Abstract
Infective endocarditis (IE) is a persistent health issue, particularly among intravenous drug users. We discussed a case of infective endocarditis in a patient who uses IV drugs, which had some unusual features such as unusual presentation, polymicrobial infection, left-sided valve involvement, coronary embolism, and an uncommon pathogen for IE.
Keywords: Infective endocarditis, Polymicrobial infections, cardiac embolism, acute limb ischemia, streptococcus agalactiae
Introduction
Despite improved diagnostic and treatment options, infectious endocarditis continues to be a major cause of morbidity and mortality in recent decades. Intravenous drug abuse (IVDA) is a well-known risk factor for infective endocarditis (IE). It increases the risk of IE 100-fold when compared to the general population [1]. The incidence of infective endocarditis caused by IVDA has increased in the United States over the last decade as a result of the heroin epidemic. IVDA-IE is linked to a high rate of early survival, followed by poor long-term outcomes [1,2]. We present a case of infective endocarditis with some unusual characteristics.
Case Presentation
A 45-year-old man with a history of active intravenous drug abuse (methamphetamine) presented to the emergency department (ED) with acute onset left lower extremity pain of one day duration. The pain was severe, burning in nature, increasing over time, exacerbated by activity and bearing weight on his left leg, and there were no relieving factors. It was accompanied by numbness, difficulty moving his left foot, and loss of sensation below his left knee. The patient denied having any other complaints, a history of leg trauma, or a history of thrombotic disease.
His vital signs were as follows: (Temperature was 99.6 F, heart rate was 132 beats per minute, blood pressure was 127/83 mmHg, respiratory rate was 20 breaths per minute, and oxygen saturation was 96% on room air).
Physical examination revealed a grade three holosystolic murmur at the apex, but no signs of acute heart failure. The left lower limb was mottled and cold to the touch. From the knee below, there was lack of tactile perception. The left femoral pulse was present, but the left posterior tibial and dorsalis pedis pulses were not. The patient was able to flex and extend his left knee but unable to plantar flex and dorsiflex his left foot or flex and extend his toes. The examination of the right lower extremity was normal.
White blood cell count was 23.83 K/uL with an 85 percent neutrophilia, hemoglobin 15.8 g/dl, platelet count 63,000 k/ul, blood urea nitrogen 40 mg/dl, creatinine 1.6 mg/dl, troponin 21.49 ng/ml, CPK 14649 U/L, CPK MB 200.7 ng/ml, and myoglobin >20,000 ng/ml.
An urgent computed tomography (CT) angiogram was performed, which revealed an occlusion of the left common femoral artery at the bifurcation with an extension into the superficial and deep femoral arteries, as well as multiple wedge-shaped hypodensities in the left kidney and spleen, indicating acute ischemia. (Figs. 1, 2 and 3).
Fig. 1.
Axial contrast enhanced CT shows wedge shaped hypodense foci in the peripheral aspect of the spleen, consistent with acute infarction (black arrows). Partially visualized hypodensity involving the left upper kidney, consistent with acute infarction (white arrow).
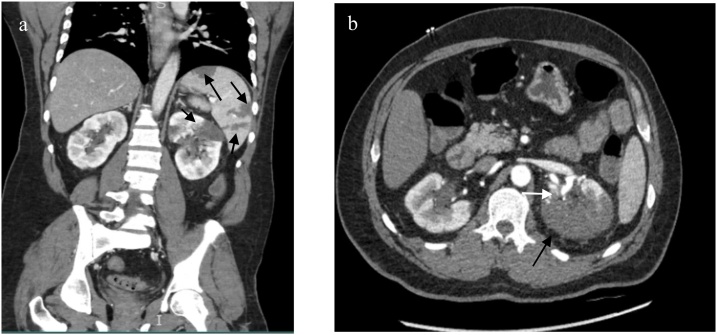
Fig. 2.
Coronal (a) and axial (b) contrast enhanced CT show hypodense foci (black arrows) within the left kidney and spleen parenchyma consistent with acute infarction. On the axial image, hypodense foci is present within part of the left renal artery (white arrow) consistent with thrombus.
Fig. 3.
Axial (a) and Coronal contrast enhanced CT (b) as well as 3D rendered reconstruction (c) show a filling defect in the left common femoral artery consistent with thrombus (arrow).
Based on these findings, infective endocarditis with disseminated emboli was the most likely diagnosis; therefore Vancomycin and Gentamicin were administered in the emergency department.
An urgent embolectomy of the left common femoral artery, profunda, and superficial femoral arteries was performed, as well as a four-compartment fasciotomy (anterior, lateral, medial, and posterior compartments). The post-operative exam revealed a warm left leg with a good dorsalis pedis pulse, improved motor function, and sensation.
Transthoracic echocardiogram (TTE) revealed a large, mobile vegetation measuring 2.3 cm x 1.9 cm on the anterior mitral leaflet, with a possible flail/perforated segment (A2) causing severe posteriorly directed mitral regurgitation and a normal systolic function with an estimated ejection fraction of 65%.
Despite the fact that the patient had no chest pain or ECG abnormalities, he was started on aspirin, clopidogrel, and heparin drip due to persistently rising troponin levels of up to 86 ng/ml, raising the possibility of cardiac ischemia.
On the second day of admission, a left heart catheterization revealed distal embolization to the left circumflex artery (LCx) with abrupt closure. Aspiration thrombectomy was performed, which was complicated by severe vasospasm of the left circumflex and left anterior descending arteries, resulting in cardiac arrest for a few minutes. During resuscitation, an Impella CP was inserted via the right femoral access into the left ventricle. Finally, the vasospasm was resolved, and blood flow in these two arteries returned to normal. The impella was removed on the fourth day of admission, and TTE revealed normal LV systolic function with an estimated ejection fraction of 70%.
Streptococcus agalactiae was found in four blood culture bottles. Streptococcus agalactiae and Methicillin-resistant Staphylococcus aureus were both found in the embolus retrieved from the left common femoral artery (MRSA). MRSA was grown in urine culture.
Ceftriaxone was added to the above regimen for streptococcus coverage at the recommendation of the infectious diseases service. Gentamicin was stopped on the seventh day of treatment due to rising creatinine levels, so Vancomycin and Ceftriaxone were continued.
On the 12th day of his hospitalization, the patient underwent mitral valve replacement surgery. Vegetation had nearly destroyed the posterior leaflet of the mitral valve, according to intraoperative findings. A portion of the anterior leaflet and chordae tendineae were also destroyed. A mechanical valve was installed in its place. Histology revealed fragments of the mitral valve with endocarditis, degenerative myxoid changes with fibrinoid necrosis, and gram-positive bacterial colonies.
The patient recovered quickly, and he was able to move his left lower extremity without pain or restriction. After 22 days in the hospital, the patient was transferred to a Long-Term Acute Care Unit (LTAC) to finish the six-week vancomycin and ceftriaxone course.
On the second and sixth weeks after discharge, the patient was seen in the out-patient clinic. He was doing well and had no complaints; he was able to move his left leg freely and painlessly. The sternum and left groin wounds had completely healed.
Discussion
The presence of several rare features of infective endocarditis, many of which are associated with poor outcomes, in a single patient distinguishes our case.
Polymicrobial infective endocarditis is a rare condition in which more than one pathogens are isolated from the bloodstream or infected tissues related to endocarditis, such as valves, prostheses, and embolic material retracted during surgery [3]. This entity was frequently linked to IVDA, but it has recently been associated with other risk factors such as intravenous catheters [3]. When compared to monomicrobial infections, polymicrobial infections are associated with a higher mortality rate and more surgical interventions [4]. Our patient exhibited a polymicrobial infection, as MRSA was isolated from embolic material of the left common femoral artery and Streptococcus agalactiae was isolated from blood and embolic material.
Streptococcus agalactiae is an uncommon cause of infective endocarditis. It is known to cause invasive diseases in pregnant women and neonates, but in recent decades, more invasive diseases in older age groups have been observed [5]. Streptococcus agalactiae infective endocarditis is associated with larger vegetations, more valve destruction, more embolic events, and a higher mortality rate than other streptococcal infective endocarditis [5].
The majority of cases of infective endocarditis in intravenous drug users are right-sided; however, involvement of left-sided valves has recently increased, with a more severe clinical course associated with higher morbidity and mortality [4].
Vascular complications are a common complication of infective endocarditis. In one study, 22.7% of patients with infective endocarditis developed vascular complications [6]. The most common vascular complications were neurovascular, followed by lower limb arteries [6]. In our patient, the arteries of the left lower extremity, spleen, left kidney, and left circumflex coronary artery were all involved.
Cardiac embolization is a rare complication of infective endocarditis. Extrinsic compression of coronary arteries by vegetations, rather than coronary embolic phenomena, was the cause of the majority of reported cases of acute coronary syndromes associated with IE [7]. Our patient had a left circumflex artery embolism that was treated with aspiration thrombectomy. According to a systematic review of 147 documented cases of coronary embolism, the author advocated for aspiration thrombectomy as the best treatment option for coronary embolism [8]. Despite having a coronary embolism, our patient did not exhibit any symptoms of cardiac ischemia, such as chest pain or shortness of breath.
In the aforementioned case, there are numerous poor prognostic factors, including polymicrobial infection, invasive pathogens (MRSA and S.agalactiae), large destructive vegetation, left-sided involvement, and multiple septic embolizations.
Conclusion
This case emphasizes the importance of recognizing the new emerging entity of infective endocarditis among intravenous drug users, which is left-sided valve involvement and polymicrobial infection, both of which are linked to increased morbidity and mortality.
Author statement
All individuals who meet the criteria for authorship are listed as authors, and all authors certify that they participated in the work and accept public responsibility for the content.
Consent
Written informed consent for publication was taken from the patient.
Data availability
All data are available from the corresponding author upon request.
Disclosures
The authors have no financial disclosures or conflicts of interest to report related to this work.
Funding
None.
CRediT authorship contribution statement
Nour Daoud: Conceptualization, Data curation, Writing - original draft, Writing - review & editing. Kiran Malikayil: Conceptualization, Data curation, Resources, Writing - review & editing. Dinesh Regalla: Conceptualization, Visualization, Writing - review & editing. Mohammad Alam: Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Nour Daoud, Email: nour.daoud@lsuhs.edu.
Kiran Malikayil, Email: kiran.malikayil@lsuhs.edu.
Dinesh Regalla, Email: dinesh.regalla@lsuhs.edu.
Mohammad Alam, Email: mohammad.alam@lsuhs.edu.
References
- 1.Rudasill S.E., Sanaiha Y., Mardock A.L., Khoury H., Xing H., Antonios J.W. Clinical Outcomes of Infective Endocarditis in Injection Drug Users. J Am Coll Cardiol. 2019;73(February 12 (5)):559–570. doi: 10.1016/j.jacc.2018.10.082. PMID: 30732709. [DOI] [PubMed] [Google Scholar]
- 2.Straw S., Baig M.W., Gillott R., Wu J., Witte K.K., O’regan D.J. Long-term Outcomes Are Poor in Intravenous Drug Users Following Infective Endocarditis, Even After Surgery. Clin Infect Dis. 2020;71(July 27 (3)):564–571. doi: 10.1093/cid/ciz869. PMID: 31504326. [DOI] [PubMed] [Google Scholar]
- 3.García-Granja P.E., López J., Vilacosta I., Ortiz-Bautista C., Sevilla T., Olmos C. Polymicrobial Infective Endocarditis: Clinical Features and Prognosis. Medicine (Baltimore). 2015;94(December (49)):e2000. doi: 10.1097/MD.0000000000002000. PMID: 26656328; PMCID: PMC5008473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sousa C., Botelho C., Rodrigues D., Azeredo J., Oliveira R. Infective endocarditis in intravenous drug abusers: an update. Eur J Clin Microbiol Infect Dis. 2012;31(November (11)):2905–2910. doi: 10.1007/s10096-012-1675-x. Epub 2012 Jun 20. PMID: 22714640. [DOI] [PubMed] [Google Scholar]
- 5.Sambola A., Miro J.M., Tornos M.P., Almirante B., Moreno-Torrico A., Gurgui M. Streptococcus agalactiae infective endocarditis: analysis of 30 cases and review of the literature, 1962-1998. Clin Infect Dis. 2002;34(June 15 (12)):1576–1584. doi: 10.1086/340538. Epub 2002 May 24. PMID: 12032892. [DOI] [PubMed] [Google Scholar]
- 6.Yameogo N.V., Baptiste Tougouma S.J., Kagambega L.J., Diallo I., Christian Millogo G.R. "Vascular complications of infective endocarditis in Burkina Faso.". J Cardiovasc Med Cardiol. 2018;5(3):020–023. [Google Scholar]
- 7.Manzano María Carmen, Vilacosta Isidre, San Román José A., Aragoncillo Paloma, Sarriá Cristina, López Daniel. "Acute coronary syndrome in infective endocarditis.". Revista Española de Cardiología (English Edition) 2007;60(1):24–31. [PubMed] [Google Scholar]
- 8.Lacey M.J., Raza S., Rehman H., Puri R., Bhatt D.L., Kalra A. Coronary Embolism: A Systematic Review. Cardiovasc Revasc Med. 2020;21(March (3)):367–374. doi: 10.1016/j.carrev.2019.05.012. Epub 2019 May 22. PMID: 31178350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available from the corresponding author upon request.