Abstract
Background
the solitary plasmacytomas entities characterized by the neoplastic proliferation of a single clone of plasma cells, typically producing a monoclonal immunoglobulin. It represents less than 5% of plasma cell dyscrasias. The most common sites of solitary plasmacytomas are long bones. The jaws location remains extremely rare, only 4.4% of solitary plasmacytomas of bone occur in the mandible, the diagnosis is based on the biopsy evidence of plasma cell proliferation and absence of evidence of involvement of other bones.
Case presentation
The authors report the case of a healthy 49-year-old man with no general history, presented with a painless slow-growing lesion of the left jaw that had persisted and increased in size for one month. Clinical examination revealed a large lesion 4 × 4 cm with irregular borders of the retro-molar area on the left jaw, infiltrated into underlying tissue, with sensory disturbances and facial asymmetry.
Conclusion
Treatment methods of plasmacytomas of the jaw include local surgery (curettage of the lesion), local irradiation, systemic chemotherapy, or a combining therapy. Early diagnosis and treatment are crucial for better resolution of the disease.
Keywords: Solitary Plasmacytoma, Mandible, Diagnosis, Management
Highlights
-
•
The solitary plasmacytoma of the jaw is rare.
-
•
The clinical behavior is very diverse.
-
•
The main treatment is surgical excision associated with local irradiation or systemic chemotherapy.
-
•
Follow-up is necessary to reveal multiple myeloma transformation.
1. Introduction
Solitary plasmacytomas represent less than 5% of plasma cell dyscrasias and are further subclassified as solitary bone plasmacytoma (SBP) or solitary extramedullary plasmacytoma (SEP) depending on whether the site of involvement in the bone versus soft tissue [3]. Plasma cell dyscrasias are a group of entities characterized by the neoplastic proliferation of a single clone of plasma cells, typically producing a monoclonal immunoglobulin [2]. In solitary plasmacytoma, it is important to make the distinction of whether or not minimal bone marrow clonal plasmacytosis is present: bone marrow plasma cells absent, single lesion of bone or soft tissue, negative whole-body imaging for additional lesions, absence of other organ damage: hypercalcemia, renal dysfunction, anemia [3], 50% of the cases transforming into multiple myeloma, SPB primarily affects the axial skeleton, especially vertebrae and secondarily long bones. However, jaw involvement is very rare, and when it does, it shows a predilection for mandible than maxilla [4]. Most of the cases reported in the literature are in the bone marrow-rich areas of the mandible that is in the body, angle, ramus, and also retromolar trigone [5], The authors report the case of a patient with solitary plasmacytoma of the mandible.
2. Case presentation
This work has been reported in line with the SCARE 2020 criteria [1].
A -67-year-old man patient with no general history, presented with painful swelling and fast-growing lesion over the left mandibular region, the symptoms were evolving for 1 month. The dental history revealed an extraction of 38 one month ago. There was no history of local trauma, radiotherapy, HPV infections, or family history of malignancy. He was referred to our department's consultation for specialized care.
Extra-oral examination revealed a facial asymmetry on the left side of the face. On inspection, swelling over the inferior-third region of the face was noticed [Fig. 1]. On palpation, the swelling was soft to firm in consistency, painful, with paresthesia of the territory of the mandibular branch of the trigeminal nerve, without facial paralysis. There were no palpable neck lymph nodes on the left side.
Fig. 1.

Extraoral examination revealing facial asymmetry on the left side of the face.
Intraoral examination [Fig. 2] revealed a well-defined flesh-colored swelling in the left molar and retromolar regions. The lesion was extending posteriorly from the distal aspect of 35 to the retromolar pad, measuring approximately 3 cm × 3 cm. On palpation, the mass was soft to firm in consistency with surface and well-defined margins, not bleeding on contact, without any disorder of the dental articulation or limitation of the mouth opening.
Fig. 2.
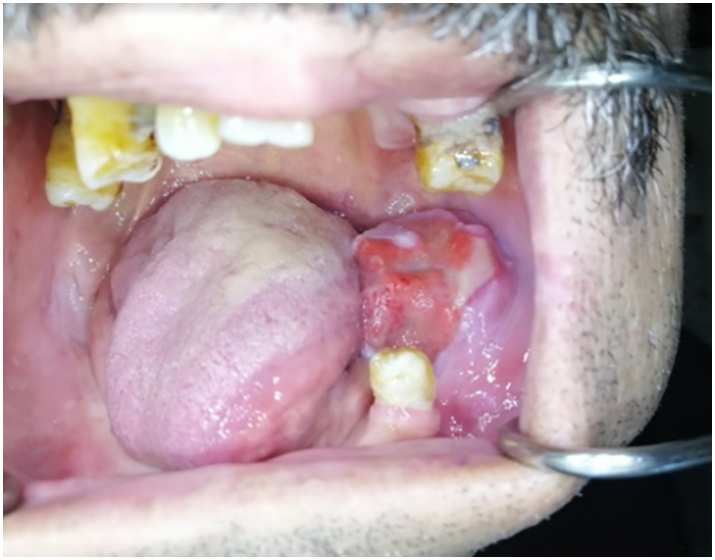
Intraoral examination revealing a well-defined solitary erythematous growth in the left retromolar region.
Orthopantamography [Fig. 3] revealed a poorly defined, multi-loculated, radiolucent lesion on the left side of the mandible, extending posteriorly from 34 to the mandibular angle. A reconstructed CT scan revealed a large expansive lytic lesion with irregular margins in the posterior region of the left mandible [Fig. 4A] with rupture of both cortices and without extension to the soft tissue [Fig. 4B]. The patient had previously undergone a biopsy which concluded to a fibroma.
Fig. 3.
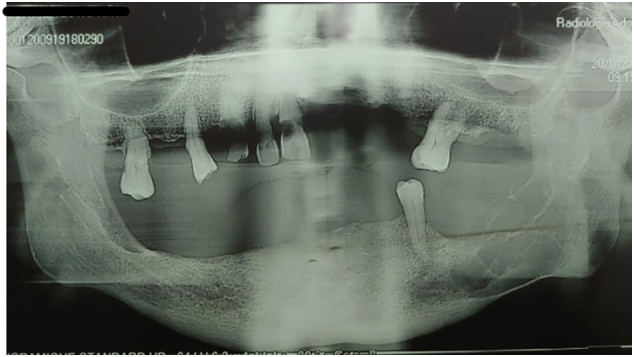
Orthopantamograph revealing a multilocular lesion in the left side of the mandible.
Fig. 4.
A: Reconstructed computed tomography scan revealing large expansile lytic on the left mandible region
B: osteolytic lesion with cortical rupture.
Based on the above clinical and radiological findings, a tentative diagnosis of intraosseous carcinoma of the left retromolar area was made. However, central giant cell lesions and osteosarcoma were considered in the differential diagnosis.
The patient has received under general anesthesia; the intra-oral approach an enucleation of the lesion and, the specimen was sent for histopathologic examination.
Duration of surgery: 40 min; estimation of blood loss: 50 ml; duration of hospital stay: 2 days.
The patient received amoxicillin/clavulanic acid 1 g twice daily and antalgics for 8 days.
Histopathology revealed plasmacytoid mononuclear cells in contact with amyloid material [Fig. 5] with an exclusive expression of anti-Lambda light chain antibody (polyclonal antibody) proving monotypy [Fig. 6A], and absence of expression of anti-Kappa light chain antibody (polyclonal antibody) [Fig. 6B]. Based on the above findings, a histopathologic diagnosis of plasmacytoid non-Hodgkin's lymphoma was made. A differential diagnosis of plasmacytoma and immunoblastic large cell lymphoma was considered.
Fig. 5.

Photomicrograph showing sheets of plasmacytoid mononuclear cells in contact with amyloid material.
Fig. 6.

Immunohistochemistry showing:
A: Exclusive expression of anti-Lambda light chain antibody (polyclonal antibody) proving monotypy,
B: Absence of expression of anti-Kappa light chain antibody (polyclonal antibody).
To confirm the diagnosis, routine blood investigations were within normal limits with no evidence of hypercalcemia and urinary Bence- Jones proteins. Complete body skeletal survey showed no other bony lesions. Based on laboratory findings, the final diagnosis of solitary plasmacytoma of the mandible was given. However, the presence of another bone lesion, Bence-Jones proteins, hypercalcemia, or a peak in the gamma-globulin area on protein electrophoresis will tip the diagnosis toward myeloma.
The definitive diagnosis of malignancy was made; metastatic investigations were performed, including chest radiography, abdominal echography, PET scan. The results of these exams were normal.
The multidisciplinary team deciding on the treatment options included surgeons, oncologists, radiotherapists, radiologists. The decision was made to treat the patient with the surgical approach with postoperative radiotherapy (PORT). Considering the patient's age, the size of the tumor, the prognosis was average.
Postoperative periods were favorable with the disappearance of swelling and advised oral rehabilitation. The patient was referred to the oncology center for postoperative radiotherapy. A follow-up for five years is planned every month for the first year, then every three months for the second year, and every six months. This follow-up will be done at the consultation by a clinical examination with, if necessary, blood and a radiological assessment.
3. Discussion
Plasmacytoma is a lymphoid neoplastic proliferation of B cells which may occur alone in the bone as solitary bone plasmacytoma (SPB), in soft tissue as extramedullary plasmacytoma (EMP) or a multifocal disseminated form as multiple myeloma (MM) [6]. Solitary plasmacytomas represent less than 5% of plasma cell dyscrasias [3]. The most common sites of SPB are long bones, and vertebrae. It rarely involves jaws and when it is seen, only 4.4% of SPB occur in the mandible, most commonly in the bone marrow-rich areas of the body, angulus, and ramus mandible [7]. The etiology of SPB remains uncertain, but several hypotheses were proposed which implicated the role of radiation, chemical exposure, viruses, and genetic factors. Cytogenetic studies revealed a loss in chromosome 13, 1p, 14q and gain in 19p, 9q, 1q, and interleukin-6 is considered as a principal growth factor in pathogenesis [4].
SBP occurs most frequently in patients between the ages of 50 and 80 years with a mean age of occurrence of about 60 years. It is rare before the age of 40 [8]. It is more prevalent in men with the ratio being 2:1 [6].
The clinical presentation of solitary plasmacytoma of the mandible (SPM) is not specific, facial swelling, tooth mobility, and sensory disturbances are the most common clinical signs. Rarely, solitary plasmacytoma of the mandible may be discovered in pathologic fracture, post-extraction bleeding, or dental joint disorder for condylar locations.[9]. Our case was in harmony with the literature since the main complaint was swelling and pain, but paresthesia was not reported.
The diagnosis of the SBP depends on the biopsy evidence of plasma cell proliferation and the absence of evidence of involvement of other bones [10]. For a diagnosis of plasmacytoma to be made, there must be an absence of others organ damage including hypercalcemia, renal dysfunction, and anemia [3]. Immunohistochemistry staining should be performed to ascertain the presence of a monoclonal plasma cell population. The panoramic radiography aspect is that of an osteolytic image which can be mono- or multilocular [11], without any sign of bone reaction [12]. The CT scan allows to refine the radiological image and to study the extension toward the cortical and soft parts. MRI remains an interesting imaging modality to detect plasmacytomas involving the spine, to discern soft tissue involvement and bone marrow infiltration, and to evaluate disease throughout the body. Solitary plasmacytoma has low signal intensity on T1-weighted imaging and high signal intensity on T2-weighted imaging. There is homogeneous enhancement with gadolinium contrast [3].
These radio clinical aspects of solitary plasmacytoma of the mandible are variable and pose the problem of differential diagnosis with cystic and pseudocysts tumors as well as metastases of malignant tumors [13].
In our case, there were signs of periosteal reaction with cortical destruction and can spread widely to the surrounding tissues.
Microscopically, the plasma cells show varying degrees of differentiation with sparse stroma. Plasmacytomas can be graded as lesions of minimal dysplasia and severe dysplasia [6]. A nucleus can be binucleated. The spherical nuclei are set eccentrically and show regular or irregular margination of chromatin often showing a cart-wheel pattern[14]. Sometimes the chromatin may be coarsely clumped showing a clock-face pattern. The cells show para nuclear globular, pale-staining, cytoplasmic space called hof 7. Pseudo-angiomatous areas, giant cell formation, amyloid deposition, and myxoid change can be seen in some cases. The plasma cells may contain intracytoplasmic (Russell body) & nuclear inclusions called Dutcher bodies. The immature plasma cells have larger or more irregular nuclei, less condensed chromatin, and occasional nuclei. The immature cells are larger and more irregular with ample slightly basophilic cytoplasm [10].
The current criteria to make a diagnosis of solitary plasmacytoma are both confirmatory histopathological and immunohistochemical (IHC) analysis with definite support of systemic investigation: Isolated area of bone destruction due to clonal plasma cells, bone marrow plasma cell infiltration not exceeding 5% of all nucleated cells, absence of further osteolytic bone lesions or other systemic plasmacytoma; absence of anemia, hypercalcemia, or renal impairment attributable to myeloma; low concentrations of serum or urine monoclonal protein; or preserved levels of immunoglobulins [8].
Single cases of plasmacytoma may be misclassified as benign lesions such as plasmacytosis or gingival plasmacytosis which can clinically excluded based on tumor size, bone involvement, evolution, and most importantly, histology which is conclusive.
A plasmacytosis is plasma cell granuloma which is polyclonal and not neoplastic. In contrast, a plasmocytoma is monoclonal and has a single kappa or lambda light chain.
There have been no therapeutic advances in the treatment of solitary plasmacytoma over the years, Treatment methods include local surgery (curettage of the lesion), local irradiation, systemic chemotherapy, or a combination of these methods. Radiation is the mainstay of therapy for plasmacytoma, which is highly radiosensitive. Local control rates can be achieved in 80–90% of cases with radiation therapy alone [3]. Surgical resection of plasmacytoma is usually not required as these malignancies are radiosensitive, for the most part, surgical excision is reserved for cases where there is loss of anatomic structural integrity or emergent decompression [15]. In many instances, patients have had surgery, with complete or partial tumor removal, as part of the diagnostic procedure. If surgery is performed, it is usually done before radiation therapy and as an adjunct to definitive radiation, and radiotherapy can be delayed but is still necessary because excision of the tumor without subsequent radiotherapy results in a very high rate of local recurrence [16]. The role of adjuvant chemotherapy after radiation therapy in the treatment of SPB remains controversial, according to the latest recommendation Adjuvant chemotherapy may be considered for patients with persistent disease after initial radiation therapy [15]. Our patient had an enucleation of the lesion followed by radiotherapy.
Despite excellent local control rates, the majority of patients with solitary plasmacytoma eventually progress to multiple myeloma. Studies have shown a 5-year overall survival rate of 70% and a 5-year disease-free survival rate of 46%, with the median time to development of multiple myeloma being 21 months, with a 5-year probability of 51% [16]. because of that, patients after definitive radiation, it is important to first assess for a complete response and follow them to detect possible recurrence, this is done with laboratory evaluation that would include a complete blood count, complete metabolic panel, LDH, beta-2-microglobulin, urine protein electrophoresis, serum protein electrophoresis, free light chains, and serum quantitative immunoglobulins. Laboratory evaluation should be performed roughly every 3 months for the first 2 years following radiation treatment and then every 6 months [3].
4. Conclusion
Mandibular plasmacytoma is a rare entity, its diagnosis requires histological and immunohistochemical evidence, and proving the solitary character, once the diagnosis is made, the management must be rapid, with careful monitoring, because SBP, despite its localized form, the literature indicates that they have a higher propensity to transform into multiple myeloma.
Funding
The authors declared that this study has received no financial support.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Registration of research studies
-
1.
Name of the registry: 6819
-
2.
Unique identifying number or registration ID: not applicable
-
3.
Hyperlink to your specific registration (must be publicly accessible and will be checked):
Guarantor
Iro salissou
CRediT authorship contribution statement
Iro salissou: Corresponding author writing the paper
Ouassime kerdoud: writing the paper
Ezzahra HMOURA: writing the paper
Dounia Sarfi: writing the paper
Meriem Regragui: Correction of the paper
Faiçal Slimani: Correction of the paper
Declaration of competing interest
Authors of this article have no conflict or competing interests. All of the authors approved the final version of the manuscript.
References
- 1.R. A. Agha, T. Franchi, C. Sohrabi, G. Mathew, A. Kerwan, and SCARE Group, “The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines,” Int. J. Surg. Lond. Engl., vol. 84, pp. 226–230, Dec. 2020, doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed]
- 2.Ng C.S.H., Lau K.K.W. Solitary bone plasmacytoma. Can. Respir. J. 2013;20(1):11. doi: 10.1155/2013/469876. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pham A., Mahindra A. Solitary plasmacytoma: a review of diagnosis and management. Curr. Hematol. Malig. Rep. 2019;14(2):63–69. doi: 10.1007/s11899-019-00499-8. Apr. [DOI] [PubMed] [Google Scholar]
- 4.S. Chittemsetti, V. R. Guttikonda, T. Sravya, and P. K. Manchikatla, “Solitary plasmacytoma of mandible: a rare entity,” J. Oral Maxillofac. Pathol. JOMFP, vol. 23, no. 1, pp. 136–139, Apr. 2019, doi: 10.4103/jomfp.JOMFP_175_18. [DOI] [PMC free article] [PubMed]
- 5.Singh: Solitary Plasmacytoma of Mandible: A rare... - Google Scholar. https://scholar.google.com/scholar_lookup?journal=Int+J+Med+Dent+Sci&title=Solitary+plasmacytoma+of+mandible:+A+rare+case+report&author=A+Singh&author=V+Singh&author=N+Sharma&volume=1&publication_year=2012&pages=28-32&
- 6.E. M. Canger, P. Çelenk, A. Alkan, and Ö. Günhan, “Mandibular involvement of solitary plasmocytoma: a case report,” Med. Oral Patol. Oral Cir. Bucal., p. 3. [PubMed]
- 7.Seoane J., Aguirre-Urizar J.M., Esparza-Gómez G., Suárez-Cunqueiro M., Campos-Trapero J., Pomareda M. The spectrum of plasma cell neoplasia in oral pathology. Med. Oral Organo Of. Soc. Espanola Med. Oral Acad. Iberoam. Patol. Med. Bucal. Oct. 2003;8(4):269–280. [PubMed] [Google Scholar]
- 8.Pisano J.J., Coupland R., Chen S.Y., Miller A.S. Plasmacytoma of the oral cavity and jaws: a clinicopathologic study of 13 cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. Feb. 1997;83(2):265–271. doi: 10.1016/s1079-2104(97)90015-9. [DOI] [PubMed] [Google Scholar]
- 9.“Florencio M, Jose L, Gil-Diez, Campano F.J, Jose R, Del Hoyo A. Mandibular lesion as the first evidence of Multiple Myeloma. J. Craniomaxillofac. Surg. 1989;17:315-317”. [DOI] [PubMed]
- 10.Rao K., N. S. P. IJCDS; Jan. 2011. Solitary bone plasmacytoma of the maxilla-a rare case report. [Google Scholar]
- 11.“Collangettes-Peyrat D, Baudet-Pommel M, Fonck Y, Meyniel P. Plasmocytome solitaire de la mandibule: étude d'un cas. Actual Odonto Stomatol 1990;169:175-183”. [PubMed]
- 12.Sham E., Leong J., Maher R., Schenberg M., Leung M., Mansour A.K. Mandibular ameloblastoma: clinical experience and literature review. ANZ J. Surg. 2009;79(10):739–744. doi: 10.1111/j.1445-2197.2009.05061.x. [DOI] [PubMed] [Google Scholar]
- 13.Z. Bellalah, A. Slama, S. Ayachi, S. Rammeh, K. Mrad-Daly, and H. Khochtali, “Plasmocytome solitaire de la mandibule. À propos de deux cas,” Actual. Odonto-Stomatol., no. 250, pp. 145–151, Jun. 2010, doi: 10.1051/aos/2010205.
- 14.“Caterian Valetti, Carlo E Grossi, Cesar Milstein, Roberto Sitia. Russell bodies: a general response of secretory cells to synthesis of a mutant immunoglobulin which can neither exit from, nor be degraded in, the endoplasmic reticulum. J. Cell Biol. 1991; 115(4): 1983-994”. [DOI] [PMC free article] [PubMed]
- 15.J. Caers et al., “Diagnosis, treatment, and response assessment in solitary plasmacytoma: updated recommendations from a European Expert Panel,” J. Hematol. Oncol.J Hematol Oncol, vol. 11, no. 1, p. 10, Jan. 2018, doi: 10.1186/s13045-017-0549-1. [DOI] [PMC free article] [PubMed]
- 16.“Knobel D, Zouhair A, Tsang RW, Poortmans P, Belkacemi Y, Bolla M, Oner FD, Landmann C, Castelain B, Ozsahin M. Prognostic factors in solitary plasmacytoma of the bone: a multicenter Rare Cancer Network study. BMC Cancer. 2006;6:118”. [DOI] [PMC free article] [PubMed]



