Abstract
Dopamine is a prototypical neuromodulator for the control of circuit function through G-protein coupled receptor signaling. Neuromodulators are volume transmitters with release followed by diffusion for widespread receptor activation on many target cells, but we are only beginning to understand the specific organization of dopamine transmission in space and time. While some roles of dopamine are mediated by slow and diffuse signaling, recent studies suggest that certain dopamine functions necessitate spatiotemporal precision. Here, we review the literature and develop a new model for dopamine transmission. We focus on dopamine signaling in the striatum and discuss release mechanisms and receptor organization. We then propose the domain-overlap model, in which release and receptors are arranged relative to one another in micrometer-scale structures. This architecture is different from point-to-point synaptic transmission or the widespread organization often proposed for neuromodulation. It enables activation of receptor subsets that are close to release sites during baseline activity, and overlap between these micrometer-scale domains with broader receptor activation when firing is synchronized across dopamine neuron populations. This signaling structure is matched to the properties of dopamine neurons, explaining how switches in firing modes support coding dynamics and may lead to distinct pathway modulation.
Introduction
Dopamine has multidimensional importance in the control of brain function and is associated with a broad spectrum of brain disorders including Parkinson’s Disease and addiction. As an evolutionarily ancient neuromodulator, roles of dopamine are preserved from C.elegans to humans1,2. In the mammalian brain, most dopamine neurons reside in two nuclei in the ventral midbrain: the pars compacta of the substantia nigra (SNc) and the ventral tegmental area (VTA). The SNc neurons project to the dorsal striatum, giving rise to the nigrostriatal pathway, important for motor control and action selection. VTA dopamine neurons predominantly project to the ventral striatum and the prefrontal cortex, forming the mesocorticolimbic pathway, and are responsible for reward processing and reinforcement learning3–6.
A central unresolved question is how dopamine signaling is organized to mediate these broad functions6–8. The classical view is that dopamine transmission lacks speed and accuracy, but recent studies have found hallmarks of precise signaling. Early work by Carlsson led to the discovery of dopamine as a neurotransmitter and suggested that dopamine functions are slow and imprecise9,10. Rabbits treated with reserpine, a vesicular monoamine transporter (VMAT) blocker that depletes brain dopamine, were paralyzed. Upon injection of L-DOPA, a dopamine precursor, movement was restored under the continued presence of VMAT blockade. Hence, the brain was able to metabolize L-DOPA and use it to drive locomotion despite the absence of vesicular dopamine loading and of precise exocytotic release. Morphological studies support the hypothesis that spatial precision is not built into dopamine signaling, with receptors and transporters being predominantly extrasynaptic11–14. Furthermore, the vertebrate dopamine receptors, which are all G-protein coupled receptors (GPCRs), operate at speeds that are orders of magnitude slower than ionotropic receptors15. These properties have led to the model that dopamine signals through volume transmission and does not rely on the high spatiotemporal precision that defines synaptic transmission (Fig. 1).
Figure 1. Modes of chemical transmission.

Overview of the fundamental modes of chemical transmission differing in release precision and organization of receptors.
a. Endocrine cells release their transmitters, generally hormones, from the cell surface. The transmitters travel over long distances through the extracellular space and the blood stream to receptors residing far away from the release sites. Often, no specialized release site architecture is evident in these cells.
b. Volume transmission relies on diffusion of transmitter in the extracellular space, and the receptors are only loosely coupled with the release sites. Often, specialized active zone-like release sites mediate neuromodulator secretion. A steep transmitter concentration gradient is built upon release, and the degree of receptor activation depends on their distance to these release sites.
c. Synaptic transmission relies on tight spatial coupling between the active zone and receptor clusters, which are often aligned with one another at a subsynaptic scale. Signal transmission is confined to the synaptic cleft to ensure accuracy and efficient receptor activation.
Striatal circuit function, however, is highly dynamic and relies on precise dopamine modulation. Important examples are that dopamine signals are local and short-lived16–18, and that dopamine-mediated behaviors and target cell modulation can correlate with dopamine neuron firing on the order of tens to hundreds of milliseconds19–27. Further supporting spatiotemporal precision, resupplying L-DOPA improves symptoms caused by dopamine decline, but learning defects are only partially alleviated28–31, consistent with a need for precise signaling.
Based on these and other findings, the field has developed a compelling framework on the importance of dopamine dynamics in behavior4–6,32, but it remains challenging to reconcile the physiology of dopamine transmission with the broad and sometimes rapid coding properties. Here, we review progress on the mechanisms of dopamine release and the functional organization of dopamine receptors to evaluate how the signaling architecture may support dopamine functions. We focus on the vertebrate striatum because of the well-established importance of dopamine in this circuit, and draw parallels to other dopamine systems when appropriate. We then develop a generalized framework for dopamine neurotransmission. We propose the domain-overlap model, which relies on rapid release followed by diffusion with micrometer-scale release-receptor organization. The presence of these signaling domains potentially explains how switches in firing modes can lead to distinct pathway modulation and broad coding dynamics.
Dopamine Release
Striatal dopamine levels are highly dynamic and fluctuate on different time scales, with sub-second transients, ramps that may last for several seconds, and oscillations on the timescale of hours20,33–37. It has long remained uncertain where dopamine is released from the extensively branched striatal axons and how the secretory mechanisms can account for these dynamic signals7,38. Because most dopamine transmission is not naturally associated with postsynaptic currents, many studies rely on electrochemical measurements that sample dopamine from a large area and either lack temporal resolution or dopamine selectivity. This has started to change with the development of fluorescent reporters and dopamine-sensitive ion channels (Fig. 2). Accumulating evidence now indicates that dopamine signaling is not only temporally dynamic but also spatially organized16–18,22,39–41.
Figure 2. Measurements of dopamine transmission.
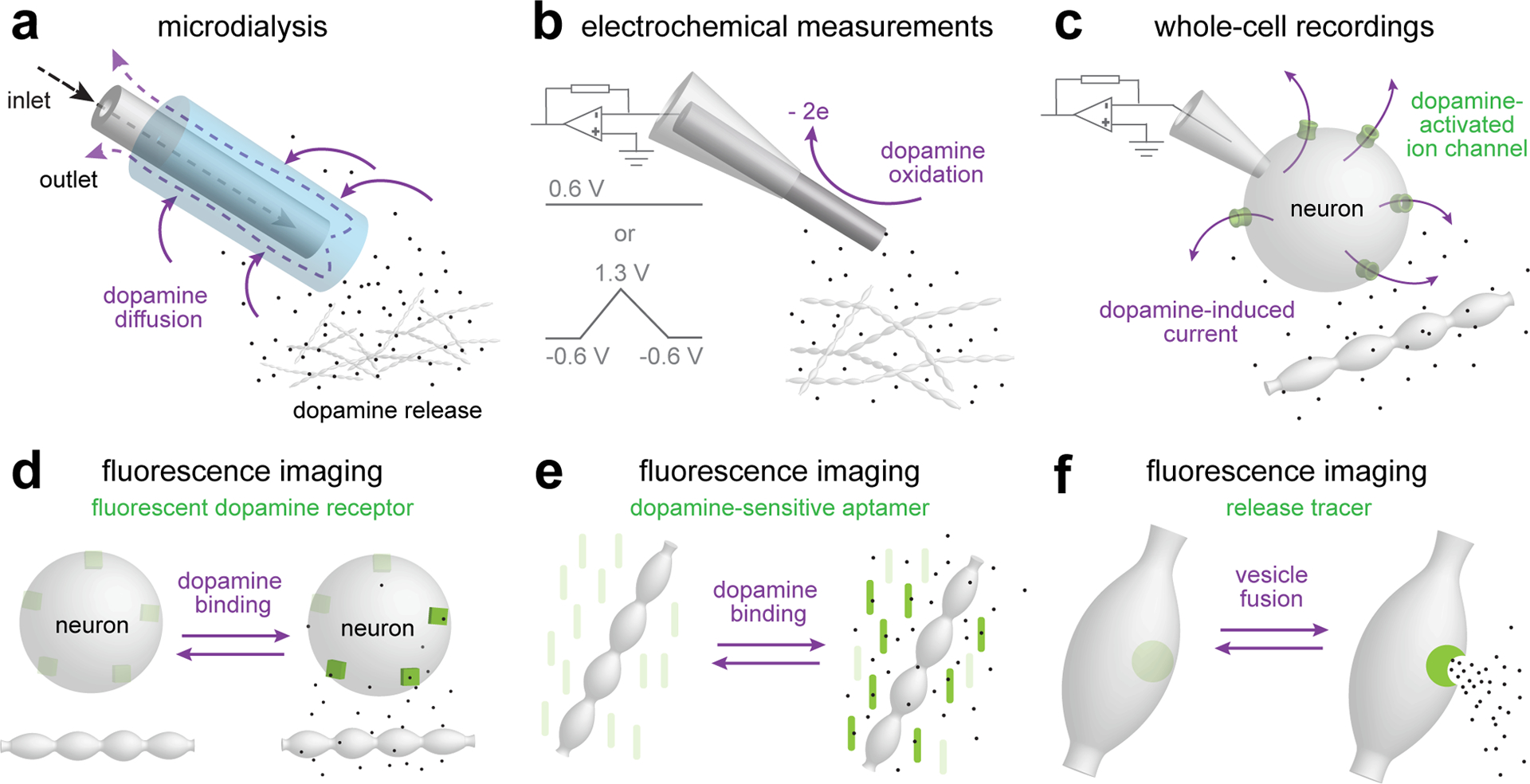
a. Microdialysis enables sampling of the chemical environment in the brain124. A semipermeable probe inserted into the brain is perfused to exchange solutes with the surrounding tissue. The method can be used to quantitatively measure multiple neurotransmitters and molecules in vivo, with a detection threshold lower than 50 fg for dopamine. Microdialysis provides the best measurement for tonic dopamine levels, but temporal (minutes) and spatial (several hundred μm) resolution are too low to report subcellular organization of dopamine transmission or to detect fast dopamine transients.
b. Electrochemical measurements rely on oxidation of dopamine at the surface of a carbon fiber electrode147. Constant-potential amperometry, typically performed at 0.6 V for dopamine, provides the best temporal resolution (sub-millisecond, limited by the sampling frequency), but suffers from low chemical selectivity as any molecule that can be oxidized at the applied voltage will contribute to the signal. Fast-scan cyclic voltammetry (FSCV) improves chemical selectivity at the cost of temporal resolution (typically one data point per ~100 ms). Different molecules are oxidized at distinct voltages and can be distinguished by scanning across holding voltages (typically with a triangular wave ranging from −0.6 V to 1.3 V and a scan speed of 400–800 V/s). Because electrochemical measurements rely on subtraction of a reference current, they are suited to measure changes, but not for assessing baseline dopamine concentration.
c. Whole-cell electrophysiology can be used to measure currents mediated by ion channels that are activated by dopamine. This method relies on natural coupling of GIRK2-channels to D2 receptors for somatodendritic dopamine release in the midbrain95, on exogenous expression of GIRK2 channels to report striatal D2 receptor activation16, or on introducing dopamine-sensitive ion channels called LGC-5349. These whole-cell recordings have high temporal precision and report dopamine at the target cell.
d-f. A range of fluorescent imaging techniques can be used to assess dopamine release. GRABDA and dLight (d) are genetically engineered dopamine receptors in which a circularly permuted GFP is inserted such that fluorescence increases upon dopamine binding79,83. The sensors exhibit good spatiotemporal resolution and can be used in vivo and in vitro. Although the sensors are engineered dopamine receptors, their expression pattern may not mimic that of endogenous dopamine receptors, and hence the signal may not report the spatial organization of dopamine transmission. Synthetic optical probes (e) are made by conjugating oligonucleotides to single-wall carbon nanotubes to gain dopamine-selectivity and -sensitivity of the infrared properties of these nanotubes. Key advantages are their resistance to photobleaching and their ability to report dopamine release with very high spatiotemporal resolution22,148. VMAT-pHluorin and FFNs (f) represent two tracing strategies for assessing vesicle fusion and content release, respectively, different from other methods that measure extracellular dopamine levels. VMAT-pHluorin contains a pH-sensitive fluorophore at the intraluminal side of the vesicular monoamine transporter 2 (VMAT2), and its fluorescence increases when the acidic vesicular lumen is neutralized upon fusion with the plasma membrane72. FFNs are VMAT2 and/or DAT substrates and can be used to monitor dopamine vesicle fusion via dye release18,149. Both methods may permit detecting quantal events, but signal-to-noise ratios are in general relatively low, and translating the measurements to absolute dopamine levels is difficult.
Dopamine varicosities
Most dopamine terminals do not form classical synapses, but contain varicosities that are not associated with defined postsynaptic structures and are packed with small, clear vesicles42–44. The findings that vesicular dopamine loading is essential45–47 and that quantal release events can be detected48–51, established that most dopamine is released through vesicle fusion7,8. Each varicosity could, in principle, embody a release site. However, a recent study suggested functional heterogeneity across varicosities, with only 20% of them secreting above detection threshold upon action potential firing18. Hence it is possible that not all varicosities are release-competent, or that there is strong heterogeneity in the release properties between varicosities.
Dopamine neurons co-release glutamate and GABA, and previous reviews provide in-depth discussions of co-release52,53. In striatal dopamine axons, glutamate release is likely segregated from dopamine release13,54–56. Symmetric synapses like the ones detected in the dopamine system43,57,58 are typically GABAergic, and optogenetic dopamine axon activation indeed robustly triggers dopamine and GABA release from the same vesicular compartment59,60. While GABA receptors and associated proteins are present at the postsynaptic sites, morphological analyses suggest that most GABAergic markers are not detected in the subset of varicosities that form asymmetric contacts with target cells13. This makes it uncertain whether the synaptic structures of dopamine axons are the source of GABA and dopamine release. Studies in cultured midbrain dopamine neurons indicate that only a small proportion of dopamine terminals form synapses, but both synaptic and non-synaptic terminals can release dopamine irrespective of the target cell contact61. Together, these studies suggest that there are specific sites for exocytosis, but that the synapse-like morphology of a subset of the dopamine varicosities does not reliably identify these sites.
Active zone-like dopamine release sites
If the varicosities themselves do not define secretory hotspots, such sites may be accounted for by specific molecular machinery. Evoked exocytosis is triggered by Ca2+ entry through voltage-gated Ca2+ channels, and mediated by SNARE complexes that drive vesicle fusion. At conventional synapses, this process is triggered by vesicular Ca2+ sensors called synaptotagmins and restricted to active zones on the target membrane (Fig. 1)62,63. Active zones are present at every synapse, they precisely target release towards postsynaptic receptors, their regulatory mechanisms shape the relationship between firing and release, and they contain the scaffolding proteins RIM, Munc13, Liprin-α, RIM-BP, ELKS and Piccolo/Bassoon62,64. In contrast to synapses, exocytosis from other secretory cells, for example chromaffin cells, occurs across broad membrane domains and may not need active zone-like specializations (Fig. 1)63,65. Until recently, it has remained unclear whether a central neuromodulator like dopamine relies on active zone machinery.
It is now established that evoked dopamine release requires active zone-like sites (Fig. 3). First, dopamine release occurs on a millisecond time scale and has a high release probability, indicating that vesicles are rendered release-ready before action potential-arrival17,40. Second, clustering of active zone proteins including Bassoon, RIM, ELKS, and Munc13 can be detected using confocal, superresolution and immuno-electron microscopy13,17,41,55,66,67. Finally, evoked dopamine release is abolished upon dopamine neuron-specific knockout of RIM or Munc13, establishing their essential roles17,41,68.
Figure 3. Sparse dopamine release sites.

a. Cellular (top) and molecular (bottom) organization of dopamine release sites. ~25% of dopamine varicosities contain functional release sites composed of active zone proteins. RIM and Munc13 are essential for action potential-triggered dopamine release, and mediate the coupling of release-ready vesicles to Ca2+ entry for fast release triggered by the fast Ca2+ sensor synaptotagmin-117,40,41,68. The exact identities and distributions of Ca2+ channels in dopamine axons are not well understood, but they may be more broadly distributed than active zone proteins, and multiple different channel subtypes contribute to release18,78. Similarly, additional Ca2+ sensors are likely present, but their identities and roles are not known40.
b. Distribution of dopamine release sites and impact area of individual dopamine release events in the striatum. The sparsity of active zone-containing varicosities, the long-lasting depression of individual sites after a release event, and the rapid dilution of dopamine into the extracellular space suggest that, at any given time, only a small fraction of the space reaches high-enough dopamine levels for a sufficient amount of time for efficient receptor activation, and large striatal areas are not within reach of these varicosities during baseline activity. If dopamine receptors reside in this distant space, they are unlikely to be activated by single vesicular release events.
Interestingly, the frequency of active zone scaffolds in dopamine axons is much lower than that of varicosities17,41. This result matches with the observation that only ~20% of varicosities have detectable release of false fluorescent neurotransmitters (FFNs), supporting the model that many varicosities are release-incompetent18. Examination of dopamine distribution using a fluorescent sensor further revealed that evoked dopamine release in the striatum is not widespread, but is concentrated around sparse hotspots22. Together, these findings have led to a model in which action potential-induced dopamine release occurs at a limited number of sites that are defined by RIM and Munc13 (Fig. 3). On average, there is one dopamine release site every ~4 μm of dopamine axon, which leads to an estimate of one site per 25 μm3 of the striatum17,41. Hence, the vast majority of striatal space is a few micrometers away from a release site (Fig. 3b). This sparsity of dopamine sources, together with the release properties discussed below, has important implications for dopamine coding and forms the foundation of the domain-overlap model that we propose.
Properties of dopamine release
The active zone of a synapse docks and primes synaptic vesicles to generate a readily releasable pool (RRP), and it positions those vesicles at defined distances of presynaptic Ca2+ channels to control their vesicular release probability (P)62,64,69–71. RRP and P control the efficacy of release; the bigger they are, the more dopamine is released when an action potential arrives. High P also leads to rapid RRP depletion during repetitive firing71.
Measurements of RRP in dopamine neuron cultures and synaptosomes revealed that only a few percent of the vesicles are part of the RRP72,73. This establishes that mechanisms to generate releasable vesicles must be present. We propose that this function is mediated by RIM and Munc13, similar to conventional synapses70,74–76, given the strong dependence of dopamine release on these proteins and their presence in dopamine axons17,41,55,68. One observation consistent across studies is that dopamine RRP replenishment is slow, with depletion of dopamine release lasting for tens of seconds after a single stimulus16,17,40,77–80. This indicates that recovery from depression is one to two orders of magnitude slower than that of fast synapses71,80,81. Since replenishment speed is critical for the frequency range at which a transmission system can operate, and since dopamine receptors are ‘slow’ GPCRs, the dopamine system is not well suited for high-frequency information transfer.
P is determined by the amount of Ca2+ entry, its localization relative to release-ready vesicles, and the Ca2+-sensitivity of the release machinery. It is often estimated by assessing responses to paired stimuli (paired-pulse ratios)71. If P is high, release triggered by the first stimulus depletes the RRP and reduces the second response when the interstimulus-interval is short. Dopamine axons exhibit strong depression even at long intervals, with the first pulse depleting ~60% of the RRP17,40,41,55,80,81, indicating that P is very high. Estimating P via paired-pulse stimulation necessitates detecting peak release, specific activation of dopamine axons, and activation of the same axon population by both stimuli, requirements that are often not met. Peak release is best assessed by measuring peak dopamine levels, which are detected within <5 ms of stimulation if the sampling rate is fast17,49,80. Hence, estimating P at short interstimulus intervals requires sampling at >400 Hz, which is typical for amperometry. Lower-frequency measurements, for example voltammetry (often performed at <10 Hz in vivo) or some imaging approaches, report dopamine levels at a specific point during the decay or average dopamine over the sampling time window, respectively. The detected dopamine amount highly depends on the exact sampling time point and on dopamine accumulation and clearance, and is not linearly correlated with release. During stimulus trains or burst firing, low sampling frequencies may further magnify apparent contributions of later responses because of prolonged dopamine decay, which has sometimes led to estimates of low P. We note, however, that when the sampling frequency is fast enough to capture the release peak, depression indicative of high P is also seen in vivo79,82,83. Ultimately, approaches that are not fast enough to measure peak release are not well suited to determine P. A further confound in early studies is that electrical stimulation coactivates striatal cholinergic interneurons, which locally drive dopamine release and dominate the evoked response17,84–87. This mechanism leads to complete depression after the first stimulus. It can be avoided through the use of pharmacology or by dopamine neuron-specific optogenetic activation. Optogenetic stimulation enables dopamine axon firing at frequencies of up to 10 Hz when a fast actuator is used17. Given the complexity of the dopamine axonal arbor and the distinct axonal vs. somatic properties, it is important that the reliability of activation is established in the area of stimulation in optogenetic experiments.
Dopamine release in the striatum is steeply dependent on extracellular Ca2+ 40,78,88, establishing the reliance on plasma membrane Ca2+ channels (Fig. 3a). A recent study used optogenetics to stimulate dopamine axons and found that CaV1 (L-type), CaV2 (P/Q-, N- and R-type), and CaV3 (T-type) channels all contribute to dopamine release78, different from classical synapses (which nearly exclusively rely on CaV2s89,90). In contrast to these mixed Ca2+ sources, synaptotagmin-1 is the single fast Ca2+ sensor for axonal dopamine release-triggering40, bolstering the model of a fast, precise and high P release mode. Remarkably, although removal of synaptotagmin-1 eliminated synchronous dopamine release, KCl-induced depolarization still triggered release, suggesting that additional release modes and Ca2+ sensors exist.
In addition to axonal release, midbrain dopamine neurons also release dopamine through vesicular exocytosis from their somata and dendrites91–94. Dopamine D2 autoreceptor activation, in turn, triggers inhibitory postsynaptic currents mediated by GIRK2 channels (D2-IPSCs), which can be measured by whole-cell recordings (Fig. 2c)95. While we are only beginning to understand the somatodendritic release machinery, a recent study established that it relies on the release site organizer RIM68, similar to axonal release17. D2-IPSCs can also be measured in the striatum when GIRK2 channels are virally expressed, and their characterization strongly supports a high P and strong depression16,55. As further discussed below, this line of work has revealed important insight into dopamine receptor activation.
Tonic and Phasic Release
Switching between firing modes is a hallmark feature of dopamine neurons (Box 1)96,97. Tonic firing at 0.2–10 Hz relies on cell-autonomous pacemaker currents98,99, while burst (or phasic) firing at >10 Hz is driven by excitatory inputs100,101 and results in synchronized dopamine neuron activity. It has been proposed that tonic firing generates steady-state dopamine concentrations, or tonic release, and burst firing leads to phasic release101,102. Switching between firing modes may account for dynamic dopamine signaling4. Somatic dopamine neuron firing rates, however, are not linearly translated into axonal dopamine release, but are subject to strong short-term depression as discussed above. Furthermore, a small amount of extracellular dopamine can be detected without somatic firing or when the protein machinery for action potential-triggered release is ablated17,33,86. For these reasons, tonic and phasic firing are not equal to tonic and phasic release (Box 1)4.
Display items.
Box 1: Tonic vs. phasic dopamine neuron activity
The terms “tonic” and “phasic” have been used for several features of dopamine neurons4. In the context of dopamine transmission, it is important to distinguish between firing, release, and signaling.
Firing:
Tonic firing refers to sustained activity at 0.2–10 Hz of a dopamine neuron, mediated by cell-autonomous pacemaker conductances98,99. It is estimated that 50–98% of dopamine neurons exhibit tonic firing in vivo101,146. Burst firing is characterized by short bursts of action potentials (3–10 spikes, >10 Hz) of a dopamine neuron. It is typically caused by activation of its NMDA receptors via excitatory inputs and embodies the response to environmental stimuli100,101. Burst firing is sometimes called phasic firing, which highlights the synchrony of activity across dopamine neurons arising from shared inputs.
Release and signaling:
Tonic release generates short-lived dopamine transients of a few milliseconds at a small, variable subset of release sites. Dopamine is quickly diluted into the extracellular space, and the balance between tonic release and reuptake via DAT determines the measured baseline dopamine levels, which are ~2–20 nM123,124. These are below the activation threshold of most dopamine receptors16,127–129, but are likely composed of many short-lived dopamine peaks that are averaged over time. Tonic signaling is most likely caused by these short-lived dopamine signals close to release sites rather than the steady-state dopamine levels. Remarkably, only ~70% of the baseline dopamine measured in microdialysis is caused by action potential firing. The remaining ~30% are independent of action potentials and the active zone proteins RIM and Munc1317,41, and may, for example, be accounted for by spontaneous vesicular fusion17,40,48,68. Phasic release is an important form of dopamine coding, and it occurs when a large number of dopamine release sites are simultaneously activated. Dopamine reuptake mechanisms are transiently overpowered, resulting in significant crosstalk between dopamine signaling domains and prolonged dopamine dwell times (Fig. 6). For phasic signaling, the rapid dopamine elevation across multi-micron sized areas may lead to activation of dopamine receptors distant from release sites. Synchrony of release across dopamine neuron populations is a prerequisite of phasic release and signaling. In experimental paradigms, low-frequency stimulation is often used to mimic tonic release. However, this does not replicate the stochastic feature of release site activation that is typical for tonic release, but instead recruits many axons simultaneously and thus mimics the essential feature of phasic release.
The notion that dopamine release machinery responds robustly to the initial activity, but rapidly depresses for tens of seconds16,17,40,77–80, makes important predictions (Fig. 4). First, tonic firing leads to depletion, and dopamine release in response to each action potential is largely determined by the recovery of these refractory sites. Therefore, neurons with lower spontaneous activity might contribute more during phasic release because their RRP is less depleted when the synchronizing stimulus arrives. Second, during burst firing, only the first few action potentials lead to significant dopamine release from a single axon. It is thus the synchrony of population firing, not the firing pattern of individual neurons, that dominates signaling during phasic release. In support of this view, burst firing is strongly impaired in mice that lack NMDA receptors, but phasic dopamine transients and behaviors mediated by them persist100,103.
Figure 4. Dopamine neuron firing and release.

a, b. Model of dopamine release of a single dopamine axon during tonic firing (black) and burst firing (purple). The amount of dopamine released from a single axon depends on how many sites release dopamine (red dots, active sites). Many sites do not release either because the initial release probability of the available sites is below 1 (black dots, inactive sites), or because sites are in a depressed state (blue dots, refractory sites). Neurons with a higher tonic firing frequency (a) will have more refractory sites and release less dopamine in response to each action potential. Neurons with a lower tonic firing frequency (b) will have less refractory sites and release more dopamine in response to each action potential. Burst firing leads to rapid depression of release from a single axon, with low-frequency neurons contributing more dopamine. Note that this speculative model presents the total amount of release from a single axon, which cannot currently be measured. Typical measurements using electrochemical methods (Fig. 2) reflect the average dopamine in a large area and from many neurons, not peak dopamine at release sites from a single axon. In addition, in vivo voltammetry may reveal a build-up of dopamine during rapid stimulation because dopamine reuptake is overwhelmed and because low frequency-measurements overestimate dopamine levels during the decay phase.
The terms phasic release and burst (or phasic) firing are widely and often interchangeably used (Box 1). We emphasize that even though they are correlated4, they represent very different aspects of dopamine coding. Phasic release depends on the simultaneous recruitment of a dopamine neuron population, which relies on synchrony across dopamine neurons and does not require burst firing33,100,103,104. In contrast, burst firing is an activity pattern of a single dopamine neuron and does not strongly enhance dopamine release from that neuron due to the prominent use-dependent depression16,17,40,77–80. In most cases, burst firing is synchronized across dopamine neurons and the first spike efficiently elevates dopamine levels, while the following activity releases less dopamine due to reduced synchrony and the presence of refractory sites79,83,105. Thus, the later spikes during bursts are likely not efficient at increasing dopamine levels further, but help maintain elevated levels caused by the first spike to increase dopamine dwell times. While high-frequency sampling reveals high initial release rates, low-frequency release measurements may detect an apparent increase late in the burst because the slow sampling frequency reports increased average levels due to the prolonged dopamine decay17,79,82,83,106.
Dopamine receptors
In the striatum, medium spiny neurons (MSNs) are the main output neurons, and they contain the majority of the dopamine receptors. The striatum displays significant regional heterogeneity and is subdivided into patches (or striosomes) and matrix107. The MSNs are localized in both areas, and we here focus on the nano- to micrometer scale organization of dopamine receptors on MSNs and how their organization could contribute to the control of dopamine signaling.
Cell-type specific dopamine receptor distribution
Dopamine receptors are large, seven-transmembrane containing GPCRs. In vertebrates, five genes encode for two major classes, the D1-like (D1, D5) and D2-like (D2, D3, and D4) receptors15. D1-like receptors are Gαs/olf coupled to activate adenylate cyclase and increase excitability. In contrast, D2-like receptors couple to Gαi/o reducing adenylate cyclase activity, having an overall inhibitory effect108–110. In the brain, dopamine receptor expression levels are correlated with the density of dopamine innervation, D1 and D2 receptors are abundant, with estimates suggesting 3–5 fold more D1 than D2 receptors, and expression of the other receptors is generally low108,111–113.
D1 and D2 receptors are most prominently found in the dorsal and ventral striata, where their presence defines MSN subtypes (D1- and D2-MSNs, respectively), and D2 receptors are also expressed on dopamine axons12,114,115 (Fig. 5). The distinct receptor localization on MSN subtypes forms the foundation of striatal dopamine regulation and defines functions of the classic model of direct and indirect pathways1,114–117. Approximately half of the MSNs predominantly express D1 receptors and form the direct pathway, facilitating movement and reinforcement learning. The other half mostly express D2 receptors and give rise to the indirect pathway, which generally inhibits the same functions25,114,117–121.
Figure 5. Dopamine receptor organization and activation.

a, b. Working model of dopamine receptor organization with overview (a) and zoom-in on a varicosity that makes a synapse-like contact (b). Dopamine is released from non-synaptic and synaptic varicosities. The main dopamine receptors are segregated over two neuron subtypes, D1-MSNs and D2-MSNs. Both MSN types sense dopamine release from non-synaptic varicosities (a, left), and can receive synaptic-like inputs from dopamine axons with appositions between dopamine varicosities and GABAergic postsynaptic assemblies (a, right, and b). Dopamine receptors are widely distributed on MSNs, and may be present in clusters. Importantly, dopamine receptors are not found in the postsynaptic specializations with the currently available tools (b). Instead, these specializations may contain gephyrin and other proteins typically found at GABA-ergic synapses13. Individual vesicular fusion events may activate both D1 and D2 receptors, and close-by receptors are more likely to be activated by dopamine than those farther away. The exact organization of dopamine receptors relative release sites is not known, but may strongly impact dopamine functions.
Receptor organization
The subcellular distribution of dopamine receptors remains largely unknown. However, their opposing functions in cell-excitability, their cell-type specific distribution, and their distinct behavioral functions revealed by pharmacological manipulations may suggest an organization for selective activation. A simple solution would be that a single dopamine axon specifically targets nearby receptors on a specific MSN. Surrounding organization for dopamine reuptake could be used to limit dopamine spread. This architecture would be similar to synapses (Fig. 1), for which a defining feature is receptor clustering within tens of nanometers of release sites62,64, but fundamentally different from classical models of volume transmission.
Most morphological studies, however, are not easily compatible with such a synapse-like organization of dopamine transmission. Ultrastructural analyses revealed that most dopamine varicosities are not apposed to postsynaptic cells and densities42–44. While antibody-labeling often suffers from unspecific signals, studies with several different dopamine receptor antibodies support one another to suggest a distributed localization. Light- and immunoelectron-microscopy revealed that D1 receptors are broadly localized on MSNs, with somatic, dendritic shaft and dendritic spine localizations, and they sometimes appear clustered13,14,122. Similarly, D2 receptors appear broadly distributed within D2-MSNs, in some studies with enhanced presence in distal dendrites12–14. A recent immuno-electron microscopy study focused on the subset of dopamine varicosities that make synaptic-like contacts13. There, D1 and D2 receptors were not commonly found in the apposed postsynaptic membrane, but were distributed perisynaptically (within 100 nm of the edges of the synaptic-like apposition), or extrasynaptically (beyond 100 nm of the synaptic-like contact). Together, these morphological studies suggest that dopamine receptors are at least partially clustered on MSNs, but fail to detect a synapse-like apposition for most receptors (Fig. 5), and the term “dopamine synapse” should not be used to describe the general striatal dopamine signaling architecture.
Receptor activation
Receptor activation is a dynamic process, relying on ligand availability and receptor-binding properties. An early observation was that D2 receptors exhibit a high-affinity state (Kd = ~25 nM), while D1 receptors are mainly in a low-affinity state (Kd = ~1 μM)112. This has led to a model in which tonic dopamine levels (~2–20 nM) and phasic dopamine release activate D2 receptors and D1 receptors, respectively. Necessary release-receptor distances were estimated based on these affinities and corresponding models were proposed123–125. However, the experimental conditions during affinity measurements may have confounded the initial results. GTP that is present within MSNs significantly reduces the affinity of D2 receptors to levels similar to D1 receptors112, and the affinity-based models have been challenged113,126. Most studies in brain slices find that the half-maximal effective dopamine concentration for activation of the two receptors is similar and in the micromolar range, suggesting that both dopamine receptors are mainly in a low-affinity state in vivo16,127–129.
A single vesicular release event generates a spreading sphere of dopamine with a steep concentration gradient surrounding this point source, and the degree of receptor activation depends on release-receptor distances and on dopamine dwell times22,123,130. In contrast to synapses, where cleft organization leads to transmitter concentrations above 1 mM64, dopamine is free to diffuse in the extracellular space and is quickly diluted upon release. If nanomolar dopamine is sufficient for receptor activation and a binding equilibrium is reached immediately, a release event might be able to recruit dopamine receptors as far as a few micrometers away. However, since dopamine transients from a single vesicle are brief (~3 ms before they are diluted to <200 nM)113,123, dopamine receptor activation necessitates micromolar dopamine16,127,128, and dopamine receptors likely have relatively slow binding kinetics131,132, it is improbable that receptors at micrometer distances are efficiently activated by a single vesicular release event. Instead, the effective distance of receptors to detect quantal release is probably below one micrometer22,113,123,130–132. An important additional consideration is the relationship between diffusion and dopamine reuptake via the dopamine transporter (DAT). Experimental data and modeling suggest that diffusion dominates dopamine levels over distances of several micrometers from release sites, and the effects of DAT may only become significant beyond that distance105,123,133. Since single release events generate a smaller receptor activation zone, this area is likely exempt from DAT regulation, but DAT activity may determine the degree of crosstalk of adjacent release sites and control spatiotemporal characteristics of phasic release22,123.
Electrophysiological studies of D2 receptors further contrast affinity-based models. They have established that D2 receptor signaling relies on close-by low-affinity receptors that are rapidly activated. D2-IPSC can be measured as GIRK currents in midbrain dopamine neurons and are triggered by somatodendritic release95. Interestingly, fluorescently tagged endogenous D2 receptors are at least partially clustered on midbrain dopamine neurons134. Somatodendritic transmission occurs in a rapid and localized manner, contrary to the classical view of tonic D2 receptor signaling. D2-IPSCs are readily evoked by a single stimulus and do not require repetitive stimulation, they have relatively fast kinetics with a lag of ~50 ms after stimulation, and they necessitate high dopamine concentrations95,135,136. Quantal events are present in the absence of action potentials, establishing efficient receptor activation by exocytosis of a single vesicle48. Together, these studies reveal that in the midbrain, release sites and receptors are organized such that individual secretory events effectively mediate somatodendritic transmission. Notably, many of these features are shared by striatal D2 transmission monitored via D2-IPSCs16. Most importantly, strong stimulation enhances D2-IPSCs over repetitive optogenetic stimulation and D2 receptors are only saturated by ~100 μM dopamine, establishing that striatal D2 receptors respond to phasic release16.
Altogether, studies on release and receptors predict that there is a steep dopamine gradient originating from a point source, that receptors within ~one micrometer are rapidly activated, and dopamine dynamics and receptor states may strongly influence their activation. Large striatal areas appear to be out of reach of individual fusion events (Figs. 3, 5), and receptors in these farther-away areas necessitate dopamine spread and pooling of multiple sites for activation, as proposed with the domain-overlap model.
A micrometer-scale framework for dopamine signaling
The domain-overlap model
Baseline dopamine signaling is often considered to rely on uniform, steady-state dopamine concentrations101,102,137, a view that arises from sampling of a large area at low frequency, for example with microdialysis or voltammetry (Fig. 2). As discussed above, most of the dopamine release sites are depleted during tonic firing. In consequence, uncoordinated tonic dopamine neuron activity leads to release from only a small and changing subset of release sites that are sparsely distributed in the striatum (Figs. 3, 4). Upon release, dopamine is diluted to sub-micromolar concentrations at micrometer distances within a matter of milliseconds50,123,138, and overlap with dopamine from adjacent sites is unlikely. Since dopamine dwell times around these sparse events are short, release during tonic firing cannot maintain steady-state receptor activation113, but instead transiently activates a changing small subset of receptors (Fig. 6a). This dynamic process may appear as steady-state activation in measurements that lack spatiotemporal resolution.
Figure 6. The domain-overlap model.

a, b. Model of dopamine signaling domains during tonic and phasic release. In a given area of the striatum, tonic release (a) generates short-lived dopamine peaks that are confined to a small domain and only recruit proximal receptors (left). After a short time interval (Δt), a distinct set of release sites is active and targets a different subset of receptors (right). The synchrony of phasic release across many release sites in a given area (b) generates significant crosstalk between signaling domains. After a brief interval, dopamine spread overwhelms the DAT, and dopamine levels increase beyond the micrometer-sized domains of active sites. This leads to augmented dopamine dwell times and activation of new receptors residing farther away from release sites. This domain-overlap model may form a basis for recruiting small variable subsets of receptors during tonic activity (arising from small dopamine domains), and recruitment of larger numbers of distant receptors during phasic activity (arising from overlap of dopamine domains). Regional heterogeneity in release site distribution within the complex dopamine axonal arbor may influence receptor activation domains, and co-release could further shape the signaling of dopamine neurons. Differential distribution of distinct dopamine receptors or cell types at micrometer-scale distances may lead to distinct pathway modulation during tonic and phasic release, respectively.
In contrast, groups of dopamine neurons are activated synchronously by reward consumption or movement initiation6,20,21,139. This simultaneous activity leads to phasic release and overlap of dopamine domains from multiple sites. As a result, more homogenous dopamine levels and receptor activation beyond the individual, micrometer-sized signaling domains are likely (Fig. 6b). Following this domain-overlap model, understanding whether there is differential dopamine receptor distribution at “long”, micrometer distances from release sites is critical as it would determine which receptors are activated during tonic vs. phasic firing. A dynamic activation mechanism combined with distinct distributions of receptor subtypes at multi-micrometer scales might contribute to pathway selection and underly the diverse roles of dopamine.
Relationship to previous models
While dopamine release and diffusion occur within a few milliseconds, dopamine receptors, like other GPCRs, require ~100 ms or more to signal, limiting signaling speeds16,17,80. The high release probability may not be necessary for rapid signaling, but instead generates fast, synchronous release needed for phasic signaling via activation of receptors beyond the individual signaling domains. Simultaneous release transiently overwhelms the DAT, leads to overall enhanced dopamine levels, and increases dopamine dwell times to reach activation thresholds of receptors residing farther away16,22,123,137. This domain-overlap model reconciles features of D2-IPSCs with morphological studies13,16,95. As long as receptors are reached within milliseconds by dopamine levels sufficient for their activation, they may not need to be precisely apposed to release sites. Localization within hundreds of nanometers may be sufficient for activation (Figs. 3, 5), and DAT blockade may enhance D2 activation because dopamine travels farther13,16,136. Rapid release with sharp rises in extracellular neuromodulator concentration followed by diffusion over distance might represent a universal mechanism for modulatory systems in the brain.
D1- and D2 MSNs define the direct and indirect pathways114,117,118, respectively, with the direct pathway facilitating movement and reinforcement learning and the indirect pathway inhibiting it25,116,119–121. In a simplified view, the role of dopamine in these pathways is twofold6,20,21,25. For movement control, dopamine modulates striatal moment-to-moment activity to mediate action selection. For learning, it couples to active ensembles of neurons to induce long-term synaptic changes and to enhance selected neural trajectories during task execution. Dopamine activates the direct and inhibits the indirect pathway, which changes the net output and triggers synaptic plasticity in this circuitry25,114,115,140,141. We propose to reconsider the affinity-based model, in which extrasynaptic D2 receptors preferably sense baseline dopamine, and D1 receptors mostly respond to high amplitude phasic dopamine in a synaptic-like structure106,112,125,142. These models assume a higher affinity of D2 receptors, do not consider receptor activation kinetics, and conclude that D2 receptors are widespread while D1 receptors are clustered in a synaptic-like fashion. As outlined throughout the review, many findings challenge these models. D2 receptors likely operate in a low-affinity state in vivo and are not efficiently activated by background dopamine levels (2–20 nM). Instead, they reliably detect phasic release and only saturate at 100 μM dopamine16, and they are markedly clustered in the midbrain134. The kinetics, affinity, abundance and distribution of D1 and D2 receptors make both receptors suited for tonic and phasic signaling13,113,126,131,132. Furthermore, tonic release does not produce steady-state dopamine levels. Instead, it is a dynamic process that transiently activates nearby receptors, and both D1 and D2 receptors can be activated if they are close enough (Fig. 5). Finally, phasic release recruits farther away receptors and the degree of activation is determined by release-receptor topographies and dopamine dwell times.
Receptor clustering at micron-scale distances is well suited to reconcile these discrepancies. While some of the receptor clusters may be in a near-synaptic organization, they do not need to be. Instead, receptor clustering is needed for compartmentalized and robust induction of intracellular signaling, and release-receptor distances that vary at a micrometer scale determine activation during tonic or phasic release (Fig. 6). Early studies likely overestimated functional dopamine release site density because varicosities instead of active zones were counted as the relevant sites (Fig. 3), and because it was not considered that spontaneous firing depresses most release sites (Fig. 4). This resulted in models in which release modes account for homogenous low concentrations of “tonic” dopamine and sharp, brief local rises of “phasic” dopamine. We propose that switches between firing modes can serve for select activation of receptor clusters through generation of separated vs. overlapping signaling domains, where the relevant parameter is not how much dopamine levels rise at release sites, but how far dopamine spreads. While isolated release events only activate close-by receptors, synchronizing firing across dopamine neuron populations recruits additional receptors due to crosstalk of active release sites (Fig. 6). If D1 and D2 receptors are distributed equally around the release sites, phasic dopamine release will not change their ratio of recruitment. Conversely, if the distances to release sites are different between receptor subtypes, distal receptors will be relatively more preferred during phasic release. The organization between dopamine release sites and its receptors not only allows a single release site to recruit multiple surrounding receptors, but may also enable receptors to receive input from distinct subsets of dopamine axons. There is currently no data on release site distribution within a single axon, but heterogeneity in their distribution within the extensively arborized axon may further impact dopamine signaling. Ultimately, the organization of dopamine release sites and distinct receptor subtypes remains an open question, but higher-order architecture on micrometer scales might be exceptionally well suited to control distinct output neuron populations with switches in firing modes.
Conclusions and outlook
With the domain-overlap model, we propose that dopamine signaling has evolved to control striatal output through the appearance of specialized architecture with relevant release-receptor assemblies at micrometer scales. This is different from synaptic transmission, and embodies a form of volume transmission that is more refined than the often proposed diffuse organization. A key feature of dopamine signaling is that tonic firing and synchronized burst firing encode distinct functions. The mesoscale signaling structure of the domain-overlap model permits differential coding. Tonic release activates variable subsets of nearby receptors through secretion from sparse sites. Burst firing triggers synchronized release from a population of neurons, which leads to overlap of the dopamine signaling domains (Fig. 6) and recruitment of additional distant receptors. The domain-overlap model, relying on fast release and diffusion to activate distant receptors, may represent a universal mechanism for volume transmission in the brain. Cholinergic transmission, for example, shares key morphological features, high P and pacemaker and burst firing modes44,143.
Future work should aim at testing this model and at mechanistically dissecting four important points. First, a precise understanding of receptor distributions at micrometer distances from release sites is essential, including their clustering, their positioning relative to the secretory hotspots, and their activation profiles during firing. Second, a majority of varicosities may release little or no dopamine in response to action potentials17,18,41. What is the benefit of having them? Material storage in the extensive axonal arbor, a reservoir that can be activated during structural plasticity, and a source for action potential independent dopamine release are possibilities that should be investigated. Third, dopamine signaling in the striatum relies on extensive local regulation8, and a remarkable feature is the striatal dopamine release triggering by cholinergic interneurons independent of ascending action potentials17,84–87. It will be important to assess how these regulatory mechanisms are embedded in the signaling architecture we describe here. Finally, at fast synapses, cell adhesion proteins are thought to provide critical signals for nanometer scale release-receptor apposition62,64. How a micrometer scale architecture between release and receptors can be set up and modulated is unclear.
Similar to other areas in neuroscience, we are at an exciting stage in understanding dopamine as new technology is developed to drive progress. Important recent advances include the development of multiple dopamine sensors22,79,83, the generation of genetic resources for inactivation of action potential-triggered dopamine release17,40,41, and advances in superresolution microscopy that enable studies of the protein composition of brain circuits at nanometer resolution over large areas144,145. We expect that these tools will enable mechanistic insight into the signaling networks of dopamine and other neuromodulatory systems with unprecedented precision.
Acknowledgements
Work on dopamine and synaptic neurotransmission in the Kaeser laboratory is supported by the National Institutes of Health (R01NS103484, R01NS083898, R01MH113349 to PSK), the Dean’s Initiative Award for Innovation (to P.S.K.), the Lefler Foundation (to P.S.K.), a Gordon postdoctoral fellowship (to C.L.), and a Damon Runyon postdoctoral fellowship (DRG-2417-20 to P.G.). We thank Dr. John Williams and Dr. Roy Wise for comments and discussions. We apologize to our colleagues that we could not cite all important work due to space restrictions.
Footnotes
Competing interests
The authors have no financial or non-financial competing interests to declare.
References
- 1.Grillner S & Robertson B The Basal Ganglia Over 500 Million Years. Curr. Biol 26, R1088–R1100 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Sawin ER, Ranganathan R & Horvitz HRC elegans Locomotory Rate Is Modulated by the Environment through a Dopaminergic Pathway and by Experience through a Serotonergic Pathway. Neuron 26, 619–631 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Surmeier DJ, Graves SM & Shen W Dopaminergic modulation of striatal networks in health and Parkinson’s disease. Curr. Opin. Neurobiol 29, 109–117 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grace AA Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci 17, 524–532 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gershman SJ & Uchida N Believing in dopamine. Nat. Rev. Neurosci 20, 703–714 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berke JD What does dopamine mean? Nature Neuroscience 21, 787–793 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu C & Kaeser PS Mechanisms and regulation of dopamine release. Curr. Opin. Neurobiol 57, 46–53 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sulzer D, Cragg SJ & Rice ME Striatal dopamine neurotransmission: regulation of release and uptake. Basal Ganglia 6, 123–148 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlsson A [On the problem of the mechanism of action of some psychopharmaca]. Psychiatr. Neurol. (Basel) 140, 220–2 (1960). [PubMed] [Google Scholar]
- 10.Carlsson A A paradigm shift in brain research. Science 294, 1021–1024 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Nirenberg MJ, Vaughan RA, Uhl GR, Kuhar MJ & Pickel VM The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J. Neurosci 16, 436–47 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sesack S, Aoki C & Pickel V Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. J. Neurosci 14, 88–106 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uchigashima M, Ohtsuka T, Kobayashi K & Watanabe M Dopamine synapse is a neuroligin-2-mediated contact between dopaminergic presynaptic and GABAergic postsynaptic structures. Proc. Natl. Acad. Sci. U. S. A 113, 201514074 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yung KK et al. Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: light and electron microscopy. Neuroscience 65, 709–30 (1995). [DOI] [PubMed] [Google Scholar]
- 15.Missale C, Nash SRS, Robinson SW, Jaber M & Caron MG Dopamine receptors: from structure to function. Physiol. Rev 78, 189–225 (1998). [DOI] [PubMed] [Google Scholar]
- 16.Marcott PF, Mamaligas AA & Ford CP Phasic Dopamine Release Drives Rapid Activation of Striatal D2-Receptors. Neuron 84, 164–176 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu C, Kershberg L, Wang J, Schneeberger S & Kaeser PS Dopamine Secretion Is Mediated by Sparse Active Zone-like Release Sites. Cell 172, 706–718 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pereira DB et al. Fluorescent false neurotransmitter reveals functionally silent dopamine vesicle clusters in the striatum. Nat. Neurosci 19, 578–586 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iino Y et al. Dopamine D2 receptors in discrimination learning and spine enlargement. Nature 579, 555–560 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Howe MW & Dombeck DA Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature 535, 505–510 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.da Silva JA, Tecuapetla F, Paixao V & Costa RM Dopamine neuron activity before action initiation gates and invigorates future movements. Nature 554, 244–248 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Beyene AG et al. Imaging striatal dopamine release using a nongenetically encoded near infrared fluorescent catecholamine nanosensor. Sci. Adv 5, eaaw3108 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwerdt HN et al. Long-term dopamine neurochemical monitoring in primates. Proc. Natl. Acad. Sci 114, 13260–13265 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menegas W, Akiti K, Amo R, Uchida N & Watabe-Uchida M Dopamine neurons projecting to the posterior striatum reinforce avoidance of threatening stimuli. Nat. Neurosci 21, 1421–1430 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen W, Flajolet M, Greengard P & Surmeier DJ Dichotomous Dopaminergic Control of Striatal Synaptic Plasticity. Science 321, 848–851 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tecuapetla F, Jin X, Lima SQ & Costa RM Complementary Contributions of Striatal Projection Pathways to Action Initiation and Execution. Cell 166, 703–715 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Yagishita S et al. A critical time window for dopamine actions on the structural plasticity of dendritic spines. Science 345, 1616–1620 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lebedev AV et al. Effects of daily L-dopa administration on learning and brain structure in older adults undergoing cognitive training: a randomised clinical trial. Sci. Rep 10, 5227 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cools R Dopaminergic modulation of cognitive function-implications for l-DOPA treatment in Parkinson’s disease. Neurosci. Biobehav. Rev 30, 1–23 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Beeler JA et al. Dopamine-dependent motor learning: insight into levodopa’s long-duration response. Ann. Neurol 67, 639–47 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frank MJ By Carrot or by Stick: Cognitive Reinforcement Learning in Parkinsonism. Science 306, 1940–1943 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Schultz W, Dayan P & Montague PR A Neural Substrate of Prediction and Reward. Science 275, 1593–1599 (1997). [DOI] [PubMed] [Google Scholar]
- 33.Mohebi A et al. Dissociable dopamine dynamics for learning and motivation. Nature 570, 65–70 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferris MJ et al. Dopamine transporters govern diurnal variation in extracellular dopamine tone. Proc. Natl. Acad. Sci 111, E2751–E2759 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamid AA, Frank MJ & Moore CI Dopamine waves as a mechanism for spatiotemporal credit assignment. bioRxiv 729640 (2019). doi: 10.1101/729640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim HR et al. A unified framework for dopamine signals across timescales. bioRxiv 803437 (2019). doi: 10.1101/803437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maimon G Parietal Area 5 and the Initiation of Self-Timed Movements versus Simple Reactions. J. Neurosci 26, 2487–2498 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuda W et al. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J. Neurosci 29, 444–53 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marcott PF et al. Regional Heterogeneity of D2-Receptor Signaling in the Dorsal Striatum and Nucleus Accumbens. Neuron 98, 575–587.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banerjee A, Lee J, Nemcova P, Liu C & Kaeser PS Synaptotagmin-1 is the Ca2+ sensor for fast striatal dopamine release. Elife 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banerjee A et al. Molecular and functional architecture of striatal dopamine release sites. bioRxiv 2020.11.25.398255 (2020). doi: 10.1101/2020.11.25.398255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Descarries L, Watkins KC, Garcia S, Bosler O & Doucet G Dual character, asynaptic and synaptic, of the dopamine innervation in adult rat neostriatum: A quantitative autoradiographic and immunocytochemical analysis. J. Comp. Neurol 375, 167–186 (1996). [DOI] [PubMed] [Google Scholar]
- 43.Freund TF, Powell JF & Smith AD Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience 13, 1189–215 (1984). [DOI] [PubMed] [Google Scholar]
- 44.Descarries L & Mechawar N Ultrastructural evidence for diffuse transmission by monoamine and acetylcholine neurons of the central nervous system. Prog. Brain Res 125, 27–47 (2000). [DOI] [PubMed] [Google Scholar]
- 45.Fon EA et al. Vesicular transport regulates monoamine storage and release but is not essential for amphetamine action. Neuron 19, 1271–1283 (1997). [DOI] [PubMed] [Google Scholar]
- 46.Tritsch NX & Sabatini BL Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron 76, 33–50 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nirenberg MJ, Chan J, Liu Y, Edwards RH & Pickel VM Ultrastructural localization of the vesicular monoamine transporter-2 in midbrain dopaminergic neurons: potential sites for somatodendritic storage and release of dopamine. J. Neurosci 16, 4135–4145 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gantz SC, Bunzow JR & Williams JT Spontaneous Inhibitory Synaptic Currents Mediated by a G Protein-Coupled Receptor. Neuron 78, 807–812 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kress GJ et al. Fast Phasic Release Properties of Dopamine Studied with a Channel Biosensor. J. Neurosci 34, 11792–11802 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borisovska M, Bensen AL, Chong G & Westbrook GL Distinct Modes of Dopamine and GABA Release in a Dual Transmitter Neuron. J. Neurosci 33, 1790–1796 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Staal RG, Mosharov EV & Sulzer D Dopamine neurons release transmitter via a flickering fusion pore. Nat. Neurosci 7, 341–346 (2004). [DOI] [PubMed] [Google Scholar]
- 52.Tritsch NX, Granger AJ & Sabatini BL Mechanisms and functions of GABA co-release. Nat. Rev. Neurosci 17, 139–145 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hnasko TS & Edwards RH Neurotransmitter corelease: mechanism and physiological role. Annu. Rev. Physiol 74, 225–243 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang S et al. Dopaminergic and glutamatergic microdomains in a subset of rodent mesoaccumbens axons. Nat. Neurosci 18, 386–392 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Silm K et al. Synaptic Vesicle Recycling Pathway Determines Neurotransmitter Content and Release Properties. Neuron 102, 786–800.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stuber GD, Hnasko TS, Britt JP, Edwards RH & Bonci A Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J. Neurosci 30, 8229–8233 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pickel VM, Beckley SC, Joh TH & Reis DJ Ultrastructural immunocytochemical localization of tyrosine hydroxylase in the neostriatum. Brain Res. 225, 373–385 (1981). [DOI] [PubMed] [Google Scholar]
- 58.Tennyson VM, Heikkila R, Mytilineou C, Côté L & Cohen G 5-Hydroxydopamine ‘tagged’ neuronal boutons in rabbit neostriatum: interrelationship between vesicles and axonal membrane. Brain Res. 82, 341–348 (1974). [DOI] [PubMed] [Google Scholar]
- 59.Tritsch NX, Ding JB & Sabatini BL Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature 490, 262–266 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stensrud MJ, Puchades M & Gundersen V GABA is localized in dopaminergic synaptic vesicles in the rodent striatum. Brain Struct. Funct 3, 1–12 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Ducrot C et al. Dopaminergic neurons establish a distinctive axonal arbor with a majority of non-synaptic terminals. bioRxiv 2020.05.11.088351 (2020). doi: 10.1101/2020.05.11.088351 [DOI] [PubMed] [Google Scholar]
- 62.Südhof TC The Presynaptic Active Zone. Neuron 75, 11–25 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pang ZP & Sudhof TC Cell biology of Ca2+-triggered exocytosis. Curr Opin Cell Biol 22, 496–505 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Biederer T, Kaeser PS & Blanpied TA Transcellular Nanoalignment of Synaptic Function. Neuron 96, 680–696 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neher E A comparison between exocytic control mechanisms in adrenal chromaffin cells and a glutamatergic synapse. Pflugers Archiv European Journal of Physiology (2006). doi: 10.1007/s00424-006-0143-9 [DOI] [PubMed] [Google Scholar]
- 66.Daniel JA, Galbraith S, Iacovitti L, Abdipranoto A & Vissel B Functional Heterogeneity at Dopamine Release Sites. J. Neurosci 29, 14670–14680 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lipton DM, Maeder CI & Shen K Rapid Assembly of Presynaptic Materials behind the Growth Cone in Dopaminergic Neurons Is Mediated by Precise Regulation of Axonal Transport. Cell Rep 24, 2709–2722 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robinson BG et al. RIM is essential for stimulated but not spontaneous somatodendritic dopamine release in the midbrain. Elife 8, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaeser PS et al. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell 144, 282–95 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaeser PS & Regehr WG The readily releasable pool of synaptic vesicles. Curr. Opin. Neurobiol 43, 63–70 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zucker RS & Regehr WG Short-term synaptic plasticity. Annu. Rev. Physiol 64, 355–405 (2002). [DOI] [PubMed] [Google Scholar]
- 72.Pan P-YY & Ryan TA Calbindin controls release probability in ventral tegmental area dopamine neurons. Nat. Neurosci 15, 813–815 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Turner TJ Nicotine Enhancement of Dopamine Release by a Calcium-Dependent Increase in the Size of the Readily Releasable Pool of Synaptic Vesicles. J. Neurosci 24, 11328–11336 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Varoqueaux F et al. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc. Natl. Acad. Sci. U. S. A 99, 9037–9042 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deng L, Kaeser PS, Xu W & Südhof TC RIM Proteins Activate Vesicle Priming by Reversing Autoinhibitory Homodimerization of Munc13. Neuron 69, 317–331 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Augustin I, Rosenmund C, Sudhof TC & Brose N Munc13–1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature 400, 457–461 (1999). [DOI] [PubMed] [Google Scholar]
- 77.Wang L et al. Modulation of dopamine release in the striatum by physiologically relevant levels of nicotine. Nat. Commun 5, 3925 (2014). [DOI] [PubMed] [Google Scholar]
- 78.Brimblecombe KR, Gracie CJ, Platt NJ & Cragg SJ Gating of dopamine transmission by calcium and axonal N-, Q-, T- and L-type voltage-gated calcium channels differs between striatal domains. J. Physiol 593, 929–946 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Patriarchi T et al. Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science 360, eaat4422 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang L et al. Temporal components of cholinergic terminal to dopaminergic terminal transmission in dorsal striatum slices of mice. J. Physiol 592, 3559–3576 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shin JH, Adrover MF & Alvarez VA Distinctive Modulation of Dopamine Release in the Nucleus Accumbens Shell Mediated by Dopamine and Acetylcholine Receptors. J. Neurosci 37, 11166–11180 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chergui K, Suaud-Chagny MF & Gonon F Nonlinear relationship between impulse flow, dopamine release and dopamine elimination in the rat brainin vivo. Neuroscience 62, 641–645 (1994). [DOI] [PubMed] [Google Scholar]
- 83.Sun F et al. A Genetically Encoded Fluorescent Sensor Enables Rapid and Specific Detection of Dopamine in Flies, Fish, and Mice. Cell 174, 481–496.e19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou F-M, Liang Y & Dani J a. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat. Neurosci 4, 1224–1229 (2001). [DOI] [PubMed] [Google Scholar]
- 85.Soliakov L & Wonnacott S Voltage-sensitive Ca2+ channels involved in nicotinic receptor-mediated [3H]dopamine release from rat striatal synaptosomes. J. Neurochem 67, 163–70 (1996). [DOI] [PubMed] [Google Scholar]
- 86.Threlfell S et al. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron 75, 58–64 (2012). [DOI] [PubMed] [Google Scholar]
- 87.Giorguieff MF, Le Floc’h ML, Westfall TC, Glowinski J & Besson MJ Nicotinic effect of acetylcholine on the release of newly synthesized [3H]dopamine in rat striatal slices and cat caudate nucleus. Brain Res 106, 117–131 (1976). [DOI] [PubMed] [Google Scholar]
- 88.Ford CP, Gantz SC, Phillips PEM & Williams JT Control of extracellular dopamine at dendrite and axon terminals. J. Neurosci 30, 6975–6983 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takahashi T & Momiyama A Different types of calcium channels mediate central synaptic transmission. Nature 366, 156–158 (1993). [DOI] [PubMed] [Google Scholar]
- 90.Held RG et al. Synapse and Active Zone Assembly in the Absence of Presynaptic Ca2+ Channels and Ca2+ Entry. Neuron 107, 667–683.e9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bergquist F, Niazi HS & Nissbrandt H Evidence for different exocytosis pathways in dendritic and terminal dopamine release in vivo. Brain Res 950, 245–253 (2002). [DOI] [PubMed] [Google Scholar]
- 92.Fortin GD, Desrosiers CC, Yamaguchi N & Trudeau LE Basal somatodendritic dopamine release requires snare proteins. J. Neurochem 96, 1740–1749 (2006). [DOI] [PubMed] [Google Scholar]
- 93.Abercrombie ED, DeBoer P & Heeringa MJ Biochemistry of Somatodendritic Dopamine Release in Substantia Nigra: An in Vivo Comparison with Striatal Dopamine Release. Adv. Pharmacol 42, 133–136 (1997). [DOI] [PubMed] [Google Scholar]
- 94.Jaffe EH, Marty A, Schulte A & Chow RH Extrasynaptic Vesicular Transmitter Release from the Somata of Substantia Nigra Neurons in Rat Midbrain Slices. J. Neurosci 18, 3548–3553 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beckstead MJ, Grandy DK, Wickman K & Williams JT Vesicular Dopamine Release Elicits an Inhibitory Postsynaptic Current in Midbrain Dopamine Neurons. Neuron 42, 939–946 (2004). [DOI] [PubMed] [Google Scholar]
- 96.Grace AA & Bunney BS The control of firing pattern in nigral dopamine neurons: single spike firing. J. Neurosci 4, 2866–2876 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grace AA & Bunney BS The Control of Firing Pattern in nigral dopamine Neurons: Burst Firing. J. Neurosci 4, 2877–2890 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chan CS et al. ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature 447, 1081–1086 (2007). [DOI] [PubMed] [Google Scholar]
- 99.Khaliq ZM & Bean BP Pacemaking in Dopaminergic Ventral Tegmental Area Neurons: Depolarizing Drive from Background and Voltage-Dependent Sodium Conductances. J. Neurosci 30, 7401–7413 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zweifel LS et al. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc. Natl. Acad. Sci 106, 7281–7288 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Floresco SB, West AR, Ash B, Moore H & Grace AA Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat. Neurosci 6, 968–973 (2003). [DOI] [PubMed] [Google Scholar]
- 102.Venton BJ et al. Real-time decoding of dopamine concentration changes in the caudate-putamen during tonic and phasic firing. J. Neurochem 87, 1284–1295 (2003). [DOI] [PubMed] [Google Scholar]
- 103.Parker JG et al. Absence of NMDA receptors in dopamine neurons attenuates dopamine release but not conditioned approach during Pavlovian conditioning. Proc. Natl. Acad. Sci 107, 13491–13496 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gao M et al. Functional Coupling between the Prefrontal Cortex and Dopamine Neurons in the Ventral Tegmental Area. J. Neurosci 27, 5414–5421 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gonon F Prolonged and Extrasynaptic Excitatory Action of Dopamine Mediated by D1 Receptors in the Rat Striatum In Vivo. J. Neurosci 17, 5972–5978 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gonon FG Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry. Neuroscience 24, 19–28 (1988). [DOI] [PubMed] [Google Scholar]
- 107.Brimblecombe KR & Cragg SJ The Striosome and Matrix Compartments of the Striatum: A Path through the Labyrinth from Neurochemistry toward Function. ACS Chem. Neurosci 8, 235–242 (2017). [DOI] [PubMed] [Google Scholar]
- 108.Beaulieu J-M & Gainetdinov RR The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev 63, 182–217 (2011). [DOI] [PubMed] [Google Scholar]
- 109.Neve KA, Seamans JK & Trantham-Davidson H Dopamine Receptor Signaling. J. Recept. Signal Transduct 24, 165–205 (2004). [DOI] [PubMed] [Google Scholar]
- 110.Gantz SC, Ford CP, Morikawa H & Williams JT The Evolving Understanding of Dopamine Neurons in the Substantia Nigra and Ventral Tegmental Area. Annu. Rev. Physiol 80, 219–241 (2018). [DOI] [PubMed] [Google Scholar]
- 111.Callier S et al. Evolution and cell biology of dopamine receptors in vertebrates. Biol Cell. 95, 489–502 (2003). [DOI] [PubMed] [Google Scholar]
- 112.Richfield EK, Penney JB & Young AB Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. Neuroscience 30, 767–777 (1989). [DOI] [PubMed] [Google Scholar]
- 113.Hunger L, Kumar A & Schmidt R Abundance Compensates Kinetics: Similar Effect of Dopamine Signals on D1 and D2 Receptor Populations. J. Neurosci 40, 2868–2881 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gerfen CR et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250, 1429–32 (1990). [DOI] [PubMed] [Google Scholar]
- 115.Gerfen CR & Surmeier DJ Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci 34, 441–66 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.DeLong MR Primate models of movement disorders of basal ganglia origin. Trends Neurosci 13, 281–285 (1990). [DOI] [PubMed] [Google Scholar]
- 117.Gong S et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425, 917–925 (2003). [DOI] [PubMed] [Google Scholar]
- 118.Wall NR, De La Parra M, Callaway EM & Kreitzer AC Differential Innervation of Direct- and Indirect-Pathway Striatal Projection Neurons. Neuron 79, 347–360 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hikida T, Kimura K, Wada N, Funabiki K & Nakanishi S Distinct Roles of Synaptic Transmission in Direct and Indirect Striatal Pathways to Reward and Aversive Behavior. Neuron 66, 896–907 (2010). [DOI] [PubMed] [Google Scholar]
- 120.Kravitz AV, Tye LD & Kreitzer AC Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat. Neurosci 15, 816–818 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kravitz AV et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466, 622–626 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Caillé I, Dumartin B & Bloch B Ultrastructural localization of D1 dopamine receptor immunoreactivity in rat striatonigral neurons and its relation with dopaminergic innervation. Brain Res. 730, 17–31 (1996). [DOI] [PubMed] [Google Scholar]
- 123.Cragg SJ & Rice ME DAncing past the DAT at a DA synapse. Trends in Neurosciences 27, 270–277 (2004). [DOI] [PubMed] [Google Scholar]
- 124.Chefer VI, Thompson AC, Zapata A & Shippenberg TS Overview of Brain Microdialysis. Curr. Protoc. Neurosci 47, 7.1.1–7.1.28 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Goto Y & Grace AA Limbic and cortical information processing in the nucleus accumbens. Trends Neurosci 31, 552–558 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Skinbjerg M, Sibley DR, Javitch JA & Abi-Dargham A Imaging the high-affinity state of the dopamine D2 receptor in vivo: Fact or fiction? Biochem. Pharmacol 83, 193–198 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bowery B, Rothwell LA & Seabrook GR Comparison between the pharmacology of dopamine receptors mediating the inhibition of cell firing in rat brain slices through the substantia nigra pars compacta and ventral tegmental area. Br. J. Pharmacol 112, 873–880 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yapo C et al. Detection of phasic dopamine by D1 and D2 striatal medium spiny neurons. J. Physiol 595, 7451–7475 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nair AG, Gutierrez-Arenas O, Eriksson O, Vincent P & Hellgren Kotaleski J Sensing Positive versus Negative Reward Signals through Adenylyl Cyclase-Coupled GPCRs in Direct and Indirect Pathway Striatal Medium Spiny Neurons. J. Neurosci 35, 14017–14030 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dreyer JK, Herrik KF, Berg RW & Hounsgaard JD Influence of Phasic and Tonic Dopamine Release on Receptor Activation. J. Neurosci 30, 14273–14283 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sano K, Noshiro O, Katsuda K, Nishikori K & Maeno H Dopamine receptors and dopamine-sensitive adenylate cyclase in canine caudate nucleus. Biochem. Pharmacol 28, 3617–3627 (1979). [DOI] [PubMed] [Google Scholar]
- 132.Klein Herenbrink C et al. The role of kinetic context in apparent biased agonism at GPCRs. Nat. Commun 7, 10842 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Garris P, Ciolkowski E, Pastore P & Wightman R Efflux of dopamine from the synaptic cleft in the nucleus accumbens of the rat brain. J. Neurosci 14, 6084–6093 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Robinson BG et al. Desensitized D2 autoreceptors are resistant to trafficking. Sci. Rep 7, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ford CP, Phillips PE & Williams JT The time course of dopamine transmission in the ventral tegmental area. J. Neurosci 29, 13344–13352 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Courtney NA & Ford CP The Timing of Dopamine- and Noradrenaline-Mediated Transmission Reflects Underlying Differences in the Extent of Spillover and Pooling. J. Neurosci 34, 7645–7656 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Arbuthnott GW & Wickens J Space, time and dopamine. Trends Neurosci. 30, 62–69 (2007). [DOI] [PubMed] [Google Scholar]
- 138.Pothos EN, Davila V & Sulzer D Presynaptic recording of quanta from midbrain dopamine neurons and modulation of the quantal size. J. Neurosci 18, 4106–4118 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Eshel N, Tian J, Bukwich M & Uchida N Dopamine neurons share common response function for reward prediction error. Nat. Neurosci 19, 479–486 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Freeze BS, Kravitz AV, Hammack N, Berke JD & Kreitzer AC Control of Basal Ganglia Output by Direct and Indirect Pathway Projection Neurons. J. Neurosci 33, 18531–18539 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cui G et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature 494, 238–242 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schultz W Multiple Dopamine Functions at Different Time Courses. Annu. Rev. Neurosci 30, 259–288 (2007). [DOI] [PubMed] [Google Scholar]
- 143.Mamaligas AA & Ford CP Spontaneous Synaptic Activation of Muscarinic Receptors by Striatal Cholinergic Neuron Firing. Neuron 91, 574–586 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Saka SK et al. Immuno-SABER enables highly multiplexed and amplified protein imaging in tissues. Nat. Biotechnol (2019). doi: 10.1038/s41587-019-0207-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gao R et al. Cortical column and whole-brain imaging with molecular contrast and nanoscale resolution. Science 363, eaau8302 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dai M & Tepper JM Do silent dopaminergic neurons exist in rat substantia nigra in vivo? Neuroscience 85, 1089–1099 (1998). [DOI] [PubMed] [Google Scholar]
- 147.Robinson DL, Venton BJ, Heien MLAV & Wightman RM Detecting Subsecond Dopamine Release with Fast-Scan Cyclic Voltammetry in Vivo. Clin. Chem 49, 1763–1773 (2003). [DOI] [PubMed] [Google Scholar]
- 148.Kruss S et al. High-resolution imaging of cellular dopamine efflux using a fluorescent nanosensor array. Proc. Natl. Acad. Sci 114, 1789–1794 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gubernator NG et al. Fluorescent false neurotransmitters visualize dopamine release from individual presynaptic terminals. Science 324, 1441–1444 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]


