Summary
The use of germ-free mice is integral to the understanding of host-gut microbiome relationships. Such models rely on faithful replication of the donor microbiome to establish causal effects of the gut microbiota on host pathophysiology. This protocol describes the preparation and transfer of donor microbiota, focusing on strict anaerobic processing methods and multiple instillations by gavage for optimal gut microbiota recovery.
For complete details on the generation and use of this protocol, please refer to Choo and Rogers (2021).
Subject areas: Sequence analysis, Microbiology, Model Organisms, Molecular Biology
Graphical abstract
Highlights
-
•
Describes preparation and transplantation of gut microbiota into germ-free mice
-
•
Strict anaerobic processing of donor cecal microbiota is essential
-
•
Faithful microbiome replication requires multiple instillations by gavage
-
•
Adaptable to different gut materials and highlights recommended validation steps
The use of germ-free mice is integral to the understanding of host-gut microbiome relationships. Such models rely on faithful replication of the donor microbiome to establish causal effects of the gut microbiota on host pathophysiology. This protocol describes the preparation and transfer of donor microbiota, focusing on strict anaerobic processing methods and multiple instillations by gavage for optimal gut microbiota recovery.
Before you begin
Reagent preparation
Timing: 1 h preparation time, 10 days wait time
-
1.
Prepare sterile 30% glycerol in phosphate-buffered saline (PBS) solution (pH 7.4).
-
2.
Prepare sterile 20% glycerol in PBS solution (pH 7.4).
-
3.
Prepare sterile PBS solution (pH 7.4), stored in a Falcon tube.
-
4.
Place all reagents in anaerobic chamber for at least 10 days prior to use.
-
5.Prepare sterile pre-reduced PBS solution (pH 7.4), stored in an anaerobic culture tube (for example, Hungate tube).
-
a.Prepare sterilized Hungate tube as detailed in Figure 1.
-
b.In an anaerobic chamber, use a sterile syringe and 23G needle to transfer sterile pre-reduced PBS solution into Hungate tube.
-
a.
-
6.
Pre-weigh a sterile, capped, 50 mL Falcon tube to at least two decimal places (for use in step-by-step 5d).
Alternatives: An anaerobic bag (for example, the Spilfyter Hands-in-Bag Atmospheric Chambers) may be considered as a cost-effective alternative to the anaerobic chamber to maintain an anaerobic environment. Equipment details are available from the supplier (https://us.vwr.com/store/product/4775838/spilfyter-hands-in-bag-atmospheric-chambers) and usage method is demonstrated in Weldy et al., 2020; Methods Video S1.
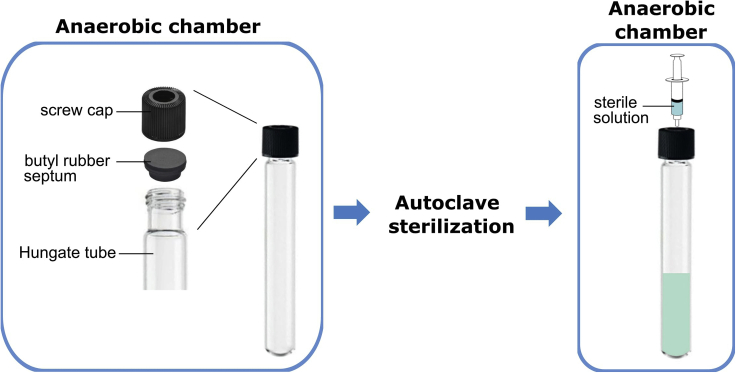
Figure 1.
Preparation of Hungate tube
Seal Hungate tube using a butyl rubber septum, followed by a screw cap in an anaerobic chamber. Sterilize the sealed Hungate tube by autoclaving. Following transport of the Hungate tube into an anaerobic chamber, transfer the appropriate solution into the Hungate tube using a sterile syringe and needle. Further information on the Hungate tube technique is accessible from the Leibniz Institute DSMZ (https://www.dsmz.de/fileadmin/Bereiche/Microbiology/Dateien/Kultivierungshinweise/Kultivierungshinweise_neu_CD/englAnaerob.pdf).
Suitability of donor and recipient mice
Timing: >10 days
-
7.
All animal procedures need to be approved by an Institutional Animal Care and Use Committee. All experiments in this protocol were approved by the South Australia Health and Medical Research Institute Animal Ethics Committee (SAM# 378).
-
8.Germ-free mice should be housed 2 to 5 animals of the same sex per cage, ensuring a random distribution of variables such as age and litter.
-
a.House mice in a temperature-controlled room (22 ± 2°C) on a 12-h light/dark cycle.
-
a.
-
9.
Acclimatize mice to facility conditions for at least 10 days prior to inoculation.
Note: Adult male mice should not be cohoused unless they were housed together before sexual maturity, which is reached between 40 to 60 days of age.
Note: Factors that are known to contribute to variation in the gut microbiota include the sex (Elderman et al., 2018), genotype (Ericsson et al., 2017), diet (Rausch et al., 2016), and bedding type (Rausch et al., 2016). These variables should be kept consistent across donor mice, wherever possible.
Note: This protocol was used for gut microbiota colonization of 6 to 7-week old female C57BL/6 germ-free mice using material from C57BL/6 donor mice. The protocol can be used for other adult donor or germ-free mouse sex, genotype and ages 6 weeks or older.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| 1× Phosphate-buffered saline (pH 7.4) | Thermo Fisher Scientific | 10010 |
| Glycerol | Thermo Fisher Scientific | 17904 |
| Experimental models: Organisms/strains | ||
| Conventional mice | The Jackson Laboratory | C57BL/6 |
| Germ-free mice | Translational Research Institute, Queensland, Australia | C57BL/6 |
| Other | ||
| Anaerobic chamber | COY | 7150220 |
| Hungate Tube (16 × 125 mm) | Bellco Glass | 2047-16125 |
| Corning 100 μm Cell Strainer | Corning | 431752 |
| Pestle for cell strainer | Sigma-Aldrich | Z742105 |
| Petri dish 90 mm | Thermo Fisher Scientific | LBS60014X |
| Sterile spatula | Sigma-Aldrich | CLS3005-100EA |
| Disposable scalpel | Adelab Scientific | LV-SCP21 |
| Syringe (1 mL, 10 mL, 30 mL) | BD | 309659, 303134, 309651 |
| Needle (23G) | Terumo | 219912 |
| Gavage needle | Instech | N/A |
| Minisart syringe filter (0.22 μm) | Sartorius | 16532 |
| Vortex mixer | Ratek | VM1-FT |
| QIAGEN DNeasy PowerLyzer PowerSoil kit | QIAGEN | 12855-100 |
| Qubit dsDNA HS Assay Kit | Thermo Fisher Scientific | Q32851 |
| Qubit Flex Fluorometer | Thermo Fisher Scientific | Q33327 |
| Tubes with screw cap (10 mL, 50 mL) | Thermo Fisher Scientific | 348224, 339653 |
| Teklad Global 18% Rodent Diet | Envigo | 2018S |
Materials and equipment
Pre-reduced 15% glycerol-phosphate buffered saline solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Glycerol | 15% | 7.5 mL |
| PBS (pH 7.4) | n/a | 42.5 mL |
| Total | Not applicable | 50 mL |
Pre-reduced 30% glycerol-phosphate buffered saline (PBS) solution
| Reagent | Final Concentration | Amount |
|---|---|---|
| Glycerol | 30% | 15 mL |
| PBS (pH 7.4) | n/a | 35 mL |
| Total | Not applicable | 50 mL |
Note: All solution can be stored at room temperature (22 ± 2°C) for up to a year.
CRITICAL: The solution should be filter-sterilized into a sterile 100 mL bottle by passing the solution through a 0.22 μm pore size syringe filter using a syringe with luer lock, or an equivalent device with a similar pore size, such as a 0.2 μm sterile bottle top filter. A bottle with a large aperture is recommended to enable rapid reduction of the solution. The bottle containing the solution should be placed with the seal cap loosened in the anaerobic chamber at room temperature (22 ± 2°C) for at least 10 days prior to use. The mixture should be swirled at least once a day to aid complete reduction.
Alternatives: Sterile PBS solution (pH7.4) can also be prepared in-house using the published protocol (Cold Spring Harbor Laboratory Press, 2006, https://doi.org/10.1101/pdb.rec8247).
Step-by-step method details
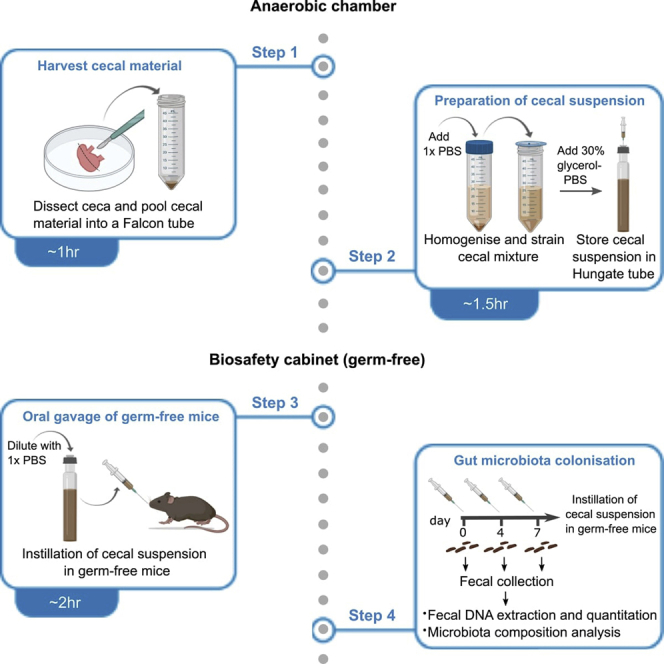
Harvest and preparation of pooled donor cecal material
Timing: 2.5 h
Preparation of cecal suspension under anaerobic conditions for inoculation into germ-free mice. The commensal gut microbiota is dominated by anaerobic bacteria. Processing of donor material under strict anaerobic conditions is therefore essential in maintaining viability across constituent taxa. The time duration indicated for this section is an approximate for processing the ceca of three to five mice of the same group.
-
1.
Perform humane killing of mice according to ethics approval.
-
2.
Position mouse in a supine position and perform a vertical midline incision to expose the gastrointestinal organs.
-
3.Dissect cecum from the gastrointestinal tract using a sterile scalpel (Figure 2).
-
a.Perform a horizontal dissection at the junction between the cecum and ileum (0.5 cm from cecum).
-
b.Perform a horizontal dissection at the junction between the cecum and colon (0.5 cm from cecum).
-
a.
-
4.
Transfer cecum into a 10 mL Falcon tube containing 5 mL of pre-reduced 15% glycerol.
-
5.In an anaerobic chamber, place the cecum onto a sterile petri dish to harvest cecal material from the tissue encasing.
-
a.Perform a longitudinal incision on the cecum using a sterile scalpel to expose cecal material.
-
b.Use a sterile spatula to scrape and transfer cecal material into a pre-weighed empty 50 mL Falcon tube.
-
c.Repeat this process for the remaining ceca from mice belonging to the same donor group.
-
d.Pool all cecal material into the pre-weighed 50 mL Falcon tube.
-
a.
-
6.
Record weight of the 50 mL Falcon tube containing the cecal material and determine net weight.
-
7.Dilute cecal sample in 4× volume of pre-reduced PBS.
-
a.For example, use 400 μL of PBS for every 100 mg cecum material.
-
a.
-
8.Vortex cecal mixture for 1 min to obtain a homogeneous suspension.
-
a.Use a pipette tip to disperse large pieces of material where necessary.
-
a.
-
9.
Place a 100 μm cell strainer onto a fresh sterile 50 mL Falcon tube (“suspension tube”).
-
10.
Pipette the cecal material onto the cell strainer.
-
11.Allow the cecal suspension (supernatant) to flow into the 50 mL Falcon tube for 5 min.
-
a.Use a sterile pestle to extract remaining supernatant in the cell strainer.
-
b.Discard the cell strainer containing the remaining cecal material.
-
a.
-
12.
Dilute the cecal suspension with 1× volume of pre-reduced 30% glycerol.
-
13.Using a sterile syringe and 23G needle, draw up the cecal suspension from the “suspension tube” and transfer cecal suspension into a sealed Hungate tube.
-
a.Aliquot the appropriate volume of cecal suspension into separate Hungate tubes.
-
b.Allocate one Hungate tube per cage for each gavage day.
-
a.
-
14.
Store cecal suspension at −80°C until use.
Note: Ideally, the dissected ceca in 15% glycerol should be processed immediately after harvest to obtain cecal suspension, in order to reduce freeze-thaw of cecal material. Freeze-thaw may affect the viability of bacteria as detailed in the limitations section. Where not possible, the dissected ceca can be stored in its entirety in 15% glycerol at −80°C, and preparation of cecal suspension should be performed as soon as possible.
Note: Use a wide-bore tip when pipetting cecal material (steps 8 and 9) to prevent blockage. All material and reagents used should be sterile and aseptic technique practiced throughout.
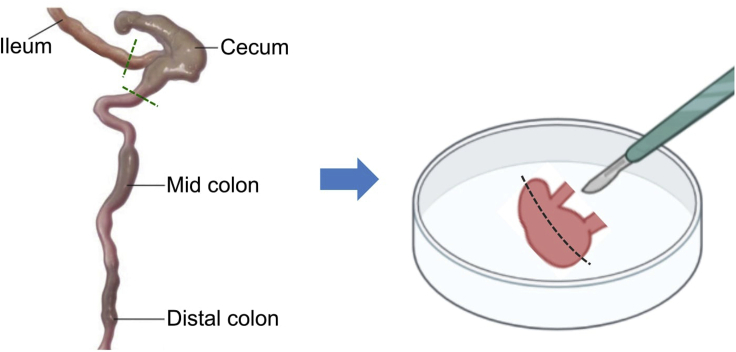
Figure 2.
Dissection of cecum in donor mice
Cecum is isolated from the gastrointestinal tract by performing a horizontal dissection at the junction between the cecum and the ileum, and between the cecum and colon, as indicated by the green dotted lines. The dissection section of the gastrointestinal tract should be within 0.5 cm of the cecum. A longitudinal incision is performed on the cecum (black dotted lines) for cecal material collection. Image of the mouse gastrointestinal tract is adapted from (Treuting et al., 2018).
Anaerobic conditions should be maintained throughout the processing of donor material.
Note: The total volume of cecal suspension to aliquot into separate Hungate tubes (step 13a) is calculated based on (n × 75 μL) + 200 μL. ‘n’ denotes the number of mice per cage. The addition of 200 μL to the total cecal suspension volume is to ensure sufficient volume of material for oral gavage per cage.
Note: Pooled material from three ceca per group should provide sufficient material to perform the gut microbiota colonization step on 15 to 20 mice per group.
Pause point: Cecal suspension in Hungate tube can be kept frozen at −80°C until required for instillation into germ-free mice.
CRITICAL: Avoid repeated freeze-thaw of cecal suspensions to prevent loss of bacterial viability.
Gut microbiota colonization of germ-free mice using cecal suspension
Timing: 7 days
Oral gavage of germ-free mice with cecal suspension is performed to achieve gut microbiota establishment.
-
15.
Thaw Hungate tubes containing aliquots of the donor cecal suspension on ice on bench top (approximately 30 mins).
-
16.
Transport germ-free mice, sterile pre-reduced PBS in Hungate tube, and cecal suspension in Hungate tube, into a sterilized biosafety cabinet in the germ-free animal facility.
-
17.Dilute cecal suspension in Hungate tube using 2× volume of sterile pre-reduced PBS.
-
a.Add the required volume of PBS (in Hungate tube) into the Hungate tube containing cecal suspension using a sterile syringe and 23G needle.
-
b.Vortex the diluted cecal suspension for 1 min.
-
a.
-
18.Perform oral gavage using 150 μL of the cecal suspension according to institutional animal research facility safety operating practices (SOPs).
-
a.Draw up cecal suspension using a sterile syringe and 23G needle.
-
b.Replace 23G needle with a gavage needle for oral gavage.
-
a.
-
19.
Repeat steps 16–18 for oral gavage at day 4 and day 7.
Optional: Collect fecal sample from individual mice prior to oral gavage at day 0, day 4, day 7, and appropriate time points thereafter for fecal DNA quantitation. Fresh fecal samples are collected using a sterile toothpick into a sterile 1.5-mL microcentrifuge tube. DNA extraction from fecal samples can be performed using the QIAGEN DNeasy PowerLyzer PowerSoil kit, or an equivalent DNA extraction kit. Quantitation of the extracted DNA can be performed using the Qubit dsDNA HS assay kit, or an equivalent fluorometric quantification assay. For high stringency bacterial DNA determination, quantitative PCR of the bacterial 16S rRNA gene can be performed on the extracted DNA samples, as described in Choo and Rogers, 2021.
Optional: Gut microbiota composition of colonized germ-free mice can be determined using 16S rRNA amplicon sequencing to determine similarity to the donor gut microbiota, as described in Choo and Rogers, 2021.
Note: Place thawed Hungate tube containing cecal suspension on ice on bench top until transported into the biosafety cabinet. Thawed cecal suspension can be kept at room temperature in the biosafety cabinet and should be used within 2.5h for oral gavage.
Note: All procedures should be performed in a cleaned biosafety cabinet according to germ-free animal techniques. Prior to transfer of equipment into the biosafety cabinet, the external surfaces of all equipment should be sterilized using a suitable disinfectant solution according to animal facility SOPs. It is recommended to use one Hungate tube per cage to minimize the risk of contamination.
Note: Where animal research facility SOP for oral gavage is not available, a reference for the procedure is available at https://ouv.vt.edu/content/dam/ouv_vt_edu/sops/small-animal-biomedical/sop-mouse-oral-gavage.pdf
Expected outcomes
The cecal suspension preparation is expected to yield approximately 105 – 106 16S rRNA copies/μL based on quantitative PCR of the 16S rRNA gene (Choo and Rogers, 2021).
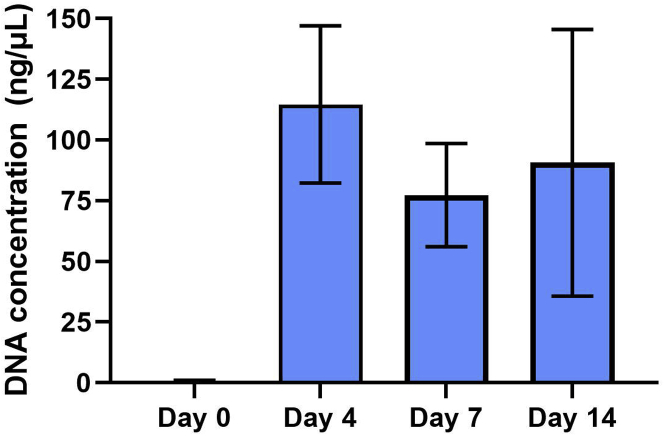
Fecal bacterial load level of colonized germ-free mice is expected to reach the maximal average ± standard deviation of 96.0 ± 32.9 ng/μL by day 4 or day 7 (Figure 3). Detailed fecal bacterial load of colonized germ-free mice from day 0 to day 70 and those of the donor mice is indicated in Choo and Rogers, 2021; Figure 1. The dissimilarity/similarity of donor-recipient microbiota composition, and bacterial relative abundance in the recipient from day 4 to day 70 compared to the donor, is indicated in Figures 4 and 6, respectively, of Choo and Rogers, 2021.
Figure 3.
Concentration of DNA extracted from fecal samples of colonized germ-free mice
DNA was extracted from fecal samples collected from colonized germ-free mice at baseline (day 0), day 4, day 7 and day 14 following the first gavage. The bar and error bars represent the mean and standard deviation, respectively.
Limitations
Several factors may influence gut microbiota colonization of germ-free mice, including the age of germ-free mice, the gut material used for oral gavage, and the microbial community of donor mice. Previous microbiota colonization studies in conventional mice suggests that microbiota engraftment may be more effective when performed on younger mice (Le Roy et al., 2019). Multiple gavages into germ-free mice is expected to increase donor-recipient compositional similarity compared to those receiving only a single gavage (Eberl et al., 2020; Choo and Rogers, 2021), although this effect is dependent on the microbial community (Choo and Rogers, 2021). Given that engraftment of the transplanted microbiota is less efficient in a stable microbial community compared to a depleted intestinal microbiota environment (Le Roy et al., 2019), the inoculation of microbiota into germ-free mice during the early time points is expected to be more effective for microbiota colonization.
The method can be adapted for preparing gavage material from fecal samples or other sections of the gastrointestinal tract. However, optimization of the dilution method will need to be performed for these samples. It should be noted that fecal samples have larger exposure to atmospheric oxygen, which can reduce viability of bacteria (Papanicolas et al., 2019). These microbiota changes can reduce the efficiency of colonization and/or skew the recapitulation of the gut microbiota. In addition, the bacterial load and composition of the donor microbial community may be altered by interventions such as antibiotic treatment. Compositional differences of the gut microbiota can influence the efficiency of gut microbiota colonization in germ-free mice (Choo and Rogers, 2021). Where significant reduction in bacterial load is anticipated, the dilution factor should be optimized. The in vitro viability of murine microbiota were not affected in fecal samples that were frozen at −20°C for up to one month (Takahashi et al., 2018), although bacterial viability have been reported to reduce by up to half in freeze-thawed human fecal samples (Papanicolas et al., 2019). Therefore, cecal suspension should be frozen at −80°C and the number of freeze-thaw cycles minimized where possible.
Troubleshooting
Problem 1
Difficulty in pipetting cecal material due to blockage in pipette tip (step 10).
Potential solution
Use a wide bore tip when pipetting cecal material. Disperse large pieces of material prior to pipetting suspension.
Problem 2
Low volume recovery of cecal suspension (step 11).
Potential solution
Use a pestle to dislodge cecal material blocking the cell strainer and to extract the remaining fluid. Use a new cell strainer if processing large volumes of cecal material.
Problem 3
Low bacterial load levels in cecal suspension (step 13 and Expected Outcomes section).
Potential solution
Where an intervention, such as antibiotic treatment, is expected to reduce the total gut bacterial load in donor mice, fecal samples should be collected from donor mice for DNA extraction and quantification prior to processing cecal material. The dilution factor and glycerol concentrations of cecal suspensions should be adjusted accordingly to achieve sufficient bacterial cells for oral gavage.
Problem 4
Cecal suspension too viscous to pass through a plastic oral gavage needle (step 18).
Potential solution
Reusable metal oral gavage needle that has been sterilized by autoclaving can be used to perform oral gavage of the cecal suspension. Personnel should be adequately trained and adhere to the SOP at all times.
Problem 5
Cecum is not available for harvesting of inoculum material (step 3).
Potential solution
Cecal material is used for the preparation of inoculum material as it yields a larger amount of material and has minimal exposure to oxygen. Where the cecum is unavailable for harvest, material from other regions of the lower gastrointestinal tract, such as the distal colon or feces, may be considered as a replacement to cecal material. Higher compositional similarity and microbiota stability is reported between the cecum, distal colon and feces, which microbiota composition differed to those of the small intestinal tract (Gu et al., 2013). However, the dilution method should be optimized and the constraints of using these material are indicated in the limitations section.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Jocelyn Choo (jocelyn.choo@sahmri.com).
Material availability
This study did not generate new unique reagents.
Data and code availability
Original data for fecal bacterial load in colonized germ-free mice based on quantitative PCR of the 16S rRNA gene is available. [https://doi.org/10.1016/j.isci.2021.102049]
Acknowledgments
We thank Samay D. Trec and Mariah Turelli from the SAHMRI-PIRL Germ-Free animal facility for contributing to the work. G.B.R. is supported by a Matthew Flinders Research Fellowship and a National Health and Medical Research Council Senior Research Fellowship (GNT1155179). Images were created using GraphPad Prism 9 and Biorender.com.
Author contributions
Conceptualization, J.M.C. and G.B.R.; methodology, J.M.C.; formal analysis, J.M.C.; investigation, J.M.C.; writing – original draft, J.M.C. and G.B.R.; writing – review & editing, J.M.C. and G.B.R.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Jocelyn M. Choo, Email: jocelyn.choo@sahmri.com.
Geraint B. Rogers, Email: geraint.rogers@sahmri.com.
References
- Choo J.M., Rogers G.B. Establishment of murine gut microbiota in gnotobiotic mice. iScience. 2021;24:102049. doi: 10.1016/j.isci.2021.102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl C., Ring D., Münch P.C., Beutler M., Basic M., Slack E.C., Schwarzer M., Srutkova D., Lange A., Frick J.S. Reproducible colonization of germ-free mice with the oligo-mouse-microbiota in different animal facilities. Front. Microbiol. 2020;10:2999. doi: 10.3389/fmicb.2019.02999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elderman M., Hugenholtz F., Belzer C., Boekschoten M., van Beek A., de Haan B., Savelkoul H., de Vos P., Faas M. Sex and strain dependent differences in mucosal immunology and microbiota composition in mice. Biol. Sex Differ. 2018;9:26. doi: 10.1186/s13293-018-0186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson A.C., Personett A.R., Turner G., Dorfmeyer R.A., Franklin C.L. Variable colonization after reciprocal fecal microbiota transfer between mice with low and high richness microbiota. Front. Microbiol. 2017;8:196. doi: 10.3389/fmicb.2017.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S., Chen D., Zhang J., Lv X., Wang K., Duan L., Nie Y., Wu X. Bacterial community mapping of the mouse gastrointestinal tract. PLoS One. 2013;8:e74957. doi: 10.1371/journal.pone.0074957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roy T., Debédat J., Marquet F., Da-Cunha C., Ichou D., Guerre-Millo M., Kapel N., Aron-Wisnewsky J., Clément K. Comparative evaluation of microbiota engraftment following fecal microbiota transfer in mice models: age, kinetic and microbial status matter. Front. Microbiol. 2019;9:3289. doi: 10.3389/fmicb.2018.03289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolas L.E., Choo J.M., Wang Y., Leong L.E.X., Costello S.P., Gordon D.L., Wesselingh S.L., Rogers G.B. Bacterial viability in faecal transplants: which bacteria survive? EBioMedicine. 2019;41:509–516. doi: 10.1016/j.ebiom.2019.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch P., Basic M., Batra A., Bischoff S.C., Blaut M., Clavel T., Glasner J., Gopalakrishnan S., Grassl G.A., Gunther C. Analysis of factors contributing to variation in the C57BL/6J fecal microbiota across German animal facilities. Int. J. Med. Microbiol. 2016;306:343–355. doi: 10.1016/j.ijmm.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Ishikawa D., Sasaki T., Lu Y.J., Kuwahra-Arai K., Kamei M., Shibuya T., Osada T., Hiramatsu K., Nagahara A. Faecal freezing preservation period influences colonization ability for faecal microbiota transplantation. J. Appl. Microbiol. 2018;126:973–984. doi: 10.1111/jam.14167. [DOI] [PubMed] [Google Scholar]
- Treuting P., Dintzis S., Montine K.S. Comparative Anatomy and Histology. Second edition. Academic Press; 2018. Lower gastrointestinal tract; pp. 213–228. [Google Scholar]
- Weldy M., Evert C., Dosa P.I., Khoruts A., Sadowsky M.J. Convenient Protocol for Production and Purification of Clostridioides difficile Spores for Germination Studies. STAR Protocols. 2020;1:100071. doi: 10.1016/j.xpro.2020.100071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data for fecal bacterial load in colonized germ-free mice based on quantitative PCR of the 16S rRNA gene is available. [https://doi.org/10.1016/j.isci.2021.102049]