Highlights
-
•
Simvastatin-romidepsin combination kills bladder cancer cells synergistically.
-
•
The combination induces histone acetylation by activating AMPK.
-
•
AMPK activation and histone acetylation are associated with ER stress induction.
-
•
Positive feedback cycle between ER stress induction and PPARγ expression.
Keywords: Romidepsin, Simvastatin, AMP-activated protein kinase (AMPK), Endoplasmic reticulum (ER) stress, Histone acetylation, Peroxisome proliferator-activated receptor (PPAR) γ
Abbreviations: 7-AAD, 7-amino-actinomycin D; AMPK, AMP-activated protein kinase; CDK, cyclin-dependent kinase; CHX, cycloheximide; CI, combination index; DHE, dihydroethidium; DMSO, dimethyl sulfoxide; ER, endoplasmic reticulum; ERp, endoplasmic reticulum resident protein; FITC, fluorescein isothiocyanate; GRP, glucose-regulated protein; HDAC, histone deacetylase; HE, hematoxylin-eosin; p-AMPK, phosphorylated AMP-activated protein kinase; p-H2AX, phosphorylated histone H2AX; PPAR, peroxisome proliferator-activated receptor; ROS, reactive oxygen species
Abstract
The HMG-CoA reductase inhibitor simvastatin activates AMP-activated protein kinase (AMPK) and thereby induces histone acetylation. We postulated that combining simvastatin with the histone deacetylase (HDAC) inhibitor romidepsin would kill bladder cancer cells by inducing histone acetylation cooperatively. The combination of romidepsin and simvastatin induced robust apoptosis and killed bladder cancer cells synergistically. In murine subcutaneous tumor models using MBT-2 cells, a 15-day treatment with 0.5 mg/kg romidepsin and 15 mg/kg simvastatin was well tolerated and inhibited tumor growth significantly. Mechanistically, the combination induced histone acetylation by activating AMPK. The combination also decreased the expression of HDACs, thus further promoting histone acetylation. This AMPK activation was essential for the combination's action because compound C, an AMPK inhibitor, suppressed the combination-induced histone acetylation and the combination's ability to induce apoptosis. We also found that the combination increased the expression of peroxisome proliferator-activated receptor (PPAR) γ, leading to reactive oxygen species production. Furthermore, the combination induced endoplasmic reticulum (ER) stress and this ER stress was shown to be associated with increased AMPK expression and histone acetylation, thus playing an important role in the combination's action. Our study also suggests there is a positive feedback cycle between ER stress induction and PPARγ expression.
Introduction
Despite the recent progress of bladder cancer treatment—such as the advent of immune checkpoint inhibitors, target therapies, and antibody-drug conjugates [1, 2]—there is still no curative treatment for metastatic bladder cancer. New treatment strategies need to be developed. AMP-activated protein kinase (AMPK) is a cellular energy sensor that is activated by impaired energy status such as glucose deprivation, ischemia, hypoxia, and oxidative stress, leading to inhibition of cellular growth to restore energy homeostasis [3]. Therefore, drugs activating AMPK have attracted much attention as novel anticancer agents [4]. HMG-CoA reductase inhibitors, which are widely used for treating dyslipidemia [5], are known to activate AMPK [6]. Simvastatin inhibits HMG-CoA reductase and has been shown to kill cancer cells in vitro [7, 8], although its anticancer efficacy has not been proven yet in clinical trials [9].
Histone acetylation is an innovative epigenetics-based cancer therapy [10] and preclinical studies showed that histone deacetylase (HDAC) inhibitors were capable of inhibiting bladder cancer growth [11, 12]. Romidepsin is a class I HDAC inhibitor clinically approved for the treatment of cutaneous T cell lymphoma [13], which acts against cancer cells at lower concentrations in vitro, but the clinical benefit against solid tumors is not satisfactory [14].
Recently, AMPK activation has been shown to induce histone acetylation [15]. We thought that simvastatin would activate AMPK and the simvastatin-romidepsin combination would kill bladder cancer cells effectively by inducing histone cooperatively. We also investigated the role of endoplasmic reticulum (ER) stress induction in the combination's anticancer activity because histone acetylation is closely related to ER stress induction [16, 17]. Furthermore, simvastatin is known to activate peroxisome proliferator-activated receptor (PPAR) γ, a regulator of fatty acid storage and glucose metabolism [18], which plays a crucial role in bladder cancer proliferation [19]. Therefore, we also evaluated the contribution of PPARγ activation to the anticancer activity of the simvastatin-romidepsin combination and its association with histone acetylation and ER stress induction.
The biological effects of the simvastatin-romidepsin combination have so far scarcely been investigated. There has been one study evaluating the combination's effect on increased γ-globin gene expression in CD34+ cells [20], but to our knowledge the present study is the first that investigated the antineoplastic effect of the combination using cancer cells.
Materials and methods
Cell cultures
Human bladder cancer cells (UMUC-3, T-24, and J-82) were purchased from the American Type Culture Collection (Rockville, MD, USA) and murine bladder cancer cells (MBT-2) were purchased from Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan). The cells were cultured in the recommended media (minimum essential medium and McCoy's 5A medium) supplemented with 10% fetal bovine serum and 1.0% penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA) at 37 °C under 5% CO2 in a humidified incubator.
Reagents
Simvastatin and vorinostat purchased from Cayman Chemical (Ann Arbor, MI, USA), romidepsin, belinostat and entinostat purchased from Selleck Chemicals (Houston, TX, USA), panobinostat purchased from LC Laboratories (Boston, MA, USA), and rosiglitazone and tunicamycin purchased from Enzo Life Sciences (Farmingdale, NY, USA) were dissolved in dimethyl sulfoxide (DMSO). Compound C dihydrochloride purchased from R&D Systems (Minneapolis, MN, USA) and cycloheximide (CHX) purchased from Enzo Life Sciences were dissolved in distilled water. These reagents were stored at −80 °C or −20 °C until use.
Cell viability assay
5 × 103 cells were seeded into each well of a 96-well culture plate one day before being treated with the indicated conditions. After treatment, cell viability was evaluated by CCK-8 assay (Dojindo, Kumamoto, Japan) according to the manufacturer's protocol.
Cell confluency assay
5 × 103 cells were seeded into each well of a 96-well culture plate one day before being treated with indicated conditions. After treatment, confluence measurements were performed at 3 h intervals over 3 days by the IncuCyte real-time video imaging system (Essen Instruments, Ann Arbor, MI, USA).
Clonogenic assay
2 or 3 × 102 cells (the number depended on the cell line) were seeded into each well of a 12-well culture plate one day before being treated for 48 h with 5 μM simvastatin and/or 20 nM romidepsin. The cells were then given fresh medium and cultured for 1 to 2 weeks. The colonies were counted after being fixed with 100% methanol and stained with Giemsa's solution.
Flow cytometry
Flow cytometry was used for analysis of annexin-V assay and cell cycle and evaluation of cellular reactive oxygen species (ROS) production. Briefly, 1.0 × 105 cells were seeded into each well of a 12-well culture plate one day before being cultured for 48 h under the indicated conditions. Cells were then washed with phosphate-buffered saline and harvested by trypsinization. For annexin-V assay, cells were subjected to annexin V and 7-amino-actinomycin D (7-AAD) double staining following the protocol of the assay kit's manufacturer (Beckman Coulter, Marseille, France). For cell cycle analysis, cells were resuspended in citrate buffer and stained with propidium iodide. For evaluation of cellular ROS production, cells were stained with dihydroethidium (DHE) (Cayman Chemical) according to the manufacturer's protocol. The cells were then analyzed using a flow cytometer (FACSCalibur, BD Biosciences, San Jose, CA, USA) and CellQuest ProSoftware (BD Biosciences). Three independent tests were performed.
Western blotting
After treating bladder cancer cells under the indicated conditions for 48 h, whole cell lysates were obtained using radioimmunoprecipitation assay buffer. Equal amounts of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. After the membranes were blocked with 5% skimmed milk, they were incubated with the primary antibodies: anti-AMPK (1:1000) and anti-PPARγ (1:1000) from Proteintech (Rosemont, IL, USA); anti-cleaved poly(ADP-ribose) polymerase (PARP) (1:1000), anti-phosphorylated AMPK (p-AMPK) (1:1000), anti-phosphorylated histone H2AX (p-H2AX) (1:1000), and anti-endoplasmic reticulum resident protein (ERp) 44 (1:1000) from Cell Signaling Technology (Danvers, MA, USA); anti-glucose-regulated protein (GRP) 78 (1:1000), anti-cyclin D1 (1:1000), anti-cyclin E (1:1000), anti-cyclin-dependent kinase (CDK) 2 (1:1000), anti-CDK4 (1:1000), anti-HDAC1 (1:1000), anti-HDAC3 (1:1000), and anti-HDAC6 (1:1000) from Santa Cruz Biotechnology (Santa Cruz, CA, USA); anti-acetylated histone (1:1000) and anti-active caspase 3 (1:1000) from Abcam (Cambridge, UK); anti-acetylated α-tubulin (1:5000) from Novus (Centennial, CO, USA); and anti-actin (1:5000) from Millipore (Billerica, MA, USA). Then the protein was detected by reaction with recommended secondary antibody (horseradish-tagged goat anti-rabbit or goat anti-mouse antibody (GE Healthcare UK, Amersham, UK)) and staining with chemiluminescence solution (Clarity Western ECL Substrate, Bio-Rad) and imaged with ChemiDoc Touch Imaging System (Bio-Rad). Densitometry for bands was performed, using the NIH ImageJ software, with data normalization to the mean density of actin.
In-vivo study
The in-vivo efficacy of the simvastatin-romidepsin combination was assessed using murine subcutaneous allograft models. Animal studies were conducted in compliance with Japanese animal use regulations and approval for these studies was obtained from the institutional Animal Care and Use Committee of National Defense Medical College. 1 × 107 MBT-2 cells were implanted subcutaneously into C3H/HeN Slc mice purchased from Japan SLC (Shizuoka, Japan) and treatment was initiated five days later (day 1), when all the mice exhibited measurable tumors. The mice were divided into the vehicle group and the treatment groups (n = 5 per group). The vehicle group received intraperitoneal injections of DMSO, and the treatment groups received 0.5 mg/kg romidepsin or 15 mg/kg simvastatin or both. The injections of romidepsin were given twice per week and the injections of simvastatin were given once a day for 15 days (5 days on 2 days off). Tumor volume and body weight were measured every 2 or 3 days. Tumor volumes were estimated using the following formula: volume = 0.5 × length × width2. After 15 days of treatment, the animals were euthanized in compliance with the United Kingdom National Cancer Research Institute's ethical policy [21] and the subcutaneous tumors were harvested.
Statistical analysis
CalcuSyn software (Biosoft, Cambridge, UK) was used for calculating the combination indexes according to the method developed by Chou and Talalay [22]. The statistical significance of observed differences between samples was evaluated using the Mann-Whitney U test (JMP Pro14 software, SAS Institute, Cary, NC, USA), and differences for which p < 0.05 were considered statistically significant.
Results
Anticancer activity of simvastatin and romidepsin in bladder cancer cells
Simvastatin inhibited the growth of bladder cancer cells in a dose- and time-dependent manner (Fig. 1A-B and Table S1). Mechanistically, it increased both the phosphorylation and expression of AMPK, thus activating AMPK, and induced histone acetylation (Fig. 1C). Furthermore, simvastatin induced ER stress evidenced by the increased expression of GRP78 and ERp44 (Fig. 1C). We also found that simvastatin increased the expression of PPARγ (Fig. 1C), a transcriptional regulator of glucose and lipid metabolism [18]. Interestingly, The Cancer Genome Atlas data analysis by using the UCSC Cancer Browser UCSC Xena (https://xena.ucsc.edu/welcome-to-ucsc-xena/) revealed that bladder cancer patients with higher expression of PPARγ genes had longer overall survival time than those with lower expression (Fig. S1).
Fig. 1.
Anticancer activity of simvastatin and romidepsin in bladder cancer cells. (A) Cells were treated for 48 h with 2.5–40 μM simvastatin and cell viability was measured using CCK-8 assay. Mean ± SD, n = 6. (B) Cells were given 2.5–10 μM simvastatin and confluence measurements were performed at 3-hour intervals over 3 days. Mean ± SD, n = 6. (C) Western blotting for AMP-activated protein kinase (AMPK), acetylated histone, glucose-regulated protein (GRP) 78, endoplasmic reticulum resident protein (ERp) 44, and peroxisome proliferator-activated receptor (PPAR) γ. Cells were treated for 48 h with 2.5–20 μM simvastatin. Actin was used for the loading control. Representative blots are shown. (D) Cells were treated for 48 h with different concentrations of various histone deacetylase (HDAC) inhibitors, and cell viability was measured using CCK-8 assay. Mean ± SD, n = 6. (E) Cells were treated for 48 h with 10–160 nM romidepsin and cell viability was measured using CCK-8 assay. Mean ± SD, n = 6. (F) Cells were given 10–40 nM romidepsin and confluence measurements were performed at 3-hour intervals over 3 days. Mean ± SD, n = 6. (G) Western blotting for acetylated histone, GRP78, and ERp44. Cells were treated for 48 h with 10–40 nM romidepsin. Actin was used for the loading control. Representative blots are shown.
We then tested the antiproliferative activity of various HDAC inhibitors in bladder cancer cells and found that romidepsin had the lowest IC50 value among them (Fig. 1D and Table S2). We therefore used romidepsin in the subsequent experiments. Romidepsin inhibited bladder cancer proliferation in a dose- and time-dependent manner (Fig. 1E-F). Mechanistically, it induced not only histone acetylation but also ER stress (Fig. 1G).
Anticancer activity of the simvastatin-romidepsin combination in bladder cancer cells
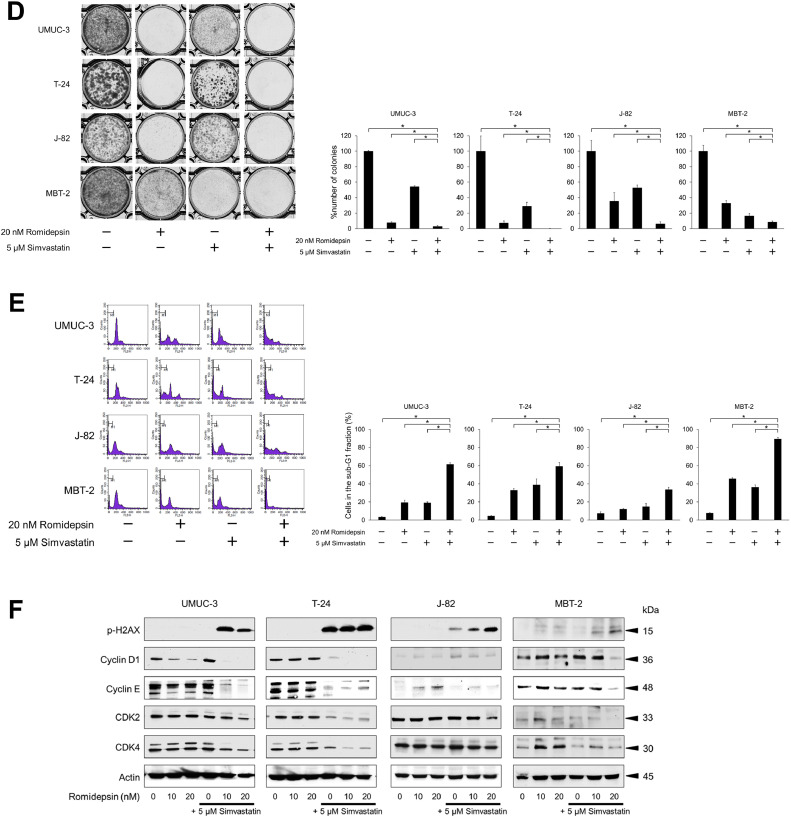
A 48-hour treatment with the combination of simvastatin and romidepsin inhibited bladder cancer growth effectively (Fig. 2A-B), and the synergism of the combination's effect was confirmed in all the treatment conditions (Table S3 and Fig. S2). We also found that the combination's antiproliferative effect was time-dependent (Fig. 2C and S3). Furthermore, the combination inhibited the clonogenic survival of bladder cancer cells significantly (Fig. 2D). Thus, the combination of simvastatin and romidepsin was shown to inhibit bladder cancer growth effectively.
Fig. 2.
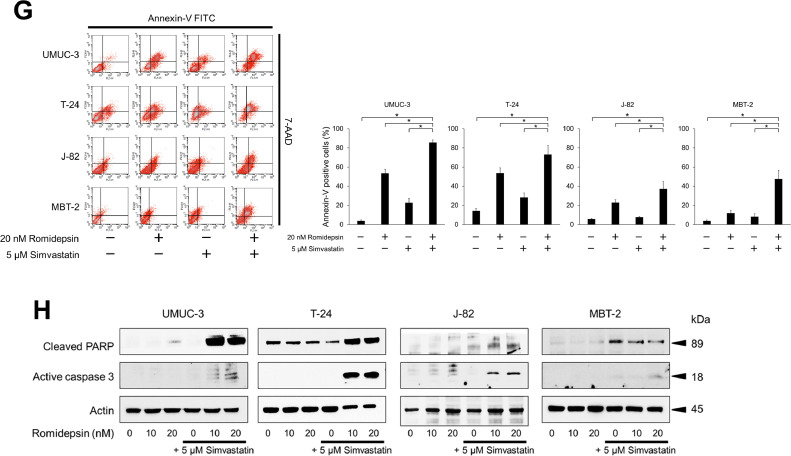
Anticancer activity of the simvastatin-romidepsin combination in bladder cancer cells. (A) Cells were treated for 48 h with 2.5–5 μM simvastatin and/or 5–20 nM romidepsin and cell viability was measured using CCK-8 assay. Bars represent mean ± SD, n = 6. (B) Photomicrographs showing morphological changes of the cells after 48-hour treatment with 5 μM simvastatin and/or 20 nM romidepsin. Scale bar = 300 μm. (C) Cells were given 5 μM simvastatin and/or 20 nM romidepsin and confluence measurements were performed at 3-hour intervals over 3 days. Mean ± SD, n = 6. (D) Clonogenic assay. 200–300 cells were treated for 48 h with 5 μM simvastatin and/or 20 nM romidepsin. The cells were then given fresh media and incubated for 1–2 weeks. Bar graphs show the%number of colonies relative to the untreated control. Mean ± SD, n = 3. *p = 0.0495. (E) Cells were treated for 48 h with 5 μM simvastatin and/or 20 nM romidepsin. Changes in the cell cycle were evaluated using flow cytometry. 10,000 cells were counted. Bar graphs show the percentages of the cells in the sub-G1 fraction. Data are expressed as mean ± SD from three independent experiments. *p = 0.0495. (F) Western blotting for phosphorylated histone H2AX (p-H2AX), cyclin D1, cyclin E, cyclin-dependent kinase (CDK) 2, and CDK4. Cells were treated with 5 μM simvastatin and/or 10–20 nM romidepsin for 48 h. Actin was used for the loading control. Representative blots are shown. (G) Cells were treated for 48 h with 5 μM simvastatin and/or 20 nM romidepsin. Apoptotic cells were detected by annexin-V assay using flow cytometry. 10,000 cells were counted. Bar graphs show the percentages of apoptotic cells. Data are expressed as mean ± SD from three independent experiments. FITC, fluorescein isothiocyanate; 7-AAD, 7-amino-actinomycin D. *p = 0.0495. (H) Western blotting for cleaved poly(ADP-ribose) polymerase (PARP) and active caspase 3. Cells were treated with 5 μM simvastatin and/or 10–20 nM romidepsin for 48 h. Actin was used for the loading control. Representative blots are shown. (I) Western blotting for AMP-activated protein kinase (AMPK), acetylated histone, and peroxisome proliferator-activated receptor (PPAR) γ. Cells were treated with 5 μM simvastatin and/or 10–20 nM romidepsin for 48 h. Actin was used for the loading control. Representative blots are shown. (J) Western blotting for glucose-regulated protein (GRP) 78, endoplasmic reticulum resident protein (ERp) 44, histone deacetylase (HDAC) 1, HDAC3, HDAC6, and acetylated α-tubulin. Cells were treated with 5 μM simvastatin and/or 10–20 nM romidepsin for 48 h. Actin was used for the loading control. Representative blots are shown. (K) Cells were treated with 5 μM simvastatin and/or 20 nM romidepsin for 48 h and reactive oxygen species production was measured by dihydroethidium (DHE) staining using flow cytometry. 10,000 cells were counted. Bar graphs show the relative DHE fluorescence intensity. Data are expressed as mean ± SD from three independent experiments. *p = 0.0495.
We then evaluated changes in the cell cycle and apoptosis caused by the combination of simvastatin and romidepsin. The combination perturbed the cell cycle and significantly increased the number of the cells in the sub-G1 fraction (Fig. 2E), suggesting that it caused DNA fragmentation and induced apoptosis. The increased expression of p-H2AX proved that the combination caused DNA double strand breaks (Fig. 2F). The combination decreased the expression of the cell cycle regulators, cyclin D1, cyclin E, CDK2, and CDK4 (Fig. 2F), which was consistent with the perturbation of the cell cycle. Interestingly, in UMUC-3, J-82, and MBT-2 cells, the expression of cyclin D1 was increased by simvastatin alone but decreased by romidepsin in combination with simvastatin, which is consistent with the antiproliferative effect of the combination. Furthermore, the combination significantly increased the percentage of the cell population that was annexin-V positive (Fig. 2G) and the expression of active caspase 3 and cleaved PARP (Fig. 2H), confirming that the combination induced apoptosis cooperatively.
In consistence with our hypothesis, simvastatin enhanced romidepsin-induced histone acetylation (Fig. 2I). Simvastatin activated AMPK and, interestingly, this activation was further promoted by romidepsin (Fig. 2I). Our previous studies showed that ER stress activates AMPK [16, 17], so we thought that the combination of romidepsin and simvastatin would also induce ER stress and thereby enhance AMPK activation. As expected, the combination induced ER stress cooperatively (Fig. 2J). We also found that the combination decreased the expression of HDAC1, 3, and 6 (Fig. 2J), which might further enhance the histone acetylation and ER stress. HDAC6 inhibition was confirmed by increased α-tubulin acetylation (Fig. 2J). Furthermore, the simvastatin-romidepsin combination increased the expression of PPARγ cooperatively (Fig. 2I), which was consistent with the combination-increased ROS production (Fig. 2K) because PPARγ activation triggers a metabolic switch that inhibits pyruvate oxidation resulting in an increase of cellular ROS levels [23].
AMPK activation was responsible for the enhanced histone acetylation and cytotoxicity caused by the simvastatin-romidepsin combination
The most combination-sensitive cells (T-24) and the least combination-sensitive cells (UMUC-3) were chosen for the subsequent in-vitro experiments to explore the combination's mechanism of action. First, we investigated the role of AMPK activation. The cells were treated with the simvastatin-romidepsin combination with or without the AMPK inhibitor compound C. Compound C significantly decreased the combination-induced increase of annexin-V positive cells, showing that inhibition of AMPK attenuated the combination-induced apoptosis (Fig. 3A). Furthermore, compound C suppressed the combination-enhanced histone acetylation (Fig. 3B). Thus, the AMPK activation was shown to be responsible for the enhanced histone acetylation and cytotoxicity caused by the combination.
Fig. 3.
AMP-activated protein kinase (AMPK) activation was responsible for the enhanced histone acetylation and cytotoxicity by the combination of simvastatin and romidepsin. (A) Cells were treated for 48 h with 5 μM simvastatin and 20 nM romidepsin with or without 5 μM compound C. Apoptotic cells were detected by annexin-V assay using flow cytometry. 10,000 cells were counted. Bar graphs show the increase in annexin-V positive cells. Data are expressed as mean ± SD from three independent experiments. FITC, fluorescein isothiocyanate; 7-AAD, 7-amino-actinomycin D. *p = 0.0495. (B) Western blotting for AMPK and acetylated histone. Cells were treated for 48 h with 5 μM simvastatin and 20 nM romidepsin with or without 5 μM compound C. Actin was used for the loading control. Representative blots are shown.
PPARγ activation played a pivotal role in killing bladder cancer cells
Romidepsin and simvastatin increased the expression of PPARγ cooperatively (Fig. 2I). To further investigate the role of PPARγ activation in killing bladder cancer cells, we treated the cells with the PPARγ activator rosiglitazone. Rosiglitazone inhibited the proliferation of bladder cancer cells in a dose-dependent manner (Fig. 4A), showing that PPARγ activation actually had an antiproliferative effect in bladder cancer cells. Although rosiglitazone is essentially a PPARγ agonist, not a transcription activator, it increased the expression of PPARγ in T-24 cells (Fig. 4B). Interestingly, rosiglitazone also induced ER stress and histone acetylation in a dose-dependent manner (Fig. 4B), suggesting that PPARγ activation regulates ER stress and histone acetylation.
Fig. 4.
Peroxisome proliferator-activated receptor (PPAR) γ activation played a pivotal role in killing bladder cancer cells. (A) Cells were treated for 48 h with 25–400 μM rosiglitazone and cell viability was measured using CCK-8 assay. Mean ± SD, n = 6. (B) Western blotting for PPARγ, glucose-regulated protein (GRP) 78, endoplasmic reticulum resident protein (ERp) 44, and acetylated histone. Cells were treated for 48 h with 50–200 μM rosiglitazone. Actin was used for the loading control. Representative blots are shown. (C) Cells were treated for 48 h with 50–100 μM rosiglitazone and/or 5–20 nM romidepsin and cell viability was measured using CCK-8 assay. Bars represent mean ± SD, n = 6. (D) Cells were treated for 48 h with 100 μM rosiglitazone and/or 20 nM romidepsin and reactive oxygen species production was measured by dihydroethidium (DHE) staining using flow cytometry. 10,000 cells were counted. Bar graphs show the relative DHE fluorescence intensity. Data are expressed as mean ± SD from three independent experiments. *p = 0.0495; N. S., not significant. (E) Cells were treated for 48 h with 100 μM rosiglitazone and/or 20 nM romidepsin. Apoptotic cells were detected by annexin-V assay using flow cytometry. 10,000 cells were counted. Bar graphs show the percentages of apoptotic cells. Data are expressed as mean ± SD from three independent experiments. FITC, fluorescein isothiocyanate; 7-AAD, 7-amino-actinomycin D. *p = 0.0495. (F) Western blotting for PPARγ, GRP78, ERp44, and acetylated histone. Cells were treated for 48 h with 100 μM rosiglitazone and/or 20 nM romidepsin. Actin was used for the loading control. Representative blots are shown.
We then treated the cells with rosiglitazone in combination with romidepsin to investigate romidepsin's ability to enhance PPARγ activator activity. The rosiglitazone-romidepsin combination inhibited the growth of bladder cancer cells synergistically (Fig. 4C, Table S4, and Fig. S4). It also cooperatively increased ROS production (Fig. 4D) and induced apoptosis (Fig. 4E). Mechanistically, the combination induced ER stress and histone acetylation cooperatively (Fig. 4F). These results suggested that PPARγ activation and consequent ER stress induction and histone acetylation played a pivotal role in killing bladder cancer cells exposed to the simvastatin-romidepsin combination.
ER stress induction is also an important mechanism of the combination's action
We next evaluated the contribution of ER stress induction to the combination's action. CHX is a protein synthesis inhibitor and a suppressor of ER stress induction [24], so we evaluated whether it attenuated the combination's antineoplastic activity. CHX significantly decreased the combination-induced increase in the number of the annexin-V positive cells (Fig. 5A), showing that ER stress induction also played an important role in the combination's antineoplastic effect. Mechanistically, CHX inhibited the combination-induced ER stress and histone acetylation (Fig. 5B), confirming that the histone acetylation was a consequence of the ER stress induction. Unexpectedly, CHX also inhibited the combination-increased PPARγ expression (Fig. 5B) and ROS production (Fig. 5C), suggesting that ER stress induction also regulates the PPARγ expression. To confirm the mechanism that ER stress induction kills bladder cancer cells, we then treated the cells with the ER stress inducer tunicamycin [25]. Tunicamycin inhibited the viability of bladder cancer cells in a dose-dependent manner (Fig. 5D) and increased the expression of AMPK, acetylated histone, and PPARγ (Fig. 5E). Thus, ER stress induction was also shown to be an important mechanism of the combination's action, regulating the expression of AMPK, acetylated histone, and even PPARγ.
Fig. 5.
Endoplasmic reticulum stress induction is also an important mechanism of the combination's action. (A) Cells were treated for 48 h with 5 μM simvastatin and 20 nM romidepsin with or without 5 μg/ml cycloheximide (CHX). Apoptotic cells were detected by annexin-V assay using flow cytometry. 10,000 cells were counted. Bar graphs show the increase in annexin-V positive cells. Data are expressed as mean ± SD from three independent experiments. FITC, fluorescein isothiocyanate; 7-AAD, 7-amino-actinomycin D. *p = 0.0495. (B) Western blotting for glucose-regulated protein (GRP) 78, endoplasmic reticulum resident protein (ERp) 44, acetylated histone, and peroxisome proliferator-activated receptor (PPAR) γ. Cells were treated for 48 h with 5 μM simvastatin and 20 nM romidepsin with or without 5 μg/ml CHX. Actin was used for the loading control. Representative blots are shown. (C) Cells were treated for 48 h with 5 μM simvastatin and 20 nM romidepsin with or without 5 μg/ml CHX and reactive oxygen species production was measured by dihydroethidium (DHE) staining using flow cytometry. 10,000 cells were counted. Bar graphs show the increase in relative DHE fluorescence intensity. Data are expressed as mean ± SD from three independent experiments. *p = 0.0495. (D) Cells were treated for 48 h with 0.1–2 μM tunicamycin and cell viability was measured using CCK-8 assay. Mean ± SD, n = 6. (E) Western blotting for GRP78, ERp44, AMP-activated protein kinase (AMPK), acetylated histone, and PPARγ. Cells were treated for 48 h with 0.5–2 μM tunicamycin. Actin was used for the loading control. Representative blots are shown.
The simvastatin-romidepsin combination inhibited bladder cancer growth in vivo
Finally, the in-vivo anticancer activity of the simvastatin-romidepsin combination was evaluated using mice MBT-2 allograft models. A 15-day treatment with the combination of simvastatin and romidepsin inhibited tumor growth significantly (Fig. 6A-B). Absence of significant loss of body weight suggested that the combination had no severe side effects leading to weight loss (Fig. 6B). Hematoxylin-eosin (HE) staining of the in-vivo tumor specimens showed that the combination caused marked tumor necrosis (Fig. 6C). We then analyzed the tumor specimens by western blotting and found that the combination of simvastatin and romidepsin increased the phosphorylation of AMPK and the expression of AMPK, acetylated histone, GRP78, and PPARγ (Fig. 6D), confirming that the combination has the same mechanism of action in vivo that it does in vitro.
Fig. 6.
The simvastatin-romidepsin combination inhibited bladder cancer growth in vivo. (A) A murine allograft model was established using MBT-2 cells. The vehicle group received intraperitoneal injections of dimethyl sulfoxide (DMSO) and the treatment groups received 15 mg/kg simvastatin or 0.5 mg/kg romidepsin or both. The injections of romidepsin were given twice per week and the injections of DMSO and simvastatin were given once a day for 15 days (5 days on, 2 days off). Mean ± SE, n = 5. *p = 0.0079 at day 15. (B) Changes in the body weight. Mean ± SD, n = 5. Note that there is no significant difference in the body weight among each group at day 15. N. S., not significant. (C) Hematoxylin eosin (HE) staining of the tumors. After 15 days of treatment, the animals were euthanized and the subcutaneous tumors were harvested and evaluated by microscopy using HE staining. (D) Western blotting for AMP-activated protein kinase (AMPK), acetylated histone, glucose-regulated protein (GRP) 78, and peroxisome proliferator-activated receptor (PPAR) γ. After 15 days of treatment, the animals were euthanized and the subcutaneous tumors were harvested, lysed, and subjected to western blotting. Actin was used for the loading control. Representative blots are shown. Box-plot graphs show the relative densities of bands normalized to actin. n = 5. *p = 0.0079. V, vehicle-treated mice; R, romidepsin-treated mice; S, simvastatin-treated mice; R + S, combination-treated mice.
Discussion
HMG-CoA reductase inhibitors have various physiological properties such as regulating metabolisms [5], inhibiting inflammation [26], modulating the immune system [27], and inhibiting cancer growth [7, 8]. Furthermore, they have been reported to act against cancer in an additive or synergistic way when combined with other anticancer agents [28, 29]. Simvastatin is a clinically available HMG-CoA reductase inhibitor. Preclinical studies demonstrated that it induces apoptosis and inhibits tumor growth in a variety of cancer cells [7, 8]. Combinations of simvastatin and an anticancer agent or radiotherapy have not been effective in patients with advanced cancer (Table S5) [30], [31], [32], [33], [34], [35]; however, given simvastatin's wide-ranging physiological properties, there is still a need to evaluate the anticancer effect of simvastatin in combination with other types of anticancer agents.
AMPK is a molecule which controls cellular energy homeostasis and metabolism essential for cancer proliferation [36] and therefore its activation is considered to be a novel anticancer mechanism [4]. Several studies demonstrated that simvastatin inhibits tumor growth by activating AMPK [7, 8] and AMPK activation has been shown to induce histone acetylation [15], [16], [17]. We therefore thought that combining simvastatin with an HDAC inhibitor would kill cancer cells effectively by inducing histone acetylation cooperatively.
HDAC inhibitors have emerged as innovative anticancer drugs because they can influence chromatin structure by acetylating histone, leading to gene upregulation inducing apoptosis and inhibiting cell proliferation [37]. In addition, they are known to increase the amount of unfolded proteins by suppressing the molecular chaperone function, thereby inducing ER stress and killing cancer cells [38]. In the present study, we tested several HDAC inhibitors for their antiproliferative effect in bladder cancer cells and found that romidepsin had the lowest IC50 value.
Simvastatin enhanced romidepsin-induced histone acetylation and effectively inhibited the growth of bladder cancer cells. In consistence with our hypothesis, AMPK activation was shown to be responsible for the enhanced histone acetylation and cytotoxicity caused by the combination. Unexpectedly, the simvastatin-induced AMPK activation was further promoted by romidepsin, which might also play a role in enhancing the histone acetylation. This AMPK activation was thought to be due to ER stress induction by the combination because the ER stressor tunicamycin increased the expression of AMPK, which is consistent with the previous reports [16, 17, 39, 40]. ER stress is caused by the accumulation and aggregation of unfolded proteins, and excessive ER stress causes apoptosis and kills cancer cells [41]. We have demonstrated that ER stress-inducing drugs or drug combinations killed urological cancers effectively [39, 40, 42, 43] and, in fact, tunicamycin actually inhibited bladder cancer growth in a dose-dependent fashion. In the present study, inhibition of ER stress by cycloheximide markedly impaired the combination's ability to cause histone acetylation and induce apoptosis, suggesting that the ER stress induction played a pivotal role in the combination's action. This ER stress-histone acetylation sequence is also consistent with our previous results that there is a crosstalk between histone acetylation and ER stress induction [16, 17, 39, 40, 43]. The decreased expression of HDACs is thought to be a consequence of the ER stress induction according to the previous studies [16, 17, 39, 40, 43]. This HDAC suppression might further enhance the histone acetylation and even the ER stress because HDAC suppression abrogates molecular chaperone function, causing the accumulation of unfolded proteins [38]. Thus, the combination forms a vicious cycle of ER stress induction and histone acetylation, killing cancer cells effectively.
In the present study, the combination of simvastatin and romidepsin increased the expression of PPARγ cooperatively. PPAR is a member of a superfamily of nuclear hormone receptors and regulates lipid metabolism as a lipid sensor [18]. Interestingly, activation of PPARγ has been shown to exert both anti-inflammatory and antineoplastic effects [18, 23, 44] and the efficacy of PPARγ agonists has been evaluated in preclinical studies and clinical trials in various cancer patients [45]. Also in the present study, the PPARγ agonist rosiglitazone actually inhibited bladder cancer growth. Furthermore, the bladder cancer patients with higher expression of PPARγ genes had longer overall survival time than those with lower expression (Fig. S1). Thus, activation of PPARγ would be an attractive approach to killing bladder cancer cells. HDAC inhibitors were reported to interact cooperatively with PPARγ agonists to kill cancer cells [46, 47], and romidepsin actually enhanced the activity of rosiglitazone in the present study. Therefore, we inferred that romidepsin enhanced the ability of simvastatin to increase the expression of PPARγ. ER stress and histone acetylation caused by the combination of simvastatin and romidepsin was thought to be a consequence of the increased PPARγ expression because rosiglitazone itself also induced both ER stress and histone acetylation. On the other hand, inhibition of the combination-caused ER stress decreased the combination-increased PPARγ expression and ER stress induction by tunicamycin increased the PPARγ expression. These results suggest that there is a positive feedback cycle between ER stress induction and PPARγ expression. To our knowledge, this is the first study to show a dual regulation of ER stress induction and PPARγ expression. The possible mechanism of the combination's action is summarized in Fig. S5.
The in-vivo efficacy and safety of the simvastatin-romidepsin combination were proved by the 15-day treatment in the present study, and clinical application would be the next step. One concern is that the combination's efficacy might substantially differ among patients because muscle-invasive bladder cancer is a heterogeneous disease with different molecular subtypes [48]. We think that the combination would act similarly in bladder cancer patients with different subtypes because the antiproliferative effect of the long-term (72 h) treatment with the combination was very similar among the cell lines (Fig. 2C). Furthermore, the combination of an HMG-CoA reductase inhibitor and an HDAC inhibitor has been shown to be effective in renal cancer cells [17], which supports our hypothesis that the simvastatin-romidepsin combination would be effective irrespective of cell type.
In the present study, we proved the combined anti-bladder cancer effects of the clinically feasible drugs simvastatin and romidepsin. Developing new drugs takes so much time and money that, using clinically feasible drugs for other purposes, i.e., drug repositioning, has emerged because it could lower the cost of developing new drugs and introduce them into the market quickly [49]. It has also been applied to find therapeutic agents against coronavirus disease 2019 in the midst of a global emergency when there is no time to conduct phase-III trials [50]. We believe that the present study would contribute to delivering novel anti-bladder cancer therapy to our patients more quickly than would developing new drugs. Furthermore, as mentioned elsewhere, our previous study showed that the combination of the HMG-CoA reductase inhibitor fluvastatin and the HDAC inhibitor vorinostat was effective in renal cancer which has biological properties completely different from those of bladder cancer [17]. Thus, combining an HMG-CoA reductase inhibitor and an HDAC inhibitor might be capable of killing cancer of any type. Pursuing the most effective combination and expanding the research to other types of cancer would be our next step. Our study has limitations, however. First, we investigated the in-vivo efficacy of the combination using the mice allograft model. Fortunately, we could prove the same mechanism of action both in vitro and in vivo using the same mouse cells (MBT-2), but xenograft models would be preferable to show the antineoplastic effects of the combination more precisely on human bladder cancer. Secondly, we did not evaluate the interaction that the drugs may have on their blood concentrations. In-vivo experiments using mice models without tumor burden would be the appropriate method and might be the next step. Although the safety of each drug has been established, what interaction they might have is unknown. Careful drug monitoring should therefore be performed when testing the combination in clinical settings.
Conclusions
The combination of simvastatin and romidepsin kills bladder cancer cells synergistically. Its mechanism of action includes ER stress induction, AMPK activation, histone acetylation, and increased PPARγ expression.
CRediT authorship contribution statement
Kazuki Okubo: Conceptualization, Methodology, Investigation, Writing – original draft. Kosuke Miyai: Validation, Investigation, Visualization. Kimi Kato: Validation, Investigation. Takako Asano: Methodology, Resources. Akinori Sato: Validation, Supervision, Writing – review & editing.
Declaration of Competing Interests
The authors declare that they have no competing interests.
Acknowledgments
This work was supported by JSPS KAKENHI Grant No. JP18K09183.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2021.101154.
Appendix. Supplementary materials
References
- 1.Mollica V., Rizzo A., Montironi R., Cheng L., Giunchi F., Schiavina R. Current strategies and novel therapeutic approaches for metastatic urothelial carcinoma. Cancers (Basel) 2020;12:1449. doi: 10.3390/cancers12061449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rizzo A., Mollica V., Massari F. Expression of programmed cell death ligand 1 as a predictive biomarker in metastatic urothelial carcinoma patients treated with first-line Immune checkpoint inhibitors versus chemotherapy: a systematic review and meta-analysis. Eur. Urol. Focus. 2021 doi: 10.1016/j.euf.2021.01.003. S2405-4569(21)00004–3. [DOI] [PubMed] [Google Scholar]
- 3.Mihaylova M.M., Shaw R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faubert B., Vincent E., Poffenberger M., Jones R.G. The AMP-activated protein kinase (AMPK) and cancer: many faces of a metabolic regulator. Cancer Lett. 2015;356:165–170. doi: 10.1016/j.canlet.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 5.Istvan E.S., Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001;292:1160–1164. doi: 10.1126/science.1059344. [DOI] [PubMed] [Google Scholar]
- 6.Sun W., Lee T.S., Zhu M., Gu C., Wang Y., Zhu Y. Statins activate AMP-activated protein kinase in vitro and in vivo. Circulation. 2006;114:2655–2662. doi: 10.1161/CIRCULATIONAHA.106.630194. [DOI] [PubMed] [Google Scholar]
- 7.Kamel W.A., Sugihara E., Nobusue H., Yamaguchi-Iwai S., Onishi N., Maki K. Simvastatin-induced apoptosis in osteosarcoma cells: a key role of RhoA-AMPK/p38 MAPK signaling in antitumor activity. Mol. Cancer Ther. 2017;16:182–192. doi: 10.1158/1535-7163.MCT-16-0499. [DOI] [PubMed] [Google Scholar]
- 8.Wang S.T., Ho H.J., Lin J.T., Shieh J.J., Wu C.Y. Simvastatin-induced cell cycle arrest through inhibition of STAT3/SKP2 axis and activation of AMPK to promote p27 and p21 accumulation in hepatocellular carcinoma cells. Cell Death. Dis. 2017;8:e2626. doi: 10.1038/cddis.2016.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strandberg T.E., Pyörälä K., Cook T.J., Wilhelmsen L., Faergeman O., Thorgeirsson G. Mortality and incidence of cancer during 10-year follow-up of the Scandinavian Simvastatin Survival Study (4S) Lancet. 2004;364:771–777. doi: 10.1016/S0140-6736(04)16936-5. [DOI] [PubMed] [Google Scholar]
- 10.Olzscha H., Sheikh S., La Thangue N.B. Deacetylation of chromatin and gene expression regulation: a new target for epigenetic therapy. Crit. Rev. Oncog. 2015;20:1–17. doi: 10.1615/critrevoncog.2014012463. [DOI] [PubMed] [Google Scholar]
- 11.Pinkerneil M., Hoffmann M.J., Deenen R., Köhrer K., Arent T., Schulz W.A. Inhibition of class I histone deacetylases 1 and 2 promotes urothelial carcinoma cell death by various mechanisms. Mol. Cancer Ther. 2016;15:299–312. doi: 10.1158/1535-7163.MCT-15-0618. [DOI] [PubMed] [Google Scholar]
- 12.Kaletsch A., Pinkerneil M., Hoffmann M.J., Jaguva Vasudevan A.A., Wang C., Hansen F.K. Effects of novel HDAC inhibitors on urothelial carcinoma cells. Clin. Epigenet. 2018;10:100. doi: 10.1186/s13148-018-0531-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whittaker S.J., Demierre M.F., Kim E.J., Rook A.H., Lerner A., Duvic M. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J. Clin. Oncol. 2010;28:4485–4491. doi: 10.1200/JCO.2010.28.9066. [DOI] [PubMed] [Google Scholar]
- 14.Amiri-Kordestani L., Luchenko V., Peer C.J., Ghafourian K., Reynolds J., Draper D. Phase I trial of a new schedule of romidepsin in patients with advanced cancers. Clin. Cancer Res. 2013;19:4499–4507. doi: 10.1158/1078-0432.CCR-13-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang M., Galdieri L., Vancura A. The yeast AMPK homolog SNF1 regulates acetyl coenzyme A homeostasis and histone acetylation. Mol. Cell. Biol. 2013;33:4701–4717. doi: 10.1128/MCB.00198-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okubo K., Isono M., Asano T., Sato A. Metformin augments panobinostat's anti-bladder cancer activity by activating AMP-activated protein kinase. Transl. Oncol. 2019;12:669–682. doi: 10.1016/j.tranon.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okubo K., Isono M., Miyai K., Asano T., Sato A. Fluvastatin potentiates anticancer activity of vorinostat in renal cancer cells. Cancer Sci. 2020;111:112–126. doi: 10.1111/cas.14225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berger J., Moller D.E. The mechanisms of action of PPARs. Annu. Rev. Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 19.Mansure J.J., Nassim R., Kassouf W. Peroxisome proliferator-activated receptor gamma in bladder cancer: a promising therapeutic target. Cancer Biol. Ther. 2009;8:6–15. doi: 10.4161/cbt.8.7.7853. [DOI] [PubMed] [Google Scholar]
- 20.Habibi H., Atashi A., Abroun S., Noruzinia M. Synergistic effect of simvastatin and romidepsin on gamma-globin gene induction. Cell J. 2019;20:576–583. doi: 10.22074/cellj.2019.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Workman P., Aboagye E.O., Balkwill F., Balmain A., Bruder G., Chaplin D.J. Guidelines for the welfare and use of animals in cancer research. Br. J. Cancer. 2010;102:1555–1577. doi: 10.1038/sj.bjc.6605642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chou T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 23.Srivastava N., Kollipara R.K., Singh D.K., Sudderth J., Hu Z., Nguyen H. Inhibition of cancer cell proliferation by PPARγ is mediated by a metabolic switch that increases reactive oxygen species levels. Cell Metab. 2014;20:650–661. doi: 10.1016/j.cmet.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ram B.M., Ramakrishna G. Endoplasmic reticulum vacuolation and unfolded protein response leading to paraptosis like cell death in cyclosporine A treated cancer cervix cells is mediated by cyclophilin B inhibition. Biochim. Biophys. Acta. 2014;1843:2497–2512. doi: 10.1016/j.bbamcr.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 25.Wu J., Chen S., Liu H., Zhang Z., Ni Z., Chen J. Tunicamycin specifically aggravates ER stress and overcomes chemoresistance in multidrug-resistant gastric cancer cells by inhibiting N-glycosylation. J. Exp. Clin. Cancer Res. 2018;37:272. doi: 10.1186/s13046-018-0935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forrester J.S., Libby P. The inflammation hypothesis and its potential relevance to statin therapy. Am. J. Cardiol. 2007;99:732–738. doi: 10.1016/j.amjcard.2006.09.125. [DOI] [PubMed] [Google Scholar]
- 27.Zeiser R. Immune modulatory effects of statins. Immunology. 2018;154:69–75. doi: 10.1111/imm.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agarwal B., Bhendwal S., Halmos B., Moss S.F., Ramey W.G., Holt P.R. Lovastatin augments apoptosis induced by chemotherapeutic agents in colon cancer cells. Clin. Cancer Res. 1999;5:2223–2229. [PubMed] [Google Scholar]
- 29.Kou X., Yang Y., Jiang X., Liu H., Sun F., Wang X. Vorinostat and simvastatin have synergistic effects on triple-negative breast cancer cells via abrogating Rab7 prenylation. Eur. J. Pharmacol. 2017;813:161–171. doi: 10.1016/j.ejphar.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 30.Lee Y., Lee K.H., Lee G.K., Lee S.H., Lim K.Y., Joo J. Randomized phase II study of afatinib plus simvastatin versus afatinib alone in previously treated patients with advanced nonadenocarcinomatous non-small cell lung cancer. Cancer Res. Treat. 2017;49:1001–1011. doi: 10.4143/crt.2016.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Hamamsy M., Elwakil H., Saad A.S., Shawki M.A. A randomized controlled open-label pilot study of simvastatin addition to whole-brain radiation therapy in patients with brain metastases. Oncol. Res. 2016;24:521–528. doi: 10.3727/096504016X14719078133528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim S.H., Kim T.W., Hong Y.S., Han S.W., Lee K.H., Kang H.J. A randomised, double-blind, placebo-controlled multi-centre phase III trial of XELIRI/FOLFIRI plus simvastatin for patients with metastatic colorectal cancer. Br. J. Cancer. 2015;113:1421–1426. doi: 10.1038/bjc.2015.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim S.T., Kang J.H., Lee J., Park S.H., Park J.O., Park Y.S. Simvastatin plus capecitabine-cisplatin versus placebo plus capecitabine-cisplatin in patients with previously untreated advanced gastric cancer: a double-blind randomised phase 3 study. Eur. J. Cancer. 2014;50:2822–2830. doi: 10.1016/j.ejca.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Hong J.Y., Nam E.M., Lee J., Park J.O., Lee S.C., Song S.Y. Randomized double-blinded, placebo-controlled phase II trial of simvastatin and gemcitabine in advanced pancreatic cancer patients. Cancer Chemother. Pharmacol. 2014;73:125–130. doi: 10.1007/s00280-013-2328-1. [DOI] [PubMed] [Google Scholar]
- 35.Han J.Y., Lee S.H., Yoo N.J., Hyung L.S., Moon Y.J., Yun T. A randomized phase II study of gefitinib plus simvastatin versus gefitinib alone in previously treated patients with advanced non-small cell lung cancer. Clin. Cancer Res. 2011;17:1553–1560. doi: 10.1158/1078-0432.CCR-10-2525. [DOI] [PubMed] [Google Scholar]
- 36.Cantor J.R., Sabatini D.M. Cancer cell metabolism: one hallmark, many faces. Cancer Discov. 2012;2:881–898. doi: 10.1158/2159-8290.CD-12-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gallinari P., Di Marco S., Jones P., Pallaoro M., Steinkühler C. HDACs, histone deacetylation and gene transcription: from molecular biology to cancer therapeutics. Cell Res. 2007;17:195–211. doi: 10.1038/sj.cr.7310149. [DOI] [PubMed] [Google Scholar]
- 38.Bali P., Pranpat M., Bradner J., Balasis M., Fiskus W., Guo F. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J. Biol. Chem. 2005;280:26729–26734. doi: 10.1074/jbc.C500186200. [DOI] [PubMed] [Google Scholar]
- 39.Sato A., Asano T., Okubo K., Isono M., Asano T. Nelfinavir and ritonavir kill bladder cancer cells synergistically by inducing endoplasmic reticulum stress. Oncol. Res. 2018;26:323–332. doi: 10.3727/096504017X14957929842972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okubo K., Isono M., Asano T., Sato A. Panobinostat and nelfinavir inhibit renal cancer growth by inducing endoplasmic reticulum stress. Anticancer Res. 2018;38:5615–5626. doi: 10.21873/anticanres.12896. [DOI] [PubMed] [Google Scholar]
- 41.Mimnaugh E.G., Xu W., Vos M., Yuan X., Isaacs J.S., Bisht K.S. Simultaneous inhibition of hsp 90 and the proteasome promotes protein ubiquitination, causes endoplasmic reticulum-derived cytosolic vacuolization, and enhances antitumor activity. Mol. Cancer Ther. 2004;3:551–566. [PubMed] [Google Scholar]
- 42.Okubo K., Sato A., Isono M., Asano T., Asano T. Nelfinavir induces endoplasmic reticulum stress and sensitizes renal cancer cells to TRAIL. Anticancer Res. 2018;38:4505–4514. doi: 10.21873/anticanres.12754. [DOI] [PubMed] [Google Scholar]
- 43.Sato A., Asano T., Okubo K., Isono M., Asano T. Ritonavir and ixazomib kill bladder cancer cells by causing ubiquitinated protein accumulation. Cancer Sci. 2017;108:1194–1202. doi: 10.1111/cas.13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grommes C., Landreth G.E., Heneka M.T. Antineoplastic effects of peroxisome proliferator-activated receptor gamma agonists. Lancet Oncol. 2004;5:419–429. doi: 10.1016/S1470-2045(04)01509-8. [DOI] [PubMed] [Google Scholar]
- 45.Burstein H.J., Demetri G.D., Mueller E., Sarraf P., Spiegelman B.M., Winer E.P. Use of the peroxisome proliferator-activated receptor (PPAR) gamma ligand troglitazone as treatment for refractory breast cancer: a phase II study. Breast Cancer Res. Treat. 2003;79:391–397. doi: 10.1023/a:1024038127156. [DOI] [PubMed] [Google Scholar]
- 46.Aouali N., Palissot V., El-Khoury V., Moussay E., Janji B., Pierson S. Peroxisome proliferator-activated receptor gamma agonists potentiate the cytotoxic effect of valproic acid in multiple myeloma cells. Br. J. Haematol. 2009;147:662–671. doi: 10.1111/j.1365-2141.2009.07902.x. [DOI] [PubMed] [Google Scholar]
- 47.Davies G.F., Ross A.R., Arnason T.G., Juurlink B.H., Harkness T.A. Troglitazone inhibits histone deacetylase activity in breast cancer cells. Cancer Lett. 2010;288:236–250. doi: 10.1016/j.canlet.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 48.Kamoun A., de Reyniès A., Allory Y. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. Eur. Urol. 2020;77:420–433. doi: 10.1016/j.eururo.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ashburn T.T., Thor K.B. Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004;3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 50.Ciliberto G., Mancini R., Paggi M.G. Drug repurposing against COVID-19: focus on anticancer agents. J. Exp. Clin. Cancer Res. 2020;39:86. doi: 10.1186/s13046-020-01590-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.