Highlights
-
•
Screening is a major public health strategy in disease prevention.
-
•
Uptake remains suboptimal and current approaches fail to engage the most vulnerable.
-
•
Interventions to improve equitable access are urgently required.
-
•
A coherent model of screening behaviour is yet to be established.
-
•
The Integrated Screening Action Model (I-SAM) proposes an integrated theoretical model aiming to improve screening access for all.
Keywords: Screening, Theory-based, Model, Framework, Intervention development, Cancer, Diabetic retinopathy, Abdominal aortic aneurysm
Abstract
Screening can reduce deaths if the people invited participate. However, good uptake is hard to achieve, and our current approaches are failing to engage the most vulnerable. A coherent model of screening behaviour to guide our understanding and intervention development is yet to be established. The present aim was to propose an Integrated Screening Action Model (I-SAM) to improve screening access.
The I-SAM synthesises existing models of health behaviour and empirical evidence. The I-SAM was developed following: i) an appraisal of the predominant models used within the screening literature; ii) the integration of the latest knowledge on behaviour change; with iii) the empirical literature, to inform the development of a theory-based approach to intervention development.
There are three key aspects to the I-SAM: i) a sequence of stages that people pass through in engaging in screening behaviour (based on the Precaution Adoption Process Model); ii) screening behaviour is shaped by the interaction between participant and environmental influences (drawing from the Access Framework); and iii) targets for intervention should focus on the sources of behaviour - ‘capability’, ‘opportunity’, and ‘motivation’ (based on the COM-B Model).
The I-SAM proposes an integrated model to support our understanding of screening behaviour and to identify targets for intervention. It will be an iterative process to test and refine the I-SAM and establish its value in supporting effective interventions to improve screening for all.
1. Introduction
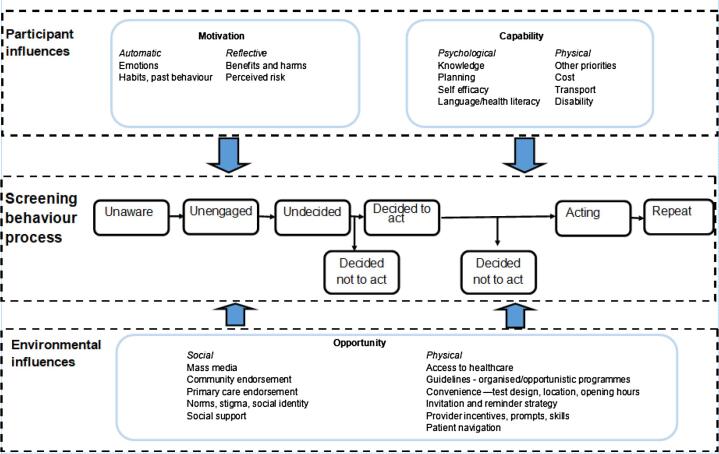
Screening can reduce deaths if the people invited participate (Ronco et al., 2014, Myers et al., 2015, National Lung Screening Trial Research Team, 2011, Lin et al., 2016, Guirguis-Blake et al., 2019, Leese et al., 2015). Future technological advances will lead to more accurate, and stratified screening tests, offering improvements in early diagnosis and survival. However, no matter how state-of-the-art the test, it will only be effective if people are willing to do it. Years of experience of cancer screening suggest that good uptake is hard to achieve, and our current approaches fail to engage the most vulnerable (McCowan et al., 2019). The existing literature on screening behaviour while informed, in some cases, by theory, has not yet established a coherent action model of screening behaviour to guide our understanding of the determinants of screening behaviour and identify targets for intervention (Rakowski and Breslau, 2004, Kobrin et al., 2015). The aim of this paper is to describe the development of an Integrated Screening Action Model (I-SAM: Fig. 1), which draws on theoretical models from behavioural science and empirical evidence, to provide a unifying structure to improve the translation of research into practice to increase the efficacy of existing and future screening tests.
Fig. 1.
Integrated Screening Action Model (I-SAM).
Participation in screening programmes (breast, colorectal, cervical, lung, diabetic retinopathy, abdominal aortic aneurysm) remains suboptimal, with persistent inequalities in uptake such that people living in more socioeconomically deprived areas, ethnic minorities, people with comorbidities, and people with intellectual disabilities are less likely to participate (Campbell et al., 2020, McCowan et al., 2019, Szczepura et al., 2008, Crilly et al., 2015, Leese et al., 2008). There is an urgent need to improve screening participation and develop effective interventions (Duffy et al., 2017).
One of the key principles of intervention development is that it should draw on existing theory (O’Cathain et al., 2019). A theoretical model can help to guide intervention research from conceptualisation to analysis and clarify why, how, and for whom an intervention may work (Kobrin et al., 2015). To date, researchers have drawn on a range of health behaviour models and theories to study screening behaviour yet despite calls for the need for multilevel theoretical and conceptual approaches (Rakowski and Breslau, 2004, Curry and Emmons, 1994) little progress has been made.
2. Approach to model development
The development of the I-SAM was guided by three key principles: i) the model should account for people being at different stages of the screening behaviour process i.e. some people are unaware, some people have formed an intention to screen; ii) screening behaviour is influenced at multiple, interacting levels; and iii) existing models of behaviour change and empirical evidence should inform the development of the I-SAM. Following these three principles, the application of the I-SAM should improve the prediction of screening behaviour and support the identification of intervention targets to enhance the screening process.
The I-SAM was developed following: i) an appraisal of the predominant models used within the screening literature; ii) integration of the latest knowledge on behaviour change; with iii) the empirical literature to inform the development of a theory-based approach to intervention development. Following the approach of von Wagner et al., (2011), the aim was not to conduct a systematic review of the diverse literature on the use of theory or constructs related to screening access (e.g. Cooke and French, 2008, Priaulx et al., 2020, Graham-Rowe et al., 2018, Sabatino et al., 2012), but to provide a novel synthesis of the theoretical and empirical literature to inform researchers and practitioners tasked with improving access to screening. Theories and models are well established in the behavioural science literature but have not been applied extensively to the screening context nor integrated with the empirical literature. This approach therefore aimed to bring together in a unifying structure the state of research to aid intervention design, outcome measures and process evaluation (Redman et al., 2015).
The predominant models used within screening research have been in the context of cancer screening and reflect the key models within Health Psychology (Table 1). These same models broadly align with the use of health behaviour theory in cancer screening in funded grant applications (Kobrin et al., 2015). There are merits in each of the existing models of health behaviour identified in Table 1. Other models and frameworks have also been used, to a lesser extent, in studying screening behaviour e.g. Attitude Social Influence Self-efficacy Model (Vries and Mudde, 1998), Preventive Health Model (Watts et al., 2003), Psychosocial Determinants of Socioeconomic Inequalities in Cancer Screening (von Wagner et al., 2011), Theoretical Domains Framework (Graham-Rowe et al., 2018). However, it is clear from Table 1 that no single model is routinely used in screening behaviour research, and typically, researchers incorporate elements from different models (e.g. Kobrin et al., 2015). This approach of incorporating components from different models can work well for those fluent in health behaviour models and screening research, but can be ad hoc, and it can be challenging for health professionals with little background in behaviour change models. Therefore, the aim of proposing a new integrated model was to build on and synthesise the key components of existing models of health behaviour and empirical evidence, to develop a parsimonious model of screening behaviour that would be helpful to those wishing to understand screening behaviour and how to intervene.
Table 1.
Selected predominant models used in screening research.
| Model | Basic premise | Example studies |
|---|---|---|
| Health Belief Model (Rosenstock, 1974) |
Behaviour result of beliefs about: perceived susceptibility; perceived severity; benefits and barriers; cues to action | Wardle et al., 2000, Yarbrough and Braden, 2001, Orbell et al., 1996 |
| Theory of Reasoned Action (Ajzen and Fishbein, 1980), Theory of Planned Behaviour (Ajzen, 1991) | Behaviour result of attitudes, subjective norm, and perceived behavioural control* predicting intention and then behaviour. *can directly impact behaviour |
Cooke and French, 2008, Orbell et al., 2006, Drossaert et al., 2003, Rutter, 2000, Devellis et al., 1990 |
| Protection Motivation Theory (Rogers, 1975) | Behaviour determined by threat appraisal and coping appraisal including key components of: perceived severity; perceived susceptibility; response efficacy and self-efficacy | Orbell and Sheeran, 1998, Li et al., 2020 |
| Precaution adoption process model (Weinstein and Sandman, 1992) | Stage model explaining how a person decides to take action and how that decision translates into action | Costanza et al., 2005, Costanza et al., 2009, Ferrer et al., 2011, Marlow et al., 2018 |
| Transtheoretical Model (Prochaska and DiClemente, 1982) |
Stage model synthesising 18 therapies to elicit and maintain behaviour change. Key stages include: pre-contemplation; contemplation; preparation; action; maintenance | Rakowski et al., 1996, Lipkus et al., 1996, Trauth et al., 2003, Kelaher et al., 1999 |
| Social Cognitive Theory (Bandura, 1986) |
An extension of Social Learning Theory proposing a dynamic and reciprocal interaction of the person, environment, and behaviour. Key components include: outcome expectancies; reciprocal determinism; behavioural capacity; modelling; social reinforcement; self-efficacy | Suarez et al., 1993, Braun et al., 2005 |
3. The integrated screening action model (I-SAM)
The I-SAM (Fig. 1) is an integrated and theoretically informed model to support our understanding of screening behaviour and identify targets to increase access to screening. There are three key aspects to the I-SAM: i) a progressive sequence of stages that people pass through in engaging in screening behaviour; ii) screening behaviour is shaped by the synergistic interaction between participant and environmental influences; iii) targets for intervention should focus on the sources of behaviour including ‘capability’, ‘opportunity’, and ‘motivation.’ Because the I-SAM integrates existing models of behaviour and behaviour change and empirical evidence, it begins with some supporting evidence for their potential utility.
3.1. Screening behaviour process
The central component of the I-SAM, the Screening behaviour process, is based on the Precaution Adoption Process Model (PAPM; Weinstein et al., 2008). The PAPM identifies seven stages in the process of precaution adoption and was initially applied to home radon testing (Weinstein and Sandman, 1992). Just as some diseases such as cancer can develop sequentially (e.g. the colorectal adenoma carcinoma sequence, Leslie et al., 2002) so too can screening behaviour, developing through a sequence of stages or steps. Dividing screening behaviour development into distinct stages is helpful in identifying stage-specific targets for intervention, again in a similar manner to how cancer itself can be targeted based on stage e.g. premalignant vs. metastatic. The PAPM therefore offers a framework for understanding screening behaviour that can be readily understood from multidisciplinary perspectives.
Stage-model approaches are advantageous because they are easily understood and they acknowledge that a ‘one-size-fits-all’ approach may have limitations (Ferrer et al., 2011); although one stage model, the Transtheoretical Model (Table 1; Prochaska and Diclemente, 1982), has attracted considerable criticism (West, 2005). A stage model offers the opportunity to target interventions to different sectors of the community based on a population’s readiness to engage with screening behaviour e.g. people living in socioeconomic deprivation, ethnic minorities, and people with comorbidities and intellectual disabilities. A more targeted approach can therefore better address inequalities in access and ensures the benefits of screening can be fully realized by all in society. More broadly, this targeted approach aligns with the concepts of proportionate universalism (Marmot and Bell, 2012) and precision medicine (Hekler et al., 2020) that recognize the need to tailor interventions based on people’s need.
The PAPM (Weinstein et al., 2008) describes the stages defined as psychological processes that people pass through in precaution adoption from ‘unaware’ to ‘unengaged’ to ‘deciding’ to ‘intending’ to ‘acting’ to ‘repeat’ (Fig. 1). Table 2 describes the various stages using colorectal cancer screening as an example. Most predominant models used within screening research (Table 1) focus on how people who get to the decision making (undecided) stage, decide what to do. However, this is to the detriment of those who fail to reach that stage (~30% in survey samples: Ferrer et al., 2011, Costanza et al., 2005) and emphasises the value in including the unaware and unengaged stages. Within behavioural science the intention-action gap is well-recognised (Orbell and Sheeran, 1998, Gollwitzer and Sheeran, 2006), and so the I-SAM acknowledges that not everyone will progress from decided to act to acting resulting in people joining the decided not to act stage. The decided not to act stage can be further broken down in to disinclined abstainers (people who are not inclined to screen and don’t) and inclined abstainers (people who are inclined to screen but fail to act), with the latter group a particularly important group when considering improving access to screening (Orbell and Sheeran, 1998, Power et al., 2008). For those who complete screening it may be necessary to attend subsequent follow up tests (e.g. colposcopy for cervical screening), which are not elaborated here. There is also the option, depending on the type of screening, to repeat screening when next invited (Table 2). Including the repeat stage in the I-SAM is important because many models focus on the initiation of behaviour (e.g. Table 1) rather than maintenance, and while screening is an infrequent behaviour, repeated screening behaviour is necessary but remains relatively understudied (Lo et al., 2015). Adopting a behaviour for the first time is different to repeating the behaviour (Weinstein et al., 2008), and intervention approaches need to reflect this.
Table 2.
Screening behaviour stages for a colorectal cancer screening example.
| Colorectal screening example | |
|---|---|
| Unaware | Never heard of colorectal screening |
| Unengaged | Never thought about colorectal screening |
| Undecided* | Undecided about colorectal screening |
| Decided to act* | Decided to colorectal screen |
| Acting | Completing colorectal screening test |
| Repeat | Complete colorectal screening when next invited |
| *Decided not to act | Decide not to colorectal screen |
Conceptualising screening behaviour in these seven stages permits the identification of distinct groups of people who may require tailored interventions to improve screening access. Weinstein et al. (1998) propose that stage theories have four key elements and assumptions. Firstly, the stages represent an ideal or ‘prototype’ to assist with intervention development. In reality, there may be overlap between stages. Secondly, stage theories assume that people progress through a sequence of stages. However, people may not progress, they may regress, or they may progress so rapidly they can be viewed as skipping stages e.g. if a woman is offered cervical screening while attending primary care for another reason, she may progress from unengaged to action with little deliberation. Thirdly, people in the same stage will face common barriers and so targeting interventions to stage can assist in supporting people to progress to the next stage. Fourthly, people in different stages will face different barriers requiring interventions targeted to their barrier and stage.
Several studies have already illustrated the value of using the Precaution Adoption Process Model to identify people at different stages in the screening process for breast (Costanza et al., 2009), cervical (Marlow et al., 2018), and colorectal (Costanza et al., 2005, Ferrer et al., 2011) screening, and that health beliefs differ across stages. The next step for research is to develop interventions to target these beliefs at the various stages.
3.2. Participant and environmental influences
Within the I-SAM, the dual impact of Participant and Environmental influences synergistically shape the central Screening behaviour process. There are multiple levels of influence on screening behaviour (Priaulx et al., 2020), and these were well-described by Taplin et al. (2012) in their description of the seven levels of influence in cancer. The I-SAM takes a more parsimonious approach with two overarching levels: participant and environmental influences. This approach of incorporating both the Participant and Environmental influences aligns with the Access Framework’s demand- and supply-side determinants (Richard et al., 2016). The Access Framework is from the Primary Care literature and has not yet been applied to screening behaviour. By simultaneously considering both Environmental (how and where screening is offered) and Participant (people’s willingness and ability to engage with screening) influences, the I-SAM provides a rigorous structure to understand the interdependent influences of environmental and participant factors in screening access. The predominant models used within screening research (Table 1) have typically focused more on participant influences on screening behaviour to the neglect of environmental influences, with the exception of Social Cognitive Theory (Bandura, 1986). By considering the Participant and Environmental influences simultaneously, this will more rapidly produce improvements in access. Marteau and colleagues powerfully argue for applying psychological evidence to the shaping of Environmental influences (e.g. ease of effort, product design) and suggest this approach has greater potential to impact behaviour than interventions encouraging people to reflect on their behaviour – the Participant influences side (Marteau et al., 2012). Furthermore, simulation model research also suggests that participant focused interventions alone are less effective than using environmental or a combination of participant and environmental interventions (Hosking et al., 2013).
3.3. Sources of behaviour: ‘capability’, ‘opportunity’, and ‘motivation’
The third component of the I-SAM draws on the COM-B Model (Michie, Stralen and West, 2011) which identifies the sources of behaviour that can be targets for interventions. The COM-B is widely used to assist behaviour change intervention developers to identify what needs to change for interventions to be effective, yet few studies have used it to support screening research (e.g. Rogers et al., 2019, Kerrison et al., 2018). The COM-B Model suggests that behaviour can be understood in terms of ‘capability’, ‘opportunity’, and ‘motivation’ and interventions need to change one or more of these constructs to effectively support screening behaviour. Within the I-SAM, ‘capability’ and ‘motivation’ have been conceived as relating to Participant influences while ‘opportunity’ relates more to Environmental influences, however in line with the COM-B Model, it is recognized that behaviour is part of an interacting system so that increasing capability or opportunity can also increase motivation (West et al., 2020).
The I-SAM also contains within ‘capability’, ‘opportunity’, and ‘motivation’ suggested targets for future interventions based on the empirical screening literature. The COM-B Model specifies that ‘motivation’ comprises both automatic motivation and reflective motivation – in line with Dual Process Theory (Kahneman, 2011, Strack and Deutsch, 2004). Within the I-SAM, automatic motivation includes negative emotional responses to screening such as fear, fatalism, disgust, embarrassment (Kotzur et al., 2020, Sarma et al., 2019, Wardle et al., 2015, Piyasena et al., 2019) as well as habits and past behaviours such as previous experience of screening and tendency to follow health recommendations. Reflective motivation involves conscious evaluations such as evaluation of the benefits and harms of screening (Hall et al., 2015, Wardle et al., 2015, Piyasena et al., 2019, Ahmad et al., 2020), and perceived risk (Katapodi et al., 2004, Vernon, 1999, Ferrer et al., 2016). There may be overlap in the extent to which motivations are automatic or reflexive. For example, emotions may be automatic in terms of a physiological fear response to the word ‘cancer’ while also eliciting more reflective thinking on the fear of cancer.
‘Capability’ comprises both psychological and physical skills to enable screening behaviour. Psychological capability for screening includes having the cognitive resources to undertake the processes involved in completing screening which could include planning where, when and how you will complete a home-based test or planning and arranging an appointment for a clinic-based test and working out how to get there (Kotzur et al., 2020). Psychological capability includes self-efficacy – the belief that you can do the action required – which is a fundamental component of behaviour change (Bandura, 1986) and has been found to influence screening behaviour (Cooke and French, 2008, Duncan et al., 2014). Psychological capability also includes having language and health literacy skills to engage with screening (Graham-Rowe et al., 2018, van Allen et al., 2021, von Wagner et al., 2009). Physical capability to perform screening includes people having other priorities (e.g. comorbidities, family responsibilities) which limits their capability to engage with screening (McCowan et al., 2019, Hall et al., 2015, Kotzur et al., 2020, Graham-Rowe et al., 2018). A person may be unable to access screening due to the financial costs of taking time off work or travelling to a screening clinic (Brown et al., 2000, Sabatino et al., 2012, Graham-Rowe et al., 2018, Ahmad et al., 2020). Physical capability also relates to disabilities, which may impede screening e.g. visual impairment may impact on self-completed screening tests while reduced mobility may impact on attending clinic-based screening (Kotzur et al., 2020).
‘Opportunity’ includes both the social opportunity and the physical opportunity and the existing literature points to several potential targets to increase access to screening in both. Social opportunity includes social cues in the environment which can influence screening behaviour such as mass media (Marlow et al., 2012, Casey et al., 2013, Macdonald et al., 2018, Durkin et al., 2019, Graham-Rowe et al., 2018), community endorsement (Martini et al., 2016, Larkey, 2006, Graham-Rowe et al., 2018), primary care endorsement (Wardle et al., 2016, Duffy et al., 2017), norms, stigma and social identify (Sieverding et al., 2010, Smith-McLallen and Fishbein, 2008, Lo et al., 2015b, Vrinten et al., 2019, Jetten et al., 2017), and social support (Katapodi et al., 2002, Larkey, 2006, Documet et al., 2015, Graham-Rowe et al., 2018). Physical opportunity relates to aspects of the physical environment which influence the opportunity to access screening such as access to healthcare and healthcare insurance (Power et al., 2009, Taplin et al., 2012, Graham-Rowe et al., 2018, Piyasena et al., 2019, Bird and Davis, 2015), whether national guidelines recommend screening, and if screening is offered as part of an organized or opportunistic programme (Wardle et al., 2015, Miles et al., 2004, Graham-Rowe et al., 2018). Convenience can also influence the physical opportunity to access screening including design of the test – such that the easier the test is to do, the more likely people are to do it, location of screening (e.g. rurality, access to public transport), opening hours, waiting time on day of appointment, one-stop-shops and side effects (Robb and O'Carroll, 2019, Sabatino et al., 2012, Graham-Rowe et al., 2018, Piyasena et al., 2019, Cavan et al., 2017, van Allen et al., 2021, Hipwell et al., 2014). Opportunity is further influenced by the invitation and reminder strategy offered by the screening provider (Duffy et al., 2017, Graham-Rowe et al., 2018, Hipwell et al., 2014, Chaudhry et al., 2012), and whether providers are incentivized or receive prompts or skills training to engage people in screening (Sabatino et al., 2012, Brouwers et al., 2011). Physical opportunity to access screening can also be influenced by the availability of patient navigators to support people through the screening process (Jandorf et al., 2005, Robinson-White et al., 2010).
The additional benefit of including the COM-B model within the I-SAM is that it forms the central hub of the broader Behaviour Change Wheel (Michie et al., 2011). Surrounding the hub is a layer of nine intervention functions (education, persuasion, incentivisation, coercion, training, enablement, modelling, environmental restructuring, restrictions) which can be used to support screening behaviour. The outer layer relates to seven policy categories (environmental/social planning, communication/marketing, legislation, service provision, regulation, fiscal measures, guidelines) that can support the delivery of these interventions.
4. Using the I-SAM to improve access to screening
An illustration of how the components of the I-SAM can be used to identify and target interventions to improve access to screening is provided in Table 3. Table 3 maps the central Screening Behaviour Process (column 1) with intervention targets derived from the COM-B and the empirical literature (column 2) with intervention functions (column 3) and policy categories (column 4) taken from the Behaviour Change Wheel to improve access to screening. Table 3 describes the various stages within the screening behaviour process and elaborates the key targets within the components of the COM-B with intervention and policy solutions. Different interventions will be required based on where someone is in the screening process. For example, among people who are unaware, unengaged, or undecided, an awareness raising campaign addressing the benefits and harms of screening could be a motivational target. Among people who have decided to act, supporting people to make a plan about how, when and where they will do screening offers a capability target.
Table 3.
Illustration of how the I-SAM components identify potential targets and policies to increase access to screening.
| Screening behaviour process | Intervention targets | Intervention function | Policy |
|---|---|---|---|
|
Preintention Unaware Unengaged Undecided |
Participant influences Motivation Knowledge of benefits and harms Perceived risk Emotions Identity Capability Self-efficacy, cost, transport, disability |
Education, persuasion Enablement |
Communication/marketing Awareness raising campaign addressing motivational factors Awareness raising campaign to ensure people perceive they can participate |
|
Environmental influences Opportunity Invitation strategy Test design, location, opening hours Primary care endorsement Provider incentives Community endorsement Mass media |
Education, persuasion Environmental restructuring Persuasion Incentivisation Modelling, education Education, persuasion |
Service provision/- environmental/social planning Engaging and evidence-based invitation materials supporting access Provide a screening test accessible to all with additional support provided where necessary Future tests should be designed to optimise ease of use Include a primary care endorsement with invitation materials Provide incentives to Primary Care providers to support access Communication/marketing Identify key figures in the community to support access Engage with mass media to create narrative on supporting access |
|
|
Intention Decided to act |
Participant influences Capability Planning Self-efficacy Environmental influences Opportunity Invitation strategy Reminder strategy Patient navigation |
Enablement Modelling Enablement, modelling Enablement/environmental restructuring Enablement, training |
Communication/marketing Support people to make a plan about how, when and where they will do screening Support people to overcome barriers Support people to believe they are capable of doing screening Service provision Accessible information to support people to reach or complete screening e.g. maps and public transport suggestions, pictures to support self-completion, narratives of people who have participated Reminders to prompt action e.g. additional letters, calls, texts, verbal reminder if attending primary care Provide additional support where necessary to navigate people through the screening process |
|
Action Acting |
Participant influences Capability Positive screening experience Environmental influences Opportunity Positive screening experience Results framing |
Enablement Environmental restructuring Environmental restructuring |
Communication/marketing Support people to feel sense of mastery/accomplishment Service provision Supportive, timely, efficient screening experience Communication/marketing Supportive and accessible communication of results and follow up |
| Repeat |
Environmental influences Opportunity Re-invitation |
Environmental restructuring/ education, persuasion |
Service provision Engaging and evidence-based invitation materials tailored to supporting repeated behaviour |
| Stages of inaction | |||
| Decided not to screen |
Participant influences Motivation Ensure knowledge of benefits and harms Address emotional beliefs and misconceptions Capability Other priorities |
Education Enablement |
Communication/marketing Support people to ensure they have made a good decision for them Service provision Ensure people have the necessary support to access screening |
It will be important to establish the application of the I-SAM in low- and middle-income countries as the majority of the theoretical and empirical evidence is from high income countries. It is anticipated that the relative influence of the different components may differ between different income settings (Piyasena et al., 2019, Chidyaonga-Maseko et al., 2015).
5. Conclusions
The I-SAM proposes an integrated theoretical model to support our understanding of screening behaviour and to identify targets for intervention. It will be an iterative process to test and refine the I-SAM to ensure we capitalise on the benefits of theory-guided approaches, as they evolve. The I-SAM aligns with a proportionate universalism and precision medicine approach which is crucial as it is clear that our current ‘one-size-fits-all’ approach to screening is failing to engage equitably all sectors of the community. More targeted approaches are required to support those less likely to engage in screening such as people living in socioeconomic deprivation, ethnic minorities, people with comorbidities and learning disabilities and in different income settings. The I-SAM aims to provide an empirically and theory-driven approach to improve screening for all.
CRediT authorship contribution statement
Kathryn A. Robb: Conceptualization, Methodology, Investigation, Writing - original draft, Writing - review & editing, Visualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
I am grateful to Professor Marie Johnston, University of Aberdeen, and Professor Rory O’Connor, University of Glasgow, for their insightful and helpful comments on an earlier draft of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Ahmad M., Reading K., Gannon M.X. Improving Abdominal Aortic Aneurysm (AAA) Screening Uptake through Patient Engagement—Analysis and Outcomes of Strategies to Improve Uptake at a Regional Program Level. Ann. Vasc. Surg. 2020;S0890–5096(20):30838–30844. doi: 10.1016/j.avsg.2020.08.146. [DOI] [PubMed] [Google Scholar]
- Ajzen I., Fishbein M. Prentice-Hall; Englewood Cliffs, NJ: 1980. Understanding attitudes and predicting social behavior. [Google Scholar]
- Ajzen I. The theory of planned behavior. Organ. Behav. Hum. Decis. Process. 1991;50(2):179–211. [Google Scholar]
- Bandura A. Social foundations of thought and action. Englewood Cliffs, NJ. 1986;1986:23–28. [Google Scholar]
- Bird A.N., Davis A.M. Screening for abdominal aortic aneurysm. JAMA. 2015;313(11):1156–1157. doi: 10.1001/jama.2015.0996. [DOI] [PubMed] [Google Scholar]
- Braun K.L., Fong M., Kaanoi M.E., Kamaka M.L., Gotay C.C. Testing a culturally appropriate, theory-based intervention to improve colorectal cancer screening among Native Hawaiians. Prev. Med. 2005;40(6):619–627. doi: 10.1016/j.ypmed.2004.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers M.C., De Vito C., Bahirathan L., Carol A., Carroll J.C., Cotterchio M., Dobbins M., Lent B., Levitt C., Lewis N., McGregor S.E., Paszat L., Rand C., Wathen N. What implementation interventions increase cancer screening rates? A systematic review. Implementation Sci. 2011;6(1) doi: 10.1186/1748-5908-6-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D.R., Fouad M.N., Basen-Engquist K., Tortolero-Luna G. Recruitment and retention of minority women in cancer screening, prevention, and treatment trials. Ann. Epidemiol. 2000;10(8):S13–S21. doi: 10.1016/s1047-2797(00)00197-6. [DOI] [PubMed] [Google Scholar]
- Campbell C., Douglas A., Williams L., Cezard G., Brewster D.H., Buchanan D., Steiner M. Are there ethnic and religious variations in uptake of bowel cancer screening? A retrospective cohort study among 1.7 million people in Scotland. BMJ Open. 2020;10(10) doi: 10.1136/bmjopen-2020-037011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey G.M., Morris B., Burnell M., Parberry A., Singh N., Rosenthal A.N. Celebrities and screening: a measurable impact on high-grade cervical neoplasia diagnosis from the ‘Jade Goody effect’ in the UK. Br. J. Cancer. 2013;109(5):1192–1197. doi: 10.1038/bjc.2013.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavan D., Makaroff L., da Rocha Fernandes J., Sylvanowicz M., Ackland P., Conlon J., Chaney D., Malhi A., Barratt J. The diabetic retinopathy barometer study: global perspectives on access to and experiences of diabetic retinopathy screening and treatment. Diabetes Res. Clin. Pract. 2017;129:16–24. doi: 10.1016/j.diabres.2017.03.023. [DOI] [PubMed] [Google Scholar]
- Chaudhry R., Tulledge-Scheitel S.M., Parks D.A., Angstman K.B., Decker L.K., Stroebel R.J. Use of a Web-based clinical decision support system to improve abdominal aortic aneurysm screening in a primary care practice. J. Eval. Clin. Pract. 2012;18(3):666–670. doi: 10.1111/j.1365-2753.2011.01661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidyaonga-Maseko F., Chirwa M.L., Muula A.S. Underutilization of cervical cancer prevention services in low and middle income countries: a review of contributing factors. Pan Afr. Med. J. 2015;21(1):231. doi: 10.11604/pamj.2015.21.231.6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., French D.P. How well do the theory of reasoned action and theory of planned behaviour predict intentions and attendance at screening programmes?A meta-analysis. Psychol. Health. 2008;23(7):745–765. doi: 10.1080/08870440701544437. [DOI] [PubMed] [Google Scholar]
- Costanza M.E., Luckmann R., Stoddard A.M., Avrunin J.S., White M.J., Stark J.R., Clemow L., Rosal M.C. Applying a stage model of behavior change to colon cancer screening. Prev. Med. 2005;41(3-4):707–719. doi: 10.1016/j.ypmed.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Costanza M.E., Luckmann R., White M.J., Rosal M.C., LaPelle N., Cranos C. Moving mammogram-reluctant women to screening: a pilot study. Ann. Behav. Med. 2009;37(3):343–349. doi: 10.1007/s12160-009-9107-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crilly M.A., Mundie A., Bachoo P., Nimmo F. Influence of rurality, deprivation and distance from clinic on uptake in men invited for abdominal aortic aneurysm screening. Br. J. Surg. 2015;102(8):916–923. doi: 10.1002/bjs.9803. [DOI] [PubMed] [Google Scholar]
- Curry S.J., Emmons K.M. Theoretical models for predicting and improving compliance with breast cancer screening. Ann. Behav. Med. 1994;16(4):302–316. [Google Scholar]
- Documet P., Bear T.M., Flatt J.D., Macia L., Trauth J., Ricci E.M. The association of social support and education with breast and cervical cancer screening. Health Educ. Behav. 2015;42(1):55–64. doi: 10.1177/1090198114557124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devellis B.M., Blalock S.J., Sandler R.S. Predicting Participation in Cancer Screening: The Role of Perceived Behavioral Control 1. J. Appl. Soc. Psychol. 1990;20(8):639–660. [Google Scholar]
- Drossaert C.H.C., Boer H., Seydel E.R. Prospective study on the determinants of repeat attendance and attendance patterns in breast cancer screening using the theory of planned behaviour. Psychol. Health. 2003;18(5):551–565. [Google Scholar]
- Duffy S.W., Myles J.P., Maroni R., Mohammad A. Rapid review of evaluation of interventions to improve participation in cancer screening services. J. Med. Screen. 2017;24(3):127–145. doi: 10.1177/0969141316664757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan A., Turnbull D., Wilson C., Osborne J.M., Cole S.R., Flight I., Young G.P. Behavioural and demographic predictors of adherence to three consecutive faecal occult blood test screening opportunities: a population study. BMC Public Health. 2014;14(1):1–13. doi: 10.1186/1471-2458-14-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkin S.J., Broun K., Spittal M.J., Wakefield M.A. Impact of a mass media campaign on participation rates in a National Bowel Cancer Screening Program: a field experiment. BMJ Open. 2019;9(1):e024267. doi: 10.1136/bmjopen-2018-024267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer R.A., Hall K.L., Portnoy D.B., Ling B.S., Han P.K.J., Klein W.M.P. Relationships among health perceptions vary depending on stage of readiness for colorectal cancer screening. Health Psychol. 2011;30(5):525–535. doi: 10.1037/a0023583. [DOI] [PubMed] [Google Scholar]
- Ferrer R.A., Klein W.M.P., Persoskie A., Avishai-Yitshak A., Sheeran P. The tripartite model of risk perception (TRIRISK): distinguishing deliberative, affective, and experiential components of perceived risk. Ann. Behav. Med. 2016;50(5):653–663. doi: 10.1007/s12160-016-9790-z. [DOI] [PubMed] [Google Scholar]
- Gollwitzer P.M., Sheeran P. Implementation intentions and goal achievement: A meta-analysis of effects and processes. Adv. Exp. Soc. Psychol. 2006;38:69–119. [Google Scholar]
- Graham-Rowe E., Lorencatto F., Lawrenson J.G., Burr J.M., Grimshaw J.M., Ivers N.M., Presseau J., Vale L., Peto T., Bunce C., Francis J. Barriers to and enablers of diabetic retinopathy screening attendance: a systematic review of published and grey literature. Diabet. Med. 2018;35(10):1308–1319. doi: 10.1111/dme.13686. [DOI] [PubMed] [Google Scholar]
- Guirguis-Blake J.M., Beil T.L., Senger C.A., Coppola E.L. Primary care screening for abdominal aortic aneurysm: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;322(22):2219–2238. doi: 10.1001/jama.2019.17021. [DOI] [PubMed] [Google Scholar]
- Hall N.J., Rubin G.P., Dobson C., Weller D., Wardle J., Ritchie M., Rees C.J. Attitudes and beliefs of non-participants in a population-based screening programme for colorectal cancer. Health Expect. 2015;18(5):1645–1657. doi: 10.1111/hex.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekler E., Tiro J.A., Hunter C.M., Nebeker C. Precision health: The role of the social and behavioral sciences in advancing the vision. Ann. Behav. Med. 2020;54(11):805–826. doi: 10.1093/abm/kaaa018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipwell A.E., Sturt J., Lindenmeyer A., Stratton I., Gadsby R., O'Hare P., Scanlon P.H. Attitudes, access and anguish: a qualitative interview study of staff and patients’ experiences of diabetic retinopathy screening. BMJ Open. 2014;4(12):e005498. doi: 10.1136/bmjopen-2014-005498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking M., Roberts S., Uzsoy R., Joseph T.M. Investigating interventions for increasing colorectal cancer screening: Insights from a simulation model. Socio-Econ. Plan. Sci. 2013;47(2):142–155. [Google Scholar]
- Jandorf L., Gutierrez Y., Lopez J., Christie J., Itzkowitz S.H. Use of a patient navigator to increase colorectal cancer screening in an urban neighborhood health clinic. J. Urban Health. 2005;82(2):216–224. doi: 10.1093/jurban/jti046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetten J., Haslam S.A., Cruwys T., Greenaway K.H., Haslam C., Steffens N.K. Advancing the social identity approach to health and well-being: Progressing the social cure research agenda. Eur. J. Soc. Psychol. 2017;47(7):789–802. [Google Scholar]
- Kahneman D. Farrar, Straus and Giroux; New York: 2011. Thinking, fast and slow. [Google Scholar]
- Katapodi M.C., Facione N.C., Miaskowski C., Dodd M.J., Waters C. The influence of social support on breast cancer screening in a multicultural community sample. Oncol. Nurs. Forum. 2002;29(5):845–852. doi: 10.1188/02.ONF.845-852. [DOI] [PubMed] [Google Scholar]
- Katapodi M.C., Lee K.A., Facione N.C., Dodd M.J. Predictors of perceived breast cancer risk and the relation between perceived risk and breast cancer screening: a meta-analytic review. Prev. Med. 2004;38(4):388–402. doi: 10.1016/j.ypmed.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Kelaher M., G. Gillespie A., Allotey P., Manderson L., Potts H., Sheldrake M., Young M. The transtheoretical model and cervical screening: Its application among culturally diverse communities in Queensland,Australia. Ethnicity and Health. 1999;4(4):259–276. doi: 10.1080/13557859998047. [DOI] [PubMed] [Google Scholar]
- Kerrison R.S., McGregor L.M., Counsell N., Marshall S., Prentice A., Isitt J., von Wagner C. Use of two self-referral reminders and a theory-based leaflet to increase the uptake of flexible sigmoidoscopy in the English bowel scope screening program: results from a randomized controlled trial in London. Ann. Behav. Med. 2018;52(11):941–951. doi: 10.1093/abm/kax068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobrin S., Ferrer R., Meissner H., Tiro J., Hall K., Shmueli-Blumberg D., Rothman A. Use of health behavior theory in funded grant proposals: cancer screening interventions as a case study. Ann. Behav. Med. 2015;49(6):809–818. doi: 10.1007/s12160-015-9714-3. [DOI] [PubMed] [Google Scholar]
- Kotzur M., McCowan C., Macdonald S., Wyke S., Gatting L., Campbell C., Weller D., Crighton E., Steele R.J.C., Robb K.A. Why colorectal screening fails to achieve the uptake rates of breast and cervical cancer screening: a comparative qualitative study. BMJ Quality & Safety. 2020;29(6):482–490. doi: 10.1136/bmjqs-2019-009998. [DOI] [PubMed] [Google Scholar]
- Larkey L. Las mujeres saludables: reaching Latinas for breast, cervical and colorectal cancer prevention and screening. J. Community Health. 2006;31(1):69–77. doi: 10.1007/s10900-005-8190-2. [DOI] [PubMed] [Google Scholar]
- Leslie A., Carey F.A., Pratt N.R., Steele R.J.C. The colorectal adenoma–carcinoma sequence. Br. J. Surg. 2002;89(7):845–860. doi: 10.1046/j.1365-2168.2002.02120.x. [DOI] [PubMed] [Google Scholar]
- Li Q., Liu Q., Chen X., Tan X., Zhang M., Tuo J., Xiang Q., Yu Q., Zhu Z. Protection motivation theory in predicting cervical cancer screening participation: A longitudinal study in rural Chinese women. Psycho-oncology. 2020;29(3):564–571. doi: 10.1002/pon.5307. [DOI] [PubMed] [Google Scholar]
- Lin J.S., Piper M.A., Perdue L.A., Rutter C.M., Webber E.M., O’Connor E., Smith N., Whitlock E.P. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315(23):2576. doi: 10.1001/jama.2016.3332. [DOI] [PubMed] [Google Scholar]
- Lipkus I.M., Rimer B.K., Strigo T.S. Relationships among objective and subjective risk for breast cancer and mammography stages of change. Cancer Epidemiol. Prev. Biomarkers. 1996;5(12):1005–1011. [PubMed] [Google Scholar]
- Lo S.H., Halloran S., Snowball J., Seaman H., Wardle J., von Wagner C. Predictors of repeat participation in the NHS bowel cancer screening programme. Br. J. Cancer. 2015;112(1):199–206. doi: 10.1038/bjc.2014.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo S.H., Waller J., Vrinten C., Kobayashi L., von Wagner C. Social cognitive mediators of sociodemographic differences in colorectal cancer screening uptake. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/165074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald S., Cunningham Y., Patterson C., Robb K., Macleod U., Anker T., Hilton S. Mass media and risk factors for cancer: the under-representation of age. BMC Public Health. 2018;18(1):490. doi: 10.1186/s12889-018-5341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow L.A., Sangha A., Patnick J., Waller J. The Jade Goody Effect: whose cervical screening decisions were influenced by her story? J. Med. Screen. 2012;19(4):184–188. doi: 10.1258/jms.2012.012095. [DOI] [PubMed] [Google Scholar]
- Marlow L.A.V., Ferrer R.A., Chorley A.J., Haddrell J.B., Waller J. Variation in health beliefs across different types of cervical screening non-participants. Prev. Med. 2018;111:204–209. doi: 10.1016/j.ypmed.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmot M., Bell R. Fair society, healthy lives. Public Health. 2012;126:S4–S10. doi: 10.1016/j.puhe.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Marteau T.M., Hollands G.J., Fletcher P.C. Changing human behavior to prevent disease: the importance of targeting automatic processes. Science. 2012;337(6101):1492–1495. doi: 10.1126/science.1226918. [DOI] [PubMed] [Google Scholar]
- Martini A., Morris J.N., Preen D. Impact of non-clinical community-based promotional campaigns on bowel cancer screening engagement: An integrative literature review. Patient Educ. Couns. 2016;99(10):1549–1557. doi: 10.1016/j.pec.2016.05.012. [DOI] [PubMed] [Google Scholar]
- McCowan C., McSkimming P., Papworth R., Kotzur M., McConnachie A., Macdonald S., Steele R.J. Comparing uptake across breast, cervical and bowel screening at an individual level: a retrospective cohort study. Br. J. Cancer. 2019;121(8):710–714. doi: 10.1038/s41416-019-0564-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie S., Van Stralen M.M., West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implementation Science. 2011;6(1):42. doi: 10.1186/1748-5908-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles A., Cockburn J., Smith R.A., Wardle J. A perspective from countries using organized screening programs. Cancer: Interdisciplinary International Journal of the American Cancer Society. 2004;101(S5):1201–1213. doi: 10.1002/cncr.20505. [DOI] [PubMed] [Google Scholar]
- Leese G.P., Boyle P., Feng Z., Emslie-Smith A., Ellis J.D. Screening uptake in a well-established diabetic retinopathy screening program: the role of geographical access and deprivation. Diabetes Care. 2008;31(11):2131–2135. doi: 10.2337/dc08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leese G.P., Stratton I.M., Land M., Bachmann M.O., Jones C., Scanlon P., Looker H.C., Ferguson B. Four Nations Diabetic Retinopathy Screening Study Group. Progression of diabetes retinal status within community screening programs and potential implications for screening intervals. Diabetes Care. 2015;38(3):488–494. doi: 10.2337/dc14-1778. [DOI] [PubMed] [Google Scholar]
- Myers E.R., Moorman P., Gierisch J.M., Havrilesky L.J., Grimm L.J., Ghate S., Davidson B., Mongtomery R.C., Crowley M.J., McCrory D.C., Kendrick A., Sanders G.D. Benefits and harms of breast cancer screening: a systematic review. JAMA. 2015;314(15):1615. doi: 10.1001/jama.2015.13183. [DOI] [PubMed] [Google Scholar]
- National Lung Screening Trial Research Team Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Cathain A., Croot L., Duncan E., Rousseau N., Sworn K., Turner K.M., Yardley L., Hoddinott P. Guidance on how to develop complex interventions to improve health and healthcare. BMJ Open. 2019;9(8):e029954. doi: 10.1136/bmjopen-2019-029954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbell S., Sheeran P. ‘Inclined abstainers’: A problem for predicting health-related behaviour. Br. J. Soc. Psychol. 1998;37(2):151–165. doi: 10.1111/j.2044-8309.1998.tb01162.x. [DOI] [PubMed] [Google Scholar]
- Orbell S., Crombie I., Johnston G. Social cognition and social structure in the prediction of cervical screening uptake. Br. J. Health Psychol. 1996;1(1):35–50. [Google Scholar]
- Orbell S., Hagger M., Brown V., Tidy J. Comparing two theories of health behavior: A prospective study of noncompletion of treatment following cervical cancer screening. Health Psychol. 2006;25(5):604–615. doi: 10.1037/0278-6133.25.5.604. [DOI] [PubMed] [Google Scholar]
- Power E., Van Jaarsveld C.H.M., McCaffery K., Miles A., Atkin W., Wardle J. Understanding intentions and action in colorectal cancer screening. Ann. Behav. Med. 2008;35(3):285–294. doi: 10.1007/s12160-008-9034-y. [DOI] [PubMed] [Google Scholar]
- Power E., Miles A., von Wagner C., Robb K., Wardle J. Uptake of colorectal cancer screening: system, provider and individual factors and strategies to improve participation. Future Oncol. 2009;5(9):1371–1388. doi: 10.2217/fon.09.134. [DOI] [PubMed] [Google Scholar]
- Priaulx J., Turnbull E., Heijnsdijk E., Csanádi M., Senore C., de Koning H.J., McKee M. The influence of health systems on breast, cervical and colorectal cancer screening: an overview of systematic reviews using health systems and implementation research frameworks. Journal of Health Services Research & Policy. 2020;25(1):49–58. doi: 10.1177/1355819619842314. [DOI] [PubMed] [Google Scholar]
- Prochaska J.O., DiClemente C.C. Transtheoretical therapy: toward a more integrative model of change. Psychotherapy: Theory, Research & Practice. 1982;19(3):276–288. [Google Scholar]
- Piyasena M.M.P.N., Murthy G.V.S., Yip J.L.Y., Gilbert C., Zuurmond M., Peto T., Gordon I., Hewage S., Kamalakannan S., Csutak A. Systematic review on barriers and enablers for access to diabetic retinopathy screening services in different income settings. PLoS ONE. 2019;14(4):e0198979. doi: 10.1371/journal.pone.0198979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowski W., Breslau E.S. Perspectives on behavioral and social science research on cancer screening. Cancer: Interdisciplinary International Journal of the American Cancer Society. 2004;101(S5):1118–1130. doi: 10.1002/cncr.20503. [DOI] [PubMed] [Google Scholar]
- Rakowski W., Dube C.A., Goldstein M.G. Considerations for extending the transtheoretical model of behavior change to screening mammography. Health Educ. Res. 1996;11(1):77–96. [Google Scholar]
- Richard L., Furler J., Densley K., Haggerty J., Russell G., Levesque J.F., Gunn J. Equity of access to primary healthcare for vulnerable populations: the IMPACT international online survey of innovations. International Journal for Equity in Health. 2016;15(1):1–20. doi: 10.1186/s12939-016-0351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman S., Turner T., Davies H., Williamson A., Haynes A., Brennan S., Milat A., O'Connor D., Blyth F., Jorm L., Green S. The SPIRIT Action Framework: A structured approach to selecting and testing strategies to increase the use of research in policy. Soc. Sci. Med. 2015;136-137:147–155. doi: 10.1016/j.socscimed.2015.05.009. [DOI] [PubMed] [Google Scholar]
- Robb, K.A., & O'Carroll, R. (2019). Simpler is better—the case of colorectal cancer screening. BMJ Opinion. https://blogs.bmj.com/bmj/2019/10/11/simpler-is-better-the-case-of-colorectal-cancer-screening/.
- Robinson-White S., Conroy B., Slavish K.H., Rosenzweig M. Patient navigation in breast cancer: a systematic review. Cancer Nurs. 2010;33(2):127–140. doi: 10.1097/NCC.0b013e3181c40401. [DOI] [PubMed] [Google Scholar]
- Rogers R.W. A protection motivation theory of fear appeals and attitude change1. J. Psychol. 1975;91(1):93–114. doi: 10.1080/00223980.1975.9915803. [DOI] [PubMed] [Google Scholar]
- Rogers C.R., Okuyemi K., Paskett E.D., Thorpe R.J., Rogers T.N., Hung M., Zickmund S., Riley C., Fetters M.D. Study protocol for developing# CuttingCRC: a barbershop-based trial on masculinity barriers to care and colorectal cancer screening uptake among African-American men using an exploratory sequential mixed-methods design. BMJ Open. 2019;9(7):e030000. doi: 10.1136/bmjopen-2019-030000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronco G., Dillner J., Elfström K.M., Tunesi S., Snijders P.J.F., Arbyn M., Kitchener H., Segnan N., Gilham C., Giorgi-Rossi P., Berkhof J., Peto J., Meijer C.J.L.M. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. The Lancet. 2014;383(9916):524–532. doi: 10.1016/S0140-6736(13)62218-7. [DOI] [PubMed] [Google Scholar]
- Rosenstock I.M. Historical origins of the health belief model. Health Education Monographs. 1974;2(4):328–335. doi: 10.1177/109019817800600406. [DOI] [PubMed] [Google Scholar]
- Rutter D.R. Attendance and reattendance for breast cancer screening: A prospective 3-year test of the Theory of Planned Behaviour. British Journal of Health Psychology. 2000;5(1):1–13. [Google Scholar]
- Sabatino S.A., Lawrence B., Elder R., Mercer S.L., Wilson K.M., DeVinney B., Melillo S., Carvalho M., Taplin S., Bastani R., Rimer B.K., Vernon S.W., Melvin C.L., Taylor V., Fernandez M., Glanz K. Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers: nine updated systematic reviews for the guide to community preventive services. Am. J. Prev. Med. 2012;43(1):97–118. doi: 10.1016/j.amepre.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Sarma E.A., Silver M.I., Kobrin S.C., Marcus P.M., Ferrer R.A. Cancer screening: health impact, prevalence, correlates, and interventions. Psychology & Health. 2019;34(9):1036–1072. doi: 10.1080/08870446.2019.1584673. [DOI] [PubMed] [Google Scholar]
- Sieverding M., Matterne U., Ciccarello L. What role do social norms play in the context of men’s cancer screening intention and behavior? Application of an extended theory of planned behavior. Health Psychol. 2010;29(1):72–81. doi: 10.1037/a0016941. [DOI] [PubMed] [Google Scholar]
- Smith-McLallen A., Fishbein M. Predictors of intentions to perform six cancer-related behaviours: roles for injunctive and descriptive norms. Psychology, Health and Medicine. 2008;13(4):389–401. doi: 10.1080/13548500701842933. [DOI] [PubMed] [Google Scholar]
- Strack F., Deutsch R. Reflective and impulsive determinants of social behavior. Personality and Social Psychology Review. 2004;8(3):220–247. doi: 10.1207/s15327957pspr0803_1. [DOI] [PubMed] [Google Scholar]
- Suarez L., Nichols D.C., Pulley L., Brady C.A., McAlister A. Local health departments implement a theory-based model to increase breast and cervical cancer screening. Public Health Rep. 1993;108(4):477. [PMC free article] [PubMed] [Google Scholar]
- Szczepura A., Price C., Gumber A. Breast and bowel cancer screening uptake patterns over 15 years for UK south Asian ethnic minority populations, corrected for differences in socio-demographic characteristics. BMC Public Health. 2008;8(1):346. doi: 10.1186/1471-2458-8-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taplin S.H., Anhang Price R., Edwards H.M., Foster M.K., Breslau E.S., Chollette V., Prabhu Das I., Clauser S.B., Fennell M.L., Zapka J. Introduction: understanding and influencing multilevel factors across the cancer care continuum. Journal of the National Cancer Institute Monographs. 2012;2012(44):2–10. doi: 10.1093/jncimonographs/lgs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauth J.M., Ling B.S., Weissfeld J.L., Schoen R.E., Hayran M. Using the transtheoretical model to stage screening behavior for colorectal cancer. Health Education & Behavior. 2003;30(3):322–336. doi: 10.1177/1090198103030003007. [DOI] [PubMed] [Google Scholar]
- van Allen Z., Dogba M.J., Brent M.H., Bach C., Grimshaw J.M., Ivers N.M., Wang X., McCleary N., Asad S., Chorghay Z., Hakim H., Sutakovic O., Drescher O., Légaré F., Witteman H.O., Zettl M., Squires J., Tremblay M., Randhawa A., Lopez G., Ben Guiza A., Presseau J. Barriers to and enablers of attendance at diabetic retinopathy screening experienced by immigrants to Canada from multiple cultural and linguistic minority groups. Diabet. Med. 2021;38(4) doi: 10.1111/dme.v38.410.1111/dme.14429. [DOI] [PubMed] [Google Scholar]
- Vernon S.W. Risk perception and risk communication for cancer screening behaviors: a review. JNCI Monographs. 1999;1999(25):101–119. doi: 10.1093/oxfordjournals.jncimonographs.a024184. [DOI] [PubMed] [Google Scholar]
- von Wagner C., Good A., Whitaker K.L., Wardle J. Psychosocial determinants of socioeconomic inequalities in cancer screening participation: a conceptual framework. Epidemiol. Rev. 2011;33(1):135–147. doi: 10.1093/epirev/mxq018. [DOI] [PubMed] [Google Scholar]
- von Wagner C., Semmler C., Good A., Wardle J. Health literacy and self-efficacy for participating in colorectal cancer screening: the role of information processing. Patient Educ. Couns. 2009;75(3):352–357. doi: 10.1016/j.pec.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Vries H.D., Mudde A.N. Predicting stage transitions for smoking cessation applying the attitude-social influence-efficacy model. Psychology and Health. 1998;13(2):369–385. [Google Scholar]
- Vrinten C., Gallagher A., Waller J., Marlow L.A. Cancer stigma and cancer screening attendance: a population based survey in England. BMC cancer. 2019;19(1):1–10. doi: 10.1186/s12885-019-5787-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle J., Sutton S., Williamson S., Taylor T., McCaffery K., Cuzick J., Hart A., Atkin W. Psychosocial influences on older adults' interest in participating in bowel cancer screening. Prev. Med. 2000;31(4):323–334. doi: 10.1006/pmed.2000.0725. [DOI] [PubMed] [Google Scholar]
- Wardle J., Robb K., Vernon S., Waller J. Screening for prevention and early diagnosis of cancer. Am. Psychol. 2015;70(2):119–133. doi: 10.1037/a0037357. [DOI] [PubMed] [Google Scholar]
- Wardle J., von Wagner C., Kralj-Hans I., Halloran S.P., Smith S.G., McGregor L.M., Vart G., Howe R., Snowball J., Handley G., Logan R.F., Rainbow S., Smith S., Thomas M.C., Counsell N., Morris S., Duffy S.W., Hackshaw A., Moss S., Atkin W., Raine R. Effects of evidence-based strategies to reduce the socioeconomic gradient of uptake in the English NHS Bowel Cancer Screening Programme (ASCEND): four cluster-randomised controlled trials. The Lancet. 2016;387(10020):751–759. doi: 10.1016/S0140-6736(15)01154-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts B.G., Vernon S.W., Myers R.E., Tilley B.C. Intention to be screened over time for colorectal cancer in male automotive workers. Cancer Epidemiology and Prevention Biomarkers. 2003;12(4):339–349. [PubMed] [Google Scholar]
- Weinstein N.D., Rothman A.J., Sutton S.R. Stage theories of health behavior: conceptual and methodological issues. Health Psychol. 1998;17(3):290–299. doi: 10.1037//0278-6133.17.3.290. [DOI] [PubMed] [Google Scholar]
- Weinstein N.D., Sandman P.M. A model of the precaution adoption process: evidence from home radon testing. Health Psychol. 1992;11(3):170–180. doi: 10.1037//0278-6133.11.3.170. [DOI] [PubMed] [Google Scholar]
- Weinstein N.D., Sandman P.M., Blalock S.J. 4th ed. Jossey-Bass, A Wiley Imprint; San Francisco: 2008. Chapter 6. The precaution adoption process model. Health behavior and health education: theory, research and practice. [Google Scholar]
- West R. Time for a change: putting the Transtheoretical (Stages of Change) Model to rest. Addiction. 2005;100(8):1036–1039. doi: 10.1111/j.1360-0443.2005.01139.x. [DOI] [PubMed] [Google Scholar]
- West R., Michie S., Rubin G.J., Amlôt R. Applying principles of behaviour change to reduce SARS-CoV-2 transmission. Nat. Hum. Behav. 2020;4(5):451–459. doi: 10.1038/s41562-020-0887-9. [DOI] [PubMed] [Google Scholar]
- Yarbrough S.S., Braden C.J. Utility of health belief model as a guide for explaining or predicting breast cancer screening behaviours. J. Adv. Nurs. 2001;33(5):677–688. doi: 10.1046/j.1365-2648.2001.01699.x. [DOI] [PubMed] [Google Scholar]