Abstract
Background
Revesz syndrome is a rare type of the dyskeratosis congenita spectrum disorder that is characterized by nail dystrophy, oral leukoplakia, and abnormal skin pigmentation. The retinal features are similar to those of exudative retinopathy with avascular areas of the peripheral retina. There are only a few publications describing patients with Revesz syndrome who underwent ocular treatments for the retinal complications. We report a Case of Revesz syndrome with bilateral retinal detachments that were successfully reattached by pars plana vitrectomy.
Observations
A 3-year-old Japanese girl with Revesz Syndrome had progressive vitreal hemorrhages and tractional retinal detachments in both eyes. She underwent pars plana vitrectomy with lensectomy on both eyes. A retinal attachment with vision improvement was achieved by a single surgery for the right eye and after repeated surgeries for the left eye. Postoperative electroretinographic (ERG) examinations of the right eye showed a negative type ERG with the b-wave/a-wave ratio <1.0. There were extensive areas of avascular retina detected by fluorescein angiography and a thinning of the inner and outer retina detected by optical coherence tomography.
Conclusion and importance
Pars plana vitrectomy can effectively treat the extensive retinal detachment in an eye with Revesz syndrome. However, postoperative retinal ischemia can be detected by careful imaging.
Keywords: Revesz syndrome; Dyskeratosis congenita; TINF2, retinal detachment; familial exudative vitreoretinopathy
1. Introduction
Revesz syndrome is a rare disorder that is the most severe type of inherited bone marrow failure disorder. It is characterized by abnormal shortening of the chromosomal telomeres, and is referred to as dyskeratosis congenita.1 Revesz syndrome has an autosomal dominant inheritance and is caused by mutations of the TINF2 gene. This gene encodes one of the six components of the telomere-associated shelterin complex that protects against a shortening of the telomeres.2 A shortening of the telomeres causes genomic instability and apoptosis of specific cell types that lead to abnormal growth of the bone marrow, skin, and retinal vasculature.3
Revesz syndrome presents with a triad of abnormalities including nail dystrophy, oral leukoplakia, and abnormal skin pigmentation. In addition, exudative retinopathy, intracranial calcifications, and cerebellar hypoplasia are commonly present.1,4,5 The retinal features include avascular areas in the peripheral retina with telangiectatic vessels, arterio-venous anastomotic loops, neovascularizations, retinal exudates, and exudative and/or tractional retinal detachments.3,6, 7, 8, 9 So far, six cases of Revesz syndrome that underwent ocular treatments for the retinal complications have been reported.3,6, 7, 8, 9 We present our findings in a Case of Revesz syndrome with bilateral retinal detachments that were successfully treated by pars plana vitrectomy.
2. Case report
A 3-year-old Japanese girl was born at 36 weeks of gestation as a dizygotic twin. She was noted to have a mild intellectual disability that required medical interventions at 2-years-of-age. At the same age, purple spots were noted on the surface of her skin of her neck and extremities. Pancytopenia was detected by blood tests, and a diagnosis of an inherited bone marrow failure syndrome was made by analyses of a bone marrow specimen. She then underwent bone marrow transplantation from her unaffected sibling. Dysplasia of the fingernails and toenails was noted (Fig. 1A and B), and magnetic resonance imaging of the brain revealed cerebellar hypoplasia (Fig. 1C). A flow-fluorescent in-situ hybridization examination showed that the telomere length was shorter than the first percentile of the age-matched standard for all leukocyte subsets. Genetic testing by targeted next generation sequencing detected a known missense mutation in the TINF2 gene, c.845G > A, (NM_001099274) predicting p.(Arg282His).2 A diagnosis of Revesz syndrome was made.
Fig. 1.
Findings in a patient with the Revesz syndrome.
A: and B:Photographs of the patient's right hand and the right foot showing dyskeratotic nails at 3-years-of-age. C: Magnetic resonance (MR) image of the brain at 3-years-of-age. Saggital T1-weighted MR image of the brain shows cerebellar hypoplasia.
Ocular examinations showed exotropia, and her best-corrected visual acuity (BCVA) was light perception in the right eye and 20/50 in the left eye by the dot card acuity test. Although vitreous hemorrhage and tractional retinal detachment were detected by ultrasonography in the right eye, surgical treatment was not considered at that time. Preretinal hemorrhage in the temporal macular area and vitreal and preretinal fibrosis with exudation was found in the left eye (Fig. 2A). The retinal detachment with the vitreous hemorrhage was found to be progressive in the left eye, and she was referred to the University of Occupational and Environmental Health Hospital for surgery.
Fig. 2.
Pre-, intra-, and postoperative findings of the left eye of a patient with Revesz syndrome.
A: Preoperative fundus photograph at 2-years-of-age showing preretinal macular fibrosis with hard exudates and preretinal hemorrhage in the temporal macular region. B: Preoperative ultrasonogram showing dense vitreous hemorrhage and tractional retinal detachment.
D: and E: Intraoperative images showing vitreoretinal adhesion and tractional detachment extending from temporal to inferior retina. Note an iatrogenic retinal break at the tight adhesion on the inferior retina (asterisk).
E: and F: Fundus photograph and optical coherence tomography (OCT) image showing the final retinal attachment at 9-years-of-age. Note that the OCT image shows a thinning of the retina especially temporal to the macula and an enlarged optic disc cup.
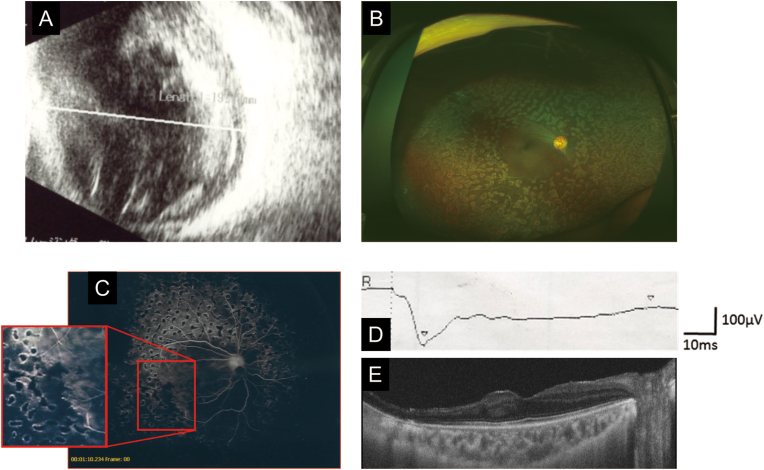
Our initial ultrasonic examination showed that vitreous hemorrhages and tractional retinal detachment were present in both eyes (Fig. 2, Fig. 3A). Her BCVA had decreased to hand motion in the left eye.
Fig. 3.
Pre- and postoperative findings of the right eye of the patient with Revesz syndrome.
A: Preoperative ultrasonogram of the right eye at 3-years-of-age showing dense vitreous hemorrhage and tractional retinal detachment.
B: Postoperative ultra-widefield fundus photograph showing retinal reattachment with scattered laser scars.
C: Fluorescein angiogram after surgery showing arterio-venous anastomotic loops and extensive avascular retina treated by laser photocoagulation.
D: Dark-adapted electroretinogram demonstrating a negative b/a pattern elicited by a stimulus intensity of 200 cd・s/m2.
E: Optical coherence tomographic image showing preserved macular structure and inner retinal thinning of the retina temporal to the macula.
She underwent pars plana vitrectomy with lensectomy on the left eye at 3-years-of-age. When the dense vitreous opacity was removed, fibrovascular proliferation was observed extending from the posterior retina to the peripheral retina (Fig. 2C and D). The proliferation led to a firm vitreoretinal adhesion and tractional retinal detachment. During the delamination of the membrane, an iatrogenic retinal break was created by the tight adhesion on the inferior retina that progressed to a cystic retinal detachment. Then, silicone oil was injected into the vitreous cavity to tamponade the retina.
Two weeks after the surgery, the intraocular pressure (IOP) was found to be elevated due to the migration of the silicone oil into the anterior chamber. An oil-air exchange was performed to normalize the IOP. A re-detachment occurred from the retinal break one week after the air-exchange, and an additional radial relaxing retinotomy was performed around the retinal break and a further membrane peeling was performed on the posterior pole. Subsequently, three retinal detachments occurred at the edge of the retinotomy that was widened to release the retinal traction. This was treated by gas or silicone oil tamponade. A retinal attachment was obtained by extracting the silicone oil and adding two rows of radial buckling silicone sponge at age of five-years (Fig. 2E). The final BCVA was 20/100 with a refractive error of +11.5 diopters (D) at 10-years-of-age. Optical coherence tomographic (OCT) imaging showed a thinning of the retina especially temporal to the macula, and a glaucomatous optic disc cup enlargement due to the long duration silicone oil tamponade and elevated IOP (Fig. 2F).
Pars plana vitrectomy with lensectomy also performed on the right eye at 3-years-of-age. Although the vitreous opacity was denser than that in the left eye, all vitreoretinal adhesions were successfully released without creating a retinal break, and laser photocoagulation was applied to the areas of nonperfusion. The retinal detachment was resolved by fluid-air exchange. No additional surgery was needed in the right eye (Fig. 3B). The final BCVA was 20/22 with a refraction of +14 D at the age of ten-years.
Electroretinography (ERG) and fluorescein angiography (FA) were performed prior to the fourth surgery on the right eye (Fig. 3C and D). FA showed wide areas of avasculariztion, arterio-venous anastomotic loops in the peripheral retina, and scattered laser photocoagulation scars in both eyes (Fig. 3C). The ERGs were recorded from only the right eye because silicone oil was retained in the left eye. The dark-adapted mixed rod and cone response had a negative b/a pattern in the right eye with a flash intensity of 200 cd・s/m2 (Fig. 3D). The OCT images showed a preserved macular structure and both inner and outer retinal thinning temporal to the macula (Fig. 3E).
3. Discussion
Our patient did not have any signs of the short telomere disorder which are essential for diagnosing Revesz syndrome, and the diagnosis was finally made by genetic testing. She had dense vitreous hemorrhage, extensive vitreoretinal adhesions, and tractional retinal detachment in both eyes. In the left eye, the preretinal hemorrhage and retinal exudation temporal to the macula progressed to a vitreal hemorrhage and retinal detachment. The avascularity of the peripheral retina and arterio-venous anastomotic loops in the FA images were consistent with the earlier reports of eyes with Revesz syndrome.
This is the seventh Case of Revesz syndrome that underwent ocular treatments for the retinal complications and the fourth case that underwent vitreous surgery.3,6, 7, 8, 9 McElena et al.3 studied an 11 year-old boy who had leukocoria with retinal detachments in both eyes. He underwent a lens-sparing vitrectomy, membrane dissection, and endolaser photocoagulation of the right eye. Three months later, the patient failed to fixate and follow a moving target. Unfortunately, a more extensive description of the surgical outcome was not reported. Gupta et al.6 reported on a 13-month-old twin girl who had a vitreous hemorrhage and retinal detachment, and she underwent pars plana vitrectomy with lensectomy of the left eye. A retinal reattachment was obtained by a temporal perfluorocarbon liquid followed by silicone oil replacement with endolaser photocoagulation to the retinal tear and areas of peripheral avascular retina as well as intravitreal injection of bevacizumab. Five months later, the eye had a massive subretinal exudation and hemorrhage with a vitreoretinal traction. Tomickova et al.7 reported on a 2-year-old boy who had a total retinal detachment in the left eye which was enucleated because of neovascular glaucoma after unsuccessful vitrectomy. The long-term follow-ups were not reported in these cases, and the outcomes in the eyes with vitrectomy was poor with no useful vision. On the other hand, the prognosis of the other seven eyes treated with laser photocoagulation with or without intravitreal bevacizumab was promising although these eyes had less extensive peripheral avascular retina than the eyes requiring vitreous surgery.3,6, 7, 8, 9
Our patient obtained relatively good visual acuities after successful vitrectomies. However, both eyes were not treated until her vision had become hand motion to light perception due to the dense vitreous hemorrhages and retinal detachments. Extensive retinal detachments with vitreoretinal adhesion can make the surgery more difficult. As in other ischemic proliferative retinopathies such as advanced diabetic retinopathy, retinopathy of prematurity, and familial exudative vitreoretinopathy, creating a retinal break around the extensive vitreoretinal adhesions and tractions can cause a recurrence of the proliferation and retinal detachment leading to limited restoration of vision due to the extensive mechanical damage or toxicity of the retinal tissues by repeated surgeries. Retinal photocoagulation appeared to be effective in preventing the retinal proliferative changes in Revesz syndrome, and an earlier intervention is recommended.
The TINF2 mutations in patients with Revesz syndrome can cause apoptosis of the developing vascular endothelium that is presumed to be associated with retinal ischemia.3 ERGs have not been recorded from eyes with the Revesz syndrome, and we found that the dark-adapted mixed rod and cone response had a b/a ratio <1.0 in the right eye. This was possibly due to the retinal ischemia associated as well as iatrogenic laser and surgical trauma shown by FA and OCT.
The retinal findings of Revesz syndrome has some similarities to those of familial exudative vitreoretinopathy (FEVR), e.g., retinal avascularization and neovascularization in the periphery and the subsequent retinal hemorrhages and retinal detachments.6,7 Thus, it was not rare that the initial diagnosis of FEVR was made in patients with dyskeratosis congenita including Revesz syndrome.6,10 However, the negative ERGs in our patient appears to be different from eyes with FEVR in which the ERG responses have been reported to be normal unless it is accompanied by severe retinal damage.11,12 Our findings indicated that the eye had severe retinal damage due to the nature of Revesz syndrome and secondary trauma by laser and surgery.
4. Conclusions
Pars plana vitrectomy was effective in treating the extensive retinal detachment in an eye with Revesz syndrome. Detailed postoperative examinations showed signs of extensive retinal ischemia and possible surgical trauma.
Patient consent
Written consent to publish this Case has not been obtained. This report does not contain any personal identifying information.
Funding
Health and Labour Sciences Research Grants of Research on intractable disease, 20FC1029 to HK from the Ministry of Health, Labour and Welfare, Japan.
Authorship
All authors declare that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
Koh-Hei Sonoda received grants from Alcon, Novartis, HOYA, Santen Pharmaceutical, Senju Pharmaceutical, Otsuka Pharmaceutical and AMO Japan; and lecture fees from Santen Pharmaceutical, Alcon, AMO Japan, Bayer, Novartis, Kowa Pharmaceutical, Senju Pharmaceutical, Otsuka Pharmaceutical, and RE Medical.
Hiroyuki Kondo received grants from Alcon, Novartis, and AMO Japan; and lecture fees from Santen Pharmaceutical, Alcon, AMO Japan, Bayer, Novartis, Kowa Pharmaceutical, Senju Pharmaceutical, Otsuka Pharmaceutical, and RE Medical.
Acknowledgements
The authors thank Professor Emeritus Duco Hamasaki of the Bascom Palmer Eye Institute of the University of Miami, Florida for his critical discussion and manuscript revision.
References
- 1.Sasa G.S., Ribes-Zamora A., Nelson NDBertuch A.A. Three novel truncating TINF2 mutations causing severe dyskeratosis congenita in early childhood. Clin Genet. 2012;81(5):470–478. doi: 10.1111/j.1399-0004.2011.01658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savage S.A., Giri N., Baerlocher G.M., Orr N., Lansdorp PMAlter B.P. TINF2, a component of the shelterin telomere protection complex, is mutated in dyskeratosis congenita. Am J Hum Genet. 2008;82(2):501–509. doi: 10.1016/j.ajhg.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McElnea E.M., van der Spek N., Smith O., Fitzsimon S., Patel Cko'Marcaigh A. Revesz syndrome masquerading as bilateral cicatricial retinopathy of prematurity. J AAPOS. 2013;17(6):634–636. doi: 10.1016/j.jaapos.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Revesz T., Fletcher S., al-Gazali LIDeBuse P. Bilateral retinopathy, aplastic anaemia, and central nervous system abnormalities: a new syndrome? J Med Genet. 1992;29(9):673–675. doi: 10.1136/jmg.29.9.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kajtar P., Mehes K. Bilateral coats retinopathy associated with aplastic anaemia and mild dyskeratotic signs. Am J Med Genet. 1994;49(4):374–377. doi: 10.1002/ajmg.1320490404. [DOI] [PubMed] [Google Scholar]
- 6.Gupta M.P., Talcott K.E., Kim D.Y., Agarwal SMukai S. Retinal findings and a novel TINF2 mutation in Revesz syndrome: clinical and molecular correlations with pediatric retinal vasculopathies. Ophthalmic Genet. 2017;38(1):51–60. doi: 10.1080/13816810.2016.1275019. [DOI] [PubMed] [Google Scholar]
- 7.Tomcikova D., Gerinec A., Busanyova B., Gresikova M., Biskup SHortnagel K. Why is it necessary to examine retina when the patient suffers from aplastic anemia? Bratisl Lek Listy. 2018;119(5):275–277. doi: 10.4149/BLL_2018_051. [DOI] [PubMed] [Google Scholar]
- 8.Gleeson M., O'Marcaigh A., Cotter M., Brosnahan D., Vulliamy TSmith O.P. Retinal vasculopathy in autosomal dominant dyskeratosis congenita due to TINF2 mutation. Br J Haematol. 2012;159(5):498. doi: 10.1111/bjh.12088. [DOI] [PubMed] [Google Scholar]
- 9.Moussa K., Huang JNMoore A.T. Revesz syndrome masquerading as traumatic retinal detachment. J AAPOS. 2017;21(5):422–425. doi: 10.1016/j.jaapos.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Sharma A., Myers K., Ye Zd'Orazio J. Dyskeratosis congenita caused by a novel TERT point mutation in siblings with pancytopenia and exudative retinopathy. Pediatr Blood Canc. 2014;61(12):2302–2304. doi: 10.1002/pbc.25161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohkubo H., Tanino T. Electrophysiological findings in familial exudative vitreoretinopathy. Doc Ophthalmol. 1987;65(4):461–469. doi: 10.1007/BF00143048. [DOI] [PubMed] [Google Scholar]
- 12.Yaguchi Y., Katagiri S., Fukushima Y. Electroretinographic effects of retinal dragging and retinal folds in eyes with familial exudative vitreoretinopathy. Sci Rep. 2016;6:30523. doi: 10.1038/srep30523. [DOI] [PMC free article] [PubMed] [Google Scholar]