Summary
The global burden of snakebites is growing, particularly its nonfatal sequelae. Therefore, the World Health Organization reinstated snakebites to its list of Neglected Tropical Diseases. We describe the case of a 4.5-year-old boy who was bitten by a spitting cobra, resulting in considerable local swelling accompanied by a right-sided facial paralysis due to neurotoxicity by cobra venom. Presently, surgical methods to recover facial paralysis include nerve repair, nerve grafting, nerve transfers, static slings, muscle transfers, and functional muscle transplantations. However, mime therapy consisting of neuromuscular retraining resulted in a good functional result with a moderate contour deficiency of the right cheek and a subtle paresis of the zygomatic muscles at 1 year and 9 months follow-up. The natural history of facial paralysis in our case shows that this condition can be transient and may resolve with mime therapy as a conservative measure.
Key words [Mesh]: Facial paralysis, Snake bites, Venoms, Neurological rehabilitation
Background
The global incidence of snakebites may be as high as 1.8 - 2.5 million per year, with 94,000 - 125,000 deaths annually. An unknown proportion of patients survives with permanent disability caused by local tissue necrosis and organ dysfunction1. Most victims are farmers and children living in isolated rural areas, resulting in a high socioeconomic impact on their surroundings. Therefore, the World Health Organization reinstated snakebites to its list of Neglected Tropical Diseases2, 3. The case presented here shows that the increase in safaris to Southern Africa may confront more tourists with the effects of snake bites, which could be survived with only transient disability.
Case presentation
A 4.5-year-old boy with no medical history presented with a cheek abscess after he was bitten by a spitting cobra during a safari in South Africa, 12 days earlier. Shortly after the bite, he was admitted to the Intensive Care unit of a rural hospital where he was resuscitated and successfully treated with an antidote: polyvalent snake antivenom (South African Institute for Medical Research, Johannesburg, South Africa).
Investigations
Examination at our outpatient clinic revealed a paralysis of the right cheek and lower eyelid muscles and a fluctuating painful swelling, which was palpable intraorally (Figure 1). A clinical diagnosis of an incomplete facial paralysis caused by snake venom neurotoxins was made, with subsequent fat necrosis and abscess formation.
Figure 1.
Twelve days after the cobra bite. Presentation to our hospital with fluctuating swelling and right-sided facial paralysis.
Treatment
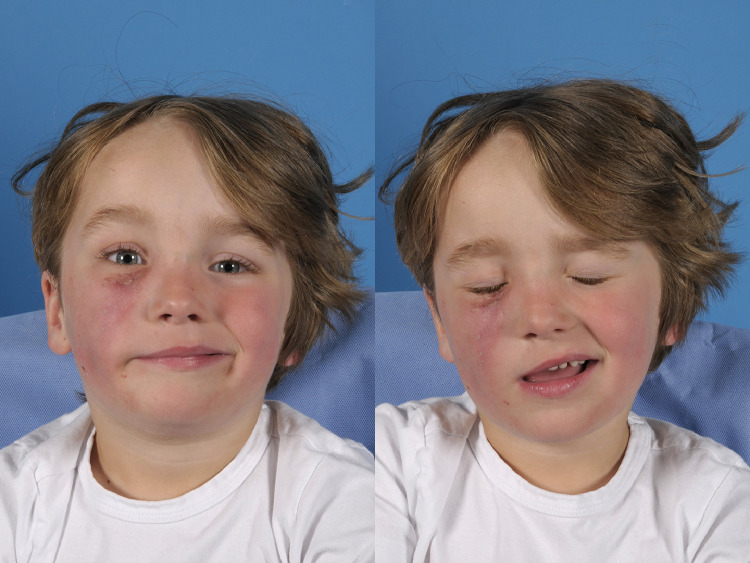
Two incisions were made to drain the abscess, which was filled with a milky, sterile fluid. A Swanson drain was left enabling flushing of the wound. One week postoperatively, the right cheek showed less erythema but was indurated with decreased sensation in the area of the infraorbital nerve. An absent nasolabial fold was observed, with a deviation of the philtrum to the left and a severe palsy of the orbicularis oculi muscle of the lower eyelid, right upper lip orbicularis oris, and right zygomaticus minor and major muscles (Figure 2). Subsequently, mime therapy was started.
Figure 2.
One week after incision and drainage. Left panel: smiling. Right panel: close eyes. An absent nasolabial fold was observed, with a deviation of the philtrum to the left and a severe palsy of the orbicularis oculi muscle of the lower eyelid, right upper lip orbicularis oris, and right zygomaticus minor and major muscles.
Outcome and follow-up
After 4 months, a significant improvement in facial nerve function was observed, with a slight residual hissing. Seven months after the attack, the child was able to shut the right eye again, with little asymmetry in orbicularis oris and zygomatic muscle function (Figure 3). The scar tissue was softer and no synkinesis was reported. At 1 year and 9 months follow-up, the asymmetry had been subsided.
Figure 3.
Seven months later, the patient was asked to close the eyes. Little asymmetry in orbicularis oris and zygomatic muscle function was observed.
Discussion
Belonging to the family of Elapidae, cobras rank high among animals in the toxin world. Other elapids are mambas, coral snakes, kraits, and Australian elapids, all of which are venomous. Their maxillae bare enlarged fangs that form hollow needles to inject venom1,3,4. Cobras are mostly known for the hoods that they display by actively expanding their ribs laterally4. Spitting cobras are found in both Africa and Asia. Their name is derived from the fact that they spit highly toxic venom in the eyes of their enemies from an orifice in the front of their luminal fangs with which they can be accurate from a distance of up to 3 m5. They have a large venom reserve and can repeatedly spit. The Mozambican spitting cobra in this case (Latin name Naja mossambica, Tsongan name m'Fezi) is commonly found around permanent water in the savannah and lowland forests of tropical and subtropical southeast Africa. It is described as gray to olive brown with black-edged scales and can reach a length of 1.5 m (Figure 4). Their venom is used to paralyze other snakes, various small vertebrates, toads, and lizards. They mainly devour rodents and are therefore found near houses. It is considered a nocturnal animal, but juveniles can forage during the day. Most literature on bites report attacks during nighttime, with many of their victims asleep6,7. The boy described in this case was also bitten when sleeping, leaving a small wound from a single fang, under his right eye. The fact that he was asleep presumably saved his eyes.
Figure 4.
The Mozambican spitting cobra.
When they eject defensively in the eyes of potential predators, a condition called venom ophthalmia can occur, characterized by severe pain, conjunctivitis, photophobia, and blepharitis. The treatment of choice is extensive rinsing of the eyes with clean water. Long-term sequelae include corneal scarring and keratitis7. While some of the Naja species have purely neurotoxic venom, most cause local tissue damage in the form of necrosis, skin blistering, and varying degrees of neurotoxicity when bitten8. It is difficult to differentiate between the local effects of inflammation, tissue necrosis, and neurotoxicity from the first hours to days after the bite. However, in the case presented here, the mid- and long-term follow-up of the facial expressions gave evidence of venom-induced neurotoxicity to the facial nerve. Local geographical differences and differences in genus, species, and subspecies exist regarding the degree of neurotoxicity, highlighting the rich diversity of snake neurotoxins9. Data are limited on the many neurotoxic manifestations, particularly delayed neurotoxicity and its treatments; however, the antidote, neostigmine, can be administrated as an anticholinesterase drug to treat postsynaptic neurotoxicity in the acute setting.
Facial paralysis can be treated surgically and nonsurgically. In general, the goal is to protect the eye, provide symmetry in the neutral face, to obtain movement of facial muscles, and restore spontaneous facial expression. Incomplete facial nerve paralysis may be improved with mime therapy by training neuromuscular activity, for which biofeedback, mirror exercises, and electromyography are used10. Surgical methods to recover facial paralysis include nerve repair, nerve grafting, nerve transfer, static slings, muscle transfers, and functional muscle transplantation. Our case illustrates that patience and conservative treatment, possibly supported by the beneficial effects of mime therapy, resulted in virtually a full recovery of a cobra bite-induced facial paralysis.
Written consent has been obtained for the publication of the case report and the use of photographs.
No Ethical Committee approval was necessary.
Declaration of Competing Interest
None.
Funding
None.
Footnotes
Manuscript prepared in accordance with the STROBE and STARD guidelines.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jpra.2021.05.007.
Appendix. Supplementary materials
References
- 1.Kasturiratne A, Wickremasinghe AR, de Silva N. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008;(11):e218. doi: 10.1371/journal.pmed.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chippaux JP. Snakebite envenomation turns again into a neglected tropical disease! J Venom Anim Toxins Incl Trop Dis. 2017:38. doi: 10.1186/s40409-017-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warrell DA. Snake bite. Lancet. 2010;(9708):77–88. doi: 10.1016/S0140-6736(09)61754-2. [DOI] [PubMed] [Google Scholar]
- 4.Young BA, Kardong KV. The functional morphology of hooding in cobras. The Journal of Experimental Biology. 2010:1521–1528. doi: 10.1242/jeb.034447. [DOI] [PubMed] [Google Scholar]
- 5.Hayes AW. Principles and Methods of Toxicology. 2007 [Google Scholar]
- 6.Blaylock R. Epidemiology of snakebite in Eshowe, KwaZulu-Natal, South Africa. Toxicon. 2004;(2):159–166. doi: 10.1016/j.toxicon.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman J. Venom ophthalmia from Naja mossambica in KwaZulu Natal, South Africa: a reminder to all that for ocular chemical injury, dilution is the solution. Trop Doct. 2015;(4):250–251. doi: 10.1177/0049475514564695. [DOI] [PubMed] [Google Scholar]
- 8.Petras D, Sanz L, Segura A. Snake venomics of African spitting cobras: toxin composition and assessment of congeneric cross-reactivity of the pan-African EchiTAb-Plus-ICP antivenom by antivenomics and neutralization approaches. J Proteome Res. 2011;(3):1266–1280. doi: 10.1021/pr101040f. [DOI] [PubMed] [Google Scholar]
- 9.Ranawaka UK, Lalloo DG, de Silva HJ. Neurotoxicity in snakebite–the limits of our knowledge. PLoS Negl Trop Dis. 2013;(10):e2302. doi: 10.1371/journal.pntd.0002302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pereira LM, Obara K, Dias JM. Facial exercise therapy for facial palsy: systematic review and meta-analysis. Clin Rehabil. 2011;(7):649–658. doi: 10.1177/0269215510395634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.