Abstract
We report about the rare case of a patient who developed large soft-tissue mass formations related with revision total knee arthroplasty which was implanted 4 years prior. Owing to suspected periprosthetic joint infection, the prosthesis was removed and the lesions were resected, resulting in severe soft-tissue loss and temporary arthrodesis using a poly(methyl methacrylate) spacer. Histological analysis revealed a type VI periprosthetic membrane. The situation was further complicated by wound infection requiring multiple revision surgeries. After discussion and evaluation of the available treatment options, the decision for an above-the-knee amputation was made. The tissue reaction resulting in these soft-tissue lesions is referred to as an “adverse local tissue reaction.” Wear-induced lesions after total knee arthroplasty, especially of this magnitude, are very rare and difficult to treat.
Keywords: ALTR, Total knee arthroplasty, Revision, Periprosthetic joint infection, Metalosis
Introduction
In metal-on-metal (MoM) bearings, metal ions might be eluted into the surrounding tissues and induce adverse local tissue reactions (ALTRs) [1]. While this phenomenon, also referred to as adverse reaction to metal debris, metallosis, or trunnionosis, has been primarily observed with total hip arthroplasty (THA) [2,3], it has also been found similarly in failed total knee arthroplasty (TKA) [[4], [5], [6]].
It is known that ALTR may lead to soft-tissue mass, or formerly described as pseudotumor, formation [7]. These fluid-filled or solid soft-tissue lesions result from an innate and adaptive immune response secondary to wear, particle toxicity, and host immunological hypersensitivity or allergy [8,9]. In the following section, we present a rare case of a soft-tissue mass formation related with revision TKA.
Case history
This is a case report describing a 60-year-old female patient, who—due to a severe varus deformity—received a primary TKA using a varus/valgus constrained implant in 2009. The patient first presented to our clinic in 2015 with pain and instability of the knee joint. C-reactive protein was 24 mg/L, however, diagnostic arthrocentesis was not performed at that time. We performed revision TKA with a rotating hinge knee implant (Fig. 1). Intraoperatively, the implant showed signs of loosening, however, there were no macroscopic signs of wear. The histological workup revealed a Krenn type III periprosthetic membrane with mixed wear particles and a prominent granuloma formation with necrosis. Owing to this granuloma formation, a polymerase chain reaction test was performed to exclude mycobacterial infection, which was negative. In addition, the tissue cultures retrieved intraoperatively were negative after the standard incubation duration of 14 days.
Figure 1.
Failure of initial TKA leading to revision surgery with a rotating hinge prosthesis (Solution RT Modular; Smith&Nephew).
Right thereafter, the patient did well initially but started developing progressive swelling of the knee joint, mild instability, and associated pain (Fig. 2) starting in 2017. When she presented again to our outpatient department in 2018, diagnostic arthrocentesis and microbiological analysis of the aspirate had been undertaken in her home country and revealed a positive culture for methicillin-sensitive Staphylococcus epidermidis. Repeated aspiration of the knee joint in our clinic revealed an inconclusive result concerning periprosthetic joint infection (PJI) for both cell count (2200 cells/μL) and culture (negative) [10]. A subsequent conventional radiograph (Fig. 3) and eventually a CT scan demonstrated 2 large pseudocysts, one located anterolaterally and one in the popliteal region. In addition, osteolytic lesions and fractured femoral epicondyles were identified on the scans (Fig. 4).
Figure 2.
Presentation of the patient with 2 large pseudocysts popliteally and anterolaterally.
Figure 3.
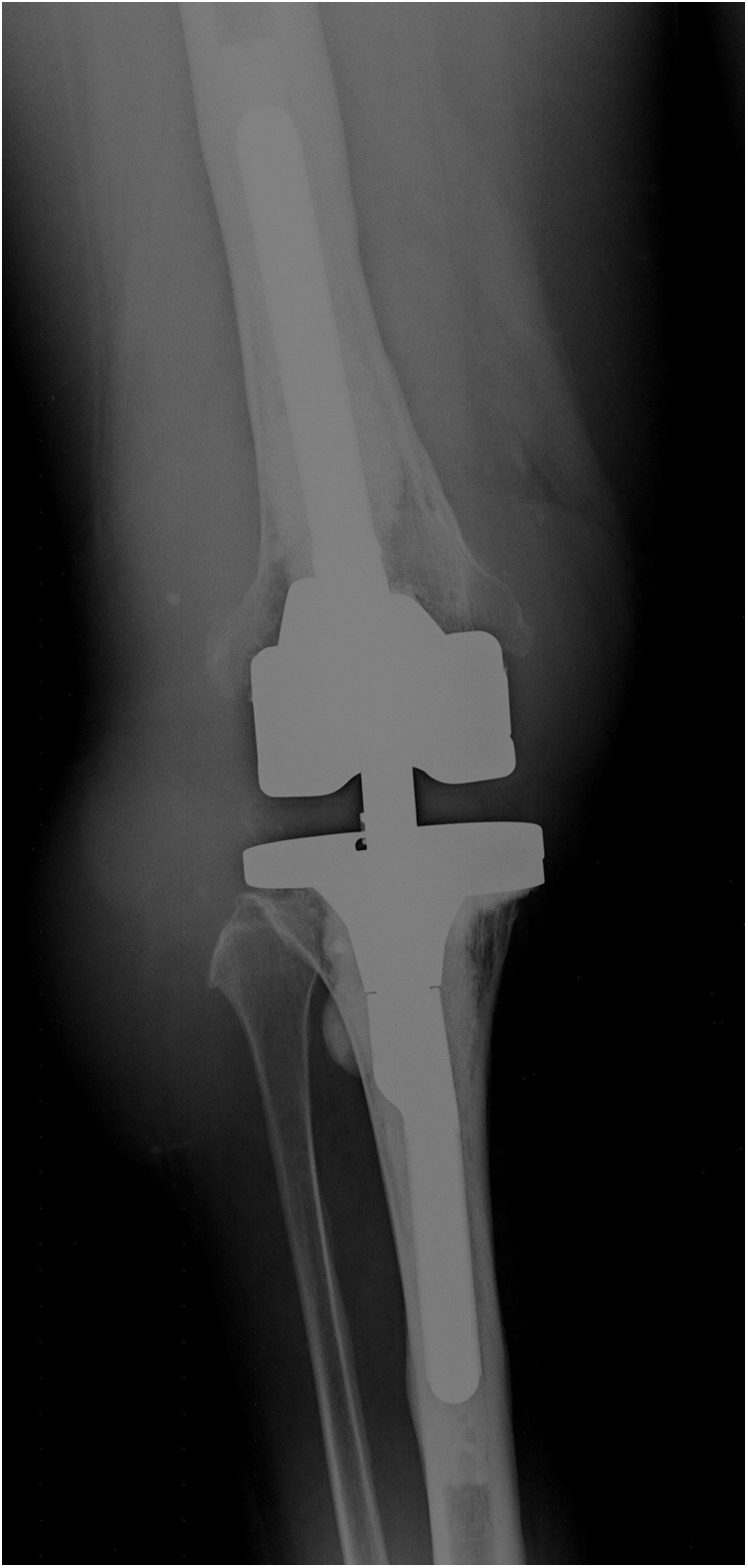
Conventional radiograph of right knee at patient presentation in our outpatient department in 2018 showing a well-fixed implant but osteolysis around the femoral epicondyles.
Figure 4.
CT scan of the right knee: osteolysis of the femoral epicondyles leading to the erosion and fracture. Soft-tissue masses popliteally (depicted with “∗”) and anterolaterally (depicted with “#”).
Unfortunately, owing to unresolved funding and visa issues, the patient was neither able to be followed up continuously nor to undergo surgery at that time. Due to the possible PJI, a critical popliteal soft-tissue situation, and the unknown etiology of the cysts, we decided to perform a two-stage revision surgery upon re-presentation of the patient more than 1 year later. A plastic surgeon (A.D.) was present at all stages, including at the time when the decision for surgery was made, as well as at all surgeries that followed.
During the first surgery in 2020, implant removal and a complete synovectomy were performed. The soft-tissue lesions and large parts of the knee joint capsule were resected through 2 separate incisions. The lesions presented themselves intraoperatively as giant, grey-yellowish, solid masses, which communicated directly with the joint. Here, the peroneal nerve, which ran directly through the popliteal lesion, was preserved (Fig. 5). Nonetheless, resection of the lesions resulted in severe soft-tissue loss in the popliteal region and a critical skin condition. Macroscopically, neither the polyethylene nor the metallic total joint components showed any visible signs of wear. In addition, severe bone loss and fractures of the femoral epicondyles were identified, as already preoperatively indicated by the CT scan. Furthermore, the patella showed extensive bone loss and appeared very thin. Both the tibial and femoral well-fixed components were removed. After explantation, thorough debridement and irrigation, a nonarticulating, static spacer was implanted using poly(methyl methacrylate) cement, 2 carbon rods, and a tube-to-tube connector.
Figure 5.
Intraoperative finding of the soft-tissue mass (yellow-gray structure) and neurovascular structures during resection through a posterior approach in the lateral decubitus position. The upper vessel loop marks the peroneal nerve, whereas the lower loop depicts the sural nerve.
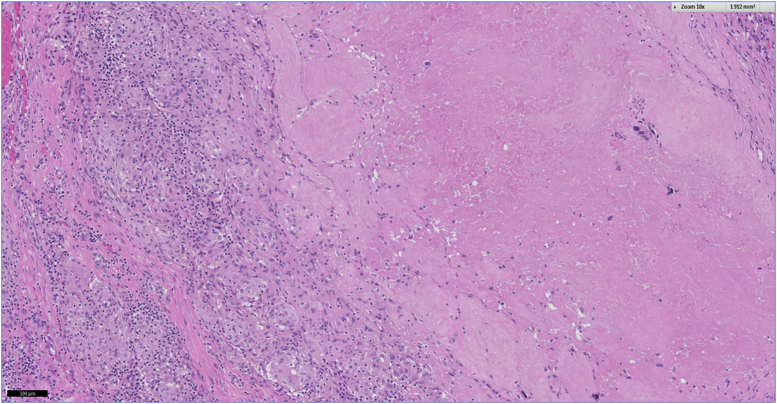
Intraoperative microbiological samples remained negative. Histological sampling of the resected lesions revealed wear particles mostly originating from the metal and to a smaller portion from the polyethylene. In summary, the histological finding was classified as a Krenn type VI periprosthetic membrane (adverse inflammatory local tissue reaction) which showed a predominantly granulomatous pattern, associated with a mixed inflammatory infiltrate of lymphocytic and plasma cells, necrosis, and foreign body giant cells (Figure 6, Figure 7, Figure 8). Owing to a suspected allergic cause of the condition, skin patch testing was undertaken which was positive for cobalt and copper.
Figure 6.
SLIM type VI: granulomatous pattern with accumulation of wear particles in cytoplasm of macrophages and giant cells (10x magnification); H&E.
Figure 7.
SLIM type VI: granulomatous pattern with accumulation of wear particles in cytoplasm of macrophages and giant cells (40x magnification); H&E.
Figure 8.
SLIM type VI: Fibrinoid necrosis; H&E.
In the further course, however, additional complications occurred. The patient developed a bilateral deep vein thrombosis, which required treatment with anticoagulants. Furthermore, a wound necrosis first and then a deep infection (initially in the popliteal and later in the lateral region) with Escherichia coli and Enterococcus faecalis occurred, which required multiple revision surgeries, negative pressure wound dressings, and i.v. antibiotics. In conclusion, the adverse tissue reaction, allergic skin reaction to cobalt (a major component of TKA alloys), massive soft-tissue and joint capsule defects, infection, and the need for anticoagulation had led to a rather complex situation. Treatment options included joint reconstruction with a coated mega-prosthesis, arthrodesis, or above-the-knee amputation. Considering the need for additional surgeries to eliminate the current infection and the risk for recurrence of an ALTR (as coating cannot completely prevent the release of cobalt particles), the decision for an above-the-knee amputation was made. After amputation, the wound healing process occurred without further complication.
Twelve months after surgery, the patient reported to be in good health. The wound had healed, and she was able to walk short distances on a walker or on crutches with a lower limb exoprosthesis.
The patient and her family gave their informed consent for this report to be published.
Discussion
ALTRs are being diagnosed using the expanded SLIM consensus classification (Fig. 9). These can be identified and classified by the presence of characteristic histological patterns. The “type VI membrane” was only added to the existing classification system in 2017 [9] and was defined as an “adverse local tissue reactions to implant wear particles”. This type of reaction includes a group of inflammatory reactions secondary to wear, particle toxicity, and/or host immunological hypersensitivity/allergy which can lead to the formation of soft-tissue masses.
Figure 9.
Expanded SLIM classification according to Krenn and Perino [9].
Histologically, these masses exhibit a pronounced macrophage response to metal wear particles, which are cytotoxic, and result in cell and tissue necrosis. In addition, they often contain a pronounced lymphoid infiltrate composed of lymphocytes, plasma cells, occasionally eosinophil polymorphs, and typically numerous perivascular lymphoid aggregates. This feature has been termed “aseptic lymphocyte-dominated vascular-associated lesion” [8].
It was observed that all arthroplasties, including TKA, expose the patient to released metal ions [11]. Exposure to metal ions may lead to an ALTR which has been primarily linked with MoM bearings in THA [7]. After TKA, ALTR is a rare condition, and the formation of large soft-tissue masses has only rarely been described in this context.
Current case reports in the literature on soft-tissue lesions or “pseudotumors” linked with TKA outline the lesions as a result of a heavy innate foreign body macrophage response (and consequent osteolysis) to polyethylene debris [12,13]. Other case presentations include lesions originating from mixed metal and polyethylene wear [14,15], while in one case, the lesion resulted from poly(methyl methacrylate) debris [16].
Harvie et al. reported about a MoM debris-induced pseudotumor after TKA with an associated common peroneal nerve palsy [17]. Intraoperatively, massive metallosis was seen within all soft tissues as well as erosion of the bone immediately adjacent to the prosthesis. Furthermore, full-thickness wear was visible in both the tibial and patellar polyethylene components [17].
In contrast, previous case reports entailed rather small lesions, which were solely found radiologically or even intraoperatively [18]. To our knowledge, there have been no previous reports about such large wear-induced lesions. Large enough not only to be clinically visible but also to result in early-stage peroneal nerve palsy. The clinical presentation of the lesion was only comparable to one case presented by Harvie et al. [17]. However, the significant differences to the current case are the integrity of the polyethylene as well as a complete lack of visible metallosis.
It is recognized that malpositioning of the prosthesis and implant instability can lead to metal-to-metal contact. In the present case, however, neither the polyethylene bearing nor the metal components showed any intraoperative signs of wear. Intraoperatively, there was no sign that direct contact between femoral and tibial components could have occurred. Possible sources of metal ion release may have incurred because of the large surface of the femoral component or the rotating hinge. The corrosion process of the hinge has recently been introduced as “hingiosis” [19]—a condition with a multifactorial pathogenesis. It is speculated that contributing factors to this may be the prosthesis model, material, lifetime of the prosthesis, dislocation or malpositioning, and the number of revisions. In the current case, corrosion particles were not detected. Likewise, the pathogenesis of the ALTR has not yet been fully understood. Apart from a direct toxicity caused by wear particles, a secondary hypersensitivity response to metal-protein complexes might play an essential role [20].
A biomechanical study by Kretzer et al. [21] found that under experimental conditions, 12% of the weight of wear in TKA was metallic, especially cobalt. The ALTR (Krenn VI) in this case was possibly further triggered by the cobalt allergy which was detected by the patch test.
As swelling had been reported soon after the revision surgery in 2015, the synovitis was assumed to be chronic. At that time, the presence of polyethylene and metal debris particles, which brought about a pronounced inflammatory reaction, was already described histopathologically—a finding that might have been the cause for the persisting swelling. Retrospectively, a low-grade infection might have also contributed to the initial TKA failure (Krenn type III membrane) in 2015 and the subsequent failure in 2020.
Summary
This case impressively demonstrated that ALTRs are not limited to MoM bearings in THA. It should therefore also be considered in cases of chronic postoperative swelling after TKA or suspected low-grade PJI. ALTRs around the knee can infiltrate adjacent vessels and nerves and need to be dissected carefully. Moreover, large soft-tissue defects are to be expected and thus must be handled with utmost caution. Commonly, such cases warrant a close interdisciplinary liaison between orthopedic surgeons, plastic surgeons, and pathologists.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Acknowledgments
The authors acknowledge support by the Open Access Funding by the Publication Fund of the TU Dresden. The authors would like to thank Frederik Brückner (cand. med.) for his proof reading services as a native speaker of the English language.
Informed Patient Consent: The author(s) confirm that informed consent has been obtained from the involved patient(s) or if appropriate from the parent, guardian, power of attorney of the involved patient(s); and, they have given approval for this information to be published in this case report (series).
Appendix A. Supplementary data
References
- 1.McGrory B.J., Jacobs J.J., Kwon Y.-M., Fillingham Y. Standardizing terms for tribocorrosion-associated adverse local tissue reaction in total hip arthroplasty. Arthroplast Today. 2020;6:196. doi: 10.1016/j.artd.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith A.J., Dieppe P., Vernon K., Porter M., Blom A.W. Failure rates of stemmed metal-on-metal hip replacements: analysis of data from the National Joint Registry of England and Wales. Lancet. 2012;379:1199. doi: 10.1016/S0140-6736(12)60353-5. [DOI] [PubMed] [Google Scholar]
- 3.Pandit H., Glyn-Jones S., McLardy-Smith P. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2008;90:847. doi: 10.1302/0301-620X.90B7.20213. [DOI] [PubMed] [Google Scholar]
- 4.Klontz K.C., Smith W.I., Jonathan C.K. Acute metallosis following total knee replacement - a case report. J Orthop Case Rep. 2014;4:21. doi: 10.13107/jocr.2250-0685.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thakur R.R., Ast M.P., McGraw M., Bostrom M.P., Rodriguez J.A., Parks M.L. Severe persistent synovitis after cobalt-chromium total knee arthroplasty requiring revision. Orthopedics. 2013;36:e520. doi: 10.3928/01477447-20130327-34. [DOI] [PubMed] [Google Scholar]
- 6.Thomsen M., Krenn V., Thomas P. Adverse Reaktionen gegenüber orthopädisch-chirurgischen Metallimplantaten nach Kniegelenkersatz [Adverse reactions to metal orthopedic implants after knee arthroplasty] Hautarzt. 2016;67:347. doi: 10.1007/s00105-016-3793-3. [DOI] [PubMed] [Google Scholar]
- 7.Hartmann A., Kieback J.-D., Lützner J., Günther K.-P., Goronzy J. Adverse reaction to metal debris in a consecutive series of DUROM™ hip resurfacing: pseudotumour incidence and metal ion concentration. Hip Int. 2017;27:343. doi: 10.5301/hipint.5000468. [DOI] [PubMed] [Google Scholar]
- 8.Athanasou N.A. The pathobiology and pathology of aseptic implant failure. Bone Joint Res. 2016;5:162. doi: 10.1302/2046-3758.55.BJR-2016-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krenn V., Perino G. Springer Berlin Heidelberg; Berlin, Heidelberg: 2017. Histological diagnosis of implant-associated pathologies. [Google Scholar]
- 10.McNally M., Sousa R., Wouthuyzen-Bakker M. The EBJIS definition of periprosthetic joint infection. Bone Joint J. 2021;103-B:18. doi: 10.1302/0301-620X.103B1.BJJ-2020-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luetzner J., Krummenauer F., Lengel A.M., Ziegler J., Witzleb W.-C. Serum metal ion exposure after total knee arthroplasty. Clin Orthop Relat Res. 2007;461:136. doi: 10.1097/BLO.0b013e31806450ef. [DOI] [PubMed] [Google Scholar]
- 12.Benevenia J., Lee F.Y.-I., Buechel F., Parsons J.R. Pathologic supracondylar fracture due to osteolytic pseudotumor of knee following cementless total knee replacement. J Biomed Mater Res. 1998;43:473. doi: 10.1002/(sici)1097-4636(199824)43:4<473::aid-jbm16>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Nadlacan L.M., Freemont A.J., Paul A.S. Wear debris-induced pseudotumour in a cemented total knee replacement. Knee. 2000;7:183. doi: 10.1016/s0968-0160(00)00049-1. [DOI] [PubMed] [Google Scholar]
- 14.Sivananthan S., Pirapat R., Goodman S.B. A rare case of pseudotumor formation following total knee arthroplasty. Malays Orthop J. 2015;9:44. doi: 10.5704/MOJ.1503.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zee M.J.M., van Bemmel B.C., van Raay J.J.A.M. Massive osteolysis due to galvanic corrosion after total knee arthroplasty: a rare cause for early revision? J Surg Case Rep. 2020;2020:rjaa002. doi: 10.1093/jscr/rjaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenan S., Kahn L., Haramati N., Kenan S. A rare case of pseudotumor formation associated with methyl methacrylate hypersensitivity in a patient following cemented total knee arthroplasty. Skeletal Radiol. 2016;45:1115. doi: 10.1007/s00256-016-2372-0. [DOI] [PubMed] [Google Scholar]
- 17.Harvie P., Torres-Grau J., Beaver R.J. Common peroneal nerve palsy associated with pseudotumour after total knee arthroplasty. The Knee. 2012;19:148. doi: 10.1016/j.knee.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Craig R., Vlychou M., McCarthy C.L., Gibbons C.L.M.H., Athanasou N.A. Metal wear-induced pseudotumour following an endoprosthetic knee replacement for Ewing sarcoma. Skeletal Radiol. 2017;46:967. doi: 10.1007/s00256-017-2610-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirchen N., Reich L., Waldstein W. ARMD-Reaktionsmuster bei Kniegelenkendoprothesen: ein neuer hypothetischer Mechanismus: Hingiose [ARMD reaction patterns in knee arthroplasty: a novel hypothetical mechanism: hingiosis] Orthopade. 2020;49:183. doi: 10.1007/s00132-019-03776-9. [DOI] [PubMed] [Google Scholar]
- 20.Mahendra G., Pandit H., Kliskey K., Murray D., Gill H.S., Athanasou N. Necrotic and inflammatory changes in metal-on-metal resurfacing hip arthroplasties. Acta Orthop. 2009;80:653. doi: 10.3109/17453670903473016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kretzer J.P., Reinders J., Sonntag R. Wear in total knee arthroplasty--just a question of polyethylene?: metal ion release in total knee arthroplasty. Int Orthop. 2014;38:335. doi: 10.1007/s00264-013-2162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.