Highlights
-
•
The relative importance for serious side effects was the highest among all attributes.
-
•
Mothers express more WTP for the quadrivalent vaccine compared to bivalent due to its protection against genital warts.
-
•
Quadrivalent vaccination could be the most suitable candidate for implementation in the national immunization schedule.
Keywords: HPV, Vaccine, Discrete choice experiment, Willingness-to-pay, Iran
Abstract
This study aimed to identify mothers’ preferences and willingness-to-pay (WTP) for human papillomavirus (HPV) vaccines (in this case, bivalent and quadrivalent) in Iran. We used a discrete choice experiment (DCE) method to present mothers with choices between two hypothetical profiles of vaccines, described by combinations of five attributes, each with two or three levels. We analyzed the DCE results using conditional logistic regression and measured WTP estimates for each attribute. Our response rate was 53.96%, while the completion rate for questioner was 93.57%. We identified protection against cervical cancer, protection against genital warts, protection duration, serious side effects, and cost to influence mothers’ preferences for HPV vaccination. The relative importance for serious side effects was the highest among all attributes. Mothers’ WTP for bivalent and quadrivalent HPV vaccines was in US $ −432 (US $1 = IRR 42,000) and US $ 380, respectively. Quadrivalent vaccination could be the most suitable candidate for implementing the national immunization schedule. The reason is that mothers express more WTP for the quadrivalent vaccine than bivalent due to its protection against genital warts.
1. Introduction
Human papillomavirus (HPV), with a global prevalence estimated at 11.7%, is one of the most common sexually transmitted infections worldwide (Serrano et al., 2018). HPV infects almost 80% of women in their lifetime (Organization, 2020). Moreover, its prevalence has been reported 9.3% among females in the Eastern Mediterranean Region (EMR) (Farahmand et al., 2019), while its prevalence was estimated to be around 9.4% (Malary et al., 2016) among females in Iran, the third most populous country in this region. Approximately 70% of cervical cancers originate from high-risk types of persistent HPV infections, i.e. HPV type16, and 18 (Crosbie et al., 2013).
Cervical cancer is the fourth most common cancer among women worldwide, with an estimated 569,847 new cases and 311,365 deaths in 2018 (Arbyn et al., 2020). Of these deaths, almost 90% occurs in low- and middle-income countries (LMICs) (Organization, 2019). The age-standardized incidence rate (ASR) and mortality rates of cervical cancer in Iran is estimated to be around 2.2 and 1.2 per 100,000 females, respectively, in 2018. (Arbyn et al., 2020) While these rates are lower than in many other countries, the incidence rate is predicted to increase to 2040. By the highest assumption (i.e. 5% annual change), we may have 475% excess in the number of cervical cancer per year in Iran (Cancer).
Cervical cancer is mainly prevented by HPV vaccination or early detection and treatment of precancerous lesions (Organization, 2018). HPV immunization can also prevent up to almost 70% of all cervical cancers. In Iran, two accessible types, including bivalent and quadrivalent, exist among the three available methods of HPV vaccination. The bivalent vaccine prevents HPV16, 18, while the quadrivalent vaccine prevents HPV6 and 11, associated with the development of genital warts (Hoz Restrepo et al., 2018).
The HPV vaccine is cost-effective in many countries. Ninety-six countries have applied the HPV vaccine into their national immunization curriculum. (SAGE, 2019) Nevertheless, most EMR countries have not incorporated HPV into their vaccination program (Division, 2018). Various studies have not shown the cost-effectiveness of the HPV vaccine in Iran yet, which is why perhaps the national program vaccination of Iran does not include HPV (Khatibi et al., 2014, Yaghoubi et al., 2018). Although cost-effective analysis (CEA) is helpful to decide the inclusion of a vaccine into a country's national vaccination program, the CEA studies do not take into account the preferences of individuals, which might be different from policymakers. In addition, it is necessary to bring the non-medical and societal impact of the HPV vaccine into consideration in these studies (Park et al., 2018) when deciding to include them in the national immunization program in any country. This study aimed to investigate mothers' preferences and their willingness-to-pay (WTP) for the HPV vaccines for their girls by using a discrete choice experiment (DCE). Our findings will provide, we envisage, insight and valuable evidence regarding HPV vaccination for policymakers in Iran and perhaps similar settings.
2. Methods
We conducted a DCE study to identify mothers’ preferences and WTP. Widely used in health economics, DCEs is a method to elicit individuals’ preferences and WTP by observing their selections within hypothetical choice sets. The choice sets can be described by two or more alternatives, which can vary in attribute levels. Individuals have to indicate the option they like the most (Ryan et al., 2007). Our study had the following steps: 1) Identification of attributes and attribute levels, 2) Experimental design, 3) Participants and data collection and 4) Data analysis.
2.1. Identification of attributes and attribute levels
We identified the relevant attributes for DCE by a literature review on the HPV vaccine and DCE (Asiedu et al., 2015, Assefa, 2017, Brown et al., 2010, Brown et al., 2014, de Bekker-Grob and Chorus, 2013, de Bekker-Grob et al., 2010, Hofman et al., 2014, Hofman et al., 2014b, Ngorsuraches et al., 2015b, Oteng et al., 2011, Stockwell et al., 2011). We included 14 articles from Hong Kong, America, Netherlands, Thailand, Vietnam, Ethiopia, and Canada in this study. The research team refined and discussed a list of possible vaccine attributes. We evaluated fourteen attributes by online interviews with relevant experts, including medical staff, policymakers, oncologists, epidemiologists, virologists, pharmacies, economics, and statisticians (n = 9). We also established the final sets of attributes through the expert panel: literature reviews and experts' consultation assigned attribute levels. There are two official and one free-market exchange rates for US$ in Iran. Government supports the vaccine by subsidy and pays the lowest (government rate, US $1 = IRR 42,000). At the same time, people's monthly income needs to be converted by free-market rate (the US $1 = IRR 124,500) at the time of data collection. Table1 presents the attributes and the levels used in this study (US $1 = IRR 42,000).
Table 1.
Attributes and levels for the HPV vaccine.
| Attribute | Level |
|---|---|
| Protection against cervical cancer | 50, 70, 90 (%) |
| Protection against genital warts | 0, 90 (%) |
| Protection duration | 6 ,25, 100 (year) |
| Serious side effects | 1:750,000, 1:150.000, 1:30,000 |
| Cost | 0, 95, 167 ($ US) |
2.2. Experimental design
Five attributes and their corresponding levels resulted in 162 profiles (four attributes at three levels and one attribute at two levels = 81*2), and a total of 13,041 possible pairwise choices ((161*162)/2) in a complete factorial design. Since it is not feasible to present a single individual with all these scenarios, we created a D-optimal fractional factorial in SAS 9.4 using %mktruns, %mktex, %mktlab, %choiceff and %mktdups macros to estimate the main effects. It jointly maximizes the principles of orthogonality, level balance, minimal overlap, and utility (Kuhfeld, 2003). The final design consisted of 36 choice sets divided into four blocks of 9 choice pairs and allocated each participant to one of the four blocks randomly (%mktblock). To test respondents’ understanding of DCE choice sets within each block, one choice set was a dominant option. In this specially designed choice set, one vaccine option was better than the other unambiguously. For those who failed in the rationality tests, we excluded their data from the final analysis. Table 2 shows an example of a choice set.
Table 2.
Sample of the choice set used in this study.
| Vaccine B | Vaccine A | Attributes |
|---|---|---|
| 70% | 50% | Protection against cervical cancer |
| 0% | 90% | Protection against genital warts |
| 25 year | 100 year | Protection duration |
| 1:150000 | 1:150000 | Serious side effects |
| US $ 0 | US $ 167 | Cost |
| Which vaccine do you prefer? |
2.3. Participants and data collection
Our study participants were mothers who had at least one 9–14-year-old daughter. We used a consecutive sampling to recruit our participants, five hospitals (two general and three specialty hospitals) affiliated with the Tehran University of Medical Sciences (TUMS) in the megacity of Tehran. We conducted face-to-face interviews in October and November 2019. We determined the optimal sample size for the DCE by several attributes and their levels. Studies review shows that the sample sizes between 300 and 400 participants or up to 300 participants are sufficient for reliable statistical analysis (Brown et al., 2014, Hofman et al., 2014). Therefore, we strived to collect at least 320 completed questionnaires. In addition, we also obtained the DCE data through socio-demographic information, participants’ knowledge of cervical cancer, genital wart, and information related to vaccine HPV.
2.4. Data analysis
We used a conditional logit regression model to analyze mothers’ preferences and calculate the WTP value for each attribute level. We defined the desirability of the HPV vaccine for mothers by a random utility model expressed through the following equations:
| V = β0 + β1 protection against cervical cancer_70% + β2 protection against cervical ancer_90% + β3 protection against genital warts_90%+ β4 protection duration_25 + β5 protection duration _100 + β6 serious side effects_1:150000 + β7 serious side effects_1:30000 + β8_ cost + ε | (1) |
V represents the utility from each choice. β1 to β8 are coefficients of the attributes indicating the relative weight that individuals place on attribute levels. In this model, more preferred outcomes have higher weights, and its sign indicates whether the attribute level has a positive or negative effect on participants' utility. Also, ε is a random error term that represents the unmeasured effects in participants' preferences. The choice data was dummy coded for all attributes, except for the cost, which was entered into the model as a continuous variable. We assessed the relative importance of each feature by part-worth utilities (Orme, 2010). The part-worth utility values or preference weights is a calculated preference weight for each attribute and its respective levels (Hauber et al., 2016). A feature with a more significant difference between its lowest level part-worth and its highest level part-worth has greater relative importance in the mothers' choice (Burnett et al., 2012, Pickard et al., 2018) (Eq. (2)).
| Relative importance of attributes % = (Maximum part-worth attribute level − Minimum part-worth attribute level)/Σ (Maximum part-worth attribute level − Minimum part-worth attribute level) | (2) |
Additionally, WTP values are calculated by dividing each attribute levels coefficient into the price attribute (Eq. (3)) (Ryan et al., 2012).
| (3) |
We conducted a sensitivity analysis by including participants who failed the rationality tests and the conditional logistic regression analysis using the SAS 9.4 software Proc Logistic function.
3. Results
This study revealed the mother's preferences and WTP to the HPV vaccine in Iran. All vaccination attributes identified to influence mother's preferences for HPV vaccination. The serious side effects were the most important attributes. The mothers' WTP for the bivalent and quadrivalent HPV vaccines were at US $ −432 and the US $ 380, respectively. In this section, we first present characteristics of respondents, then preferences for vaccine attributes and relative importance of attributes, and finally WTP for HPV vaccine.
3.1. Characteristics of respondents
The response rate was 306/567 (53.96%). A total of 327 participants completed the questionnaire, of whom 21 were excluded due to incorrect responses to the choice set for the rationality test. Therefore, the final sample included 306 participants, and the completion rate was 93.57%.
The mothers’ demographic characteristics are in Table 3. 67% of respondents were between 31 and 40 years, 41% had a diploma, 97% were married and housewife (88%). Most participants reported their monthly income less than US $ 321(US $1 = IRR 124,500, based on free-market exchange rate). 57% of mothers refused to use HPV vaccination for their daughters in response to the open question regarding selecting HPV vaccine.
Table 3.
Characteristics of respondents (n = 306).
| Variables | N (%) |
|---|---|
| Age (y) | |
| 20–30 | 37 (12.1) |
| 31–40 | 206 (67.3) |
| 41–50 | 58 (19) |
| 51–60 | 5 (1.6) |
| Marital status | |
| Married | 296 (96.7) |
| Widowed/Divorced/Separated | 10 (3.3) |
| Education | |
| Elementary education ≤ 5 years | 52 (17) |
| Secondary education ≤ 8 years | 62 (20.3) |
| High school diploma ≤ 12 years | 134 (43.8) |
| University education | 58 (18.9) |
| Employment status | |
| Civil servant | 20 (6.5) |
| Private firm | 17 (5.6) |
| Homemaker | 269 (87.9) |
| Monthly household income, US $ | |
| >80 | 69 (22.6) |
| 80–161 | 120 (39.2) |
| 161–321 | 91 (29.7) |
| 321–562 | 17 (5.6) |
| 562> | 9 (2.9) |
| Family size | |
| ≤4 | 208 (68) |
| ≥5 | 98 (32) |
| Head of household | |
| Yes | 15 (4.9) |
| No | 291 (95.1) |
3.2. Preferences for vaccine attributes
Results from the conditional logit regression estimation are in Table 4. All vaccination characteristics influence mothers’ preferences for HPV vaccination (P < 0.05). The positive sign of coefficients protection duration, protection against cervical cancer and protection against genital warts indicated that mothers preferred an HPV vaccination due to a more extended protection duration and expected a higher level of protection. In addition, serious side effects and cost coefficient were both adverse, demonstrating that mothers preferred to choose a vaccine with lower serious side effects and cost. Our conditional logit regression had a pseudo R2 of 0.1727, which indicates that the model is acceptable. We performed a sensitivity analysis, including mothers who failed the rationality tests, and we did not observe a significant impact.
Table 4.
Coefficients from conditional logit vaccine preference model.
| Attribute | Coefficient | P value | Odd Ratio | 95% confidence interval |
|---|---|---|---|---|
| Protection against cervical cancer | ||||
| 70% | 0.3986 | <0.001 | 1.49 | 1.27–1.73 |
| 90% | 0.596 | <0.001 | 1.81 | 1.47–2.23 |
| Protection against genital warts | ||||
| 90% | 0.9179 | <0.001 | 2.50 | 2.24–2.79 |
| Protection duration | ||||
| 25 year | 0.508 | <0.001 | 1.66 | 1.42–1.94 |
| 100 year | 0.8272 | <0.001 | 2.28 | 1.92–2.71 |
| Serious side effects | <0.001 | |||
| 1:150000 | − 0.7079 | <0.001 | 0.49 | 0.42–0.57 |
| 1:30000 | − 1.3946 | <0.001 | 0.24 | 0.20–0.29 |
| Cost | −0.00113 | 0.033 | 0.99 | 0.99–1 |
3.3. The relative importance of attributes
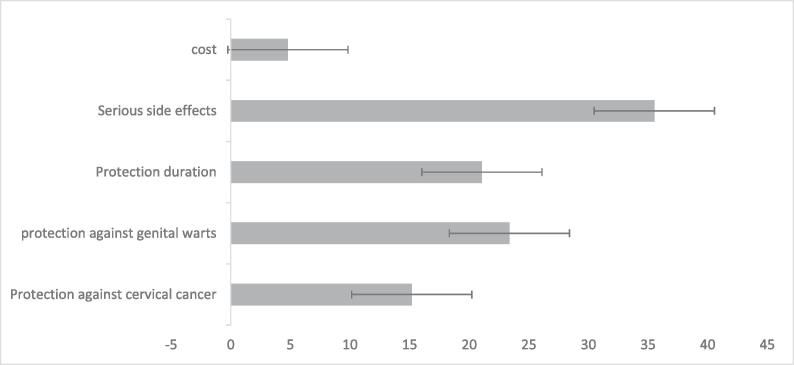
Importance scores represent the relative weight that an attribute had on mothers’ choice. After serious side effects, which were the most important attributes, protection against genital warts, protection duration, protection against cervical cancer, and cost are in the following categories. Fig. 1 shows the relative importance of attributes.
Fig. 1.
Relative importance of attributes.
3.4. Willingness to pay
Table 5 shows the WTP for attributes of HPV vaccines. Mothers’ WTP was US $1234 (US $1 = IRR 42000) to avoid serious side effects from getting vaccinated. On the other hand, they were willing to pay the US $ 527 and the US $ 812 for protection against getting cervical cancer and genital warts, respectively. Table 6 shows the WTP for bivalent and quadrivalent HPV vaccines. We obtained the attribute levels of vaccines from literature reviews (Brown et al., 2014, Wong et al., 2018), Centers for Disease Control and Prevention (CDC) (Prevention, Accessed [June 2020]) and consultation with an expert to estimate the WTP for both vaccines. The results showed that mothers’ WTP for the bivalent and quadrivalent HPV vaccines were at the US $ −432 and the US $ 380, respectively.
Table 5.
WTP for the attributes of HPV vaccine.
| Attribute | Coefficient | Average WTP, US $ |
|---|---|---|
| Protection against cervical cancer | 0.596 | 527.43 |
| Protection against genital warts | 0.9179 | 812.3 |
| Protection duration | 0.8272 | 732.03 |
| Serious side effects | 1.3946 | −1234.15 |
Table 6.
WTP for bivalent and quadrivalent vaccines.
| Attribute | WTP for bivalent vaccine, US $ | WTP for quadrivalent vaccine, US $ |
|---|---|---|
| Protection against cervical cancer (70%) | 352.74 | 352.74 |
| Protection against genital warts (90%) | 0 | 812.3 |
| Protection duration (25 year) | 449.55 | 449.55 |
| Serious side effects (1:30000) | −1234.15 | −1234.15 |
| Total WTP | −431.85 | 380.44 |
4. Discussion
This study elicited mothers’ preferences and WTP to vaccinate their 9–14-year-old daughters against HPV in Iran using the discrete choice experiment (DCE) method. Mothers prefer vaccines with fewer serious side effects and cost and higher vaccine protection. Our findings revealed that while serious side effects have the highest impact on the mothers’ preference for getting HPV vaccines, protection against genital warts and protection duration is also influential. Our results are similar to the experience in Hong Kong (Wong et al., 2018), which suggest that serious side effects compared to other vaccine characteristics are the most important issues for policymakers to decide about vaccinations. According to the coefficient strengths, mothers weighted protection against genital warts more than protection against cervical cancer (0.25 VS 0.16). It might be due to the low incidence of cervical cancer in Iran. Over half of our study participants were unsure about HPV vaccines, which might cause vaccines' side effects (Chan et al., 2012) and their cultural and religious boundaries. In contrary, studies in Vietnam and Thailand showed that most participants were willing to get the HPV vaccine, which might result from the high incidence of cervical cancer in those countries (Ngorsuraches et al., 2015a, Tran et al., 2018).
Willingness to pay for the quadrivalent vaccine was US$ 380 in Iran, while it was US$ 684 in Thailand and US$758 in Chile (Cerda et al., 2013, Ngorsuraches et al., 2015a). The calculated WTP assists in determining perceived vaccination value among mothers. Lack of mothers’ WTP for the bivalent vaccine (US $ − 432) may be related to the generally low incidence of cervical cancer in Iran, lack of awareness and weak attitude towards the importance of prevention, as well as religious and cultural barriers to effective communications of sexually related diseases in families. The fragile economic situation might have also reduced mothers’ priority to pay for the bivalent vaccine (Reza and Abdi Zhaleh, 2017). Our findings might change as a result of campaigns to increase mothers’ motivation. WTP for the quadrivalent vaccine was US $ 380, which suggests that protection against genital warts is important, and mothers prefer to pay for having additional protection. The mean maternal WTP for the quadrivalent HPV vaccine was high compared to its current price in Iran, reflecting people’s fear of genital wart. Our findings are consistent with those of Thailand and The United States (Brown et al., 2014, Ngorsuraches et al., 2015b).
Despite the substantial effort and the heated debate to include the HPV vaccine in the national immunization program in Iran, current evidence shows that the vaccine is not cost-effective 13,14. Knowing people’s preference regarding vaccination, including concerns about the serious side effects and people’s desire for quadrivalent over bivalent vaccine and WTP for vaccination, might help policymakers take public demand into consideration when deciding about the HPV vaccine.
Despite its strengths, our study has some limitations. First, we used the multinomial logit model, which does not allow interaction between socio-economic characteristics and attributes of the alternatives (Boyle et al., 2017). Second, our study participants were mothers. However, decision-making about girl’s vaccinations may be also influenced by fathers or other family members. Finally, the samples in our study only consist of mothers from the capital city of Tehran, which is overall more affluent than other cities. As a result, our findings may overestimate the acceptance, preference, and WTP for the HPV vaccine and might be nationally non-representative. Despite these limitations, our study offers substantial evidence for policymakers about the preferences and the monetary value placed on HPV vaccines. Our research presents empirical evidence in specific cases, where CEA does not provide appropriate answers. Finally, WTP can benefit from cost-benefit analysis by national HPV vaccination programs (Buchanan, 2015).
5. Conclusions
Our study revealed that serious side effects are the most important attribute in determining the acceptability of HPV vaccines in Iran. Mothers express more WTP for the quadrivalent vaccine compared to bivalent due to its protection against genital warts. Successful implementation of HPV vaccination within the national programs would require adequate consideration of people's preferences. While the Ministry of Health and Medical Education of Iran is deciding to include appropriate HPV vaccination in the national curriculum, understanding and concerning public preference is crucial in determining such decisions.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
We thank our anonymous respondents who completed the survey. We are also grateful to Somayeh Jalilvand. PhD (TUMS) for her insightful advice and assistance during data collection.
Funding
This research is a part of an MSc thesis in health economics at the Tehran University of Medical Sciences (TUMS), which benefited from the support of the TUMS, Iran. Registration code: IR.TUMS.SPH.REC.1398.121.
Authors’ contribution
AT and NS conceived the study. AT supervised all phases of evaluation and critically revised the manuscript; he is the guarantor. MY, RAD, were advisors in methodology, analysis, and interpretation of data and equally contributed to the intellectual development of the manuscript. NS collected and conducted primary data analysis. All authors contributed to the development and approval of the final manuscript.
References
- Arbyn M., Weiderpass E., Bruni L., de Sanjosé S., Saraiya M., Ferlay J., Bray F. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Global Health. 2020;8(2):e191–e203. doi: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asiedu G.B., Breitkopf C.R., Kremers W.K., Ngo Q.V., Nguyen N.V., Barenberg B.J., Tran V.D., Dinh T.A. Vietnamese health care providers’ preferences regarding recommendation of HPV vaccines. Asian Pac. J. Cancer Prev. 2015;16(12):4895–4900. doi: 10.7314/apjcp.2015.16.12.4895. [DOI] [PubMed] [Google Scholar]
- Assefa, R., 2017. Girl’s Preference for Human Papilloma Virus Vaccination in secondary schools in Addis Ababa, Ethiopia, 2017: Discrete Choice Experiment. Addis Abeba Universty.
- Boyle K.J., Brown T.C., Champ P.A. Springer; Netherlands: 2017. A Primer on Nonmarket Valuation. [Google Scholar]
- Brown D.S., Johnson F.R., Poulos C., Messonnier M.L. Mothers’ preferences and willingness to pay for vaccinating daughters against human papillomavirus. Vaccine. 2010;28(7):1702–1708. doi: 10.1016/j.vaccine.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Brown D.S., Poulos C., Johnson F.R., Chamiec-Case L., Messonnier M.L. Adolescent girls’ preferences for HPV vaccines: a discrete choice experiment. Adv. Health Econ. Health Serv. Res. 2014;24:93–121. [PubMed] [Google Scholar]
- Buchanan J. University of Oxford; 2015. Issues Related to the Economic Analysis of Genomic Diagnostic Technologies in the UK National Health Service: An Exploration of Methods. [Google Scholar]
- Burnett H.F., Regier D.A., Feldman B.M., Miller F.A., Ungar W.J. Parents' preferences for drug treatments in juvenile idiopathic arthritis: a discrete choice experiment. Arthritis Care Res. 2012;64(9):1382–1391. doi: 10.1002/acr.21698. [DOI] [PubMed] [Google Scholar]
- Cerda A.A., Garcia L.Y., Gaete F.I., Pizarro T.H. Willingness to pay for human papillomavirus vaccine in Metropolitan Santiago, Chile. Rev. Med. Chil. 2013;141:167–172. doi: 10.4067/S0034-98872013000200004. [DOI] [PubMed] [Google Scholar]
- Chan K.K., Kwan T.T., Yao T.J., Tam K.F., Cheung A.N., Ngan H.Y. Human papillomavirus vaccine: what are women most concerned about? J. Obstetrics Gynaecol. Res. 2012;38:23–30. doi: 10.1111/j.1447-0756.2011.01639.x. [DOI] [PubMed] [Google Scholar]
- Crosbie E.J., Einstein M.H., Franceschi S., Kitchener H.C. Human papillomavirus and cervical cancer. Lancet. 2013;382(9895):889–899. doi: 10.1016/S0140-6736(13)60022-7. [DOI] [PubMed] [Google Scholar]
- de Bekker-Grob E.W., Chorus C.G. Random regret-based discrete-choice modelling: an application to healthcare. PharmacoEconomics. 2013;31(7):623–634. doi: 10.1007/s40273-013-0059-0. [DOI] [PubMed] [Google Scholar]
- de Bekker-Grob E.W., Hofman R., Donkers B., van Ballegooijen M., Helmerhorst T.J.M., Raat H., Korfage I.J. Girls’ preferences for HPV vaccination: a discrete choice experiment. Vaccine. 2010;28(41):6692–6697. doi: 10.1016/j.vaccine.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Division, U.S., 2018. Human papillomavirus vaccine: supply and demand update.
- Farahmand M., Shoja Z., Arashkia A., Salavatiha Z., Jalilvand S. Systematic review and meta-analysis of human papillomavirus prevalence and types among women with normal cervical cytology in the Eastern Mediterranean Region. Future Virol. 2019;14(11):761–777. [Google Scholar]
- Hauber A.B., González J.M., Groothuis-Oudshoorn C.G.M., Prior T., Marshall D.A., Cunningham C., IJzerman M.J., Bridges J.F.P. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value Health. 2016;19(4):300–315. doi: 10.1016/j.jval.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Hofman R., de Bekker-Grob E.W., Raat H., Helmerhorst T.J., van Ballegooijen M., Korfage I.J. Parents’ preferences for vaccinating daughters against human papillomavirus in the Netherlands: a discrete choice experiment. BMC Public Health. 2014;14:454. doi: 10.1186/1471-2458-14-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman, R., de Bekker-Grob, E.W., Richardus, J.H., de Koning, H.J., van Ballegooijen, M., Korfage, I.J., 2014b. Have preferences of girls changed almost 3 years after the much debated start of the HPV vaccination program in The Netherlands? A discrete choice experiment. PloS one 9. [DOI] [PMC free article] [PubMed]
- Restrepo, F.D.l.H., Guzman, N.A., Gomez, A.D.l.H., Ruiz, C., 2018. Policies and processes for human papillomavirus vaccination in Latin America and the Caribbean. Revista Panamericana de Salud Pública 41:e124. [DOI] [PMC free article] [PubMed]
- Khatibi M., Rasekh H.R., Shahverdi Z. Cost-effectiveness evaluation of quadrivalent human papilloma virus vaccine for HPV-related disease in Iran. Iran. J. Pharm. Res.: IJPR. 2014;13:225. [PMC free article] [PubMed] [Google Scholar]
- Kuhfeld W.F. Marketing Research Methods in SAS. Citeseer. 2003 [Google Scholar]
- Malary, M., Moosazadeh, M., Hamzehgardeshi, Z., Afshari, M., Moghaddasifar, I., Afsharimoghaddam, A., 2016. The prevalence of cervical human papillomavirus infection and the most at-risk genotypes among Iranian healthy women: A systematic review and meta-analysis. International journal of preventive medicine 7. [DOI] [PMC free article] [PubMed]
- Ngorsuraches S., Nawanukool K., Petcharamanee K., Poopantrakool U. Parents’ preferences and willingness-to-pay for human papilloma virus vaccines in Thailand. J. Pharm. Policy Pract. 2015;8:1–9. doi: 10.1186/s40545-015-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngorsuraches S., Nawanukool K., Petcharamanee K., Poopantrakool U. Parents’ preferences and willingness-to-pay for human papilloma virus vaccines in Thailand. J. Pharm. Policy Pract. 2015;8:20. doi: 10.1186/s40545-015-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization, W.H., 2018. Improving data for decision-making: a toolkit for cervical cancer prevention and control programmes.
- Organization, W.H., 2019. WHO guidelines for the use of thermal ablation for cervical pre-cancer lesions. [PubMed]
- Organization, W.H., 2020. WHO technical guidance and specifications of medical devices for screening and treatment of precancerous lesions in the prevention of cervical cancer.
- Orme, B., 2010. Interpreting the results of conjoint analysis. Getting Started with Conjoint Analyis: Strategies for Product Design and Pricing Research 2:77-88.
- Oteng B., Marra F., Lynd L.D., Ogilvie G., Patrick D., Marra C.A. Evaluating societal preferences for human papillomavirus vaccine and cervical smear test screening programme. Sexually Transmitted Infections. 2011;87(1):52–57. doi: 10.1136/sti.2009.041392. [DOI] [PubMed] [Google Scholar]
- Park M., Jit M., Wu J.T. Cost-benefit analysis of vaccination: a comparative analysis of eight approaches for valuing changes to mortality and morbidity risks. BMC Med. 2018;16:139. doi: 10.1186/s12916-018-1130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard A.S., Huynh L., Ivanova J.I., Totev T., Graham S., Mühlbacher A.C., Roy A., Duh M.S. Value of transfusion independence in severe aplastic anemia from patients’ perspectives–a discrete choice experiment. J. Patient-reported Outcomes. 2018;2:13. doi: 10.1186/s41687-018-0032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevention, C.f.D.C.a., Accessed [June, 2020]. Availablefrom: https://www.cdc.gov/hpv/hcp/vaccine-safety-data.html.
- Majdzadeh Seyed Reza, A.E., Abdi Zhaleh, 2017. Observatory on Health System, Islamic Republic of Iran. National Institute for Health Research.
- Ryan M., Gerard K., Amaya-Amaya M. Springer Science & Business Media; 2007. Using Discrete Choice Experiments to Value Health and Health Care. [Google Scholar]
- Ryan M., Kolstad J., Rockers P., Dolea C. World Health Organization & CapacityPlus: World Bank; 2012. How to Conduct a Discrete Choice Experiment for Health Workforce Recruitment and Retention in Remote and Rural Areas: A User Guide With Case Studies. [Google Scholar]
- SAGE, B.D.a.R.t., 2019. Strategic Advisory Group of Experts (SAGE) on Immunization Working Group onpotential contribution of Human Papillomavirus (HPV) vaccines and immunization towards cervical cancer elimination.
- Serrano Beatriz, Brotons María, Bosch Francesc Xavier, Bruni Laia. Epidemiology and burden of HPV-related disease. Best Pract. Res. Clin. Obstetr. Gynaecol. 2018;47:14–26. doi: 10.1016/j.bpobgyn.2017.08.006. [DOI] [PubMed] [Google Scholar]
- Stockwell Melissa S., Rosenthal Susan L., Sturm Lynne A., Mays Rose M., Bair Rita M., Zimet Gregory D. The effects of vaccine characteristics on adult women's attitudes about vaccination: a conjoint analysis study. Vaccine. 2011;29(27):4507–4511. doi: 10.1016/j.vaccine.2011.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran B.X., Than P.T.Q., Doan T.T.N., Nguyen H.L.T., Mai H.T., Nguyen T.H.T., Le H.T., Latkin C.A., Zhang M.W. Knowledge, attitude, and practice on and willingness to pay for human papillomavirus vaccine: a cross-sectional study in Hanoi, Vietnam. Patient Preference Adherence. 2018;12:945. doi: 10.2147/PPA.S165357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Carlos K.H., Man Kenneth K.C., Ip Patrick, Kwan Mike, McGhee Sarah M. Mothers’ Preferences and Willingness to Pay for Human Papillomavirus Vaccination for Their Daughters: A Discrete Choice Experiment in Hong Kong. Value Health. 2018;21(5):622–629. doi: 10.1016/j.jval.2017.10.012. [DOI] [PubMed] [Google Scholar]
- Yaghoubi Mohsen, Nojomi Marzieh, Vaezi Atefeh, Erfani Vida, Mahmoudi Susan, Ezoji Khadijeh, Zahraei Seyed Mohsen, Chaudhri Irtaza, Moradi-Lakeh Maziar. Cost-effectiveness analysis of the introduction of HPV vaccination of 9-year-old-girls in Iran. Value Health Regional Issues. 2018;15:112–119. doi: 10.1016/j.vhri.2018.03.001. [DOI] [PubMed] [Google Scholar]