Abstract
Human Granulocytic Anaplasmosis (HGA) is an acute febrile tick-borne illness caused by the organism Anaplasma phagocytophilum. Patients often present with fever and a flu-like symptoms following a tick bite. In this case, the patient presented with subacute abdominal pain and severe hyponatremia consistent with SIADH. The patient was started on appropriate empiric antibiotics given the patient’s tick exposure. Blood smear confirmed findings consistent with HGA and the patient continued antibiotic treatment with resolution of his symptoms. This case is unique in that the patient presented with severe hyponatremia that improved with treatment of the HGA. He also had subacute abdominal pain which is also a rare presentation of HGA. Our hope is that our case highlights the value of empiric treatment with appropriate monitoring to prevent downstream, severe sequelae from undiagnosed HGA. In the setting of climate change, increased duration of Ixodes spp. tick life cycles with emerging regional distribution of the ticks, coinfections with Borrelia burgdorferi and increased incidence of HGA in the last two decades, it is important to recognize this entity.
Keywords: Anaplasmosis, Hyponatremia, Abdominal pain, tick-borne, HGA
Introduction
Human Granulocytic Anaplasmosis (HGA) is an acute febrile tick-borne illness caused by obligate intracellular Gram negative bacteria Anaplasma phagocytophilum (A. phagocytophilum) [1]. Infections occur commonly in the spring and summer months [2]. Patients will report exposure to a tick about 1-2 weeks prior to presentation. Patients often present with flu-like symptoms, including fever, malaise, myalgias, and headache following a tick bite by Ixodes spp. Initial lab work will often show leukopenia, thrombocytopenia, and/or elevated transaminases [3]. It is rare for patients with HGA to present with subacute abdominal pain and confusion as their chief concern [3]. Since 2008, HGA has become a reportable disease with the Center for Disease Control (CDC) tracking the number of cases each year. In 2017, the number of cases peaked at 5,762 in the United States [4]. Recognition of HGA is imperative, as ticks are now living longer with an increased ability to spread regionally and resultant increased rates of tick-borne illnesses in humans [5,6]. In this case we discuss HGA presenting as subacute abdominal pain, confusion, and hyponatremia in a patient following recent tick exposure.
Case Presentation
A 71-year-old vegetarian male naïve to medical care presented with the chief complaint of abdominal pain and confusion. The left lower quadrant and periumbilical abdominal pain started after he attended an outdoor party 4 days prior to presentation, where he consumed grilled squash and cold potato salad. He did not experience any nausea, emesis, or diarrhea, hematochezia, or melena. Further, he reported his bowel movements remained normal. The patient had also noticed that he had been bruising easily recently, with several areas of bruising on his arms. He attributed his new bruising to the use of his garlic supplement that he had been taking. The patient denied new animal exposures but did note that he recently had a tick exposure about 2 weeks prior to admission where he found a tick on both he and his wife. He was unclear as to whether or not they had been bitten by the ticks. He also expressed that approximately one year prior to presentation he was bitten by a tick and later had a bullseye like rash on his arm. The rash eventually went away after he rubbed a garlic compound on the rash. Of note, the patient frequently travelled to the woods in Michigan and Florida, where he hunted and consumed wild morel and oyster mushrooms.
At the time of admission, his vital signs were significant for a maximum temperature of 39.4 degrees Celsius, blood pressure of 151/79 mmHg, and heart rate of 106 beats/min, but otherwise stable. His eyes had no icterus. His mouth showed poor dentition, with a left buccal mucosa with nonbleeding ulcer ∼5 mm. His neck had no lymphadenopathy. His cardiovascular exam was remarkable for tachycardia, but no murmur or gallops. His abdomen was soft, non-tender, non-distended, with no hepatosplenomegaly. His neurologic exam found the patient to be alert, oriented to name, era only (not oriented to date or reason for hospitalization), but was otherwise negative for sensorimotor or cerebellar abnormalities. His skin was remarkable for purpura on his left upper extremity, but no petechiae, and no lesions on palms or soles or trunk. All other portions of the patient’s physical exam were unremarkable.
A complete blood count with differential was notable for a white blood cell count (WBC) of 2.5 × 10*9/L (4.5-11.0 × 10*9/L and platelets of 22 × 10*9/L (150-400 × 10*9/L). A complete metabolic panel on presentation was notable for a sodium of 117 mmol/L (135-145 mmol/L), a chloride of 86 mmol/L (101-111 mmol/L), alkaline phosphatase 40 U/L (45-115 U/L), aspartate aminotransferase 158 U/L (13-36 U/L), and alanine transaminase at 36 U/L (6-40 U/L). A disseminated intravascular coagulation (DIC) panel was normal with the exception of an elevated D-dimer of 56.72 ug/mL (<0.5 ug/mL). Lactic acid was elevated at 3.5 mmol/L (0.7-2.5 mmol/L). As part of the workup for the patient’s hyponatremia, after being given 1 liter of 0.9% normal saline, a serum osmolality, urine osmolality, and urine sodium were remarkable for 267 mosm/kg (275-295 mosm/kg), 229 mosm/kg (300-1,100 mosm/kg), and 42 mmol/L respectively.
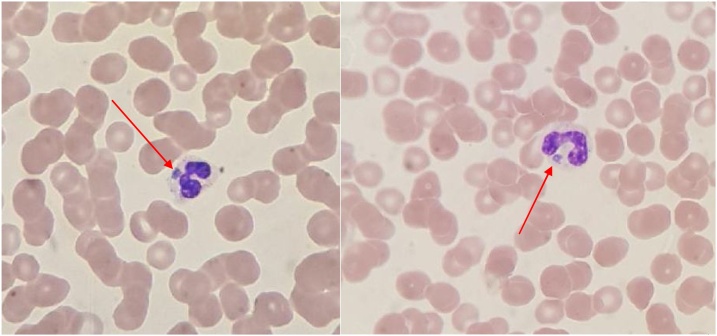
The patient underwent a computed tomography of his abdomen which showed no acute pathology or abscesses. Due to the strong suspicion of an acute infection due to his recent tick exposure, the patient was started on broad-spectrum antibiotics with vancomycin, ceftriaxone, and doxycycline. On presentation, a tick-borne PCR panel including testing for ehrlichiosis, anaplasmosis, Lyme disease, and babesia was drawn and was sent to an outside lab. In the interim, further infectious and autoimmune disease workup was negative. Infectious diseases was consulted, at which time vancomycin and ceftriaxone were discontinued, but doxycycline was continued given the ongoing concern for a tick-borne illness. His blood smear showed intracytoplasmic granular basophilic inclusions suspicious for morulae consistent with Anaplasma or Ehrlichia microcolonies (see Fig. 1). Eventually, the PCR panel was positive for A. phagocytophilum. The patient was discharged with oral doxycycline to complete a 2-week course of treatment. He responded well to treatment and was seen at outpatient follow up 5 days later where he was no longer experiencing fever, confusion, or abdominal pain. His lab work at that time also showed improvement of his hyponatremia to 136 mmol/L, elevated transaminases to alanine transferase 17 U/L and aspartate aminotransferase 21 U/L. His leukopenia improved to WBC 4.71 × 10*9 U/L, and thrombocytopenia to 301 × 10*9 U/L.
Fig. 1.
images from the patient’s peripheral smear from the day of admission, prior to initiation of antibiotics, showing morulae (intracytoplasmic basophilic inclusions) within the neutrophils.
Discussion
Both human anaplasmosis and ehrlichiosis are acute tick-borne illnesses. While human granulocytic anaplasmosis (HGA) is caused by Anaplasma phagocytophilum, human monocytotrophic ehrlichiosis (HME) is caused by Ehrlichia chaffeensis. Both are obligatory Gram negative bacteria belonging to the family Anaplasmataceae [1]. Previously, both of these infections were considered to be under the umbrella entity of HME, however it is now known that Anaplasma infects granulocytes and is a separate genus [3]. The Anaplasma bacteria is first introduced into human hosts through a bite from the Ixodes spp. ticks. The bacteria itself lives in salivary glands and midgut cells of the tick [7]. Once bitten by the tick, the bacteria disseminates intravascularly to infect host granulocytes, with a predilection for neutrophils. While in the neutrophil it creates membrane compartments intracellularly to avoid the cell’s defenses [7]. The bacteria inhibits part of the host’s innate immunity, including apoptosis of infected cells and down-regulates the production of reactive oxygen species (ROS). It also causes the upregulation of intracellular cholesterol to ensure its survival [7].
One of the initial findings in our patient was a significant pancytopenia. A unified pathogenesis resulting in this finding in patients with HGA is not well understood at this time. Our patient’s subacute HGA infection resulted in lymphopenia and severe thrombocytopenia. Notably, multilineage cytopenias are not unique to HGA, thus having a clear understanding of the findings expected on a CBC may differentiate HGA from other tick-borne illnesses (see Table 1). As the infection was treated with the correct antibiotic, the patient had significant and accelerated improvement with his cytopenias at the time of discharge, avoiding additional considerations for diagnostic testing.
Table 1.
A table demonstrating common lab findings in tick-borne illnesses.[18]
| Lab Test | Lyme Disease | Babesiosis | Anaplasmosis | Ehrlichiosis | RMSF |
|---|---|---|---|---|---|
| WBC | Normal, abnormality suggest coinfection | Elevated, normal, or decreased | Leukopenia | Leukopenia | Normal or slightly increased |
| RBC | Normal, abnormality suggest coinfection | Hemolytic Anemia |
Mild Anemia | Anemia late in illness (50%) | Anemia in 15% |
| Platelets | Normal, abnormality suggest coinfection | Low | Low | Low | Low initially |
| Liver Function Tests | Mild elevation | Mild elevation | Moderate Elevation | Mild to moderate elevation | Slight Elevations |
| Blood Smear | Normal | Maltese Crosses, parasites within erythrocytes | Commonly morulae within granulocytes | Rarely morulae present | Usually Normal |
One of the most unique and significant findings in our patient was his severe hyponatremia on presentation. Few studies have been done to investigate hyponatremia in tick-borne illnesses. In a study examining HME in children, 55% of children demonstrated hyponatremia [11]. This percentage was found to be even smaller in the adult population [1]. Most cases of hyponatremia with in HGA have been defined as mild. In our patient’s case, his hyponatremia could be defined as severe, which is atypical as a finding in HGA. Intriguingly, hyponatremia is not exactly an unusual finding in tick-borne illnesses. Although the pathophysiology is not quite understood, in a study looking at the incidence of hyponatremia in tick-borne encephalitis, 41% of patients in the study showed some form of hyponatremia, with only 3% showing severe hyponatremia defined as <125 mmol/L [12,13]. The most common cause of hyponatremia in this study was hypovolemia, while all of the patients with severe hyponatremia fulfilled criteria for syndrome of inappropriate secretion of antidiuretic hormone (SIADH). While this study was limited by the number of patients and was looking at tick-borne encephalitis as opposed to HGA, it is possible that the same conclusions could be applied to patients with hyponatremia and HGA. Our patient’s hyponatremia was consistent with hypotonic, euvolemic hyponatremia. His hyponatremic workup on presentation showed a urine osmolality > 100 mosm/kg with an appropriate urine sodium of >40 mmol/L, which can be interpreted as an inappropriate urine osmolality resulting in an increase in total body water relative to total body sodium, or SIADH. Following the diagnosis of SIADH, the patient’s sodium levels were monitored closely and as the patient had treatment for HGA, his sodium levels return to normal, which suggests HGA as a rare cause of his SIADH. While our patient did not receive a lumbar puncture when he presented, it can be postulated that his SIADH and his altered mentation could have been a result of tick-borne encephalitis (TBE), with the TBE causing the SIADH [14].
A third significant finding in our patient’s presentation was his complaint of abdominal pain and gastrointestinal (GI) symptoms. Johan Bakken was one of the first clinicians to recognize HGA as a separate disease from ehrlichiosis in Duluth, MN. Bakken later went on to treat these patients in their Duluth clinic and include them in studies to further elucidate HGA as a diagnostic entity. One such study involved studying the patient’s initial presenting complaints based on prevalence [3]. One of the least common presenting symptoms was that of abdominal pain. According to Bakken, in published cases at that time, only 20% of patients presented with abdominal pain. In Bakken’s own patient clinic in Duluth, abdominal pain was only noted to be present 4% of the time [3]. Similar to other rare findings in HGA, the cause of abdominal pain in these patients is not well understood. One hypothesis in these patients is progressive splenomegaly, which is commonly seen in HGA [8]. In our patient, he did not have splenomegaly on physical exam or on imaging. Gastrointestinal hemorrhage has been shown to be a cause of abdominal pain in HME, however this again was not seen in our patient [15]. HME has also been found to be the cause of febrile diarrhea in 10% of patients [16]. Our patient did not complain of diarrhea. Finally, hepatic involvement certainly could have contributed to our patient’s abdominal pain. Both AST and ALT were elevated suggesting hepatic involvement as a result of direct infection of the organism in hepatocytes and indirectly through pro-inflammatory mechanisms often seen in any infection [3,17]. Focal hepatic necrosis can be seen along with cholestatic hepatitis on histology in these cases.
Conclusion
In this case, a 71-year-old man originally presented with subacute abdominal pain, confusion, fever, elevated liver enzymes and hyponatremia. He was diagnosed with HGA and treated successfully with antibiotics with resolution of his symptoms. Through this case, we hope to emphasize that HGA’s atypical presenting signs, including abdominal pain along with severe hyponatremia, may make diagnosis difficult. However, with a high index of suspicion in the setting of the appropriate risk factors and tick exposure, our hope is that our case highlights the value of empiric treatment with appropriate monitoring to prevent downstream, severe sequelae from undiagnosed HGA. In the setting of climate change, increased duration of Ixodes spp. tick life cycles with emerging regional distribution of the ticks, coinfections with Borrelia burgdorferi and increased incidence of HGA in the last two decades, it is important to recognize this entity [6].
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Written and verbal consent was obtained from the patient. All information is anonymous.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Authorship contribution
Category 1: Conception and design of study: Prashant P. Patel, Melissa Baker, Adam Timothy Ladzinski.
Acquisition of data: Prashant P. Patel, Melissa Baker, Adam Timothy Ladzinski.
Analysis and/or interpretation of data: Prashant P. Patel, Melissa Baker, Adam Timothy Ladzinski.
Category 2: Drafting the manuscript: Prashant P. Patel, Melissa Baker, Adam Timothy Ladzinski.
Revising the manuscript critically for important intellectual content: Prashant P. Patel, Melissa Baker, Adam Timothy Ladzinski.
Category 3: Approval of the version of the manuscript to be published (the name of all authors must be listed): Prashant P. Patel, Melissa Baker, Adam Timothy Ladzinski.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgment
None.
Contributor Information
Adam Timothy Ladzinski, Email: adam.ladzinski@med.wmich.edu.
Melissa Baker, Email: melissa.baker@med.wmich.edu.
Karla Dunning, Email: dunningk@bronsonhg.org.
Prashant P. Patel, Email: prashant.patel@med.wmich.edu.
References
- 1.Ismail N., McBride J.W. Tick-Borne Emerging Infections: Ehrlichiosis and Anaplasmosis. Clin Lab Med. 2017;37(2):317–340. doi: 10.1016/j.cll.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez E., Vannier E., Wormser G.P., Hu L.T. Diagnosis, Treatment, and Prevention of Lyme Disease, Human Granulocytic Anaplasmosis, and Babesiosis: A Review. JAMA. 2016;315(16):1767–1777. doi: 10.1001/jama.2016.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakken J.S., Dumler J.S. Clinical diagnosis and treatment of human granulocytotropic anaplasmosis. Ann N Y Acad Sci. 2006;1078:236–247. doi: 10.1196/annals.1374.042. [DOI] [PubMed] [Google Scholar]
- 4.Anaplasmosis. CDC; 2020. Epidemiology and Statistics. Published March 26. [Accessed November 28, 2020] [Google Scholar]
- 5.Matei I.A., Estrada-Peña A., Cutler S.J., Vayssier-Taussat M., Varela-Castro L., Potkonjak A. A review on the eco-epidemiology and clinical management of human granulocytic anaplasmosis and its agent in Europe. Parasit Vectors. 2019;12(1):599. doi: 10.1186/s13071-019-3852-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dumic I., Severnini E. “Ticking Bomb”: The Impact of Climate Change on the Incidence of Lyme Disease. Canadian Journal of Infectious Diseases and Medical Microbiology. 2018 doi: 10.1155/2018/5719081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rikihisa Y. Mechanisms of obligatory intracellular infection with Anaplasma phagocytophilum. Clin Microbiol Rev. 2011;24(3):469–489. doi: 10.1128/CMR.00064-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johns J.L., MacNamara K.C., Walker N.J., Winslow G.M., Borjesson D.L. Infection with Anaplasma phagocytophilum Induces Multilineage Alterations in Hematopoietic Progenitor Cells and Peripheral Blood Cells. Infect Immun. 2009;77(9):4070–4080. doi: 10.1128/IAI.00570-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schutze G.E., Buckingham S.C., Marshall G.S., Woods C.R., Jackson M.A., Patterson L.E. Human monocytic ehrlichiosis in children. Pediatr Infect Dis J. 2007;26(6):475–479. doi: 10.1097/INF.0b013e318042b66c. [DOI] [PubMed] [Google Scholar]
- 12.Czupryna P., Moniuszko A., Garkowski A., Pancewicz S., Guziejko K., Zajkowska J. Evaluation of hyponatraemia in patients with tick-borne encephalitis--a preliminary study. Ticks Tick-Borne Dis. 2014;5(3):284–286. doi: 10.1016/j.ttbdis.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Czupryna P., Moniuszko A., Garkowski A., Pancewicz S., Zajkowska J. Comparison of hyponatremia and SIADH frequency in patients with tick borne encephalitis and meningitis of other origin. Scand J Clin Lab Invest. 2016;76(2):159–164. doi: 10.3109/00365513.2015.1129669. [DOI] [PubMed] [Google Scholar]
- 14.Da Porto A., Battellino M., Colussi G., Di Piazza V., Sechi L. Hiccups and Inappropriate ADH Secretion Syndrome as Presentations of Tick-Borne Disease. Eur J Case Rep Intern Med. 2019;6(8) doi: 10.12890/2019_001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace B.J., Brady G., Ackman D.M., Wong S.J., Jacquette G., Lloyd E.E., Birkhead G.S. Human granulocytic ehrlichiosis in New York. Arch Intern Med. 1998;158(7):769–773. doi: 10.1001/archinte.158.7.769. [DOI] [PubMed] [Google Scholar]
- 16.Devereaux C.E. Human monocytic ehrlichiosis presenting as febrile diarrhea. J Clin Gastroenterol. 1997;25(3):544–545. doi: 10.1097/00004836-199710000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Zaidi S.A., Singer C. Gastrointestinal and Hepatic Manifestations of Tickborne Diseases in the United States. Clin Infect Dis. 2002;34(9):1206–1212. doi: 10.1086/339871. [DOI] [PubMed] [Google Scholar]
- 18.Biggs H.M. Diagnosis and Management of Tickborne Rickettsial Diseases: Rocky Mountain Spotted Fever and Other Spotted Fever Group Rickettsioses, Ehrlichioses, and Anaplasmosis — United States. MMWR Recomm Rep. 2016:65. doi: 10.15585/mmwr.rr6502a1. [DOI] [PubMed] [Google Scholar]