Abstract
Purpose
Norrie disease is a rare X-linked recessive vitreoretinopathy. Variants of the NDP gene are associated with this condition. This case reports aims to demonstrate the variations of clinical presentations and exam findings of this disease.
Observations
A retrospective chart review of the patient's ocular and systemic findings and imaging results was performed. The patient had received genetic testing, including mutational analysis of targeted genes associated with retrolental masses. The patient had a comprehensive eye exam for bilateral leukocoria, demonstrating large retrolental masses, anterior polar cataracts, stretched ciliary processes, and roving eye movements. B-scan ultrasonography and magnetic resonance imaging indicated total, funnel-shaped retinal detachments, which is a unique retinal configuration in Norrie disease. Genetic testing confirmed deletion of the coding region of all three exons in the NDP gene, which confirmed Norrie disease. He has not shown any extraocular involvement to date.
Conclusions and Importance
This is a case demonstrating the association between deletion of the coding region NDP gene and Norrie disease. The phenotypical variation of this disease warrants further studies of genotype-phenotype correlations and mutations of the NDP gene.
Keywords: Norrie disease, NDP, Norrin, Leukocoria, Retinal detachment
1. Introduction
Norrie disease is a rare X-linked recessive disorder that is characterized by a varying degree of fibrovascular changes within the eyes.1 Encoded by the “Norrie disease (pseudoglioma)” (NDP) gene (MIM: 300658; cytogenetic location: Xp11.3), Norrin is a protein that is thought to be important in retinal neuroprotection and retinal angiogenesis.2,3 Pathogenic variants of NDP are associated with Norrie disease and related vitreoretinopathies of milder phenotypes. To date, more than 100 mutations of NDP have been identified — which include missense, null, splice, and deletions — leading to an abnormal gene product.4 Patients with Norrie disease may have congenital blindness associated with abnormal findings such as leukocoria, iris atrophy, corneal opacity, cataract, and retinal dysgenesis5 with an associated fibrovascular mass (a.k.a. pseudoglioma).1,4 Secondary tractional retinal detachment is very common at birth,6 and thus, there is a high risk of developing phthisis bulbi with age.7 In addition, there may be extraocular manifestations such as sensorineural hearing loss and mental cognitive and behavioral impairments.4 Herein, we present a case of Norrie disease due to a deletion of the coding region of NDP gene and the associated clinical findings, including some unique retinal configurations.
2. Case report
2.1. Clinical findings
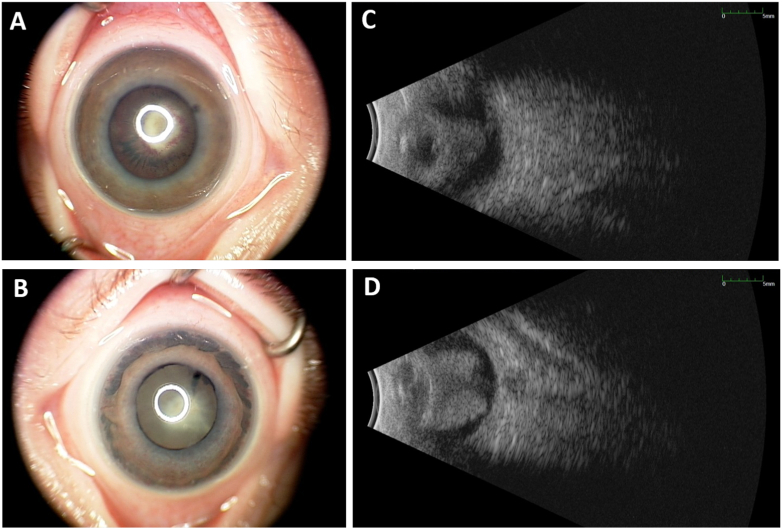
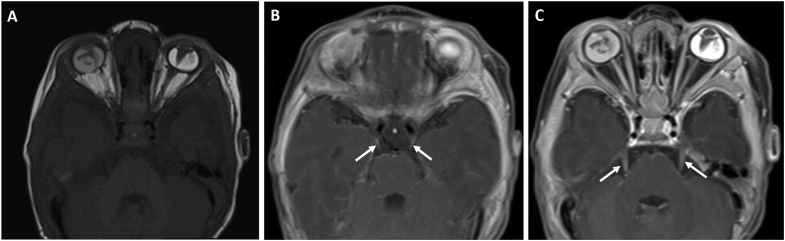
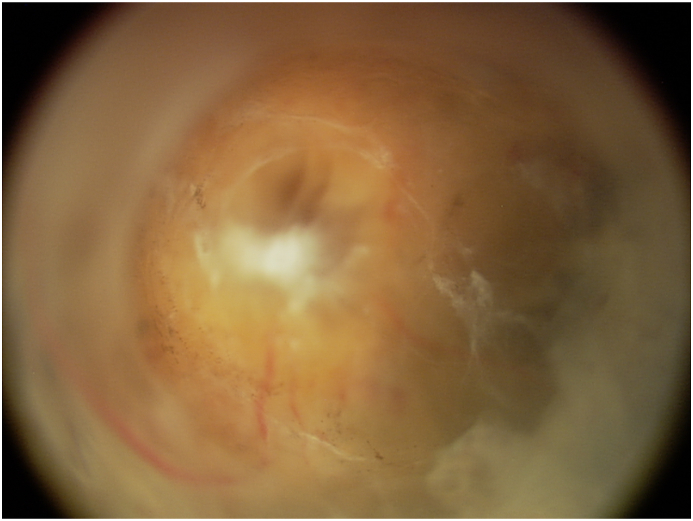
A 7-week-old, African-American male was referred to our institution for bilateral leukocoria. His mother and primary care provider noted normal red reflexes until 5 weeks of age. His family history was significant for a paternal great uncle who had unilateral blindness causing strabismus at 3 years old and his paternal grandfather who had high myopia. On exam, the patient had roving eye movements and did not blink or wince to light in either eye. The anterior chamber of both eyes appeared quiet but shallow. The anterior segment was otherwise notable for an anterior polar cataract with fine persistent pupillary membrane and peripheral maldevelopment of the anterior leaflet of the iris. A large, white retrolental mass could be seen in each eye, which had an irregular surface but no prominent vascular dilatation or tortuosity. The mass appeared to be stretching the ciliary processes, particularly in the right eye (Fig. 1A and B). B-scan of each eye demonstrated a funnel shaped retinal detachment but no hyperechoic spots within the intraocular mass that would be consistent with calcifications (Fig. 1C and D). Magnetic resonance imaging (MRI) of the brain and orbits (Fig. 2A) showed heterogeneous masses in each globe and retinal detachment with mild microphthalmia. The optic nerves were hypoplastic with markedly diminutive prechiasmatic size and a barely discernible optic chiasm. In addition, bilateral enhancement of the intraorbital optic nerves, oculomotor nerves, and trigeminal nerves was noted — albeit partially visualized in the case of cranial nerves III (Fig. 2B) and V (Fig. 2C). The patient was followed with serial exams and B-scans to ensure there was no calcification formation, and a normal RB1 analysis helped rule out retinoblastoma. Cranial nerve enhancement raised the possibility of an infectious or inflammatory condition. TORCH titers, including for lymphocytic choriomeningitis virus (LCMV), were unremarkable. Lumbar puncture, complete blood count, and complete metabolic panel were also unremarkable. At the age of 3 months, his right eye had a funnel-shaped retinal detachment; his left eye showed central hyperechoic area with globe disorganization, posterior synechiae, retinal detachment in the periphery, and probable funnel detachment. Subsequently, he had vitrectomy of both eyes after which he was able to track light with both eyes. At surgery, the retinal detachment was directly viewed, confirming the funnel shape (Fig. 3). Genetic analysis ultimately revealed complete deletion of the NDP gene, confirming the diagnosis of Norrie disease. He underwent additional vitrectomy, membranectomy, and retinal repair of the right eye. The structural outcome resulted in an open funnel-shaped retinal detachment. His most recent visit at the age of 3.5 years old indicated no measurable vision in either eye, and his left eye was phthisical.
Fig. 1.
External color photographs and B-scans.
A–B: External color photographs of the right (A) and left (B) eyes highlight the large, white retrolental mass in each eye with stretched ciliary processes (right > left eye) and anterior iris atrophy (left > right eye).
C–D: B-scan ultrasonography of right (C) and left (D) eyes show a retrolental mass taking up the majority of the posterior segment and absent of discrete hyperechoic spots that would indicate calcifications. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
Magnetic resonance imaging of the brain and orbits
A: Axial T1-weighted image showing heterogeneous masses in both eyes, funnel-shaped retinal detachment in the left eye, and mild microphthalmia of both eyes
B–C: Axial fat saturated, T2-weighted image highlighting bilateral enhancement of cranial nerves III (arrows in B) and V (arrows in C).
Fig. 3.
Post-vitrectomy color fundus photograph demonstrating funnel-shaped configuration of retinal detachment. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.2. Genetic testing
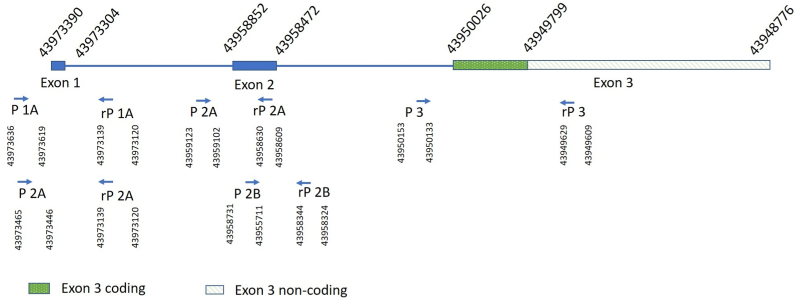
Initial whole-genome microarray analysis at the Cytogenetics Laboratory of Lurie Children's Hospital was normal. Given the early onset of bilateral leukocoria with retrolental masses and the inability of the microarray to detect small or single gene deletions, targeted gene analysis was performed at GeneDx to evaluate for retinoblastoma (RB1), Norrie disease (NDP), familial exudative vitreoretinopathy (FEVR) (FZD4, TSPAN12), and osteoporosis-pseudoglioma syndrome (LRP5). NDP analysis indicated complete deletion of the gene, confirming the diagnosis of Norrie disease. Exons 1–3 of NDP and their flanking splice sites were analyzed using PCR-amplification of genomic DNA extracted from the patient's peripheral blood. The test was designed to amplify and sequence the coding region of the gene, using cDNA as the reference. Table 1 shows the primer sequences that were used for PCR amplification of the NDP gene, and Fig. 4 shows the coordinates of the primers in relation to the exons. There was no amplification of the entire coding region of NDP (entire exons 1–2 and coding region of exon 3) and attempted amplification with a new preparation of DNA failed with repeat PCR. A control gene (PTEN) was able to be successfully amplified from the provided sample. Alternate primers were not found to be needed as allele drop-out was not a concern. The patient continued to have outside follow-up, so specific information on familial testing was unfortunately unclear. There was reported family history of “blindness” in the patient's sibling and uncle, but specific testing results were not available to confirm this.
Table 1.
Primer sequences used for PCR amplification of the NDP gene.
| Exon | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| 1 (A) | CTGAGTCCCCGATAACGA | GACTTCAAAGACCCGTTCAT |
| 1 (B) | CCCTGTGTCGCTTAAACAAC | GACTTCAAAGACCCGTTCAT |
| 2 (A) | CTATTCTTGGCCCTAGGAACAT | GCATAGAAAAGGATGCAGCTAG |
| 2 (B) | CTGGATCCTAGGAGGTGAAGC | AAAAGCCTCATTCTCCCACAA |
| 3 | GCATTGAGCCACTGGTCTAAT | CTGCAAAGAAGTTCCCAGAGT |
Fig. 4.
Coordinates of primers used in PCR amplification and exons of NDPgene
P1A: Forward Primer 1A. rP 1A: Reverse Primer 1A. P1B: Forward Primer 1B. rP 1B Reverse Primer 1B. P2A: Forward Primer 2A. rP 2A: Reverse Primer 2A. P2B: Forward Primer 1B. rP 2B: Reverse Primer 2B. P3: Forward Primer 3. rP 3: Reverse Primer 3.
3. Discussion
Norrie disease is caused by pathogenic variants of the NDP gene. NDP is comprised of three exons located on chromosome Xp11.3, which spans 28kb.8,9 Exons 2 and 3 are coding regions of NDP, while exon 1 is untranslated and may act as the promoter for gene transcription.4,10 As the protein product of NDP, Norrin is involved in regulating retinal development and regression of hyaloid vessels in the eye.3,11 It contains a cysteine knot motif, which is a highly conserved region in many growth factors and is important for the stable structure and proper folding of the Norrin protein.7 Mutations in the initiation and stop codons of NDP that cause Norrie disease have been identified.7,12,13 Non-cysteine mutations may also be associated with familial exudative vitreoretinopathy, Coats disease, and retinopathy of prematurity.14
Norrin is expressed on endothelial cells of the developing retina to provide protection for ocular capillaries and retinal neurons; in turn, the retinal neurons are involved in normal expression and processing of Norrin.15,16 Norrin binds to frizzled-4 (Fzd4) receptors as a dimer with a co-receptor, LRP5, which activates the Wnt/β-catenin signaling pathway, acting on the developing endothelial cells to regulate retinal vasculature development.17,18 Disruption of this pathway may lead to dysgenesis of the retina, as well as abnormal angiogenesis in the eye, ear, or brain.17,18 Evidence has suggested that the neuroprotective effects of Norrin are also mediated by the Wnt/β-catenin pathway, leading to an increased production of neuroprotective factors that is considered an independent process from Norrin's role in vascular development and protection.16 Persistent fetal vasculature (PFV) syndrome also has been reported in previous studies in patients with Norrie disease. It is a result of abnormal regression of the hyaloid vasculature and may share a common molecular mechanism with Norrie disease.5,18
Existing literature indicates that NDP deletions are associated with more severe disease manifestations than other types of mutations.5,11 Our patient had many of the ocular features of Norrie disease: bilateral fibrovascular retrolental masses, cataract, PFV stigmata, and retinal detachment — causing severe vision impairment. However, his retinal detachment was similar to stage 5 retinopathy of prematurity (i.e., anterior and posterior, narrow-funnel retinal detachment) rather than the retinal dysgenesis hypothesized to be associated with more severe Norrie disease deletions.7
Larger deletions that extend beyond NDP may lead to more neurological abnormalities and other complications.10,19 Since our patient had deletion of the entire coding sequences of NDP, it is expected that he did not have any Norrin protein product and would have more profound impairments. Interestingly, our patient has not shown any signs of hearing loss or mental retardation, which are two common non-ocular features of Norrie disease. Although there was enhancement of the cranial nerves on the MRI, he did not have any corresponding neurological deficits. Hearing loss can be gradual and manifest later in life.12 In mouse models of Norrie disease, Rehm et al. found that the hearing impairment purportedly has complete penetrance, but it may have variable phenotypic expression.20 Previous reports have presented patients who had deletions of the coding regions of NDP (exons 2 and 3) who only had ocular manifestations. Epigenetic factors are thought to play a role in genotype-phenotype correlations, which is probably why even the same mutation in the NDP gene can result in different phenotypes.10,12,21 It has also been suggested that the severity of Norrie disease is more correlated with the age of the patient than the type of NDP mutation.21
4. Conclusions
In conclusion, we have reported a patient with deletion of the entire coding region of NDP gene that resulted in Norrie disease of a non-dysplastic ocular phenotype with neuroimaging findings but no clinically neurologic or other systemic issues thus far. There is no clear mechanism to explain the phenotypic variation of this disease, and it is difficult to discern genotype-phenotype correlations. Broader investigation of the role of the NDP gene and Norrin are required to understand their roles in Norrie disease and other vitreoretinopathies.
Patient consent
Written consent was obtained from the patient's mother for the personal and/or clinical details along with the relevant images to be published in this study.
Institutional review board approval
The study was approved by Institutional Review Board of Ann & Robert H. Lurie Children's Hospital of Chicago.
Funding
No funding or grant support.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
The following authors have no financial disclosures: YZ, MJS, BKB, SK, MBM.
Acknowledgment
The authors would like to thank Hanta Ralay-Ranaivo, PhD for her assistance with the coordination of manuscript production, Katrin M. Carlson Leuer, PhD for her insight on genetic testing and GeneDx for the providing details and interpretation about the genetic testing.
Contributor Information
Yujia Zhou, Email: yujia8838@gmail.com.
Michael J. Shapiro, Email: michaeljshapiro@gmail.com.
Barbara K. Burton, Email: BBurton@luriechildrens.org.
Marilyn B. Mets, Email: MMets@luriechildrens.org.
Sudhi P. Kurup, Email: SKurup@luriechildrens.org.
References
- 1.Warburg M. Norrie's disease--differential diagnosis and treatment. Acta Ophthalmol. 1975;53:217–236. doi: 10.1111/j.1755-3768.1975.tb01156.x. [DOI] [PubMed] [Google Scholar]
- 2.Braunger B.M., Tamm E.R. The different functions of Norrin. Adv Exp Med Biol. 2012;723:679–683. doi: 10.1007/978-1-4614-0631-0_86. [DOI] [PubMed] [Google Scholar]
- 3.Luhmann U.F., Lin J., Acar N. Role of the Norrie disease pseudoglioma gene in sprouting angiogenesis during development of the retinal vasculature. Invest Ophthalmol Vis Sci. 2005;46:3372–3382. doi: 10.1167/iovs.05-0174. [DOI] [PubMed] [Google Scholar]
- 4.Sims K.B. In: NDP-related Retinopathies. Pagon R.A., Adam M.P., Ardinger H.H., editors. GeneReviews(R); Seattle (WA): 1993. [Google Scholar]
- 5.Wu W., Drenser K., Trese M., Capone A., Jr., Dailey W. Retinal phenotype–genotype correlation of pediatric patients expressing mutations in the norrie disease gene. Arch Ophthalmol. 2007;125:225–230. doi: 10.1001/archopht.125.2.225. [DOI] [PubMed] [Google Scholar]
- 6.Walsh M.K., Drenser K.A., Capone A., Jr., Trese M.T. Early vitrectomy effective for Norrie disease. Arch Ophthalmol. 2010;128:456–460. doi: 10.1001/archophthalmol.2009.403. [DOI] [PubMed] [Google Scholar]
- 7.Drenser K.A., Fecko A., Dailey W., Trese M.T. A characteristic phenotypic retinal appearance in Norrie disease. Retina. 2007;27:243–246. doi: 10.1097/01.iae.0000231380.29644.c3. [DOI] [PubMed] [Google Scholar]
- 8.Sims K.B., Lebo R.V., Benson G. The Norrie disease gene maps to a 150 kb region on chromosome Xp11.3. Hum Mol Genet. 1992;1:83–89. doi: 10.1093/hmg/1.2.83. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z.Y., Battinelli E.M., Hendriks R.W. Norrie disease gene: characterization of deletions and possible function. Genomics. 1993;16:533–535. doi: 10.1006/geno.1993.1224. [DOI] [PubMed] [Google Scholar]
- 10.Schuback D.E., Chen Z.Y., Craig I.W., Breakefield X.O., Sims K.B. Mutations in the Norrie disease gene. Hum Mutat. 1995;5:285–292. doi: 10.1002/humu.1380050403. [DOI] [PubMed] [Google Scholar]
- 11.Arai E., Fujimaki T., Yanagawa A. Familial cases of Norrie disease detected by copy number analysis. Jpn J Ophthalmol. 2014;58:448–454. doi: 10.1007/s10384-014-0334-4. [DOI] [PubMed] [Google Scholar]
- 12.Riveiro-Alvarez R., Trujillo-Tiebas M.J., Gimenez-Pardo A. Genotype-phenotype variations in five Spanish families with Norrie disease or X-linked FEVR. Mol Vis. 2005;11:705–712. [PubMed] [Google Scholar]
- 13.Zhang X.Y., Jiang W.Y., Chen L.M., Chen S.Q. A novel Norrie disease pseudoglioma gene mutation, c.-1_2delAAT, responsible for Norrie disease in a Chinese family. Int J Ophthalmol. 2013;6:739–743. doi: 10.3980/j.issn.2222-3959.2013.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow C.C., Kiernan D.F., Chau F.Y. Laser photocoagulation at birth prevents blindness in Norrie's disease diagnosed using amniocentesis. Ophthalmology. 2010;117:2402–2406. doi: 10.1016/j.ophtha.2010.03.057. [DOI] [PubMed] [Google Scholar]
- 15.Lee H., Jo D.H., Kim J.H. Norrin expression in endothelial cells in the developing mouse retina. Acta Histochem. 2013;115:447–451. doi: 10.1016/j.acthis.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Seitz R., Hackl S., Seibuchner T., Tamm E.R., Ohlmann A. Norrin mediates neuroprotective effects on retinal ganglion cells via activation of the Wnt/beta-catenin signaling pathway and the induction of neuroprotective growth factors in Muller cells. J Neurosci. 2010;30:5998–6010. doi: 10.1523/JNEUROSCI.0730-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye X., Wang Y., Nathans J. The Norrin/Frizzled4 signaling pathway in retinal vascular development and disease. Trends Mol Med. 2010;16:417–425. doi: 10.1016/j.molmed.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Payabvash S., Anderson J.S., Nascene D.R. Bilateral persistent fetal vasculature due to a mutation in the Norrie disease protein gene. NeuroRadiol J. 2015;28:623–627. doi: 10.1177/1971400915609350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez-Revenga L., Madrigal I., Alkhalidi L.S. Contiguous deletion of the NDP, MAOA, MAOB, and EFHC2 genes in a patient with Norrie disease, severe psychomotor retardation and myoclonic epilepsy. Am J Med Genet. 2007;143A:916–920. doi: 10.1002/ajmg.a.31521. [DOI] [PubMed] [Google Scholar]
- 20.Rehm H.L., Zhang D.-S., Brown M.C. Vascular defects and sensorineural deafness in a mouse model of norrie disease. J Neurosci. 2002;22:4286–4292. doi: 10.1523/JNEUROSCI.22-11-04286.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Royer G., Hanein S., Raclin V. NDP gene mutations in 14 French families with Norrie disease. Hum Mutat. 2003;22:499–504. doi: 10.1002/humu.9204. [DOI] [PubMed] [Google Scholar]