Abstract
The world is currently engaged in a race of vaccination versus infection in an effort to control the COVID-19 pandemic. Some countries have already achieved high vaccination rates, offering a glimpse into the so-called “post-vaccination” world. We describe here a striking comparison between the similar-sized and neighboring countries of Bahrain and Qatar. While both countries have achieved impressive vaccination rates, cases increased to unprecedented levels in one country while decreasing steadily in the other. Although this could be attributed to a number of factors, we argue here that the heavy reliance on alum-adjuvanted inactivated virus vaccines may have contributed to these discrepant outcomes. We then expand the analysis to compare the outcomes of the top 10 vaccinated countries based on their reliance on inactivated virus vaccines. The results remarkably align with the initial findings seen in Bahrain and Qatar. Countries that did not use inactivated virus vaccines achieved steady declines in daily COVID-19 deaths, while other countries did not. This work highlights the urgent need to further study the effectiveness of alum-adjuvanted inactivated virus vaccines for COVID-19 before expanding their use.
Keywords: COVID-19 vaccines, BIBP vaccine, alum adjuvant, Coronavac, inactivated virus vaccines, mRNA vaccines, adenovirus-vector vaccines
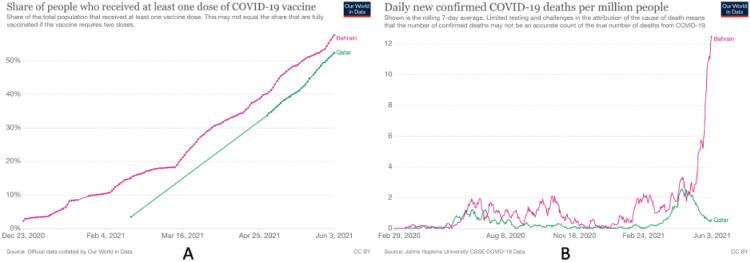
With the race to vaccinate the world against SARS-CoV-2 well on its way, many countries have already achieved impressive vaccination rates and are quickly reaping the benefits. However, not all highly vaccinated populations are seeing the same results. A striking example of such divergent outcomes is currently being seen in the neighboring states of Qatar and Bahrain. In an impressive feat, both nations have vaccinated more than 50% of their population. However, while in Qatar, infection rates have decreased, infection rates have increased to unprecedented and alarming levels in Bahrain (Figure 1 ).
Figure 1.
Excellent vaccination rates, yet divergent outcomes. A: Percentage of the population who received at least one dose of COVID-19 vaccine in Qatar and Bahrain between December 23, 2020, and June 3, 2021. B: Daily confirmed COVID-19 deaths per million people in Qatar and Bahrain, shown as a rolling 7-day average of reported figures (Hannah et al., 2021).
What could explain such divergent outcomes? While multiple factors are likely at play here, there is a major difference between the 2 countries in the types of vaccines being deployed. In Bahrain, the most commonly used vaccine has been the BIBP (Sinopharm) vaccine, accounting for approximately 60% of its inoculations (Trofimov and Said, 2021). Qatar, on the other hand, has relied solely on the BNT162b2 (Pfizer/BioNTech) and mRNA-1273 (Moderna) vaccines (Qatar, 2021).
There is a significant difference in the immune responses elicited by the different vaccines. The BIBP vaccine's mechanism is based on an inactivated SARS-CoV-2 virus with an alum adjuvant. It has been shown to efficiently induce the production of neutralizing antibodies (Xia et al., 2021), but there are no studies that directly compare the neutralizing antibody activity from this vaccine to others. One study by Khoury et al used modeling to predict the protective efficacy of different vaccines in preventing symptomatic SARS-CoV-2 infection based on neutralizing antibody titers (Khoury et al., 2021). While this study did not directly address the BIBP vaccine, it showed that Coronavac, an alum-adjuvanted inactivated virus vaccine, had less protective efficacy than mRNA adenovirus-vector vaccines and that lower protective efficacy correlated strongly with lower neutralizing antibody titers. This study also predicted that a lower starting efficacy would result in a larger reduction in efficacy over time and against variants (Khoury et al., 2021).
Another difference between the BIBP vaccine and mRNA and adenovirus-vector vaccines is cellular immune responses. Data on the cellular immune responses to the BIBP vaccine are lacking. However, it is generally accepted that alum-adjuvanted inactivated virus vaccines induce no or weak cellular responses, specifically cytotoxic CD8 T cell responses (Jeyanathan et al., 2020). On the other hand, mRNA and adenovirus-vector vaccines have shown robust, Th1 predominant cellular adaptive immune responses, with the production of antigen-specific CD4 and CD8 T cells (Anderson et al., 2020; Ewer et al., 2021; Jeyanathan et al., 2020; Walsh et al., 2020). The presence of cellular immune responses in combination with neutralizing antibodies is thought to confer long-lasting immunity and improve outcomes in the case of acute infection (Dan et al., 2021; Rydyznski Moderbacher et al., 2020). In addition, while emerging SARS-CoV-2 variants of concern have shown significant escape from neutralizing antibody activity, the activity of cellular immune responses against variants appears to be preserved (Geers et al., 2021).
As such, a potential hypothesis to explain the divergent epidemiologic outcomes observed between Qatar and Bahrain is that the BIBP vaccine used heavily in Bahrain induces weaker humoral responses, cellular responses, or both. While the BIBP vaccine showed efficacy of 73%–78% against symptomatic infection, according to interim analysis of a phase 3 randomized clinical trial of the vaccine (Al Kaabi et al., 2021), it should be noted that the data were collected on healthy young adults (mean age 36.1 years), and for a median follow-up of only 77 days, ending on December 20, 2020. As such, we do not have conclusive data on the duration of protection conferred by the BIBP vaccine or its efficacy in vulnerable populations, against variants of concern, in preventing severe disease or in preventing transmission of infection. On the other hand, efficacy in the above domains has been demonstrated for mRNA and adenovirus-vector vaccines (Public Health England, 2021).
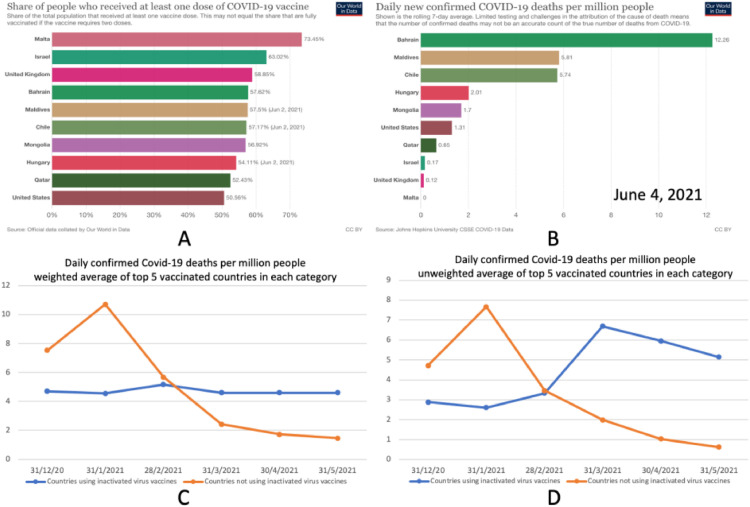
Although the divergent outcomes observed in Qatar and Bahrain are potentially the most striking, the trend also seems to hold in other countries. Looking at countries with a population exceeding 500 000 and with vaccination data updated as of June 2, 2021, among the 10 countries with the highest doses of COVID-19 vaccines administered per capita, 5 relied in part on alum-adjuvanted inactivated virus vaccines: Bahrain, Chile, Hungary, Maldives, and Mongolia (Hannah et al., 2021; Snyder and Root-Wiley, 2021). The other 5 countries among the top 10—Israel, Malta, Qatar, United Kingdom and the United States—relied only on mRNA and/or adenovirus-vector vaccines (Hannah et al., 2021; Snyder and Root-Wiley, 2021). Figure 2 shows data from these 10 countries. While the 10 countries have achieved high vaccination rates, on June 4, 2021, all 5 countries that relied on inactivated virus vaccines had higher daily COVID-19 deaths per million people (Figure 2, A and B). Additionally, the trend for the average daily COVID-19 deaths per million population across the 5 nations that did not rely on inactivated virus vaccines showed a steady decline after January 31, 2021. However, for the other 5 nations, which relied on inactivated virus vaccines, such a decline was not observed (Figure 2, C and D). Thus, across highly vaccinated countries, the reliance on inactivated virus vaccines seems to be associated with worse outcomes.
Figure 2.
Comparing daily confirmed COVID-19 deaths among the top 10 vaccinated countries. Bahrain, Maldives, Chile, Hungary, and Mongolia relied heavily on inactivated virus vaccines while the United States, Qatar, Israel, United Kingdom and Malta did not. A: Percentage of people in each of the top 10 countries who received at least one dose of COVID-19 vaccine. B: Daily confirmed COVID-19 deaths per million people in each of the 10 countries, on June 4, 2021; a rolling 7-day average of reported figures is shown. C: Population-weighted average of daily confirmed COVID-19 deaths per million people among the top 5 vaccinated countries in each category (top 5 vaccinated countries that relied on inactivated virus vaccines vs top 5 vaccinated countries that did not rely on inactivated virus vaccines). D: Unweighted average of the daily confirmed COVID-19 deaths per million people. Daily confirmed COVID-19 deaths at 6 specific dates shown; numbers reflect a rolling 7-day average on each date. Countries were included if their population exceeded 500 000 and had updated vaccination data as of June 2, 2021. Source: Our World in Data (Hannah et al., 2021).
An alternative explanation for such discrepancies in outcomes is that countries with high or increasing mortality rates relaxed social distancing measures too quickly. However, what is the likelihood that all 5 countries that relied on inactivated virus vaccines made that same error? There is also a difference in the socioeconomic status of the countries being compared. The combined GDP per capita of the 5 countries that relied on inactivated virus vaccines was US $14 069, compared with US $56 187 for the other countries (GDP by Country, 2017). This factor may contribute to a country's ability to control the spread of infection. However, it should be noted that the 5 countries with lower GDP per capita had, on average, better control of the pandemic at the start of their vaccination campaigns compared with the 5 other countries (Figure 2, C and D). Additionally, if there was no drop in the effectiveness of the vaccines, then why are some countries now offering an extra dose of an mRNA vaccine to those who already received 2 doses of an inactivated virus vaccine (Trofimov and Said, 2021)?
The evidence presented in this short communication is circumstantial and not conclusive. Scientific studies on the long-term efficacy of alum-adjuvanted inactivated virus vaccines and their efficacy in vulnerable populations, in preventing severe disease, in preventing transmission of infection and against variants of concern are urgently needed. Such evidence should be considered before more resources are allocated towards the deployment of these vaccines. The danger of deploying vaccines that may not provide sufficient long-lasting protection or protection against variants of concern is that new waves of infection may return, and more variants of concern may emerge. Additionally, the deployment of sub-optimal vaccines may threaten trust in vaccination campaigns overall.
Conflict of interest: The authors certify that they have no affiliations with or involvement in any organization or entity with any financial or non-financial interest in the subject matter or materials discussed in this article.
Funding source: No funding was provided for this research.
Ethical approval: This research, relying exclusively on publicly available information, did not require ethical review.
Footnotes
Authors’ contributions: All authors made substantial contributions to this paper based on the ICMJE criteria.
Zaid Alhinai: Contributed to the writing of the manuscript, design of figures, and analysis of results.
Nagi Elsidig: Contributed to the writing and editing of the manuscript.
References
- Anderson EJ, Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M, et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N Engl J Med. 2020;383:2427–2438. doi: 10.1056/nejmoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;(80-):371. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewer KJ, Barrett JR, Belij-Rammerstorfer S, Sharpe H, Makinson R, Morter R, et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat Med. 2021;27:270–278. doi: 10.1038/s41591-020-01194-5. [DOI] [PubMed] [Google Scholar]
- Geers D, Immunol S, Daryl Geers, Shamier MC, Bogers S, Den Hartog G, et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. 2021;1750:1–22. doi: 10.1126/sciimmunol.abj1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah R, Ortiz-Ospina E, Beltekian D, Mathieu E, Hasell J, Macdonald B, et al. Coronavirus (COVID-19) Vaccinations - Statistics and Research - Our World in Data. Ourworldindata. 2021 https://ourworldindata.org/covid-vaccinations (accessed June 7, 2021) [Google Scholar]
- Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. 2020;20:615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Kaabi N, Zhang Yuntao, Xia S, Yang Y, Al Qahtani MM, Abdulrazzaq N, et al. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults: A Randomized Clinical Trial. Jama. 2021 doi: 10.1001/jama.2021.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021 doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- Worldometer . Worldometer; 2017. GDP by Country.https://www.worldometers.info/gdp/gdp-by-country/ [Google Scholar]
- Public Health England. COVID-19 vaccine surveillance report - week 22 2021:1–23.
- Rydyznski Moderbacher C, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell. 2020;183:996–1012. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qatar COVID-19 Vaccine. Minist Public Heal State Qatar 2021. https://covid19.moph.gov.qa/EN/Covid19-Vaccine/Pages/default.aspx (accessed June 7, 2021).
- Snyder J, Root-Wiley M. COVID-19 Vaccine Tracker. COVID-19 Vaccine Tracker 2021. https://covid19.trackvaccines.org/ (accessed June 7, 2021).
- Trofimov Y, Said S.Bahrain. Facing a Covid Surge, Starts Giving Pfizer Boosters to Recipients of Chinese Vaccine - WSJ. Wall Str J. 2021 [Google Scholar]
- Walsh EE, Frenck RW, Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/nejmoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Zhang Y, Wang Y, Wang Hui, Yang Yunkai, Gao GF, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]