Abstract
Objectives
Epileptic seizures and epilepsy in urban settings of low-income and middle-income countries (LMIC) are largely under-researched, but their prevalence is necessary for good healthcare planning. This study aimed to determine the lifetime prevalence of epileptic seizures and epilepsy in urban Dar es Salaam.
Methods
Nearly 50,000 people in former Kinondoni district, Dar es Salaam, were screened for epileptic seizures using a set of nine questions. Answers to these nine questions were categorized into generalized, focal, and unspecified seizures. Screening positivity rates were adjusted for questionnaire inaccuracy using two scenarios to analyse true epilepsy prevalences.
Results
Overall, 1085 (2.23%) people fulfilled the criteria for ever having had at least one type of epileptic seizure. Two-hundred-ninety-six (0.60%) people screened positive for generalized seizures, 986 (2.02%) for focal seizures, and 32 (0.07%) for unspecified seizures. Women more commonly screened positive than men (2.61% versus 1.72%, p < 0.001), particularly for focal seizures (p < 0.001). Adjusting for different degrees of accuracy of the screening questionnaire yielded true lifetime prevalences for epilepsy of any type between 1.59% and 2.41%. We furthermore observed a considerable variation of screening positivity rates between wards in Kinondoni district (p < 0.001).
Conclusion
The prevalence of epilepsy, based on a questionnaire survey in urban Tanzania, was higher than previously observed, probably due to the screening questionnaire, which contained questions specifically designed to identify focal seizures. Further studies on epileptic seizures/epilepsy are needed for urban settings in LMIC, preferably with an integrated follow-up of positive cases.
Keywords: Epilepsy, Global health, Epidemiology, Census, Seizures
Highlights
-
•
Data on epilepsy in urban African populations are scarce
-
•
Reliable prevalence estimates for epilepsy are important for healthcare planning but difficult to obtain
-
•
Epilepsy screening tools need to be developed carefully in a participatory approach
-
•
Focal and non-convulsive seizures are often overlooked in studies
-
•
Epilepsy prevalence in urban Tanzania is similar to other parts of Africa
1. Introduction
Epilepsy is one of the commonest neurological disorders worldwide. In 2018, nearly 50 million people were estimated to suffer from active convulsive epilepsy, half of them fall into the category of secondary epilepsy [1]. Around 85% of people with epilepsy live in low-income and middle-income countries (LMIC) where age-standardized epilepsy prevalence is rising faster than in high-income countries (HIC) [2]. Reasons are higher prevalences of risk factors for secondary epilepsy such as perinatal complications, parasitic infections (e.g. cerebral malaria, neurocysticercosis) or road traffic accidents [1,3]. Risk factors for epileptic seizures most likely are similar to those of epilepsy [4]. Epileptic seizures are largely under-investigated, especially in urban settings of LMIC.
Planning of healthcare facilities and dispensaries is important because people with epileptic seizures/epilepsy (PWE) need to have prompt and reliable access to healthcare services without any barriers. That is why accurate prevalence estimates for epileptic seizures/epilepsy are necessary to ensure appropriate planning and sufficient allocation of funds. Knowledge of epilepsy causes is also important for targeting prevention programmes and to ensure best possible treatment for PWE.
Diagnosis of epileptic seizures/epilepsy is seldomly based on imaging, electroencephalogram (EEG) or direct observation of seizures and mostly on description of events by the PWE themselves or observers. This makes it challenging to report reliable prevalence estimates. Moreover, other medical conditions like syncope, movement or sleep disorders may present similarly and may therefore be misdiagnosed as epileptic seizure. In addition, stigma and discrimination against PWE may cause underreporting of seizures.
A meta-analysis on epilepsy prevalences yielded higher rates in LMIC compared with HIC [5]. Furthermore, prevalences were higher in rural than urban areas in LMIC. A recent meta-analysis for sub-Saharan Africa found a lifetime prevalence for epilepsy of 1.60% (95%CI 1.23 to 1.97%) and a prevalence of active epilepsy of 0.90% (95%CI 0.80 to 0.99%). Great variation of prevalences was observed between African sub-regions with the highest rates in Central Africa and lowest rates in Eastern Africa. Furthermore, prevalence of epilepsy was higher in rural African areas (0.98% vs 0.49%) [6]. The estimate for urban areas, however, is based on only three studies, all from Nigeria [[7], [8], [9]]. Yet, the association of higher prevalences in rural areas has been shown also for LMIC outside Africa [5]. Furthermore, most studies assessed the prevalence of epilepsy (lifetime or active), but only few studies assessed the lifetime prevalence of epileptic seizures. This proportion can be considerably higher, as e.g. found in a study in Nigeria where only 16% of people who had an epileptic seizure in their life, had active epilepsy [9].
Although cheap and effective antiepileptic medication is available, many PWE in LMIC do not have access to sustained medical treatment [10,11]. Also, considerable treatment gaps were highlighted before [11,12]. A meta-analysis showed that in Africa nearly 50% of PWE do not receive the treatment they should [11]. This gap is partly due to patient-related reasons such as cultural beliefs and stigma about epilepsy; it is also due to e.g. financial constraints impacting on access to treatment or stock-out of medication. It can be assumed that the lack of healthcare facilities offering counselling for epilepsy in the first place contributes to the undertreatment of PWE. The fast-growing population of Dar es Salaam which is expected to increase from less than 4 million in 2010 to more than 13 million by 2030, poses an equal need for an expansion of healthcare services with expertise on management of PWE to ensure quality patient care [13]. For planning of healthcare services, a good assessment of the need is crucial. That is why this study aims to analyse the proportion of people who have had at least one epileptic seizure in their lifetime and to estimate the lifetime prevalence of epilepsy in an urban population of Dar-es-Salaam, Tanzania.
2. Methods
This study originally had two phases and aimed to assess community lifetime prevalence of epileptic seizures/epilepsy and clinical characteristics/causes of epileptic seizures. In phase one, a large door-to-door survey including 50,000 people was conducted in 2012, to analyse the community-based prevalence of different types of epileptic seizure in Kinondoni District, Dar es Salaam, Tanzania. In phase two, the people screening positive were supposed to be further clinically evaluated and examined to confirm diagnosis. Due to unexpected lack of funding, phase two could not be started directly after phase one, but only one year after the initial screening. Since there is high mobility within the population of Dar es Salaam, it was not possible to track a representative sample of people that screened positive in phase one. We overcame this challenge by applying different sets of previously published parameters to account for the inaccuracy of the screening questionnaire. Due to the very large sample size of phase one and the lack of knowledge about epileptic seizures/epilepsy in an urban LMIC setting, it was deemed worthwhile reporting on the phase one results, nonetheless.
2.1. Study site
The study was conducted in the former Kinondoni district, one of the three districts of Dar es Salaam, Tanzania, with a population of nearly 1.8 million people. The district consists of 27 wards, of which 23 are urban, two semi-urban and two rural. The two rural wards were excluded from the study.
2.2. Questionnaire and diagnostic criteria
In the absence of a standardized screening questionnaire for epileptic seizures/epilepsy, a set of nine questions was selected consisting of questions from epilepsy questionnaires that had been used in other community-based studies for screening [15], and also of newly developed questions on focal onset seizures and non-motor seizures. The nine questions are listed in the appendix (appendix A. Table A.1). The questionnaire was first translated from English into Kiswahili and then translated back to ensure preservation of meaning throughout languages. The final version of the questionnaire in English as well as in Kiswahili was crosschecked for accuracy and clarity by both bilingual principal investigators (ASW and WM). We removed question five from our original questionnaire (“Have you ever had attacks of numbness, tingling in one arm or leg without losing consciousness?”) during data analysis as it yielded to be not very specific. This question was answered with “yes” by 7.1% of women and 2.9% of men (appendix B. Table A.2).
After collection of data, results were grouped based on the combination of answers to different questions, yielding categories of generalized, focal and unspecified epileptic seizures. Focal seizures were then also further differentiated into focal motor and focal impaired awareness seizures according to the latest International League against Epilepsy definition [16]. The necessary combination of answers for a specific seizure type is outlined in the appendix (appendix A. Table A.1). The overall prevalence was determined by screening positive for at least one of the three types of epileptic seizure.
Together with the screening questionnaire, demographic and socioeconomic data were collected from each participant. For children under the age of 18 years, assent was obtained, and the parent or guardian was asked the questionnaire on their behalf.
2.3. Sampling procedure and study population
The sampling procedure has previously been described in detail [17,18]. In short, the administrative system in Tanzania consists of regions and districts. Every district is divided into wards consisting of villages and sub-villages that are further divided into so called ‘ten-cells’; ten-cells are the smallest administrative unit in Tanzania and comprise around 10 to 20 households. In this study, the population of the Kinondoni district was divided into 2886 clusters each consisting of 5 to 10 ten-cells. As the study was not only designed to assess community-based lifetime prevalence of epileptic seizures/epilepsy, but also to assess underlying causes of epileptic seizures, a sample size of 300 patients with epileptic seizures was envisioned. Assuming a prevalence of 12/1000 people for seizures and a drop-out of 50%, yielded the target sample size of 50,000 people necessary for a representative sample of the urban and semi-urban wards of Kinondoni to assess our objectives. For the sampling, 137 clusters were selected following the probability-proportional-to-size method. In each cluster, all households were visited, and all people present were interviewed. If a person was not present, the head of household or another adult was asked on their behalf. In our survey, overall 13,759 households including 49,697 people were recruited.
The screening of the study population was performed between May and July 2012. Fifteen enumerators were involved in interviewing all members of the selected households. Numerators were trained before the study initiation by WM in general aspects of the disease, in the guidelines for data collection and how to use the questionnaire in both languages.
2.4. Statistical analyses
Prevalences by type of seizure were analysed for males and females, by age group, ward, and education. Prevalences were standardized according to 2012 Tanzanian census data by sex and age group (in 5-year bands) and summed up for overall prevalences to make them representative for the population of Kinondoni. Ninety-five percent confidence intervals (95% CI) were calculated based on a binomial distribution. Since phase two of the study could not be carried out as planned, we accounted for inaccuracy of the screening questionnaire by adjusting the results using a Bayesian approach (executed with the R package ‘epiR’). By using Bayesian analyses, we were able to report lifetime prevalence of epilepsy, instead of lifetime prevalence of epileptic seizures. We analysed two scenarios: 1) assuming a sensitivity of 76.3% and a specificity of 99.6%, and 2) assuming a positive predictive value of 73% and a negative predictive value of 100%. The values for the two scenarios were derived from two studies assessing sensitivity and specificity, and positive and negative predictive value of a similar screening questionnaire for lifetime epilepsy [19,20]. In the questionnaire by Hunter et al., questions to identify focal epileptic seizures were also included [20]. Categorical data were compared using Chi square tests and Chi square tests for trend, where appropriate.
2.5. Ethical approval and informed consent
Both phases of the study were approved by the ethics committees of the Muhimbili University of Health and Allied Sciences, Tanzania (Ref. No.: MU/DRP/REC/Vol.I/36, MU/RP/AEC/Vol.Xii/86 and MU/DRP/AEC/Vol.XVI/91) and the Ludwig-Maximilians-University of Munich. During the screening phase, each head of household gave oral informed consent, after receiving appropriate information, on behalf of all family members for the participation in the study. In addition, before performing the interview all interviewees were consented orally and children below the age of 18 years were asked for their assent, while their parents consented officially.
3. Results
49,679 people were screened with our screening questionnaire. The median age in our study sample was 23 years (interquartile range: 11–35) compared with 22 years (interquartile range: 11–33) from the census data for Kinondoni; 51.7% were females (51.4% in the census data of Kinondoni). Most people had no formal (20.7%) or only primary education (54.1%), lived in houses without own water supply (65.5%) and did not have their own toilet but used pit latrines (74.2%). For more detail, refer to Table 1.
Table 1.
Baseline data of the study population.
| All |
Females |
Males |
||
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Study participants | 49,697 | 25,689 (51.7) | 24,008 (48.3) | |
| Age in years | median (interquartile range) | 23 (11–35) | 23 (11–35) | 23 (11–35) |
| Ward | Bunju | 4097 (8.2) | 2120 (8.3) | 1977 (8.2) |
| Hananasif | 425 (0.9) | 236 (0.9) | 189 (0.8) | |
| Kawe | 2026 (4.1) | 1042 (4.1) | 984 (4.1) | |
| Kibamba | 4032 (8.1) | 2043 (8.0) | 1989 (8.3) | |
| Kigogo | 3395 (6.8) | 1729 (6.7) | 1666 (6.9) | |
| Kijitonyama | 452 (0.9) | 236 (0.9) | 216 (0.9) | |
| Kimara | 8272 (16.6) | 4289 (16.7) | 3983 (16.6) | |
| Kinondoni | 667 (1.3) | 366 (1.4) | 301 (1.3) | |
| Kunduchi | 1844 (3.7) | 958 (3.7) | 886 (3.7) | |
| Mabibo | 5680 (11.4) | 2930 (11.4) | 2750 (11.5) | |
| Magomeni | 1525 (3.1) | 791 (3.1) | 734 (3.1) | |
| Makuburi | 476 (1.0) | 251 (1.0) | 225 (0.9) | |
| Makumbusho | 1178 (2.4) | 593 (2.3) | 585 (2.4) | |
| Makurumla | 594 (1.2) | 300 (1.2) | 294 (1.2) | |
| Manzese | 6552 (13.2) | 3405 (13.3) | 3147 (13.1) | |
| Mbezi | 1379 (2.8) | 740 (2.9) | 639 (2.7) | |
| Mburahati | 617 (1.2) | 302 (1.2) | 315 (1.3) | |
| Msasani | 723 (1.5) | 377 (1.5) | 346 (1.4) | |
| Mwananyamala | 2081 (4.2) | 1030 (4.0) | 1051 (4.4) | |
| Ndugumbi | 680 (1.4) | 335 (1.3) | 345 (1.4) | |
| Sinza | 1516 (3.1) | 824 (3.2) | 692 (2.9) | |
| Tandale | 582 (1.2) | 299 (1.2) | 283 (1.2) | |
| Ubungo | 904 (1.8) | 493 (1.9) | 411 (1.7) | |
| Relation to household head | Head of household | 12,697 (25.5) | 2746 (10.7) | 9951 (41.4) |
| Spouse | 8279 (16.7) | 8136 (31.7) | 143 (0.6) | |
| Child/stepchild | 18,664 (37.6) | 9247 (36.0) | 9417 (39.2) | |
| Other relative | 8873 (17.9) | 4755 (18.5) | 4118 (17.2) | |
| No blood relationship | 991 (2.0) | 711 (2.8) | 280 (1.2) | |
| Missing | 193 (0.4) | 94 (0.4) | 99 (0.4) | |
| Education | No formal education | 10,311 (20.7) | 5336 (20.8) | 4975 (20.7) |
| Primary education | 26,865 (54.1) | 14,496 (56.4) | 12,369 (51.5) | |
| Secondary education | 9396 (18.9) | 4535 (17.7) | 4861 (20.2) | |
| Tertiary education | 2839 (5.7) | 1168 (4.5) | 1671 (7.0) | |
| Missing | 286 (0.6) | 154 (0.6) | 132 (0.5) | |
| Marital status | Single | 29,763 (59.9) | 14,988 (58.3) | 14,775 (61.5) |
| Married | 16,961 (34.1) | 8460 (32.9) | 8501 (35.4) | |
| Seperated | 2760 (5.6) | 2123 (8.3) | 637 (2.7) | |
| Missing | 213 (0.4) | 118 (0.5) | 95 (0.4) | |
| Smoking | Does not smoke | 37,650 (75.8) | 19,841 (77.2) | 17,809 (74.2) |
| Smokes | 11,847 (23.8) | 5739 (22.3) | 6108 (25.4) | |
| Missing | 200 (0.4) | 109 (0.4) | 91 (0.4) | |
| Alcohol drinking behaviour | Does not drink | 43,518 (87.6) | 23,425 (91.2) | 20,093 (83.7) |
| Drinks seldomly | 2118 (4.3) | 918 (3.6) | 1200 (5.0) | |
| Drinks frequently | 3878 (7.8) | 1241 (4.8) | 2637 (11.0) | |
| Missing | 183 (0.4) | 105 (0.4) | 78 (0.3) |
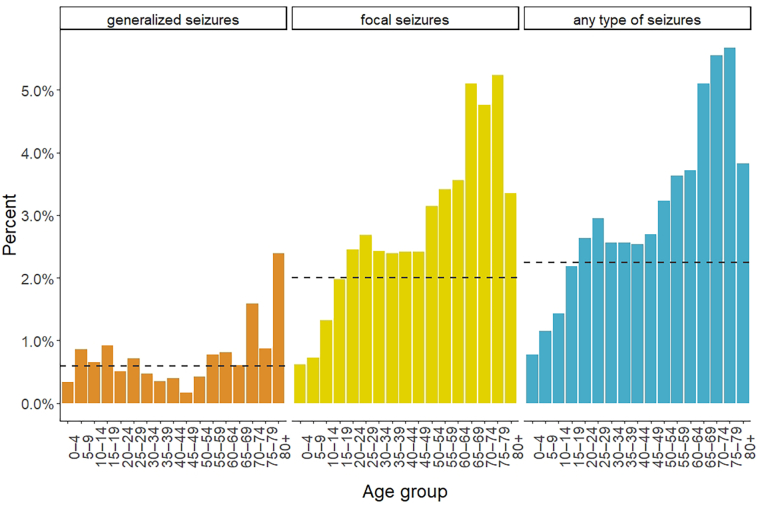
Overall, 1085 (2.23%) people fulfilled the criteria for at least one type of epileptic seizure. 293 (0.60%) people screened positive for generalized seizures, 986 (2.02%) for focal seizures, and 32 (0.07%) for unspecified seizures (Table 2, Table 3). Screening positivity increased with age for all types of seizure, but stronger for focal seizures (Fig. 1). Among those who screened positive, 226 screened positive for both, generalized and focal seizures. Seventy-seven percent of those who screened positive for generalized seizures also screened positive for focal seizures, and 23% of those who screened positive for focal seizures also screened positive for generalized seizures. Every fifth patient who screened positive for focal seizures, screened positive for both – focal motor seizures and focal impaired awareness seizures (203; 21%). Four hundred fifty-seven people (46%) only had focal motor seizures and 326 (33%) only had focal impaired awareness seizures (Table 3).
Table 2.
Screening positivity rates by type of epileptic seizure and estimated lifetime epilepsy prevalences by scenario and type of seizure.
| n | Type of epileptic seizure |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| generalized |
focal |
unspecified |
any type |
||||||
| n | % | n | % | n | % | n | % | ||
| Both sexes | 49,697 | 293 | 0.60 [0.53–0.67] | 986 | 2.02 [1.90–2.15] | 32 | 0.07 [0.04–0.09] | 1085 | 2.23 [2.10–2.36] |
| scenario 1 | 0.27 [0.18–0.36] | 2.14 [1.98–2.31] | NA † | 2.41 [2.24–2.59] | |||||
| scenario 2 | 0.43 [0.37–0.49] | 1.45 [1.34–1.55] | 0.05 [0.03–0.07] | 1.59 [1.48–1.70] | |||||
| Females | 25,689 | 151 | 0.60 [0.51–0.70] | 613 | 2.43 [2.24–2.63] | 24 | 0.10 [0.06–0.14] | 671 | 2.66 [2.46–2.86] |
| scenario 1 | 0.26 [0.14–0.40] | 2.67 [2.43–2.93] | NA † | 2.98 [2.72–3.25] | |||||
| scenario 2 | 0.43 [0.35–0.51] | 1.74 [1.58–1.90] | 0.07 [0.04–0.1] | 1.91 [1.74–2.07] | |||||
| Males | 24,008 | 142 | 0.60 [0.51–0.71] | 373 | 1.59 [1.43–1.74] | 8 | 0.03 [0.01–0.07] | 414 | 1.76 [1.60–1.94] |
| scenario 1 | 0.27 [0.14–0.41] | 1.54 [1.34–1.76] | NA † | 1.80 [1.58–2.03] | |||||
| scenario 2 | 0.43 [0.35–0.51] | 1.13 [1.00–1.27] | 0.02 [0.00–0.04] | 1.26 [1.12–1.40] | |||||
Adjusting for inaccuracy of screening questionnaire using Bayesian statistics.
Scenario 1: sensitivity 76.3%, specificity 99.6%.
Scenario 2: positive predictive value 73%, negative predictive value 100%.
† Bayesian analysis yielded negative results.
Table 3.
Number of people screening positive by classification of type of epileptic seizure.
| All | Proportion | |
|---|---|---|
| Generalized seizures | 293 | (67 uniquely | 226 [77%] also focal seizures) |
| Focal seizures | 986 | (660 uniquely| 226 [23%] also generalized seizures) |
| focal motor | 457 | 46% |
| focal impaired awareness | 326 | 33% |
| both | 203 | 21% |
| Unspecified seizures | 32 |
Fig. 1.
Screening positivity rates by type of epileptic seizure and age group.
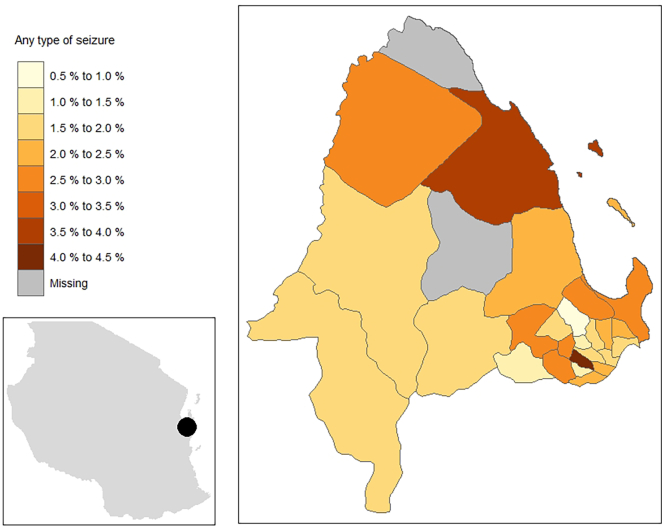
Overall, women more commonly screened positive for any type of seizure than men (2.61% versus 1.72%, p < 0.001). The difference was mainly observed for focal seizures (2.39% versus 1.55%, p < 0.001). Prevalence of any type of epileptic seizure differed significantly by degree of education (p = 0.04). People with higher education, more commonly screened positive for any type of seizure. On the contrary, prevalence of generalized seizures was inversely associated with degree of education (p = 0.001). Screening positivity varied by ward, ranging from 0.8% in Kijitonyama to 4.4% in Makurumla (Table 1, Table 2; Fig. 2).
Fig. 2.
Screening positivity rates for any type of epileptic seizure, by ward in Kinondoni district.
Our two scenarios, assuming different positive/negative predictive values and different values for sensitivity/specificity of our screening questionnaire, yielded true prevalence midpoint estimates of 0.27 to 0.43% for epilepsy with generalized seizures (unadjusted estimate: 0.60%), 1.45 to 2.14% for epilepsy with focal seizures (unadjusted estimate: 2.02%) and 1.59 to 2.41% for epilepsy with any type of epileptic seizure (unadjusted estimate: 2.23%; Table 2).
4. Discussion
In this large community-based door-to-door survey in Dar-es-Salaam, Tanzania, we found a community lifetime prevalence for any type of epileptic seizure that was comparable to other studies in similar settings [7,21,22]. After adjusting for inaccuracy of our screening questionnaire, the lifetime prevalences of epilepsy were within similar range yet mostly higher than previously reported [5,6]. Reason for the higher prevalence in our study is likely the inclusion of questions for focal seizures. One study from Burkina Faso, however, observed even higher rates of lifetime epilepsy (3.5%, 95%CI 3.0–4.0%) that increased up to 7.9% (95%CI 5.9–10.6%) when adjusting for different degrees for sensitivity and specificity of the screening questionnaire [23]. In this study, 535 people who screened positive for epileptic seizures using a four-item screening questionnaire, were examined by a physician and only 153 (28.6%) were confirmed to have epilepsy. At the same time, of 231 people who screened negative for epileptic seizures and were examined by a physician, 13 (5.6%) actually had epilepsy [23].
In two studies from urban Nigeria, the prevalence of active epilepsy was between 0.43% (95%CI 0.27–0.59%) and 0.60% (95%CI 0.59–0.60%) [7,8]. Both studies used a two-stage design for the confirmation of epilepsy, although the two studies had only a small sample size. For the study by Ezeala-Adikaibe et al., 8300 people were screened for lifetime prevalence of convulsions which yielded a positivity rate of 3.74%, which was even higher than the in our study [7]. In the second stage, all people who screened positive were evaluated whether they had epilepsy and if it was active epilepsy. As a result, only 16% of those who screened positive were found to suffer from active epilepsy. If we applied this proportion to our study, around 0.3% of people would be suffering from active epilepsy. In this context, it would be important to point out the difference between “active epilepsy”, “lifetime epilepsy” and “lifetime epileptic seizures”. Our study calculated the latter two, but did not determine active epilepsy. The proportion of people who have ever experienced an epileptic seizure in their lifetime is greater than the proportion of people who have lifetime epilepsy, which, in turn, is greater than the proportion of people with active epilepsy. The following reasons are relevant: firstly, epileptic seizures represent events that often do not recur, whereas epilepsy refers to a neurological disorder that involves recurrent seemingly unprovoked seizures. Secondly, active epilepsy includes patients who are either taking antiepileptic medication or whose last epileptic seizure occurred within a specific time period, usually the last year. Distinguishing between the three categories would require a more detailed history of epileptic seizures and preferably a clinical neurological examination, which screening questionnaire interviews alone usually cannot accommodate. A second phase after the initial screening is therefore necessary to establish the diagnosis of epilepsy as opposed to epileptic seizure.In our study, particularly the prevalence of focal seizures contributed to the increased positivity rate for any type of epileptic seizure which is likely due to us including questions specifically addressing focal epileptic seizures. As comparison, most other community-based studies used a questionnaire focussing on generalized convulsive seizures only. That is because generalized seizures can be identified more easily by community health workers than non-convulsive seizures or focal seizures [24,25]. The large excessive burden of focal seizures that we found was observed more commonly among females than males which has been reported before [26,27]. However, other studies have found a higher proportion of men suffering from epilepsy which was linked to a higher risk of secondary seizures, e.g. after head trauma or stroke [28]. On the other hand, there are also several reasons why women might have screened positive more often. These are e.g. better awareness of symptoms and consciousness about their own health, but also because women may speak more often and openly about their own health, e.g. at antenatal clinics. Another reason could be that there are lower levels of stigma and negative attitudes towards epilepsy among women. Nonetheless, overreporting is likely because the questions for focal seizures were rather unspecific.
The degree of concealment due to stigma or traditional beliefs, such as epilepsy as a form of witchcraft or supernatural influence, can only be estimated. Furthermore, unawareness of epilepsy was not taken into account in this survey. This could have been captured by adding a question whether the person has ever heard of epilepsy.
Whilst generalized seizures were similar across age groups, the prevalence of focal seizures increased with age which could be due to secondary epilepsy. In our study, 91% of those who screened positive for any type of epileptic seizure, screened positive for focal epileptic seizures. Most other studies that evaluated epileptic seizures/epilepsy in sub-Saharan Africa, found considerably smaller proportions of patients with focal epileptic seizures (mostly between 4 and 40%) [29]. However, there were also two studies in rural Kenya and rural Tanzania which found that more than 70% of patients with epilepsy had focal epilepsy [20,30]. In a study conducted in mental health clinics in the same district in Dar-es-Salaam, only 27% of patients had focal seizures – either solely focal or focal to bilateral tonic-clonic [31].
The prevalence of epileptic seizures/epilepsy in sub-Saharan Africa varies considerably which may be the result of different questionnaires, sample populations, definitions (epileptic seizure or epilepsy) or observation periods (lifetime epilepsy or active epilepsy) [29]. However, also definitions of epilepsy differ between studies. Most studies on epileptic seizures in sub-Saharan Africa did not identify people with non-convulsive seizures because the questionnaires used were designed to detect convulsive seizures only, and particularly generalized convulsive seizures [24,25]. Assessment of non-convulsive epileptic seizures requires taking a more detailed history of signs/symptoms like strange behaviour or staring. This is why we included questions designed to detect non-convulsive seizures (questions 6 to 8 in our questionnaire). If phase two of this study had taken place as planned, we could have verified or excluded a diagnosis of non-convulsive seizures. It is estimated that the actual burden of epilepsy might be up to twice as high as commonly found in epidemiological studies because of non-comprehensive questionnaires which exclude e.g. non-convulsive seizures a priori [32,33]. In our study, the prevalence of epileptic seizures more than doubled when including non-convulsive epileptic seizures.
We observed quite a profound geographic variation of screening positivity. In some wards of Kinondoni district nearly no person screened positive for epileptic seizures, whereas in others, more than 4% screened positive. A geographic heterogeneity of epilepsy has previously been reported and could be due to environmental or genetic risk factors which are often clustered too. [14,28] These could be e.g. exposure to risk factors for secondary epilepsy, such as malaria or neurocysticercosis. A study among people with epilepsy in Kinondoni district, however, did not find many cases of neurocysticercosis in this district which makes it an unlikely explanation of the geographic variation observed [31]. Also, awareness of epilepsy may differ by degree of education which may cluster too. Furthermore, distance to the next healthcare service has been hypothesised as factor influencing the likelihood of screening positive for seizures because people living closer to a healthcare facility might be more likely to receive treatment and therefore be more aware of their disorder. This, however, could not be confirmed in a study [14].
5. Limitations
Our study had several limitations. Due to lack of funding, phase two of this study could not be started until more than one year after the initial screening of patients. When we started inviting people again for neurological examination, we were not able to find most of the individuals that originally screened positive. Also, tracing back individuals to their homes was difficult because the study support team had changed and because many people had moved houses. Hence we were not able to accomplish phase two of this study and could therefore not verify whether people who screened positive had epileptic seizures or epilepsy. We tried to overcome this by analysing two scenarios of inaccuracy of our questionnaires. Whilst this approach has limitations compared with examining patients directly, we think that the results are nonetheless valid, particularly for generalized epileptic seizures, because the values for the inaccuracy of the screening tool came from studies that used questionnaires primarly designed to detect generalized convulsive seizures. For the analysis of true prevalences of focal seizures, different test accuracy parameters may be needed. It is likely that the positive predictive value from our scenario 2 is too high, because our set of questions (especially those on focal seizures) may capture several other conditions/diseases as well and, therefore, potentially less than 73% of people who screened positive for focal seizures, really had focal seizures. Likewise, the negative predictive value of 100% most likely is too high, because it does not account for unawareness of epilepsy and concealment of epileptic seizures. Estimating the true negative predictive value, however, is difficult as there is no objective test for epilepsy. It would have required questions on knowledge about epilepsy as well as detailed questions on epileptic seizures answered by the individuals themselves and also by family members. Nonetheless, we consider the scenarios we used as appropriate to estimate the true prevalence of epilepsy, because they are from studies conducted in similar settings using similar screening questionnaires.
6. Conclusion
In this study with almost 50,000 individuals contacts, we analysed the urban lifetime prevalence of epileptic seizures, estimated the urban lifetime prevalence of epilepsy and put both measures in the context of other African studies. The majority of epileptic seizures were of the focal type, which may have been due to the nature of our questionnaire that, unlike most other questionnaires, also captured non-convulsive seizures and other focal types of epileptic seizure. Whilst our results are likely overestimations, they nonetheless demonstrate that non-convulsive seizures seem to be common and hence need to be considered. Unfortunately, we were not able to follow-up on individuals that screened positive due to the reasons given above. However, our study still contributes to filling the gap of knowledge on the prevalence of epileptic seizures/epilepsy in urban settings of LMIC. Those numbers are important for healthcare planning.
Funding
This study was funded by the DFG (German Research Foundation) within the research grant (WI 3427/1–1) “Neurocysticercosis in sub-Saharan Africa”. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
Acknowledgements
We deeply acknowledge the help and support of all students, staff, translators and other parties involved in this large door-to-door survey. We are very grateful for the participants' cooperation and time to participate in this study.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Declaration of Competing Interest
None of the authors declare a conflict of interest.
Appendix A
Table A.1.
| Question 1: Have you ever lost consciousness or fallen and lost consciousness? Question 2: Have you ever been told that while you were unconscious your arms and legs shake or stretch out? Question 3: Have you ever had attacks in which you fall and bite your tongue or lost control of your bladder or bowels? Question 4: Have you ever had uncontrollable attacks of shaking or trembling in one arm or leg or in the face without losing consciousness? Question 5: Have you ever had attacks of numbness, tingling in one arm or leg without losing consciousness? Question 6: Have you ever had attacks in which you lose contact with the surroundings without losing consciousness? Question 7: Have you ever had attacks of losing awareness that was associated with a feeling of vagueness, unreality or dreaminess or experience of abnormal smells, sounds, or vision without losing consciousness? Question 8: Have you ever had episodes of strange behaviour without remembering it? Question 9: Have you ever been told that you have or had epilepsy or epileptic fits? | |
| Generalized seizures | 1 AND (2 OR 3) |
| Focal seizures† | 4 OR 6 OR 7 OR 8 |
| Simple partial seizures‡ | 4 AND NOT (6 OR 7 OR 8) |
| Complex-partial seizures | (6 OR 7 OR 8) AND NOT 4 |
| Unspecified seizures | 9 AND NOT (generalized seizures or focal seizures) |
| Any type of seizures | At least one of the above four types |
† Originally, question 5 was also considered for definition of focal seizures, but due to its low specificity, the question was removed from the analysis.
‡ Simple partial motor seizures.
Table A.2.
| All | Females | Males | |||||
|---|---|---|---|---|---|---|---|
| People screened | 49,697 | 25,689 | 24,008 | p‡ | |||
| Question 1 | 293 | 0.6% | 151 | 0.6% | 142 | 0.6% | 1.00 |
| Question 2 | 307 | 0.6% | 162 | 0.6% | 145 | 0.6% | 0.75 |
| Question 3 | 240 | 0.5% | 129 | 0.5% | 111 | 0.5% | 0.57 |
| Question 4 | 660 | 1.3% | 418 | 1.6% | 242 | 1.0% | <0.001 |
| Question 5† | 2530 | 5.1% | 1828 | 7.1% | 702 | 2.9% | <0.001 |
| Question 6 | 334 | 0.7% | 196 | 0.8% | 138 | 0.6% | 0.012 |
| Question 7 | 266 | 0.5% | 162 | 0.6% | 104 | 0.4% | 0.003 |
| Question 8 | 303 | 0.6% | 172 | 0.7% | 131 | 0.5% | 0.09 |
| Question 9 | 261 | 0.5% | 143 | 0.6% | 118 | 0.5% | 0.35 |
† Question was removed from the analysis.
‡ p-value for the difference between females and males.
References
- 1.Global, regional, and national burden of epilepsy, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(4):357–375. doi: 10.1016/S1474-4422(18)30454-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Institute for Health Metrics and Evaluation (IHME) University of Washington Global Burden of Disease Results Tool. 2020. http://ghdx.healthdata.org/gbd-results-tool (Accessed 02/05)
- 3.Winkler A.S. Neurocysticercosis in sub-Saharan Africa: a review of prevalence, clinical characteristics, diagnosis, and management. Pathog Glob. Health. 2012;106(5):261–274. doi: 10.1179/2047773212Y.0000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosser P., Schmutzhard E., Winkler A.S. The pattern of epileptic seizures in rural Tanzania. J. Neurol. Sci. 2007;258(1–2):33–38. doi: 10.1016/j.jns.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Ngugi A.K., Bottomley C., Kleinschmidt I., Sander J.W., Newton C.R. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia. 2010;51(5):883–890. doi: 10.1111/j.1528-1167.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owolabi L.F., Adamu B., Jibo A.M. Prevalence of active epilepsy, lifetime epilepsy prevalence, and burden of epilepsy in Sub-Saharan Africa from meta-analysis of door-to-door population-based surveys. Epilepsy Behav. 2020;103(Pt A):106846. doi: 10.1016/j.yebeh.2019.106846. [DOI] [PubMed] [Google Scholar]
- 7.Ezeala-Adikaibe B.A., Orjioke C., Ekenze O. Prevalence of active convulsive epilepsy in an urban slum in Enugu south East Nigeria. Seizure. 2016;35:100–105. doi: 10.1016/j.seizure.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nwani P., Nwosu M., Asomugha L., Enwereji K., Arinzechi E., Ogunniyi A. Epidemiology of active epilepsy in a suburban community in Southeast Nigeria: a door-to-door survey. Niger. J. Clin. Pract. 2015;18(4):527–533. doi: 10.4103/1119-3077.151789. [DOI] [PubMed] [Google Scholar]
- 9.Osakwe C., Otte W.M., Alo C. Epilepsy prevalence, potential causes and social beliefs in Ebonyi state and Benue state, Nigeria. Epilepsy Res. 2014;108(2):316–326. doi: 10.1016/j.eplepsyres.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Meyer A.C., Dua T., Ma J., Saxena S., Birbeck G. Global disparities in the epilepsy treatment gap: a systematic review. Bull. World Health Organ. 2010;88(4):260–266. doi: 10.2471/BLT.09.064147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mbuba C.K., Ngugi A.K., Newton C.R., Carter J.A. The epilepsy treatment gap in developing countries: a systematic review of the magnitude, causes, and intervention strategies. Epilepsia. 2008;49(9):1491–1503. doi: 10.1111/j.1528-1167.2008.01693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mbuba C.K., Ngugi A.K., Fegan G. Risk factors associated with the epilepsy treatment gap in Kilifi, Kenya: a cross-sectional study. Lancet Neurol. 2012;11(8):688–696. doi: 10.1016/S1474-4422(12)70155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.United Nations Department of Economic and Social Affairs (UN DESA) Population Division. World Urbanization Prospects. 2019. https://population.un.org/wup/ (Accessed 19/05/, 2020)
- 14.Edwards T., Scott A.G., Munyoki G. Active convulsive epilepsy in a rural district of Kenya: a study of prevalence and possible risk factors. Lancet Neurol. 2008;7(1):50–56. doi: 10.1016/S1474-4422(07)70292-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Preux P.M. Questionnaire in a study of epilepsy in tropical countries. Bull. Soc. Pathol. Exot. 2000;93(4):276–278. [PubMed] [Google Scholar]
- 16.Fisher R.S., Cross J.H., French J.A. Operational classification of seizure types by the international league against epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):522–530. doi: 10.1111/epi.13670. [DOI] [PubMed] [Google Scholar]
- 17.Burtscher C., Baxmann A., Kassubek J. Prevalence of restless legs syndrome in an urban population of eastern Africa (Tanzania) J. Neurol. Sci. 2014;346(1–2):121–127. doi: 10.1016/j.jns.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Stelzle D., Storz C., Baxmann A. Febrile seizures in an urban Tanzanian population: lessons learned from a community-based random cluster survey. Tropical Med. Int. Health. 2021;26(4):492–502. doi: 10.1111/tmi.13548. Epub 2021 Feb 18. PMID: 33415795. [DOI] [PubMed] [Google Scholar]
- 19.Giuliano L., Cicero C.E., Crespo Gomez E.B. A screening questionnaire for convulsive seizures: a three-stage field-validation in rural Bolivia. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0173945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunter E., Rogathi J., Chigudu S. Prevalence of active epilepsy in rural Tanzania: a large community-based survey in an adult population. Seizure. 2012;21(9):691–698. doi: 10.1016/j.seizure.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Balogou A.A.K., Grunitzky E.K., Belo M. Management of epilepsy patients in Batamariba district, Togo. Acta Neurol. Scand. 2007;116(4):211–216. doi: 10.1111/j.1600-0404.2007.00871.x. [DOI] [PubMed] [Google Scholar]
- 22.Sebera F., Munyandamutsa N., Teuwen D.E. Addressing the treatment gap and societal impact of epilepsy in Rwanda — results of a survey conducted in 2005 and subsequent actions. Epilepsy Behav. 2015;46:126–132. doi: 10.1016/j.yebeh.2015.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sahlu I., Bauer C., Ganaba R. The impact of imperfect screening tools on measuring the prevalence of epilepsy and headaches in Burkina Faso. PLoS Negl. Trop. Dis. 2019;13(1) doi: 10.1371/journal.pntd.0007109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization . WHO; 2015. Diagnosis of convulsive epilepsy by non-specialist health care providers. [Google Scholar]
- 25.Nicoletti A., Reggio A., Bartoloni A. Prevalence of epilepsy in rural Bolivia: a door-to-door survey. Neurology. 1999;53(9):2064–2069. doi: 10.1212/wnl.53.9.2064. [DOI] [PubMed] [Google Scholar]
- 26.Rwiza H.T., Kilonzo G.P., Haule J., Matuja W.B.P. Prevalence and incidence of epilepsy in Ulanga, a rural Tanzanian District: a community-based study. Epilepsia. 1992;33(6):1051–1056. doi: 10.1111/j.1528-1157.1992.tb01758.x. [DOI] [PubMed] [Google Scholar]
- 27.Winkler A.S., Kerschbaumsteiner K., Stelzhammer B., Meindl M., Kaaya J., Schmutzhard E. Prevalence, incidence, and clinical characteristics of epilepsy--a community-based door-to-door study in northern Tanzania. Epilepsia. 2009;50(10):2310–2313. doi: 10.1111/j.1528-1167.2009.02184.x. [DOI] [PubMed] [Google Scholar]
- 28.Preux P.M., Druet-Cabanac M. Epidemiology and aetiology of epilepsy in sub-Saharan Africa. Lancet Neurol. 2005;4(1):21–31. doi: 10.1016/S1474-4422(04)00963-9. [DOI] [PubMed] [Google Scholar]
- 29.Ba-Diop A., Marin B., Druet-Cabanac M., Ngoungou E.B., Newton C.R., Preux P.M. Epidemiology, causes, and treatment of epilepsy in sub-Saharan Africa. Lancet Neurol. 2014;13(10):1029–1044. doi: 10.1016/S1474-4422(14)70114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munyoki G., Edwards T., White S. Clinical and neurophysiologic features of active convulsive epilepsy in rural Kenya: a population-based study. Epilepsia. 2010;51(12):2370–2376. doi: 10.1111/j.1528-1167.2010.02653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt V., O’Hara M.C., Ngowi B. Taenia solium cysticercosis and taeniasis in urban settings: epidemiological evidence from a health-center based study among people with epilepsy in Dar Es Salaam, Tanzania. PLoS Negl. Trop. Dis. 2019;13(12) doi: 10.1371/journal.pntd.0007751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cockerell O.C., Eckle I., Goodridge D.M., Sander J.W., Shorvon S.D. Epilepsy in a population of 6000 re-examined: secular trends in first attendance rates, prevalence, and prognosis. J. Neurol. Neurosurg. Psychiatry. 1995;58(5):570–576. doi: 10.1136/jnnp.58.5.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banerjee P.N., Filippi D., Allen Hauser W. The descriptive epidemiology of epilepsy-a review. Epilepsy Res. 2009;85(1):31–45. doi: 10.1016/j.eplepsyres.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.