doi: 10.3109/10253890.2012.682109. [DOI] [PubMed] [Google Scholar]
- Luethi M., Meier B., Sandi C. Stress effects on working memory, explicit memory, and implicit memory for neutral and emotional stimuli in healthy men. Front. Behav. Neurosci. 2009;2:1–9. doi: 10.3389/neuro.08.005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S.J., Juster R.-P., Raymond C., Marin M.-F. The effects of chronic stress on the human brain: from neurotoxicity, to vulnerability, to opportunity. Front. Neuroendocrinol. 2018;49:91–105. doi: 10.1016/j.yfrne.2018.02.001. [DOI] [PubMed] [Google Scholar]
- Méjean C., Deschamps V., Bellin-Lestienne C., Oleko A., Darmon N., Serge H., Katia C. Associations of socioeconomic factors with inadequate dietary intake in food aid users in France (The ABENA study 2004-2005) Eur. J. Clin. Nutr. 2010;64:374–382. doi: 10.1038/ejcn.2009.153. [DOI] [PubMed] [Google Scholar]
- New B., Brysbaert M., Veronis J., Pallier C. The use of film subtitles to estimate word frequencies. Appl. Psycholinguist. 2007;28:661–677. [Google Scholar]
- Noble E.E., Olson C.A., Davis E., Tsan L., Chen Y.-W., Schade R., Liu C., Suarez A., Jones R.B., De C., Serre L., Yang X., Hsiao E.Y., Kanoski S.E. Gut microbial taxa elevated by dietary sugar disrupt memory function. Transl. Psychiatry. 2021;11:194. doi: 10.1038/s41398-021-01309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble J.M., Manly J.J., Schupf N., Tang M.X., Mayeux R., Luchsinger J.A. Association of C-reactive protein to cognitive impairment. Arch. Neurol. 2010;67:87–92. doi: 10.1001/archneurol.2009.308. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olver J.S., Pinney M., Maruff P., Norman T.R. Impairments of spatial working memory and attention following acute psychosocial stress. Stress Health. 2015;31:115–123. doi: 10.1002/smi.2533. [DOI] [PubMed] [Google Scholar]
- Prather A.A., Carroll J.E., Fury J.M., Mcdade K.K., Ross D., Marsland A.L. Gender differences in stimulated cytokine production following acute psychological stress. Brain Behav. Immun. 2008;23:622–628. doi: 10.1016/j.bbi.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provensi G., Schmidt S.D., Boehme M., Bastiaanssen T.F.S., Rani B., Costa A., Busca K., Fouhy F., Strain C., Stanton C., Blandina P., Izquierdo I., Cryan J.F., Passani M.B. Preventing adolescent stress-induced cognitive and microbiome changes by diet. Proc. Natl. Acad. Sci. U.S.A. 2019;116:9644–9651. doi: 10.1073/pnas.1820832116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahakian B.J., Owen A.M. Computerized assessment in neuropsychiatry using CANTAB: discussion paper. J. R. Soc. Med. 1992;85:399–402. [PMC free article] [PubMed] [Google Scholar]
- Samieri C., Lorrain S., Buaud B., Vaysse C., Berr C., Peuchant E., Cunnane S.C., Barberger-Gateau P. Relationship between diet and plasma long-chain n-3 PUFA in older people: impact of Apolipoprotein E genotype. J. Lipid Res. 2013;54:2559–25567. doi: 10.1194/jlr.P036475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu K.V., Sherwin E., Schellekens H., Stanton C., Dinan T.G., Cryan J.F. Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry. Transl. Res. 2017;179:223–244. doi: 10.1016/j.trsl.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Schulz M., Kroke A., Liese A.D., Hoffmann K., Bergmann M.M., Boeing H. 2002. Food Groups as Predictors for Short-Term Weight Changes in Men and Women of the EPIC-Potsdam Cohort. [DOI] [PubMed] [Google Scholar]
- Schwabe L., Höffken O., Tegenthoff M., Wolf O.T. Stress-induced enhancement of response inhibition depends on mineralocorticoid receptor activation. Psychoneuroendocrinology. 2013;38:2319–2326. doi: 10.1016/j.psyneuen.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Shields G.S., Sazma M.A., McCullough A.M., Yonelinas A.P. The effects of acute stress on episodic memory: a meta-analysis and integrative review. Psychol. Bull. 2017;143:636–675. doi: 10.1037/bul0000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivappa N., Bonaccio M., Hebert J.R., Di Castelnuovo A., Costanzo S., Ruggiero E., Pounis G., Donati M.B., de Gaetano G., Iacoviello L. Association of proinflammatory diet with low-grade inflammation: results from the Moli-sani study. Nutrition. 2018;54:182–188. doi: 10.1016/j.nut.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobesky J.L., Barrientos R.M., De May H.S., Thompson B.M., Weber M.D., Watkins L.R., Maier S.F. High-fat diet consumption disrupts memory and primes elevations in hippocampal IL-1β, an effect that can be prevented with dietary reversal or IL-1 receptor antagonism. Brain Behav. Immun. 2014;42:22–32. doi: 10.1016/j.bbi.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S.J., Korosi A., Laye S., Shukitt-Hale B., Barrientos R.M. Food for thought: how nutrition impacts cognition and emotion. NPJ Sci. Food. 2017:1–9. doi: 10.1038/s41538-017-0008-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S.J., Soch A., D'Angelo H., Watkins L.R., Maier S.F., Barrientos R.M. High-fat diet and aging interact to produce neuroinflammation and impair hippocampal-and amygdalar-dependent memory. Neurobiol. Aging. 2017;58:88–101. doi: 10.1016/j.neurobiolaging.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl F., Dörner G. Responses of salivary cortisol levels to stress-situations. Endokrinologie. 1982;80:158–162. [PubMed] [Google Scholar]
- Stawski R.S., Sliwinski M.J., Smyth J.M. The effects of an acute psychosocial stressor on episodic memory. Eur. J. Cognit. Psychol. 2008;21:897–918. doi: 10.1080/09541440802333042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson R.J., Francis H.M., Attuquayefio T., Gupta D., Yeomans M.R., Oaten M.J., Davidson T. Hippocampal-dependent appetitive control is impaired by experimental exposure to a Western-style diet. R. Soc. Open Sci. 2020;7:1–33. doi: 10.1098/rsos.191338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappy L., Morio B., Azzout-Marniche D., Champ M., Gerber M., Houdart S., Mas E., Rizkalla S., Slama G., Mariotti F., Margaritis I. French recommendations for sugar intake in adults: a novel approach chosen by anses. Nutrients. 2018;10:1–16. doi: 10.3390/nu10080989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A., Moreno-Padilla M., Garcia-Rios M.C., Lopez-Torrecillas F., Delgado-Rico E., Schmidt-Rio-Valle J., Fernandez-Serrano M.J. Social stress increases cortisol and hampers attention in adolescents with excess weight. PloS One. 2015;10 doi: 10.1371/journal.pone.0123565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Stamler J., Chan Q., Horn L. Van, Daviglus M.L., Dyer A.R., Miura K., Okuda N., Wu Y., Ueshima H., Elliott P., Zhao L. Salt intake and prevalence of overweight/obesity in Japan, China, the United Kingdom, and the United States: the INTERMAP Study. Am. J. Clin. Nutr. 2019;110:34–40. doi: 10.1093/ajcn/nqz067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.