Graphical abstract
Keywords: Coal dust, Coal microparticles, Marine terminals, Marine ecosystems, Ecotoxicology, Genotoxicology
Highlights
-
•
Coal dust in seawater is generally not an inert substance for marine organisms.
-
•
The important factor is the dose of coal particles to which organisms are exposed.
-
•
The marine fauna is more resistant to the effects of coal, than plants and protozoans.
-
•
There is a need of impact assessment of different types of coal in one experiment.
Abstract
Coal dust is a source of pollution not only for atmospheric air but also for the marine environment. In places of storage and handling of coal near water bodies, visible pollution of the water area can be observed. Coal, despite its natural origin, can be referred to as anthropogenic sources of pollution. If coal microparticles enter the marine environment, it may cause both physical and toxic effects on organisms. The purpose of this review is to assess the stage of knowledge of the impact of coal particles on marine organisms, to identify the main factors affecting them, and to define advanced research directions. The results presented in the review have shown that coal dust in seawater is generally not an inert substance for marine organisms, and there is a need for further study of the impact of coal dust particles on marine ecosystems.
1. Introduction
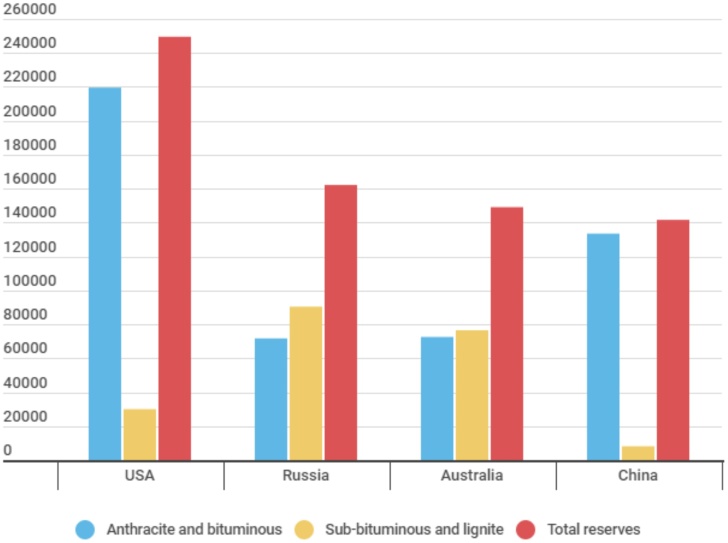
Economic and social development is accompanied by an increase in energy consumption these days. Coal is an important fossil fuel that provides heat and electricity to people around the world. According to coal reserves data, the USA ranks 1st place in the world (233% of world reserves, 249537 million tons by the end of 2019), Russia ranks 2nd place (152%, 162166 million tons), Australia and China rank 3rd and 4th place (139%, 149079 million tons and 132%, 141595 million tons, respectively) (see Fig. 1) [1].
Fig. 1.
Countries with the largest coal reserves in the world, million tons [1].
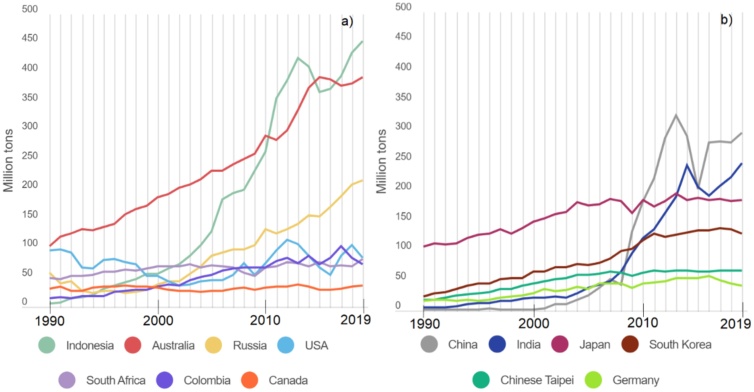
The international coal trade is heavily dependent on shipping. The leading importers of coal are China, India, the countries of Europe, and the Asia-Pacific region, while the leading exporters are Australia, Indonesia, and Russia as shown in Fig. 2 [1,2].
Fig. 2.
Export (a) and import (b) of coal in countries for the period from 1990 to 2019, million tons per year, according to [2].
Handling and transportation of bulk and dusty cargo in ports are significant sources of air dust [[3], [4], [5]] that can also pollute the marine environment. For example, the content of suspended solids in coastal waters near some coal ports in Australia, Indonesia, China, and Columbia range from 10 to 511 mg/L [[6], [7], [8], [9]]. These introduced coal particles are also a result of handling procedures of marine coal terminals (when coal is loaded onto a ship or unloaded from a ship, when using conveyors and other uncovered transport equipment) and storage procedures, where coal is placed as open piles at coal terminals (dust and particles can enter the water area due to wind, cyclones, and heavy monsoon rains) [10,11]. The influence of wind during storage and transportation of coal, can lead to emissions of pulverized coal, which has a significant impact on the climate, human health, flora, and fauna [[12], [13], [14]]. Also, coal can enter the marine environment as a result of accidents on sea vessels, as well as various operations (discharge or unloading of residues after washing the cargo compartments). Although bulk cargo losses occur much more frequently than oil spills, they usually stay unrecorded [15,16].
Well-known dust suppression technologies, such as the installation of windbreak walls, irrigation of coal, use of closed conveyors and rotary car dumper systems, reduce the formation of coal dust but do not exclude it, as these measures are aimed primarily at reducing the concentration of coal dust in the residential area. Irrigation of coal can also contribute to the washout of coal particles into the sea if the storm collector is not properly designed.
To address the concentration of released coal dust, new technologies are also applied, for example, a smart installation in the port of Huanghua, China, allows compaction of a mixture of water and coal that is collected on the territory of the port and then sold as a product. However, the drawback of such systems is the time it requires to reach a sufficient profit revenue (the payback period for this installation is 30 years) [5].
Suspended coal particles in the absence of precipitation can remain in the atmosphere for about a week [17], which will allow them to mix with other aerosol components during their movement [[18], [19], [20]] and bring additional pollutants with them, into the marine environment. A significant deposition of coal particles takes place near coal terminals, where at the same time, the concentration of coal in seawater, decreases rapidly with increasing distance. Larger coal particles (> 2.36 mm) settle near the terminal, while smaller particles (<53 μm) move much further [21].
Coal entering seawater not only changes the properties of the marine environment, but also can affect marine organisms, that are able to adapt to new habitat conditions, but possible reactions may reduce their capability to counteract any toxic effects. An important task is to assess these consequences and reactions and to determine the mechanisms that may cause them.
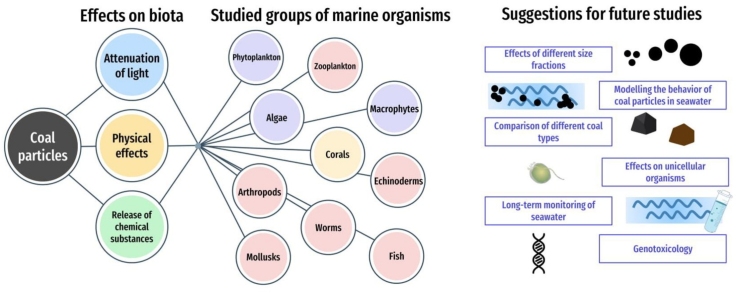
In recent years, a significant number of studies have been devoted to the effect of micro and nanosized particles of various origins on marine organisms [[22], [23], [24], [25], [26], [27]]. Amongst them, some are devoted to the effects of coal particles and are presented in this review. Since there is no consensus regarding the safety of coal for marine organisms and the experimental results differ significantly depending on the species, the summary of the data will allow determining limitations. The continuing interest in this problem allows us to conclude that it is necessary to review the results of these studies, update the current review data on the biological effects of coal on aquatic organisms [28], and define directions for further possible research and solutions. In section 2 of this review, we discussed coal characteristics and their possible integrations with marine biota. Section 3 is devoted to the impact of coal particles on different groups of organisms, from plankton to fish, and sections 4 and 5 discussed future research and conclude the presented review.
2. Coal dust, its properties, and behavior in seawater
Coal is a sedimentary rock formed during two biochemical and thermophysical processes, diagenesis and catagenesis [29]. As a sedimentary rock, coal is a complex heterogeneous mixture of organic matter and, to a lesser extent, inorganic matter of allogenic or authigenic origin. Organic matter consists mainly of non-crystalline constituents such as petrographic ingredients (lithotypes, microlitotype groups, and macerals), and in some cases, crystalline compounds (organic minerals). An inorganic substance consists of crystalline components (mineral substances from sulfides-thiosalts, oxides-hydroxides, silicates, sulfates, carbonates, phosphates, chlorides, vanadates, tungstates, etc.), to a lesser extent - semi- crystalline components (poorly crystallized mineraloids of some silicates, phosphates, and hydroxides) and, sometimes, amorphous compounds. Mineral matter, as a part of inorganic matter, includes minerals and mineraloids [30].
2.1. Chemical composition
Coal can contain various inorganic compounds of the following elements As, B, Ba, Cd, Cl, Co, Cr, Cu, F, Hg, Mn, Mo, Ni, Pb, Sb, Se, Th, U, V, Zn, and some organic compounds, with polycyclic aromatic hydrocarbons (PAHs) being especially toxic [[30], [31], [32], [33], [34], [35], [36]]. Coal can become a potential source of these substances in water due to their leaching [37]. For example, mercury (Hg) and lead (Pb) are elements lethal to organisms, arsenic (As) is potentially mutagenic, and As and Pb are both carcinogenic [38,39]. Hazardous compounds (Hg, Pb, As) are stable in the biosphere and bioaccumulate in food chains. Hg is toxic in both inorganic and elemental forms after being released into water, earth, and air [39]. Mn is important for algal growth [40], but its uptake by marine phytoplankton is insignificant due to its low affinity for metal [41]. However, it has been demonstrated that Mn accumulation in coastal marine phytoplankton can be prevented by high concentrations of metals such as Cu or Cd [42]. A study on three types of South African coal, showed that the leaching of metals such as Cu, Cr, Fe, and Pb in seawater at a pH level of 8 was insignificant due to the presence of humic substances in coal or seawater, which prevented them moving from coal to seawater [43]. At the same time, the high solubility of the compounds Ni and Mn was noted, and the that leaching efficiency is greatly reduced for coal with a high calcite content. Coal also contains (PAHS) in the number of hundreds, and some cases even thousands (mg/kg) [44]. The presence of PAHs was recorded in bottom sediments and suspended particles near the Hay Point coal terminal, Australia, in concentrations close to toxic levels for marine organisms [11]. It was reported that volatile organic compounds can inhibit the growth of algae while reducing zooplankton biomass [28,45]. Bituminous coal contains a significant quantity of PAHs [28,46], which is mentioned for coking coals [47]. PAHs bioavailability could cause toxic effects on marine organisms [48,49]. Physical and chemical properties of coal can be determined by sedimentation conditions in which peat is formed and its subsequent transformations [50]. For example, peat deposition conditions are related to elevated concentrations of some toxic components (such as Sulphur (S), and in some cases As and Hg), which have an adverse effect on the environment and human health [[50], [51], [52]]. Therefore, it is necessary to know the origin of coal and its type so as to evaluate the consequences if it enters the environment. At the same time, fossil coals are very diverse in their composition and properties [[53], [54], [55]].
The reactivity of organic compounds in seawater is certainly related to their chemical composition and structure, but the reactivity of organic carbon is explained by many factors, such as temperature, structure of the microbial community and the benthic ecosystem, type of mineral, surface area, redox potential, light, availability of nutrients, pH level, salinity, porosity, permeability, water content and time of exposure to oxygen [[56], [57], [58], [59], [60], [61]]. These variables are biophysiochemical properties of the environment, therefore the reactivity of organic substances, including coal, is determined by the interaction of matter and the ecosystem [56].
2.2. Physical properties
The behavior of particles in seawater depends on many factors, especially on the vertical movement, which contributes to the deposition of particles to the marine bottom [56,[62], [63], [64]]. The behavior of large particles is more studied, however, small particles (<1 μm) contribute to the chemical composition of organic carbon particles in the composition of marine bottom sediments [56,65], which indicates the importance of studying the behavior of particles of this size. Microparticles of coal dust (<53 μm) form agglomerates in the form of spherical particles (up to 1 cm) are under static conditions in the water, or remain on the surface in the form of a film, which indicates the hydrophobicity of coal. Resistance to particle settling can also be related to surface tension. This effect however, disappeared when coal particles were placed in a container with seawater while shaken. A thin layer of fine coal particles was observed during a sampling procedure near the coal terminal, even though no coal was being loaded onto the ship at that moment [21]. Coal was also found in bottom sediments [66]. Thus, it can be assumed that larger particles of coal will settle faster in seawater, while smaller particles will remain on the surface and prevent the penetration of light into the water column. The settling rate of micro-sized coal dust particles is much less than the settling rate of large particles, which contributes to their wider dispersion in the marine environment [21]. At the same time when the particle size decreases, they become more susceptible to aggregation caused by higher surface energy [67,68]. These aggregates settle more rapidly than coarse particles, which have fewer interactions. Important role plays the contact angle of coal particles, which associated with hydrophobicity and varies from different type of coal [69]. The interaction between coal particles and biota will depend on particles size distribution. It can be assumed that more species of flora and fauna inhabiting the marine environment will be affected by coal dust microparticles. In studies of the toxic effects of coal on marine organisms, samples of coal with different particle sizes were studied, for example, <38 μm [70] <40 μm [9], <63 μm [71,72], ≤ 425 μm [73]. Since coal has lower specific gravity than many other components of bottom sediments as the specific gravity of coal can vary, depending on the ash content (1.2–2.9 g/cm3) [74] and by the movement of coal by water flow (larger particles of coal will move and settle with smaller and denser particles of sand and gravel) [28]. The movement of seawater promotes dispersion of suspended particles, but currents and waves can destroy large particles of coal, which will lead to continuous formation of smaller suspended particles and a longer absorption of light by particles of coal dust [71]. The spread of coal dust in seawater can affect benthic plants and organisms near coal terminals, which are most susceptible to coal dust and possible hypoxia [21]. Also, coal particles in water significantly reduces light penetration into the water by 44–99 %, depending on the coal concentration (from 38 to 278 mg/L) compared to unpolluted seawater [75].
3. Impact of coal dust particles on marine biota
Marine organisms can exhibit different responses to anthropogenic pollutants. A study evaluated whether if potentially toxic components of coal have a negative effect on aquatic biota, is actually determined by their bioavailability and concentration in the aquatic environment. Additionally, effects of coal on freshwater organisms were shown, as the mechanisms of action are likely to be the same in seawater organisms [28]. The results of these researches are presented in Table 1.
Table 1.
Effects of coal particles on marine organisms. The original table from [29] with adjustment and additions. The sources marked with * are presented in the review [29]. Sources marked with ** describe effects on freshwater organisms.
| Species | Experiment type | Exposure condition | Coal type | Coal particles size | Experiment duration | Coal concentration | Assumed stressor | Observed effect | References |
|---|---|---|---|---|---|---|---|---|---|
| Green alga (Ulva lactuca) | Lab | Suspended colliery waste | NE England, U.K. | 0-2000 μm | 8 days in static and mixing conditions, 30 and 60 days in static conditions | 29% by weight in waste, 1 g/L suspended waste | Abrasion by particulates | Reduced growth in the presence of waste and water movement, but increased growth with waste in still conditions | Hyslop, Davies [99]* |
| Seagrass (Halodule uninervis) | Lab | Suspended and settled coal particles | No data | <63 μm | 28 days | 0-275 mg/L | Reduced light penetration | Reduced growth, attaching of coal particles to leaves, decreased shoot density | Berry et al. [75] |
| Mangrove (Avicennia marina) | Field | Airborne coal | No data | 5-12 μm | - | No data | Light reduction | Reduced CO2 exchange by 17–39%, reduced photosynthetic performance | Naidoo, Chirkoot [107]* |
| Coral (Acropora tenuis) | Lab | Suspended and settled coal particles | No data | <63 μm | 28 days | 0-275 mg/L | Anoxia, reduced light penetration | Dying off and sloughed off tissues from the skeleton of corals within 14 days, 100% tissue mortality at concentrations of 73, 202, and 275 mg/L for 28 days | Berry et al. [72], Berry et al. [75] |
| Coral (Acropora tenuis | Lab | Suspended and settled coal particles, leachate | No data | <63 μm | 72 hours | 12.5-800 mg/L for suspended particles and 6.25-100 v/v of coal leachate with an initial concentration of 10000 mg/L | Physical impact | Reduced survival of embryos and larvae, subsidence of larvae reduced to 50%, no effect on fertilization, minimal anomalies in the development of embryos | Berry et al. [72] |
| Corals (Acropora tenuis, Montipora spp., Porites spp.) | Lab | Sediments, suspended solids | Coking coal, Queensland, Australia | 63-125 μm | 4 weeks for chronic exposure and 1.5 hours for acute | In the sediment - until the deposition layer reaches 30 mg*cm2, in suspended form - 1250 mg /L | 1) Reduced light penetration; 2) reduced gaseous exchange; 3) increased expenditure of the coral's energy for cleaning the surface of tissues; 4) potential chemical effects of leached metals | Acute exposure led to a significant reduction in oxygen production (ranged from 112 to 135% depending on the species without prior chronic exposure and ranged from 68 to 104% with chronic exposure), reduction in light calcification rates (from 58 to 149% without chronic exposure and 72 to79 % with chronic) and dark calcification rates (from 88 to 192% without chronic exposure and from 223 to 339% with chronic). In the presence of only chronic exposure, the reactions were less pronounced | Berry [100] |
| Crab (Cancer magister) | Lab | Coal mixed with sand in suspension | Westshore terminal, Tsawwassen, Canada | <300 μm | 22 days | Up to 50% by weight mixed with sand | Smothering of gills by particulates | Accumulation of coal in gills at higher concentrations | Pearce, McBride [120]* |
| Crab (Cancer magister) | Lab | Coal mixed with sand at the bottom of the aquarium | Westshore terminal, Tsawwassen, Canada | 3,9-500 μm | 21 days | Up to 75% by weight mixed with sand | Smothering of gills by particulates | No measurable difference in ventilation and oxygen consumption relative to controls | Hillaby [121]* |
| Marine worm (Arenicola marina) | Field | Deposited colliery waste | NE England, U.K. | Average value from 209 to 283 μm | - | 11% of sediment by weight | Physical destabilization of sediment by particulates | Worms avoided ingesting coal particles during deposit feeding (possibly based on particle size); avoidance of contaminated sediments in choice tests; reduced abundance | Hyslop, Davies [122]* |
| Marine predatory snail (Hexaplex trunculus) | Lab and field | Coal sediment | No data | No data | 2 months in a lab experiment | No data | Cd from direct contact | The increased concentration of Cd in the hepatopancreas by 1.8 times, damaged outer epithelium and increased its permeability by 3.6 times, increased the level of metallothioneins by 3 times | Siboni et al. [123]* |
| Scallop (Argopecten nucleus) | Lab | Suspended coal particles | Bituminous coal, Santa Marta, Colombia | <40 μm | 12 hours | 2, 9, and 40 mg/L | Availability of coal particles (as organic matter for food), physical effects of particles, chemical effects of metals | Increased water filtration rate by gills, reduced purification rate, selectivity in the absorption of particles, reduced oxygen consumption, physiological stress | Benitez- Polo, Velasco [9] |
| Bivalve mollusk (Villosa iris) | Lab | Coal mixed with sand at the bottom of the aquarium | Sydney Mine, Kentucky, USA | ⩽425 μm | 20 weeks | Up to 50% by volume mixed with sand | Chemical effects of leached substances, the physical presence of coal particles | No significant decrease in survival, but sublethal effects were recorded: tissue necrosis in the gills, resorption of oocytes | Henley et al.[73]** |
| Bivalve mollusk (Crassostrea virginica) | Lab | Suspended coal particles, including leachate | No data | <40 μm | 28 days | 1 and 10 mg/L | PAHs | No significant adverse effect on oyster survival, shell growth, or pumping activity, no significant accumulation of PAHs in tissues of depurated oysters, assuming that coal particles have been removed from the intestine | Bender et al. [125]* |
| Bivalve mollusk (Modiolus modiolus) | Lab | Suspended coal particles | Lignite coal (rank 1B), Novoshakhtins k, Primorsky Krai | <10 μm | 6 hours | 1, 10, 100 and 1000 mg/L | Chemical effects of leached substances, the physical presence of coal particles | The number of living hemocytes’ cells did not reduce, but enzymatic activity and partial depolarization of membranes reduces | Kirichenk o et al. [126] |
| Fish (Acanthochromis polyacanthus) | Lab | Suspended and settled coal particles | No data | <63 μm | 28 days | 0-275 mg/L | Availability of coal particles (as organic matter for food), physical effects of particles | Significantly reduced growth rate, no significant effect on survival | Berry et al. [75] |
| Fish (Danio rerio) | Lab | Methanolic coal dust extract | Mine in La Loma, Department of Cesar, Colombia | No data | 48 hours | 1-5000 mg/L | PAHs | Changes in the morphology of the head, tail, body, and heart. At a concentration of 500 mg/L, the survival was less than 20%, and at 5000 mg/L - 0% in 24 hours | Guerrero- Castilla et al. [138]** |
| Fish (Danio rerio) | Lab | Aqueous extract of coal dust | Bituminous coal, Department of Cesar, Colombia | <38 μm | 72 hours | 0,1-1000 mg/L | PAHs, chemical effects of leached substances | No effect on the mortality and morphological changes in embryos. Changes in genes associated with the development and function of cells of the connective tissue, hematological system, with immunological and inflammatory diseases, with cancer were revealed | Caballero -Gallardo et al. [70]** |
| Fish (Oncorhynchus tshawytscha) | Lab | Suspended coal particles | No data | No data | 8 days | 60-500 mg/L | PAHs | Increased CYP1A1 and ribosomal protein L5 expression in liver | Campbell, Devlin [48]* |
| Fish (Acanthochromis polyacanthus) | Lab | Suspended coal particles | No data | <63 μm | 31 days | 38, 73, and 275 mg/L | Chemical effects of leached substances, the physical presence of coal particles | Increased oxygen consumption, adhesion of coal to the gills, changes in their structure | Berry [100] |
3.1. Microorganisms, bacteria, and viruses
The effect of coal dust on microorganisms, bacteria, and viruses in seawater remains currently unexplored. It is impossible to make a conclusive assumption about the mechanism of action and its consequences due to the huge variety of species of these groups of organisms. However, some research results may allow to make an approximate estimate of the interactions between coal and some organisms. For example, in the sea bottom deposits with different oxidation-reduction states, signs of the presence of viruses were found [[76], [77], [78], [79]], which can facilitate the processing of organic matter through lysis of microbial biomass [56,80,81]. It was shown that coal dust dispersed in the air after exposure to the bacterium Bacillus subtilis was exposed to overgrowth of the surface with calcium carbonate crystals [82].
3.2. Phytoplankton and zooplankton
Phytoplankton and zooplankton interact with dissolved and suspended substances in seawater through various processes, including active biological uptake, adsorption-desorption, particle aggregation, microbial decomposition, etc. [83]. In the ocean, phytoplankton forms the basis of the marine food chain [84], but under the anthropogenic impact, there is a significant decrease in its population (currently by 1% per year) [85] due to changes in water temperature, lighting intensity, increased acidity, deposition of substances from the atmosphere and stratification [86,87]. These changes affect the productivity of phytoplankton, giving an advantage to fast-growing species with increased adaptability [88], which creates conducive conditions for harmful algal blooms [87,89].
In a study of the impact of coal mines on phytoplankton, in particular on diatoms, it was noted that in the river, near the coal mining, the species diversity of phytoplankton was 24 % lower than in the river where coal is not mined [90]. However, this study does not indicate a direct relationship between the presence of coal in water and species diversity. A study investigated the impact of micro-sized coal particles (<20 μm, 20−100 μm, 100−250 μm, and 250−500 μm fractions) on the viability of zooplanktonic crustacean Artemia salina was studied where the nauplii of A. salina were exposed to particles of four different types of coal at concentrations from 100 to 5000 mg/L at static and shaking conditions. During the 96 h period of exposure, there were no pronounced toxicological effects of coal in these used concentrations. However, the observed absorption of the coal particles in the guts of A. salina may cause possible various abnormalities in a longer-term experiment [91]. Zooplankton also plays an important ecosystem function in the marine food chain, since it has a key role in the transfer of energy from primary producers to the upper trophic levels [92,93]. With the help of zooplankton, pollutants, especially persistent organic pollutants (POPs), enter the food chain [[94], [95], [96]]. In seawater, zooplankton can accumulate PAHs both during sorption from water, and when feeding through phytoplankton, which is exposed to pollutants in the water [97]. Observation of zooplankton exposed to coal ash showed that the zooplankton community had been exposed to extensive restructuring over 30 years. Only 12 species of 35 species that lived in the lake in 1985, remained by the year 2015 [98].
3.3. Algae, macrophytes, and plants
The assumption that the decrease in the distribution and biomass of the green alga Ulva lactuca L. (Chlorophyceae) near the shores where coal waste is discharged is associated with the abrasive effect on the algae leaves was tested experimentally [99]. The impact of colliery waste particles of different sizes (3 categories: <500 μm, 500−2000 μm, up to 2000 μm) on U. lactuca samples was carried out under turbulent and still conditions for 8 days. At the same time, ambiguous results were obtained: the coarse particles of colliery waste damaged macroalgae under turbulent conditions and could contribute decrease in their species diversity, however, under still conditions with the presence of coal particles, an increase in the growth of macroalgae was observed. The maximum weight loss was also observed in the presence of coal with a particle size of 500−2000 μm under turbulent conditions [99].
Metals leached from coal can also be toxic to seagrass [[100], [101], [102]]. When studying the effect of coal leachate on freshwater algae, it was found that growth inhibition occurred only in closed containers, and this effect stops when the container is aerated, which may indicate the effect of volatile organic compounds. Moreover, in an artificial ecosystem, when the concentration of coal from the leachate is from 1 to 20 vol. %, an increase of algae and bacteria and the death of zooplankton is observed [45]. Under the impact of coal waste on the sandy bottom or rocky shores, a significant decrease in the species diversity and abundance of algae is also observed [103].
The density of algae shoots and leaf growth are effective bioindicators in an environment with a lack of light [104], since reduced carbon fixation during photosynthesis reduces the amount of carbon distributed for plant growth [100,105,106]. The effect of coal particles on tropical algae (Halodule uninervis) was studied [75] where under laboratory conditions, an accidental discharge of coal was simulated, where organisms were exposed to five concentrations of coal (range, 0–275 mg/L and particle size <63 μm) for 28 days. This size of coal particles was chosen since such particles remain suspended in water for longer durations [21,75]. The attachment of coal particles to algae leaves was observed in less than 24 h and decreased growth was noted. The IC50 for algae was 275 mg/L at 28 days of exposure. Shoot density also decreased compared to the control group.
Coating the leaves directly with a layer of carbon particles can further reduce light penetration, leading to a decrease in chlorophyll a production [107], which prevents plant growth [75,105]. Thus, for algae, as noted earlier, it is possible that the decrease in the intensity of the light is due to the formation of suspended matter [108].
Also, the deposition of coal particles on the surface of plants (both marine and terrestrial) reduces the efficiency of photosynthesis, which was shown on mangroves [107] growing at one of the world's largest coal terminals in Richards Bay, South Africa. A 17–39 % reduction in photosynthesis was noted in leaves covered with coal dust.
Coal is dangerous for flora due to the duration of exposure. In experiments with the Halodule uninervis algae [75,100], the flow of water in the tanks did not promote the removal of coal particles from the leaves of the algae, just as the coal dust deposited on the leaves of mangrove trees near the port was not blown away by the wind or washed away by rain [107], thus the flora can be covered with coal for a long time [100].
3.4. Corals
An experiment similar to the H. uninervis algae was carried out for the corals Acropora tenuis [75]. In this case, coal particles settled on coral polyps and connecting tissue. Although branching corals such as Acropora tenuis are considered to be among the most resistant to sediment deposition [109], some tissues died and sloughed off the skeleton within 14 days at coal concentrations of ≥ 38 mg/L. 100 % tissue mortality was observed at a carbon concentration exceeding 73 mg/L on exposure for 28 days, and at a concentration of 275 mg/L for 14 days. The LD50 for corals was 87 mg/L at 14 days of exposure and 36 mg/L at 28 days. Presumably, anoxia [110] was a lethal factor for corals, as well as increased energy consumption for a protective reaction from deposited coal particles due to light attenuation [75]. Reproduction of organisms is an important function in assessing toxicological effects. Most reef-building corals reproduce by spawning [111], like many other invertebrates. Successful reproduction plays an important role in the growth of the coral population [111], however, a decrease in the quality of water and substrate can affect these processes [72,112]. In an experiment with corals Acropora tenuis [72], indicators such as fertilization, the survival of embryos, larvae, and larval settlement decreased under the influence of various concentrations of coal particles (12.5, 25, 50, 100, 200, 400, 500, 600, 700, 800 and 10000 mg/L) with particle size <63 μm and the duration of the experiment up to 96 h, depending on the stage of development. Early stages of development (gametes and embryos) were more sensitive to the effects of coal. However, these effects were likely caused by the physical impact of coal rather than toxic, as no effect on coral reproduction was noted in the experiment with coal leachate. Rather low concentrations of PAHs and metals were recorded in the leachate [72]. However, some studies have shown that Cu and Cd can be leached from coal and are toxic to corals [[113], [114], [115]] above threshold levels [100].
A study [100] was carried out on the effect of coal on 3 species of reef-forming corals Acropora tenuis, Montipora spp., Porites spp. to determine if there are differences in physiological changes during exposure to coal and suspended particles (carbonate deposits) with a size of 63−125 μm. In this case, 2 types of exposure were carried out, chronic (2 times a week for 4 weeks, coal and carbonate deposits were added to the container with corals in concentrations that provide a layer of sediment in the container of 30 mg*cm2) and acute (single exposure for 1.5 h of 1250 mg/L of coal and carbonate deposits). Acute impacts were assessed for both corals that were not previously exposed to any impact and for corals after chronic exposure. The experiment noted that the physiological responses of corals to coal particles differ from their responses to carbonate deposits, but these differences depend on the type of coral. Acute coal exposure resulted in a significant reduction in oxygen production and calcification that were not observed in sediment-exposed corals. Both chronic and acute exposure to coal and sediment significantly affected calcification. The author has identified the probable primary mechanisms of the effect of coal on corals: 1) attenuation of light, 2) reduced gas exchange; 3) increased expenditure of the energy for cleaning the surface of tissues, 4) potential chemical effects of leached metals [100]. Under acute exposure, a reduction in respiration rates was observed only in coal treatments for Porites spp., which is explained by the difference in physiological reactions in these species and the efficiency of tissue clearance from particles. However, Montipora spp. responded immediately by moving cilia and secreting mucus, thereby increasing the respiratory rate. Acropora tenuis accumulated fewer particles due to the vertical growth of branches. Also in the experiment, the concentrations of leached metals were determined, but they were not significant, so their effect was not evaluated [100]. However, metals can have long-term effects on biological processes in corals, such as respiration and reproduction [114,116], as well as PAHs can cause histological abnormalities [117] and affect coral growth [118].
Although the mechanisms of action of coal and carbonate deposits are similar, the characteristics of particles (color, adhesiveness) and changes in the abiotic environment caused by coal pollution (attenuation of light, leaching of metals) contribute to more serious changes in coral organisms [100].
3.5. Echinoderms
A study [119] examined the effects of coke-rich sediments (mainly PAHs) on the development of the sea urchin Dendraster excentricus (from fertilized egg to the echinopluteus stage), and no toxic effects were found. Although this study focused not on coal, but on its thermochemically modified product, the absence of mortality virtually eliminates any potential toxicity from coal that may have been present in these sediments [28].
3.6. Arthropods
As for arthropods, any suspended particles in the water can penetrate the respiratory, vision, and nutrition organs, "clogging" them, and causing various damage. Accumulation of coal in the gills was observed in the experiment with crab Cancer magister in an aquarium with mixing coal and sand [120], which could affect oxygen consumption [28]. However, a later study found no effect of coal mixed with sand on oxygen consumption by Cancer magister or gill ventilation [121], although technical problems with the experiment (the coal was not in suspension, but was mixed with sand at the bottom of the aquarium) are regarded a drawback as the effect of coal on these organisms remains unclear.
3.7. Worms
In an experiment with Arenicola marina worms in the presence of coal in the sediments in an amount of 11 % weight/ratio, it was recorded that the worms avoided contaminated areas of bottom sediments, and their number decreased [122]. Comparing the sediment, gut contents, and fecal material of Arenicola marina from the heavily coal-contaminated area and the slightly polluted area, it was found that worms in the heavily polluted area selectively fed on sand grains and coal particles. Smaller particles of coal in the intestines and feces were found in worms from both sites. Despite the rejection of larger coal particles by worms in more polluted areas, there is no evidence that the lack of suitable sediment for feeding was responsible for the observed reduction in Arenicola marina abundance in heavily polluted areas. Perhaps the more important factor was the instability of sediments due to the presence of coal [28,122].
3.8. Mollusks
A study [123] suggested that the main factor affecting organisms might be leached Cd, which was confirmed by an experiment with the mollusk Hexaplex trunculus. The study was carried out on mollusks collected in the water area contaminated with coal and in the clean water area. An experiment was also carried out in aquariums. Both experiments showed that under the influence of coal, the concentration of Cd in the hepatopancreas of mollusks increased significantly. Damage to the outer epithelium and an increase in its permeability were recorded using microfluorimetry, which the authors explain by contact with Cd from coal deposits. Metallothioneins play an important role in the detoxification of metals in bivalve mollusks, especially of Cd [124]. Elevated levels of metallothioneins in mollusk tissues can also be caused by chemical components of coal [123]. Bivalve mollusks of the Caribbean Sea Argopecten nucleus were selected to assess the effect of coal on the growth factor, which were exposed to coal particles with a diameter of <40 μm at concentrations of 2, 9, and 40 mg/L under laboratory conditions [9]. With the increasing concentration of coal, an increase in the rate of water filtration by the gills was observed, but the rate of purification was reduced. The selectivity of uptake has also been found, and it can be assumed that this species can select particles prior to feeding based on organic content or particle type, preferring coal particles with a higher organic content than microalgae cells that were added to the aquarium. The efficiency of absorption of particles at high concentrations of coal decreased, which may be related to the functional deterioration of the digestive gland caused by the greater availability of coal nanoparticles in seawater. The level of oxygen consumption also decreased due to inhibition of respiratory and metabolic functions as a result of either physical exposure to particles or chemical or biological effects of heavy metals present in coal nanoparticles [9]. Physiological stress was recorded in this experiment in the observed organism, which lies in the fact that the organism is not able to generate energy for its growth and reproduction. At the same time, for the mollusk A. nucleus, the concentration of coal and the exposure time required for the revealing of such effects were lower [9] than in corals, some bivalve mollusks, and fish [48,72,75,120,125]. The impact of pulverized coal in a sandy substrate with different percentages of coal (0, 10, 25, and 50 % vol.) on the Villosa iris mussels did not cause significant changes in the survival of this species when exposed to the substrate for 7 weeks (3 of 40 mussels died during the experiment). The sublethal changes were observed when assessing the effect on the tissues of the mussel organs during a 20-week experiment at coal concentrations of 0 and 50 % vol. in a sandy substrate. For example, tissue necrosis was recorded in the gills, and the lipofuscin content in the kidney diverticulum was higher than in the control organisms. This experiment confirmed the hypothesis that coal in the substrate has a negative effect on reproductive and physiological functions in mussels [73]. Villosa iris is a freshwater mollusk, but histological findings are important for understanding how coal affects mollusks in general. On the contrary, the effect of PAHs was not recorded in an experiment with the mollusk Crassostrea virginica at coal concentrations of 1 and 10 mg/L in suspension (there were no changes in survival rate, shell growth, or water pumping activity) [125]. However, there are suggestions that Crassostrea virginica were exposed to high PAH contamination before coal exposure since high background PAH concentrations were recorded at the site of the collection of organisms [28]. Another experiment was devoted to determining the toxic effect of coal particles up to 10 μm size on hemocytes of Modiolus modiolus mollusks at concentrations from 1 to 1000 mg/L for 6 h, and the number of alive cells in comparison with the control remained approximately the same at all concentrations. At the same time, a decrease in enzyme activity and partial depolarization of membranes under the exposure of coal particles were recorded [126].
3.9. Fish
Fish are more subjected to sublethal effects than lethal ones when it comes to exposure to suspended particles since fish can move from an area with a higher concentration of particles to an area with a lower concentration, in contrast to sedentary or less mobile species [127]. The presence of suspended matter in the water, worsens the feeding conditions for fish, since the prey becomes less noticeable due to turbidity [128], which is a positive aspect for species that are food sources for fish. Besides, water turbidity contributes to the protection of fish larvae from large predators [127,129]. Histological analysis and observation of the gills of fish and oysters showed that solid particles of coal can adhere to the gills, having a suffocating effect, and clog lamellae [120,121], which are important for gas exchange [100,130]. The adhesion of suspended particles to the gill lamellae is expected to increase the resistance to gas transport through the gills [131]. Also, various pollutants can cause cell proliferation in the fish gills [[132], [133], [134]]. This structural modification can protect the gills from abrasive damage and/or reduce the permeability of the gills to toxins [133], but it also reduces the permeability of the gills to oxygen and may affect respiratory function [100,135]. An experiment on the effect of coal dust with a particle size of <63 μm was carried out with the fish, Acanthochromis polyacanthus (the experimental setup was similar to H. uninervis and Acropora tenuis) [75]. At all coal concentrations, a decrease in the growth of fish is observed, as well as a change in their color. The IC50 was 73 mg/L with 28 days of exposure. With 14 days of exposure, growth inhibition was less than 50 %. Only two individuals died that were exposed to the maximum concentration of coal. An autopsy revealed the presence of coal in the fish's alimentary tracts, which could block digestion, contributing to starvation and exhaustion. There is also an assumption that coal, like suspended particles, influenced the respiration of fish [136,137]. The effects of coal on early fish life may be more significant. The effect of methanol extract of coal dust on embryos of freshwater fish Danio rerio at concentrations from 1 to 5000 mg/L showed changes in the development of embryos at the genetic level, and three malformed phenotypes were obtained depending on the concentration. All embryos exposed to a coal dust concentration of 5000 mg/L died within 24 h. The LC50 in this experiment was 223.68 ± 29.48 and 161.55 ± 17.16 mg/L at 24 and 48 h of exposure, respectively [138]. In a field experiment in water containing suspended solids from coal mine water, a 98–100 % mortality of Salmo gairdneri fish eggs during the incubation period was found due to a decrease in dissolved oxygen concentration [139].
In another experiment [70] with embryos of freshwater fish Danio rerio at concentrations of coal dust extract up to 1000 mg/L and exposure times up to 72 h had no increase in mortality, but at concentrations of 0.1 and 1000 mg/L, a delay in hatching was found. Also, morphological changes in embryos were studied at concentrations up to 100 mg/L, where no differences were found. The genotoxic effect of coal was investigated, which was carried out at concentrations up to 100 mg/L by quantitative real-time PCR and transcriptomic analysis. As a result, transcripts with altered expression were identified. The altered genes are associated with the development and function of connective tissue cells, hematological system, immunological and inflammatory diseases, cancer [70]. Also, other studies have noted high mortality (more than 50 % within 30 days) and a negative effect on the reproductive function when exposed to Cd on Danio rerio individuals [140] and their sperm cells [141], which could be a concomitant factor for the impact of coal particles. Even though Danio rerio is a freshwater fish, these research findings are very useful and suggest a similar effect of coal on marine fish, which is generally consistent with such studies, the results of which are presented in this review. And the results of the investigation of changes in the transcripts emission are an important step in the further development of genotoxicology concerning the effect of coal dust on aquatic organisms. The effect of PAHs leached from coal on fish Oncorhynchus tshawytscha was also established [48]. Exposure to suspended particles of coal at concentrations from 60 to 500 mg/L for 8 days increased the expression of the CYP1A1 genes and the ribosomal protein L5. CYP1A1 plays an important role in the detoxification of such xenobiotic compounds as PAHs, while L5 plays an important role in ribosome biogenesis. The enzymes encoded by these genes have been used as a sensitive biomarker to assess the impact of organic pollutants [28,48]. However, in an experiment with fish Oncorhynchus mykiss gairdnerii, exposed to aqueous leachates of coal for 28 days, no changes in the liver were recorded as compared to the control groups [49]. It should be noted that in this experiment, the leachate was centrifuged, while in the experiment with Oncorhynchus tshawytscha [48], coal dust simply precipitated during the experiment, and the final leachate contained a significant amount of insoluble solids [28,48]. When Pimephales promelas fish were exposed to non-centrifuged coal leachate, a 100 % mortality rate was recorded within 96 h [142]. The level of oxygen consumption in tropical fish Acanthochromis polyacanthus after 5, 21, and 31 days of exposure at concentrations of suspended coal of 0, 38, and 73 mg/L was studied [100]. Oxygen consumption decreased by 17 % during 5 days of exposure, however, after 21 days of exposure at a concentration of 38 mg/L, this indicator increased by 47 % and at 73 mg/L by 18 % compared to the control groups. This indicator was also higher after 31 days for fish exposed to coal compared with control groups, by 30 % at a concentration of 38 mg/L and 38 % at 73 mg/L. Oxygen consumption is an estimate of the metabolic rate, and a significant increase in this indicator may indicate that coal was a stress factor that disrupted homeostasis [100,135,143]. Increased oxygen consumption is often stimulated by the release of stress hormones [143]. In response to stress, the immune function can be suppressed, as well as energy can be redistributed from the functions of reproduction and growth [100,144]. This study also evaluated the state of the gills of A. polyacanthus after 31 days of exposure at coal concentrations of 73 and 275 mg/L. Adhesion of a large number of coal particles to the gill surface and discoloration (darkening) of the gills were visually observed [100]. The morphology of the gills in some species can adapt to altered oxygen conditions, reversibly changing the morphology of the gills [100,135,145,146]. In this experiment, significant changes in the structure of the gills were observed, but they were different from the changes observed after exposure to other forms of suspended solids [100]. The author explains this by the "stickiness" of coal and its adhesion to the gill surfaces and clogging of the lamellae, which interferes with the transport of gas. In the gills of fish under control conditions, a thick epithelium was observed in filaments and lamellae in 48 % of cases, while under the influence of coal, only in 9%, indicating that the morphology of the gills was reconstructed to increase the ability to absorb oxygen and compensate for the adhesion of coal particles and clogging of lamellas [100].
The accumulation of metals such as Fe > Zn > Mn > Cu > Pb > Ni > Cd and tissue abnormalities were shown when studying the consequences of transporting and dumping coal after catching fish Epinephelus sp., Lutjanus sp., Otolithes sp., Nemipterus sp., Thryssa sp. and Mugil sp. and carrying out histological analysis and spectroscopy of acid-destroyed tissues of the gills, liver, and muscles. Heavy metals can cause various deformities in fish, for example, Cd can cause deformities in bone tissue, Cd and Cu cause developmental delay, Pb has hematological and neurological effects, and Zn acts on the gills and can cause hypocalcemia [147]. Thus, in fish, the effect of coal particles can be a factor for the development of various pathologies. Reducing growth rates can also affect the reproductive function [75]. Direct physical interactions between fish and coal include the absorption of coal particles [75,120,142], which can lead to extensive mucus secretion by the intestinal mucosa [142] and may also contribute to reducing growth rates [75,100].
3.10. Mammals
There are no studies in the area of assessing the toxicity of coal to marine mammals, but it can be assumed that they will be indirectly affected through the food chain. It is unlikely that coal will have a direct impact, due to the more complex system of "barriers" in mammals in general. For example, in an experiment with monkeys [148], it was shown that PAHs leached from coal were unable to penetrate the skin.
4. Limitations and future research
Although various studies have been presented in the field of the effect of coal particles on marine organisms, some areas remain unexplored. There are very few studies devoted to the effect on organisms of coal particles of different size fractions, while many experiments have confirmed that the physical effect of particles is one of the most important factors. There is also insufficient information in the field of modelling the behavior of coal particles in seawater and, accordingly, of determination groups of organisms that are most susceptible to negative effects.
The limitations of the carried-out investigations allow us to point out several directions for further research. As noted earlier [28], it is necessary to investigate the bioavailability of various substances from different types of coal, which will determine the most dangerous types of coal. The study of the behavior of coal particles in seawater will reveal the most vulnerable areas, as well as elements of the marine ecosystem that may be affected. Long-term monitoring of sea areas is also promising to identify the frequency of their pollution near marine coal terminals, as well as the concentration of coal particles. Since micro-sized particles of coal (coal dust) mainly enter the marine environment, studies on the interaction of unicellular organisms and cells with such particles and their possible adaptation options are of great interest.
5. Conclusion
Coal particles in seawater may have a different effect on flora and fauna and can be divided into four reasons (the attenuation of light in the water column, the physical presence of solid matter, the release of inorganic substances and the release of organic substances) [103]. All these types of effects can be both sublethal and lethal for marine organisms [[149], [150], [151], [152]], but it will significantly depend on the duration of the impact, the type of coal, and its concentration [100]. Physical effects can be conditionally divided into direct and indirect effects. The abrasion is related to direct impact, which means that the presence of a large number of coal particles in the water leads to injury, damage to animals and plants living at the seabed, on stones, or at berthing facilities [103,153,154]. It is obvious that the presence of coal in seawater, as well as other suspended particles, will lead to the attenuation of light penetrating the water column [155], which will affect the growth of algae, seagrasses, and microalgae [71,156,157], and this will also affect the fauna that feeds on these algae [71]. Since coal from the suspended state settles on the seabed, the most direct impact is likely to be the “suffocating” effect on animals and plants [28]. The indirect physical effects consist of changing the habitat of organisms due to the deposition of coal particles at the bottom (in rock splits, etc.). But these effects can be both negative and positive [28]. For example, in naturally homogeneous muddy sediments, the presence of larger coal particles can increase the heterogeneity of the sediments, making it suitable for more animals to inhabit [155]. However, in general, the indirect effects are negative and can appear as a decrease in productivity, changes in food chains, as well as a decrease in the volume of fish and shellfish catch due to their death or biological pollution [100,158]. It was also concluded that the relevant indicator in assessing physical effects will be the dose of particles to which organisms are exposed (i.e., a function of the concentration of particles and the duration of exposure) and not the concentration of particles [159]. Different types of coal have different chemical compositions, and therefore it is difficult to provide generalized data on the chemical effects of coal on flora and fauna [100]. The content of PAHs and other toxins in different types of coal is different, and these substances can be leached out when interacting with water [37]. However, in a large volume of water, their concentrations are insignificant due to the dilution of these substances. Besides, the formation of insoluble salts upon contact with seawater, complex formation with dissolved organic substances in seawater, adsorption on particle surfaces, or redox reactions that lead to changes in mineralogical composition or solubility can cause these substances to become biologically inaccessible. Metals and metalloids that are readily-soluble under low pH conditions, such as Cd, Cu, Pb, Zn, and As, can become insoluble on contact and dilution with alkaline seawater. On the other hand, particle-bound metals and metalloids that are soluble under alkaline conditions, such as Cr and Se, can dissolve upon contact with seawater [28]. When determining the degree of leaching of substances from coal, it was shown that some chemical compounds, on the contrary, were adsorbed by coal from seawater, therefore, their concentrations in water decreased compared to control groups [160]. In general, studies have shown that the leaching of toxic trace elements [43,160] and PAHs [28,71,125] from coal is rather limited [100]. Complex mixtures of aliphatic and aromatic hydrocarbons can be leached also from open coal piles by rain [161]. The investigations have shown that coal dust is generally not an inert substance to marine biota. However, variances in the effects of exposure may be associated with a wide range of coal dust concentrations used in studies (from 1 to 1000 mg/L) and different levels of tolerance and/or sensitivity to stress factors in the studied species [9]. Several studies that have examined the effects of coal on marine organisms suggest that the physical presence of coal particles is more harmful than the effects of substances leached from coal [10]. It can be concluded that, in general, the marine fauna is more resistant to the effects of coal, than plants and protozoans. Acute toxic effects were observed more often in algae, as well as in corals. Nevertheless, an indirect effect on the fauna cannot be ruled out, due to the deterioration of conditions for feeding and reproduction.
Reactions in genes were recorded in fish at an early stage of life, which is an extremely valuable result in the field of genotoxic effects, and in the future, should be studied with other species as well. It is noted that in addition to assessing the acute impact of accidental coal discharge, studies of the impact of low coal concentrations in chronic exposure are needed [72].
The effects of coal particles on marine organisms at the cellular level remain poorly understood, although there are studies on the effects of coal on the DNA of other species cells. In this case, the main type of exposure is oxidative damage to DNA, which is caused by the production of active radicals by metals in the composition of coal particles, as well as chromosomal instability [162]. Metals can also cause genotoxicity and carcinogenicity by inhibiting DNA repair systems, which leads to genome instability and accumulation of mutations [163]. Oxidative DNA damage has also been observed in studies of effects on human cells [164].
Considerably more work will need to be done in the field of ecotoxicology and genotoxicology of the coal dust impact on marine organisms, which will allow us to assess the consequences of anthropogenic activities near the sea area and to predict possible consequences during its further development. It should also be noted that the studies do not carry out a comparative impact assessment of different types of coal in one experiment, which may cause different effects of coal particles in experiments due to different chemical compositions.
Author statement
A.M. Tsatsakis, K.S. Golokhvast: Conceptualization.
Y.O. Mezhuev: Methodology, Software.
M.O. Tretyakova, A.I. Vardavas: Data curation, Writing- Original draft preparation.
P.D. Stivaktakis, T.I. Burykina, E.I. Iatrou : Visualization, Investigation.
Jan Jansen: Supervision.
C.I. Vardavas: Software, Validation.
A.M. Tsatsakis, K.S. Golokhvast: Writing- Reviewing and Editing.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgement
This work was supported by Grant of the Russian Foundation of Basic Research (19-05-50010).
Edited by Dr. A.M Tsatsakis
References
- 1.Looney B. 2020. Statistical Review of World Energy, 2020. In Bp. [Google Scholar]
- 2.Agency I.E. 2019. Coal Information 2019. [Google Scholar]
- 3.Moreno N., Alastuey A., Querol X., Artinano B., Guerra A., Luaces J., Lorente A., Basora J. Characterisation of dust material emitted during harbour operations (HADA Project) Atmos. Environ. 2007;41(30):6331–6343. [Google Scholar]
- 4.Walker T.R. Green marine: an environmental program to establish sustainability in marine transportation. Mar. Pollut. Bull. 2016;105(1):199–207. doi: 10.1016/j.marpolbul.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 5.Zhao D., Wang T., Han H. Approach towards sustainable and smart coal port development: the case of Huanghua Port in China. Sustainability. 2020;12(9):3924. [Google Scholar]
- 6.Goonetilleke A., Egodawatta P., Kitchen B. Evaluation of pollutant build-up and wash-off from selected land uses at the Port of Brisbane, Australia. Marine Pollut. Bull. 2009;58(2):213–221. doi: 10.1016/j.marpolbul.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 7.Vivas Aguas L., Ibarra Gutierrez K. Serie de Publicaciones Periodicas del Invemar; Santa Marta: 2014. Diagnóstico Y Evaluación De La Calidad De Las Aguas Marinas Y Costeras Del Caribe Y Pacifico Colombianos; p. 4. [Google Scholar]
- 8.Song D., Gao Z., Zhang H., Xu F., Zheng X., Ai J., Hu X., Huang G., Zhang H. GIS-based health assessment of the marine ecosystem in Laizhou Bay, China. Mar. Pollut. Bull. 2017;125(1–2):242–249. doi: 10.1016/j.marpolbul.2017.08.027. [DOI] [PubMed] [Google Scholar]
- 9.Benitez-Polo Z., Velasco L. Effects of suspended mineral coal dust on the energetic physiology of the Caribbean scallop Argopecten nucleus (born, 1778) Environ. Pollut. 2020;260 doi: 10.1016/j.envpol.2020.114000. [DOI] [PubMed] [Google Scholar]
- 10.Kroon F.J., Berry K.L., Brinkman D.L., Kookana R., Leusch F.D., Melvin S.D., Neale P.A., Negri A.P., Puotinen M., Tsang J.J. Sources, presence and potential effects of contaminants of emerging concern in the marine environments of the Great Barrier Reef and Torres Strait, Australia. Sci. Total Environ. 2020;719 doi: 10.1016/j.scitotenv.2019.135140. [DOI] [PubMed] [Google Scholar]
- 11.Burns K.A. PAHs in the Great Barrier Reef Lagoon reach potentially toxic levels from coal port activities. Estuar. Coast. Shelf Sci. 2014;144:39–45. [Google Scholar]
- 12.Huertas J., Huertas M., Cervantes G., Diaz J. Assessment of the natural sources of particulate matter on the opencast mines air quality. Sci. Total Environ. 2014;493:1047–1055. doi: 10.1016/j.scitotenv.2014.05.111. [DOI] [PubMed] [Google Scholar]
- 13.Koval S., Krahenbuhl G., Warren K., O’Brien G. Optical microscopy as a new approach for characterising dust particulates in urban environment. J. Environ. Manage. 2018;223:196–202. doi: 10.1016/j.jenvman.2018.06.038. [DOI] [PubMed] [Google Scholar]
- 14.Bai C., Li Y., Liu B., Liu C., Xie F. Spectral reflection characteristics of sea ice polluted by pulverized coal in the Bohai Sea. Environ. Forensics. 2020;21(2):157–166. [Google Scholar]
- 15.Grote M., Mazurek N., Gräbsch C., Zeilinger J., Le Floch S., Wahrendorf D.-S., Höfer T. Dry bulk cargo shipping—An overlooked threat to the marine environment? Mar. Pollut. Bull. 2016;110(1):511–519. doi: 10.1016/j.marpolbul.2016.05.066. [DOI] [PubMed] [Google Scholar]
- 16.Walker T.R., Adebambo O., Feijoo M.C.D.A., Elhaimer E., Hossain T., Edwards S.J., Morrison C.E., Romo J., Sharma N., Taylor S. Elsevier; 2019. Environmental Effects of Marine Transportation, World Seas: an Environmental Evaluation; pp. 505–530. [Google Scholar]
- 17.Ramanathan V., Carmichael G. Global and regional climate changes due to black carbon. Nat. Geosci. 2008;1(4):221–227. [Google Scholar]
- 18.Wang Y., Wang X., Kondo Y., Kajino M., Munger J.W., Hao J. Black carbon and its correlation with trace gases at a rural site in Beijing: top‐down constraints from ambient measurements on bottom‐up emissions. J. Geophys. Res. Atmos. 2011;116(D24) [Google Scholar]
- 19.Cape J., Coyle M., Dumitrean P. The atmospheric lifetime of black carbon. Atmos. Environ. 2012;59:256–263. [Google Scholar]
- 20.Chen X., Zhang Z., Engling G., Zhang R., Tao J., Lin M., Sang X., Chan C., Li S., Li Y. Characterization of fine particulate black carbon in Guangzhou, a megacity of South China. Atmos. Pollut. Res. 2014;5(3):361–370. [Google Scholar]
- 21.Johnson R., Bustin R. Coal dust dispersal around a marine coal terminal (1977–1999), British Columbia: the fate of coal dust in the marine environment. Int. J. Coal Geol. 2006;68(1–2):57–69. [Google Scholar]
- 22.Gardon T., Reisser C., Soyez C., Quillien V., Le Moullac G. Microplastics affect energy balance and gametogenesis in the pearl oyster Pinctada margaritifera. Environ. Sci. Technol. 2018;52(9):5277–5286. doi: 10.1021/acs.est.8b00168. [DOI] [PubMed] [Google Scholar]
- 23.Liu W., Zeng Z., Chen A., Zeng G., Xiao R., Guo Z., Yi F., Huang Z., He K., Hu L. Toxicity effects of silver nanoparticles on the freshwater bivalve Corbicula fluminea. J. Environ. Chem. Eng. 2018;6(4):4236–4244. [Google Scholar]
- 24.Sussarellu R., Suquet M., Thomas Y., Lambert C., Fabioux C., Pernet M.E.J., Le Goïc N., Quillien V., Mingant C., Epelboin Y. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. 2016;113(9):2430–2435. doi: 10.1073/pnas.1519019113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buffet P.-E., Zalouk-Vergnoux A., Châtel A., Berthet B., Métais I., Perrein-Ettajani H., Poirier L., Luna-Acosta A., Thomas-Guyon H., Risso-de Faverney C. A marine mesocosm study on the environmental fate of silver nanoparticles and toxicity effects on two endobenthic species: the ragworm Hediste diversicolor and the bivalve mollusc Scrobicularia plana. Sci. Total Environ. 2014;470:1151–1159. doi: 10.1016/j.scitotenv.2013.10.114. [DOI] [PubMed] [Google Scholar]
- 26.Pikula K., Chaika V., Zakharenko A., Markina Z., Vedyagin A., Kuznetsov V., Gusev A., Park S., Golokhvast K. Comparison of the level and mechanisms of toxicity of carbon nanotubes, carbon nanofibers, and silicon nanotubes in bioassay with four marine microalgae. Nanomaterials. 2020;10(3):485. doi: 10.3390/nano10030485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pikula K., Mintcheva N., Kulinich S.A., Zakharenko A., Markina Z., Chaika V., Orlova T., Mezhuev Y., Kokkinakis E., Tsatsakis A. Aquatic toxicity and mode of action of CdS and ZnS nanoparticles in four microalgae species. Environ. Res. 2020;186 doi: 10.1016/j.envres.2020.109513. [DOI] [PubMed] [Google Scholar]
- 28.Ahrens M.J., Morrisey D.J. CRC Press; 2005. Biological Effects of Unburnt Coal in the Marine Environment, Oceanography and Marine Biology; pp. 79–132. [Google Scholar]
- 29.Jaffrennou C., Stephan L., Giamarchi P., Cabon J., Burel-Deschamps L., Bautin F. Direct fluorescence monitoring of coal organic matter released in seawater. J. Fluoresc. 2007;17(5):564–572. doi: 10.1007/s10895-007-0216-y. [DOI] [PubMed] [Google Scholar]
- 30.Vassilev S.V., Vassileva C.G. A new approach for the combined chemical and mineral classification of the inorganic matter in coal. 1. Chemical and mineral classification systems. Fuel. 2009;88(2):235–245. [Google Scholar]
- 31.National Research Council . The National Academies Press; Washington, DC: 1980. Trace-element Geochemistry of Coal Resource Development Related to Environmental Quality and Health. [Google Scholar]
- 32.Yoshioka H., Takeda N. Analysis of organic compounds in coal macerals by infrared laser micropyrolysis. J. Anal. Appl. Pyrolysis. 2004;71(1):137–149. [Google Scholar]
- 33.Greenwood P.F., George S.C., Pickel W., Zhu Y., Zhong N. In situ analytical pyrolysis of coal macerals and solid bitumens by laser micropyrolysis GC–MS. J. Anal. Appl. Pyrolysis. 2001;58:237–253. [Google Scholar]
- 34.Mazumdar B. Molecular structure and molar volume of organic compounds and complexes with special reference to coal. Fuel. 1999;78(9):1097–1107. [Google Scholar]
- 35.Zhao Z.-B., Liu K., Xie W., Pan W.-P., Riley J.T. Soluble polycyclic aromatic hydrocarbons in raw coals. J. Hazard. Mater. 2000;73(1):77–85. doi: 10.1016/s0304-3894(99)00178-8. [DOI] [PubMed] [Google Scholar]
- 36.Suyatna I., Adnan A., Syahrir M., Ghitarina G., Abdunnur A., Saleh S. Heavy metal levels in water and fish samples from coastal waters of Mahakam Delta, kutai Kartanegara District, East Kalimantan, Indonesia. Aquac. Aquar. Conserv. Legis. 2017;10(5):1319–1329. [Google Scholar]
- 37.Cheam V., Reynoldson T., Garbai G., Rajkumar J., Milani D. Local impacts of coal mines and power plants across Canada. II. Metals, organics and toxicity in sediments. Water Qual. Res. J. Canada. 2000;35(4):609–632. [Google Scholar]
- 38.Cheng S. Heavy metal pollution in China: origin, pattern and control. Environ. Sci. Pollut. Res. - Int. 2003;10(3):192–198. doi: 10.1065/espr2002.11.141.1. [DOI] [PubMed] [Google Scholar]
- 39.George A., Shen B., Kang D., Yang J., Luo J. Emission control strategies of hazardous trace elements from coal-fired power plants in China. J. Environ. Sci. 2020;93:66–90. doi: 10.1016/j.jes.2020.02.025. [DOI] [PubMed] [Google Scholar]
- 40.Morel F.M., Milligan A., Saito M. Marine bioinorganic chemistry: the role of trace metals in the oceanic cycles of major nutrients. Treatise Geochem. 2003;6:625. [Google Scholar]
- 41.Sunda W.G., Huntsman S.A. Control of Cd concentrations in a coastal diatom by interactions among free ionic Cd, Zn, and Mn in seawater. Environ. Sci. Technol. 1998;32(19):2961–2968. [Google Scholar]
- 42.Sunda W.G., Huntsman S.A. Antagonisms between cadmium and zinc toxicity and manganese limitation in a coastal diatom. Limnol. Oceanogr. 1996;41(3):373–387. [Google Scholar]
- 43.Cabon J.Y., Burel L., Jaffrennou C., Giamarchi P., Bautin F. Study of trace metal leaching from coals into seawater. Chemosphere. 2007;69(7):1100–1110. doi: 10.1016/j.chemosphere.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 44.Achten C., Hofmann T. Native polycyclic aromatic hydrocarbons (PAH) in coals–a hardly recognized source of environmental contamination. Sci. Total Environ. 2009;407(8):2461–2473. doi: 10.1016/j.scitotenv.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 45.Gerhart D.Z. Office of Research and Development; US: 1980. Algal Bioassays With Leachates and Distillates From Western Coal, Environmental Research Laboratory. [Google Scholar]
- 46.Wang R., Liu G., Zhang J., Chou C.-L., Liu J. Abundances of polycyclic aromatic hydrocarbons (PAHs) in 14 Chinese and American coals and their relation to coal rank and weathering. Energy Fuels. 2010;24(11):6061–6066. [Google Scholar]
- 47.Zhuravleva N., Khavivulina E., Ismagilov Z., Potokina R., Sozinov S. Studies of the interconnection of the structure of fossil coal and the concentrations of polycyclic aromatic hydrocarbons in it. Chem. Sustain. Dev. 2016;24:355–361. [Google Scholar]
- 48.Campbell P., Devlin R. Increased CYP1A1 and ribosomal protein L5 gene expression in a teleost: the response of juvenile chinook salmon to coal dust exposure. Aquat. Toxicol. 1997;38(1–3):1–15. [Google Scholar]
- 49.Carlson R.M. Environmental Research Laboratory, Office of Research and Development; US: 1979. Implications to the Aquatic Environment of Polynuclear Aromatic Hydrocarbons Liberated From Northern Great Plains Coal. [Google Scholar]
- 50.Dai S., Bechtel A., Eble C.F., Flores R.M., French D., Graham I.T., Hood M.M., Hower J.C., Korasidis V.A., Moore T.A. Recognition of peat depositional environments in coal: a review. Int. J. Coal Geol. 2020;219 [Google Scholar]
- 51.Dai S., Ren D., Chou C.-L., Finkelman R.B., Seredin V.V., Zhou Y. Geochemistry of trace elements in Chinese coals: a review of abundances, genetic types, impacts on human health, and industrial utilization. Int. J. Coal Geol. 2012;94:3–21. [Google Scholar]
- 52.Finkelman R.B., Belkin H.E., Zheng B. Health impacts of domestic coal use in China. Proc. Natl. Acad. Sci. 1999;96(7):3427–3431. doi: 10.1073/pnas.96.7.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yakovlev I.Y., Glukhov Y.S., Mikheev V.G. GATsMiZ Press; 1997. Laboratory Methods for the Study of Combustible Minerals; p. 40. [Google Scholar]
- 54.InEc А; Kemerovo: 2017. Compendium of Innovative Biodiversity Conservation Solutions for the Coal Mining Sector, Novokuznetsk. [Google Scholar]
- 55.Allison I.S. McGraw-Hill Companies; 1974. Geology: the Science of a Changing Earth. [Google Scholar]
- 56.LaRowe D., Arndt S., Bradley J., Estes E., Hoarfrost A., Lang S., Lloyd K., Mahmoudi N., Orsi W., Walter S.S. The fate of organic carbon in marine sediments-New insights from recent data and analysis. Earth. Rev. 2020;204 [Google Scholar]
- 57.Hedges J.I., Eglinton G., Hatcher P.G., Kirchman D.L., Arnosti C., Derenne S., Evershed R.P., Kögel-Knabner I., de Leeuw J.W., Littke R. The molecularly-uncharacterized component of nonliving organic matter in natural environments. Org. Geochem. 2000;31(10):945–958. [Google Scholar]
- 58.Arndt S., Jørgensen B.B., LaRowe D.E., Middelburg J., Pancost R., Regnier P. Quantifying the degradation of organic matter in marine sediments: a review and synthesis. Earth. Rev. 2013;123:53–86. [Google Scholar]
- 59.Burdige D.J. Preservation of organic matter in marine sediments: controls, mechanisms, and an imbalance in sediment organic carbon budgets? Chem. Rev. 2007;107(2):467–485. doi: 10.1021/cr050347q. [DOI] [PubMed] [Google Scholar]
- 60.Middelburg J.J. Reviews and syntheses: to the bottom of carbon processing at the seafloor. Biogeosciences. 2018;15(2):413–427. [Google Scholar]
- 61.Middelburg J.J. Springer Nature; 2019. Marine Carbon Biogeochemistry: a Primer for Earth System Scientists. [Google Scholar]
- 62.Lutz M., Dunbar R., Caldeira K. Regional variability in the vertical flux of particulate organic carbon in the ocean interior. Global Biogeochem. Cycles. 2002;16(3) 11-11-11-18. [Google Scholar]
- 63.Henson S.A., Sanders R., Madsen E. Global patterns in efficiency of particulate organic carbon export and transfer to the deep ocean. Global Biogeochem. Cycles. 2012;26(1) [Google Scholar]
- 64.Wilson J.D., Barker S., Ridgwell A. Assessment of the spatial variability in particulate organic matter and mineral sinking fluxes in the ocean interior: implications for the ballast hypothesis. Global Biogeochem. Cycles. 2012;26(4) [Google Scholar]
- 65.Close H.G., Shah S.R., Ingalls A.E., Diefendorf A.F., Brodie E.L., Hansman R.L., Freeman K.H., Aluwihare L.I., Pearson A. Export of submicron particulate organic matter to mesopelagic depth in an oligotrophic gyre. Proc. Natl. Acad. Sci. 2013;110(31):12565–12570. doi: 10.1073/pnas.1217514110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lebedev A.A., Tikhonova O.A., Blinovskaya Y.Y., Chaika V.V., Kiryanov A.V., Khristoforova N.K., Pikula K.S., Shevchenko V.P., Golokhvast K.S. Impact of the coal terminal on the composition of marine suspensions in the Nakhodka Bay (Sea of Japan) Scientific notes of the RSHU. 2017;48:195–202. [Google Scholar]
- 67.He Y.T., Wan J., Tokunaga T. Kinetic stability of hematite nanoparticles: the effect of particle sizes. J. Nanoparticle Res. 2008;10(2):321–332. [Google Scholar]
- 68.Fitch R.M. Principles of colloid and surface chemistry. In: Hiemenz Paul C., Dekker Marcel., editors. No Price Given. Wiley Online Library; New York: 1977. p. 516. 1984. [Google Scholar]
- 69.Holuszko M.E., Mastalerz M.D. Coal macerals chemistry and its implications for selectivity in coal floatability. Int. J. Coal Prep. Util. 2015;35(2):99–110. [Google Scholar]
- 70.Caballero-Gallardo K., Wirbisky-Hershberger S.E., Olivero-Verbel J., de la Rosa J., Freeman J.L. Embryonic exposure to an aqueous coal dust extract results in gene expression alterations associated with the development and function of connective tissue and the hematological system, immunological and inflammatory disease, and cancer in zebrafish. Metallomics. 2018;10(3):463–473. doi: 10.1039/c7mt00300e. [DOI] [PubMed] [Google Scholar]
- 71.Jaffrennou C., Giamarchi P., Cabon J.-Y., Stephan L., Burel-Deschamps L., Bautin F., Thomas A., Dumont J., Le Floch S. Simulations of accidental coal immersion. Mar. Pollut. Bull. 2007;54(12):1932–1939. doi: 10.1016/j.marpolbul.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 72.Berry K.L., Hoogenboom M.O., Brinkman D.L., Burns K.A., Negri A.P. Effects of coal contamination on early life history processes of a reef-building coral, Acropora tenuis. Mar. Pollut. Bull. 2017;114(1):505–514. doi: 10.1016/j.marpolbul.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 73.Henley W.F., Johnson N.G., Ciparis S., Hanlon S.D., Heffinger D.G. Effects of coal particles in aquatic sediments on organ tissues of rainbow mussels Villosa iris (Unionidae) J. Shellfish Res. 2015;34(3):1019–1027. [Google Scholar]
- 74.Alpern B. Evaluation of the energy potential of carbonaceous sediments. World Coal;(United States) 1977;3(3) [Google Scholar]
- 75.Berry K.L., Hoogenboom M.O., Flores F., Negri A.P. Simulated coal spill causes mortality and growth inhibition in tropical marine organisms. Sci. Rep. 2016;6(1):1–8. doi: 10.1038/srep25894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Engelhardt T., Orsi W.D., Jørgensen B.B. Viral activities and life cycles in deep subseafloor sediments. Environ. Microbiol. Rep. 2015;7(6):868–873. doi: 10.1111/1758-2229.12316. [DOI] [PubMed] [Google Scholar]
- 77.Tully B.J., Heidelberg J.F. Potential mechanisms for microbial energy acquisition in oxic deep-sea sediments. Appl. Environ. Microbiol. 2016;82(14):4232–4243. doi: 10.1128/AEM.01023-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bäckström D., Yutin N., Jørgensen S.L., Dharamshi J., Homa F., Zaremba-Niedwiedzka K., Spang A., Wolf Y.I., Koonin E.V., Ettema T.J. Virus genomes from deep sea sediments expand the ocean megavirome and support independent origins of viral gigantism. MBio. 2019;10(2) doi: 10.1128/mBio.02497-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cai L., Jørgensen B.B., Suttle C.A., He M., Cragg B.A., Jiao N., Zhang R. Active and diverse viruses persist in the deep sub-seafloor sediments over thousands of years. ISME J. 2019;13(7):1857–1864. doi: 10.1038/s41396-019-0397-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Danovaro R., Dell’Anno A., Corinaldesi C., Magagnini M., Noble R., Tamburini C., Weinbauer M. Major viral impact on the functioning of benthic deep-sea ecosystems. Nature. 2008;454(7208):1084–1087. doi: 10.1038/nature07268. [DOI] [PubMed] [Google Scholar]
- 81.Orsi W.D. Ecology and evolution of seafloor and subseafloor microbial communities. Nat. Rev. Microbiol. 2018;16(11):671–683. doi: 10.1038/s41579-018-0046-8. [DOI] [PubMed] [Google Scholar]
- 82.Farashahi M., Bagherpour R., Kalhori H., Ghasemi E. Application of bacteria for coal dust stabilization. Environ. Earth Sci. 2019;78(5):1–9. [Google Scholar]
- 83.Ho T.-Y., Wen L.-S., You C.-F., Lee D.-C. The trace metal composition of size‐fractionated plankton in the South China Sea: biotic versus abiotic sources. Limnol. Oceanogr. 2007;52(5):1776–1788. [Google Scholar]
- 84.Field C.B., Behrenfeld M.J., Randerson J.T., Falkowski P. Primary production of the biosphere: integrating terrestrial and oceanic components. science. 1998;281(5374):237–240. doi: 10.1126/science.281.5374.237. [DOI] [PubMed] [Google Scholar]
- 85.Boyce D.G., Lewis M.R., Worm B. Global phytoplankton decline over the past century. Nature. 2010;466(7306):591–596. doi: 10.1038/nature09268. [DOI] [PubMed] [Google Scholar]
- 86.Behrenfeld M.J., O’Malley R.T., Siegel D.A., McClain C.R., Sarmiento J.L., Feldman G.C., Milligan A.J., Falkowski P.G., Letelier R.M., Boss E.S. Climate-driven trends in contemporary ocean productivity. Nature. 2006;444(7120):752–755. doi: 10.1038/nature05317. [DOI] [PubMed] [Google Scholar]
- 87.Whiteside M., Herndon J.M. Role of aerosolized coal fly ash in the global plankton imbalance: case of florida’s toxic algae crisis. Asian J. Biol. 2019:1–24. [Google Scholar]
- 88.Vallina S.M., Follows M., Dutkiewicz S., Montoya J.M., Cermeno P., Loreau M. Global relationship between phytoplankton diversity and productivity in the ocean. Nat. Commun. 2014;5(1):1–10. doi: 10.1038/ncomms5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wells M.L., Trainer V.L., Smayda T.J., Karlson B.S., Trick C.G., Kudela R.M., Ishikawa A., Bernard S., Wulff A., Anderson D.M. Harmful algal blooms and climate change: learning from the past and present to forecast the future. Harmful Algae. 2015;49:68–93. doi: 10.1016/j.hal.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weaks T. A step-wise discriminant analysis of the effects of long term coal mine drainage and coal dredging on phytoplankton of the Guyandotte River. Hydrobiologia. 1982;97(2):97–103. [Google Scholar]
- 91.Tretyakova M., Pikula K., Kirichenko K.Y., Golokhvast K. Ecotoxicological impact assessment of micro-sized coal particles on zooplanktonic crustacean artemia Salina. IOP Publishing; 2021. p. 012082. [Google Scholar]
- 92.Beaugrand G., Brander K.M., Lindley J.A., Souissi S., Reid P.C. Plankton effect on cod recruitment in the North Sea. Nature. 2003;426(6967):661–664. doi: 10.1038/nature02164. [DOI] [PubMed] [Google Scholar]
- 93.Takahashi K., Ide K. Reproduction, grazing, and development of the large subarctic calanoid Eucalanus bungii: is the spring diatom bloom the key to controlling their recruitment? Hydrobiologia. 2011;666(1):99–109. [Google Scholar]
- 94.Swackhamer D.L., Skoglund R. Bioaccumulation of PCBs by algae: kinetics versus equilibrium. Environ. Toxicol. 1993;12(5):831–838. [Google Scholar]
- 95.Chiuchiolo A.L., Dickhut R.M., Cochran M.A., Ducklow H.W. Persistent organic pollutants at the base of the Antarctic marine food web. Environ. Sci. Technol. 2004;38(13):3551–3557. doi: 10.1021/es0351793. [DOI] [PubMed] [Google Scholar]
- 96.Hung C.-C., Ko F.-C., Gong G.-C., Chen K.-S., Wu J.-M., Chiang H.-L., Peng S.-C., Santschi P.H. Increased zooplankton PAH concentrations across hydrographic fronts in the East China Sea. Mar. Pollut. Bull. 2014;83(1):248–257. doi: 10.1016/j.marpolbul.2014.03.045. [DOI] [PubMed] [Google Scholar]
- 97.Hsieh H.-Y., Huang K.-C., Cheng J.-O., Lo W.-T., Meng P.-J., Ko F.-C. Environmental effects on the bioaccumulation of PAHs in marine zooplankton in Gaoping coastal waters, Taiwan: concentration, distribution, profile, and sources. Mar. Pollut. Bull. 2019;144:68–78. doi: 10.1016/j.marpolbul.2019.04.048. [DOI] [PubMed] [Google Scholar]
- 98.Rocha T.L., Gomes T., Sousa V.S., Mestre N.C., Bebianno M.J. Ecotoxicological impact of engineered nanomaterials in bivalve molluscs: an overview. Mar. Environ. Res. 2015;111:74–88. doi: 10.1016/j.marenvres.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 99.Hyslop B.T., Davies M.S. Evidence for abrasion and enhanced growth of Ulva lactuca L. In the presence of colliery waste particles. Environ. Pollut. 1998;101(1):117–121. doi: 10.1016/s0269-7491(98)00006-2. [DOI] [PubMed] [Google Scholar]
- 100.Berry K.L.E. James Cook University; 2017. Effects of Coal Contamination on Tropical Marine Organisms. [Google Scholar]
- 101.Macinnis-Ng C.M., Ralph P.J. Towards a more ecologically relevant assessment of the impact of heavy metals on the photosynthesis of the seagrass, Zostera capricorni. Mar. Pollut. Bull. 2002;45(1–12):100–106. doi: 10.1016/s0025-326x(01)00300-9. [DOI] [PubMed] [Google Scholar]
- 102.Macinnis-Ng C.M., Ralph P.J. Variations in sensitivity to copper and zinc among three isolated populations of the seagrass, Zostera capricorni. J. Exp. Mar. Biol. Ecol. 2004;302(1):63–83. [Google Scholar]
- 103.Hyslop B.T., Davies M.S., Arthur W., Gazey N.J., Holroyd S. Effects of colliery waste on littoral communities in north-east England. Environ. Pollut. 1997;96(3):383–400. doi: 10.1016/s0269-7491(97)00039-0. [DOI] [PubMed] [Google Scholar]
- 104.McMahon K., Collier C., Lavery P.S. Identifying robust bioindicators of light stress in seagrasses: a meta-analysis. Ecol. Indic. 2013;30:7–15. [Google Scholar]
- 105.Ralph P., Durako M.J., Enriquez S., Collier C., Doblin M. Impact of light limitation on seagrasses. J. Exp. Mar. Biol. Ecol. 2007;350(1–2):176–193. [Google Scholar]
- 106.Alcoverro T., Manzanera M., Romero J. Annual metabolic carbon balance of the seagrass Posidonia oceanica: the importance of carbohydrate reserves. Mar. Ecol. Prog. Ser. 2001;211:105–116. [Google Scholar]
- 107.Naidoo G., Chirkoot D. The effects of coal dust on photosynthetic performance of the mangrove, Avicennia marina in Richards Bay, South Africa. Environ. Pollut. 2004;127(3):359–366. doi: 10.1016/j.envpol.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 108.Collier C.J., Waycott M., Ospina A.G. Responses of four Indo-West Pacific seagrass species to shading. Mar. Pollut. Bull. 2012;65(4–9):342–354. doi: 10.1016/j.marpolbul.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 109.Stafford-Smith M., Ormond R. Sediment-rejection mechanisms of 42 species of Australian scleractinian corals. Mar. Freshw. Res. 1992;43(4):683–705. [Google Scholar]
- 110.Weber M., De Beer D., Lott C., Polerecky L., Kohls K., Abed R.M., Ferdelman T.G., Fabricius K.E. Mechanisms of damage to corals exposed to sedimentation. Proc. Natl. Acad. Sci. 2012;109(24):E1558–E1567. doi: 10.1073/pnas.1100715109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Harrison P.L., Wallace C. Reproduction, dispersal and recruitment of scleractinian corals. Ecosystems of the world. 1990;25:133–207. [Google Scholar]
- 112.Richmond R.H. Chapman & Hall; New York: 1997. Reproduction and Recruitment in Corals: Critical Links in the Persistence of Reefs. Life and Death of Coral Reefs; pp. 175–197. [Google Scholar]
- 113.Brown B. Human Impacts on Coral Reefs: Facts and Recommendations. 1987. Heavy metals pollution on coral reefs. [Google Scholar]
- 114.Negri A.P., Heyward A. Inhibition of coral fertilisation and larval metamorphosis by tributyltin and copper. Mar. Environ. Res. 2001;51(1):17–27. doi: 10.1016/s0141-1136(00)00029-5. [DOI] [PubMed] [Google Scholar]
- 115.Reichelt-Brushett A., Hudspith M. The effects of metals of emerging concern on the fertilization success of gametes of the tropical scleractinian coral Platygyra daedalea. Chemosphere. 2016;150:398–406. doi: 10.1016/j.chemosphere.2016.02.048. [DOI] [PubMed] [Google Scholar]
- 116.Howard L., Brown B. Heavy metals and coral reefs. Oceanogr Mar Biol Ann Rev. 1984;22:195–210. [Google Scholar]
- 117.Peters E.C., Meyers P.A., Yevich P.P., Blake N.J. Bioaccumulation and histopathological effects of oil on a stony coral. Mar. Pollut. Bull. 1981;12(10):333–339. [Google Scholar]
- 118.Guzmán H.M., Burns K.A., Jackson J.B. Injury, regeneration and growth of Caribbean reef corals after a major oil spill in Panama. Mar. Ecol. Prog. Ser. 1994:231–241. [Google Scholar]
- 119.Paine M.D., Chapman P.M., Allard P.J., Murdoch M.H., Minifie D. Limited bioavailability of sediment PAH near an aluminum smelter: contamination does not equal effects. Environ. Toxicol. 1996;15(11):2003–2018. [Google Scholar]
- 120.Pearce B., McBride J. Fisheries and Environment Canada, Water Quality Division; 1977. Preliminary Study on the Occurrence of Coal Dust in Roberts Banks Sediments and the Effect of Coal Dust on Selected Fauna. [Google Scholar]
- 121.Hillaby B.A. Fisheries and Oceans. Government of Canada; 1981. The effects of coal dust on ventilation and oxygen consumption in the dungeness crab, cancer magister. [Google Scholar]
- 122.Hyslop B.T., Davies M.S. The effect of colliery waste on the feeding of the lugworm Arenicola marina. J. Sea Res. 1999;42(2):147–155. [Google Scholar]
- 123.Siboni N., Fine M., Bresler V., Loya Y. Coastal coal pollution increases Cd concentrations in the predatory gastropod Hexaplex trunculus and is detrimental to its health. Mar. Pollut. Bull. 2004;49(1–2):111–118. doi: 10.1016/j.marpolbul.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 124.Zaroogian G., Jackim E. In vivo metallothionein and glutathione status in an acute response to cadmium in Mercenaria mercenaria brown cells. Comp. Biochem. Physiol. C, Pharmacol. Toxicol. Endocrinol. 2000;127(3):251–261. doi: 10.1016/s0742-8413(00)00152-3. [DOI] [PubMed] [Google Scholar]
- 125.Bender M.E., Roberts M.H., Jr., deFur P.O. Unavailability of polynuclear aromatic hydrocarbons from coal particles to the eastern oyster. Environ. Pollut. 1987;44(4):243–260. doi: 10.1016/0269-7491(87)90202-8. [DOI] [PubMed] [Google Scholar]
- 126.Kirichenko K., Pikula K., Chaika V., Zakharenko A., Kholodov A., Chernyshev V., Tretyakova M., Golokhvast K. Assessment of coal dust particles influence on marine mollusk modiolus modiolus. Advances in Raw Material Industries for Sustainable Development Goals: Proceedings of the Xii Russian-German Raw Materials Conference (Saint-Petersburg, Russia, 27-29 November 2019) 2020:230. [Google Scholar]
- 127.Kjelland M.E., Woodley C.M., Swannack T.M., Smith D.L. A review of the potential effects of suspended sediment on fishes: potential dredging-related physiological, behavioral, and transgenerational implications. Environ. Syst. Decis. 2015;35(3):334–350. [Google Scholar]
- 128.O. N. O. F. T. D. Unit, Ward N. Northwestern Ontario Forest Technology; 1992. The Problem of Sediment in Water for Fish, Ministry of Natural Resources. [Google Scholar]
- 129.Utne‐Palm A. The effect of prey mobility, prey contrast, turbidity and spectral composition on the reaction distance of Gobiusculus flavescens to its planktonic prey. J. Fish Biol. 1999;54(6):1244–1258. [Google Scholar]
- 130.Hughes G. 1 General anatomy of the gills. Fish Physiol. Biochem. 1984;10:1–72. [Google Scholar]
- 131.Hughes G.M. Respiratory responses to hypoxia in fish. Am. Zool. 1973;13(2):475–489. [Google Scholar]
- 132.Hess S., Wenger A.S., Ainsworth T.D., Rummer J.L. Exposure of clownfish larvae to suspended sediment levels found on the Great Barrier Reef: impacts on gill structure and microbiome. Sci. Rep. 2015;5(1):1–8. doi: 10.1038/srep10561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mallatt J. Fish gill structural changes induced by toxicants and other irritants: a statistical review. Can. J. Fish. Aquat. Sci. 1985;42(4):630–648. [Google Scholar]
- 134.Mueller M., Sanchez D., Bergman H., McDonald D., Rhem R., Wood C. Nature and time course of acclimation to aluminum in juvenile brook trout (Salvelinus fontinalis). II. Gill histology. Can. J. Fish. Aquat. Sci. 1991;48(10):2016–2027. [Google Scholar]
- 135.Heath A. CRC. Press. INC; Boca. Raton, Florida: 1995. Water Pollution and Fish Physiology; p. 359. [Google Scholar]
- 136.Hughes G.M. Coughing in the rainbow trout (Salmo gairdneri) and the influence of pollutants. Rev. Suisse Zool. 1975;82(1):47–64. doi: 10.5962/bhl.part.78257. [DOI] [PubMed] [Google Scholar]
- 137.Sutherland A.B., Meyer J.L. Effects of increased suspended sediment on growth rate and gill condition of two southern Appalachian minnows. Environ. Biol. Fishes. 2007;80(4):389–403. [Google Scholar]
- 138.Guerrero-Castilla A., Olivero-Verbel J., Sandoval I.T., Jones D.A. Toxic effects of a methanolic coal dust extract on fish early life stage. Chemosphere. 2019;227:100–108. doi: 10.1016/j.chemosphere.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 139.Turnpenny A., Williams R. Effects of sedimentation on the gravels of an industrial river system. J. Fish Biol. 1980;17(6):681–693. [Google Scholar]
- 140.Renieri E.A., Sfakianakis D.G., Alegakis A.A., Safenkova I.V., Buha A., Matović V., Tzardi M., Dzantiev B.B., Divanach P., Kentouri M. Nonlinear responses to waterborne cadmium exposure in zebrafish. An in vivo study. Environ. Res. 2017;157:173–181. doi: 10.1016/j.envres.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 141.Acosta I.B., Junior A.S.V., e Silva E.F., Cardoso T.F., Caldas J.S., Jardim R.D., Corcini C.D. Effects of exposure to cadmium in sperm cells of zebrafish, Danio rerio. Toxicol. Rep. 2016;3:696–700. doi: 10.1016/j.toxrep.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gerhart E.H., Liukkonen R.J., Carlson R.M., Stokes G.N., Lukasewycz M., Oyler A.R. Histological effects and bioaccumulation potential of coal particulate-bound phenanthrene in the fathead minnow Pimephales promelas. Environ. Pollut. Ser. A Ecol. Biol. 1981;25(3):165–180. [Google Scholar]
- 143.Wendelaar Bonga S.E. The stress response in fish. Physiol. Rev. 1997;77(3):591–625. doi: 10.1152/physrev.1997.77.3.591. [DOI] [PubMed] [Google Scholar]
- 144.Schreck C.B., Tort L., Farrell A.P., Brauner C.J. Academic Press; 2016. Biology of Stress in Fish. [Google Scholar]
- 145.Nilsson G.E. Gill remodeling in fish–a new fashion or an ancient secret? J. Exp. Biol. 2007;210(14):2403–2409. doi: 10.1242/jeb.000281. [DOI] [PubMed] [Google Scholar]
- 146.Nilsson G.E., Dymowska A., Stecyk J.A. New insights into the plasticity of gill structure. Respir. Physiol. Neurobiol. 2012;184(3):214–222. doi: 10.1016/j.resp.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 147.Sfakianakis D., Renieri E., Kentouri M., Tsatsakis A. Effect of heavy metals on fish larvae deformities: a review. Environ. Res. 2015;137:246–255. doi: 10.1016/j.envres.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 148.Foa V., Elia G., Schiavulli N., Sartorelli P., Schiarra G., Cenni A., Novelli M., Mangani F., Cecchetti G., Iachetta R. Non-bioavailability of carcinogenic polycyclic aromatic hydrocarbons contained in coal dust. Med. Lav. 1998;89(1):68–77. [PubMed] [Google Scholar]
- 149.Berry W., Rubinstein N., Melzian B., Hill B. Vol. 32. United States Environmental Protection Agency; Duluth: 2003. pp. 54–55. (The Biological Effects of Suspended and Bedded Sediment (SABS) in Aquatic Systems: a Review). 1. [Google Scholar]
- 150.Erftemeijer P.L., Riegl B., Hoeksema B.W., Todd P.A. Environmental impacts of dredging and other sediment disturbances on corals: a review. Mar. Pollut. Bull. 2012;64(9):1737–1765. doi: 10.1016/j.marpolbul.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 151.Jones R., Bessell-Browne P., Fisher R., Klonowski W., Slivkoff M. Assessing the impacts of sediments from dredging on corals. Mar. Pollut. Bull. 2016;102(1):9–29. doi: 10.1016/j.marpolbul.2015.10.049. [DOI] [PubMed] [Google Scholar]
- 152.Peters E.C., Gassman N.J., Firman J.C., Richmond R.H., Power E.A. Ecotoxicology of tropical marine ecosystems. Environ. Toxicol. 1997;16(1):12–40. [Google Scholar]
- 153.Emerson S., Zedler J. Recolonization of intertidal algae: an experimental study. Mar. Biol. 1978;44(4):315–324. [Google Scholar]
- 154.Kendrick G.A. Recruitment of coralline crusts and filamentous turf algae in the Galapagos archipelago: effect of simulated scour, erosion and accretion. J. Exp. Mar. Biol. Ecol. 1991;147(1):47–63. [Google Scholar]
- 155.Moore P., PG M. 1977. Inorganic Particulate Suspensions in the Sea and Their Effects on Marine Animals. [Google Scholar]
- 156.Longstaff B.J., Dennison W.C. Seagrass survival during pulsed turbidity events: the effects of light deprivation on the seagrasses Halodule pinifolia and Halophila ovalis. Aquat. Bot. 1999;65(1–4):105–121. [Google Scholar]
- 157.O’Brien K.R., Adams M.P., Ferguson A.J., Samper-Villarreal J., Maxwell P.S., Baird M.E., Collier C. Springer; 2018. Seagrass Resistance to Light Deprivation: Implications for Resilience, Seagrasses of Australia; pp. 287–311. [Google Scholar]
- 158.Johnson L., Frid C. The recovery of benthic communities along the County Durham coast after cessation of colliery spoil dumping. Mar. Pollut. Bull. 1995;30(3):215–220. [Google Scholar]
- 159.Newcombe C.P., MacDonald D.D. Effects of suspended sediments on aquatic ecosystems. N. Am. J. Fish. Manag. 1991;11(1):72–82. [Google Scholar]
- 160.Lucas S.A., Planner J. Grounded or submerged bulk carrier: the potential for leaching of coal trace elements to seawater. Mar. Pollut. Bull. 2012;64(5):1012–1017. doi: 10.1016/j.marpolbul.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 161.Dore S.Y., Laursen A.E., Kulpa J., Charles F. Polycyclic aromatic hydrocarbon degradation in soil surrounding an outdoor coal storage pile. J. Environ. Eng. Sci. 2003;2(3):227–231. [Google Scholar]
- 162.León-Mejía G., Silva L.F., Civeira M.S., Oliveira M.L., Machado M., Villela I.V., Hartmann A., Premoli S., Corrêa D.S., Da Silva J. Cytotoxicity and genotoxicity induced by coal and coal fly ash particles samples in V79 cells. Environ. Sci. Pollut. Res. - Int. 2016;23(23):24019–24031. doi: 10.1007/s11356-016-7623-z. [DOI] [PubMed] [Google Scholar]
- 163.Matzenbacher C.A., Garcia A.L.H., Dos Santos M.S., Nicolau C.C., Premoli S., Corrêa D.S., de Souza C.T., Niekraszewicz L., Dias J.F., Delgado T.V. DNA damage induced by coal dust, fly and bottom ash from coal combustion evaluated using the micronucleus test and comet assay in vitro. J. Hazard. Mater. 2017;324:781–788. doi: 10.1016/j.jhazmat.2016.11.062. [DOI] [PubMed] [Google Scholar]
- 164.Torres-Ávila J.F., Espitia-Pérez L., Bonatto D., Silva F.Rd., Oliveira I.Md., Silva L.F., Corrêa D.S., Dias J.F., Silva Jd., Henriques J.A.P. Systems chemo-biology analysis of DNA damage response and cell cycle effects induced by coal exposure. Genet. Mol. Biol. 2020;43(3) doi: 10.1590/1678-4685-GMB-2019-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]