The first half of the 20th century was marked by a shift in morbidity and mortality patterns in industrialized countries all over the world, moving from the leading role of infectious diseases to the increasing role of chronic, non-communicable diseases. In particular, cardiovascular disease (CVD) has been an important cause of morbidity and mortality, with coronary heart disease (CHD) being the leading cause of death. The primary reason for this transition was the discovery of antibiotics and vaccines, the widespread use of which has led to a decline in infectious diseases and increase in life expectancy and population aging.[1]
Initiatives to improve the understanding of CVD’s etiology led to the establishment of the first population-based cohort study for assessing the factors related to the development of CVD. It was the Framingham Heart Study, which was established in Massachusetts state, in the United States, in the middle of the 20th century, and is still continuing.[2] The results of this study have provided important insights into factors, both biological and environmental, that are independent predictors of the onset of CVD. These factors are known as “classical or traditional cardiovascular (CV) risk factors” (Table 1). Many of these factors have been considered to be consequences of the lifestyle’s changes associated with urbanization and industrialization. This assumption has led to the idea that by ameliorating these factors at both individual and population levels, it should be possible to combat the epidemic spread of CVD.[3]
Table 1. CV risk factors and biomarkers that can be found in eHRs.
| Traditional CV risk factors | Non-traditional CV risk factors | CV biomarkers that can be found in eHRs |
| CV: cardiovascular; eHRs: electronic health records. | ||
| Advancing age | Low-level inflammation | C-reactive protein |
| Smoking cigarettes | Homocysteine | Neutrophil-to-lymphocyte ratio |
| Elevated blood pressure (in particular systolic) | Fibrinogen, impaired fibrinolysis | Ventricular hypertrophy by electrocardiogram |
| Elevated total and low-density lipoprotein cholesterol | Increased platelet reactivity | Cardiac troponin T |
| Low high-density lipoprotein cholesterol | Hypercoagulability | N-terminal pro B-type natriuretic peptide |
| Overweight/obesity (in particular abdominal obesity) | Natriuretic peptides | Body composition and muscle mass measures |
| Diabetes mellitus or glucose intolerance | Lipoprotein | Glycated hemoglobin |
| Sedentary lifestyles | Small dense low-density lipoprotein cholesterol | Serum albumin (pre-albumin), serum transferin |
| Postmenopause (in women) | Albuminuria | Vitamin D |
| Chronic latent infection (helicobacter pylori, citomegalo virus) | Thyroid gland hormones | |
| The level of comorbidy and the comorbidity patterns | Haemoglobin | |
| Rheumatoid arthritis (other autoimmune diseases) | Blood lipids | |
| Chronic kidney disease | Urin albumin/creatinine ratio | |
| Depression, psychiatric diseases | Cystatine C | |
| Some drugs, polypharmacy | Estimated glomerular filtration rate | |
| Carotid intima-media thickness | ||
| Ankle-brachial index | ||
| Functional assessments | ||
The classical CV risk factors, as defined in the Framingham Heart Study, were used to create the first integrated CV risk prediction score, thus laying the foundation for the development of strategies for primary CVD prevention.[3] By using the CV risk prediction score, the absolute risk of developing CVD within a determined future period can be estimated more precisely than by simply summing the individual risk factors. This has enabled those individuals who have increased CV risk and who are likely to benefit from the application of preventive measures, to be selected from the general population. Subsequently, evidence has been emerging for the existence of different CV risk factors, other than the classical risk factors, which have been shown to add value to the prognostic accuracy of the classical CV risk factors (Table 1).[4]
So far, a number of CV risk assessment scores have been developed in European countries, the United States, and some other large countries of the world.[5] Currently, the two best known and most widely used are the American College of Cardiology/American Heart Association risk assessment score, and the European Society of Cardiology’s Systematic Coronary Risk Evaluation algorithm.[6, 7] Contrary to the high expectations of the use of CV risk assessment scores in primary prevention of CVD, the evidence indicates that their use is of no benefit in reducing CVD morbidity and mortality.[8] The level of implementation of these models in routine practice, is also low.[9] The reasons for these flaws are manifold.[10] One reason is the lack of trust in these systems’ efficiency, due to the fact that about 40% of individuals covered by the scores are misclassified. In addition, an advanced age is the strongest CV risk factor. An implication is that older people (≥ 60 years), who are otherwise free of conventional CV risk factors, could be exposed to inappropriate treatments and over-medicalization, which could do more harm than good in these people.[11, 12]
CURRENT TOOLS FOR ASSESSING CV RISK
The majority of CV risk assessment systems use the same limited set of variables, including age, sex, smoking, blood pressure and blood cholesterol measurements, to predict the absolute risk of developing CVD or of CV-related death in the next ten-year period.[5] Other predictors included are diabetes mellitus, body mass index (BMI), and information about previous vascular diseases and a family history of early-onset CHD.[13, 14] The effect of antihypertensive treatments is sometimes considered.[5]
The logic behind the selection of variables for the predictive models is to achieve the greatest possible accurate risk stratification of individuals in the general population, in order to inform cost-benefit decisions for treatment. This means using medications to treat those individuals from high-risk groups who are likely to benefit the most, and avoiding over-medicalization and its side-effects, in those who will benefit less, such as those from lower-risk groups.[15] At the same time, the predictive model should be easy to use and sufficiently robust for repeated use, both in the same population from which it was derived and in other populations. Following this logic, the best variables for modeling are those which are not influenced by coding difficulties, which include numerical variables (blood pressure or cholesterol measurements) and simple, dichotomized variables [smoking status (yes/no), sex].
Other requirements for variables are that they are important for characterizing the majority of individuals in the population (not only the minority), and are easy to use and cost-effective. The latter characteristics are important for conducting population-based surveys, giving the fact that most models used today have been derived from prospective cohort studies.[5]
As a consequence, the number of variables included in the model is quite limited, in comparison to the large range of possible determinants of CVD. Methodological issues also dictate that a limited number of variables be used in the model. The current CV risk assessment systems were derived by using statistical regression models which cannot utilize a large number of variables.[16] For the model to achieve good predictive performances, the variables should be selected on the basis of the hypothesis, that is, the existing knowledge, which restricts their scope.[17]
One advantage of such models is their good uptake in practice, in particular when they are presented visually, as a chart, or an on-line calculator.[18, 19] On the other hand, the limited number of variables mean that the model is unable to represent inter-individual variations in CV risk factors accurately, which restricts the model’s discriminative ability (its ability to separate those individuals who will develop the end point from those who will not).[16] In consequence, some individuals from the intermediate risk groups are wrongly classified, which lessens their chance of being offered adequate treatments. An attempt was made to remedy this shortcoming by adding new variables to the model, usually biomarkers indicative of different disease pathways, such as C-reactive protein (CRP), homocysteine, cystatin-C, natriuretic peptides, etc.[20] However, a “forced” enlargement of the scope of variables in the rigorous model such as the regression function, is not likely to provide a visible improvement. Namely, the regression function is unable to cope with variable imbalance (skewed distributions) or variable interactions (co-linearity).[17]
Another limitation of the current CV risk assessment systems is their low ability to cope with population diversity, with respect to sex, race, ethnicity, and the level of expression of CV risk factors. For this reason, it is necessary to recalibrate the function if it is applied to populations other than that from which it was derived.[3, 16] The American College of Cardiology/American Heart Association guidelines are based on people aged between 40 and 79 years from several cohorts, and assess hard endpoints (fatal and nonfatal myocardial infarction and stroke). The prediction equation has been calibrated for some but not all, ethnic cohorts. The equation overestimates the ten-year risk for CVD by an average of 20% across all risk groups. The advantage of the Systematic Coronary Risk Evaluation risk chart is that it is based on a large dataset, collected across a wide range of European countries, and that it makes a distinction between high-risk and low-risk regions. However, it is still used less often outside Europe.[3]
The CV risk assessment models are based on traditional risk factors that have been validated in middle-aged populations, with the relative risk coefficient being derived for older age groups, on the assumption that the risk factors’ effects are constant regardless of age.[13, 21] Since age is the strongest CVD determinant, these systems’ discriminatory power in older age groups is generally low. However, it has been shown that the strength of traditional risk factors’ effects change with age, and that there are some new factors which are important for predictions in these age groups.[22–24] An attempt was made to improve this shortcoming by creating the competing risk regression model, which is only performed on older people.[25] Recently, the function, which is similar to the Systematic Coronary Risk Evaluation, was derived and validated on part of the dataset used in the Systematic Coronary Risk Evaluation project, to improve the accuracy of CV risk assessment in the elderly population.[25] The variables contained in the model included age, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, smoking status and diabetes mellitus.
AGING AND CV RISK
Aging and CVD
Population aging has been a global and progressive trend since the middle of the 20th century, with important implications for societies and health care systems. The proportion of older individuals (> 60 years) is increasing at a faster rate in developed countries and more slowly in developing countries. It is projected that, by 2050, the proportions of older individuals will be up to one-third in developed countries and one-fifth in developing countries.
The prevalence of CVD increases in these age groups and is the leading cause of death.[26, 27] Even the incipient decline in its prevalence which had been recorded in developed countries in the last two decades, due to progress in prevention and treatment, is now stagnating.[28] In low resources countries, CVD is emerging concomitantly to the epidemics of obesity and diabetes mellitus.[29]
The high prevalence of CVD in the older population can be explained by the current understanding of the CV system as the prime target for stochastic and degenerative senescent processes.[30] In these terms, atherosclerosis, a progressive vascular lesion underlying CV incidents, is increasingly considered to be a maladaptive immune response associated with chronic inflammation, which may synergize the effect of metabolic risk factors on the formation of lipid-bearing arterial wall injuries.[31] In long-lived individuals (> 80 years), who are otherwise free from overt metabolic disturbances, the formation of lipid-rich plaques might have been overcome by dominant fibrotic degenerative processes. Therefore, these people’s vascular systems are still characterized by arterial stiffness, as a consequence of endothelial dysfunction and the effect of long-term hemodynamic stress acting on the arterial walls.[30]
Many age-related changes at the bodily, organ system, cellular, and subcellular levels, are associated with the development of fibrotic and structural changes to the vasculature, the heart and the kidneys, leading to a vicious circle of hemodynamic, structural, and functional changes within the CV system.[30, 32] Molecular mechanisms such as cell senescence, disturbed autophagy (an important mechanism for cell waste management), chronic activation of inflammasomes (intracellular defense system), increased production of reactive oxygen substances, and the imbalance between inflammatory and anti-inflammatory mediators, are only some of the mechanisms involved. In the aging heart, the impairment of aerobic metabolism due to mitochondrial dysfunction leads to local hyperproduction of reactive oxygen substances and the activation of inflammatory and fibrotic signaling, which augments structural changes and the related diastolic and systolic cardiac dysfunction.[33, 34] At the bodily level, inflammatory response and tissue resistance to insulin may be exaggerated by age-related changes to the body shape, associated with the loss of muscle mass and increase in fat tissue, in particular visceral fat tissue, predisposing older individuals to metabolic disorders, increased blood pressure, and the development of structural and hemodynamic changes to the CV system.[35, 36] Based on this discussion, it seems that those advocates who propose using biomarkers in predicting CV end points in the older population, are on the right track (Table 2).[37]
Table 2. Age-related sources of inflammation.
| The body, organs, tissue level | The cell level | The subcellular level |
| Visceral obesity (secretion of inflammatory cytokines, chemokines, adipokines) | Activation of the innate immune cells by pathogen-associated molecular patterns and damage-associated molecular patterns | Posttranslational alterations (at the level of micro-RNA) |
| Latent infections (cytomegalovirus, helicobacter pylori) | Cell senescence (a senescence-associated secretory phenotype: intracellular signaling loops and inflammatory cascade involving the nuclear factor kappa-B, IL-1α, transforming growth factor-betta and IL-6 pathway) | Mitochondrial dysfunction (oxydative-stress mediated inflammation via activation of nod-like receptor 3 inflammasome) |
| Depression, chronic stress (via activation of the hypothalamus-hypophysis-adrenal stress axis) | Cell apoptosis-transformation into necrotic cells | Impaired autophagy (impaired clearance of apoptotic cells) |
| Comorbidities (tissue infiltration with inflammatory cells, paracrine cell activation) | Cell surfice receptors density and activation alterations | Transcription factors activation in inflammatory response pathways (nuclear factor kappa-B, activation protein-1) |
| Neuro-endocrine alterations | Dysregulation of paroxisome proliferator activated receptors (nuclear hormone receptors activated by fatty acids and oic osano ides) | |
| Renal function decline | Dysregulation of pro-resolving lipid mediators (lipoxins, resolvins, maresins) | |
| Altered permeability of the gastro-intestinal system | M1 (pro-inflammatory) type macrophages | |
| Alterations in gut microbiome | Insufficient inflammation resolution | |
| Genetic predisposition | An imbalance between pro-inflammatory TNF-α, IL-6, IL-18, IL-1 cytokine family (IL-1α, IL-1β, IL-18, IL-33, IL-36α, IL-36β, and IL-36γ), IL-17A, IL-22, and anti-inflammatory cytokines IL-10, IL-37, transforming growth factor-betta, sTNFR, sIL-1R |
CVD in the Context of Aging Diseases
The current level of knowledge suggests that it is no longer plausible to consider the development of CVD in isolation, without considering other age-related diseases and chronic health conditions. This is supported by the evidence indicating that chronic diseases accumulate with age. More than half the people over the age of 60 years will have two or more chronic diseases, which is termed multimorbidity.[38] Multimorbidity accelerates aging and the decline in performance (including limitations on mobility, strength, cognition, and a person’s ability to perform everyday tasks).[39] The multiple medications (polypharmacy) given to older patients with multimorbidity, may exacerbate the situation due to drug-disease and drug-drug interactions.[40] Knowledge of the factors that interact over time to accelerate aging, is challenging the concept of chronological age. The term biological age has been introduced to indicate a person’s position on the aging trajectory and highlight the need for intervention.[32]
The number and complexity of comorbid conditions increases with age. It has been recognized that not only the number of chronic diseases but also the disease combinations (patterns), have an effect on outcome prediction.[41] Typically, CVD in older adults occurs concurrently with many comorbidities.[42] Of the different disease patterns, the combinations containing CVD were shown to have the largest effect on excess mortality.[43] This new evidence challenges the current CVD research and clinical practice, and highlights that more attention needs to be paid to the context of CVD.[44]
This new evidence is not surprising as it is known that chronic diseases, such as hypertension, metabolic syndrome (a cluster of disorders associated with abdominal obesity) and type 2 diabetes mellitus, all complex conditions the presentation of which precedes or is concurrent with CVD, increase in prevalence with age.[45] Chronic kidney disease, a major predisposing factor for CVD, was found to affect only those older individuals with hypertension and/or diabetes mellitus, and is not inherent to aging.[46] There is a close association between CVD and depression. These facts are well known. What is not known, is how elements of these disorders’ common pathways are clustered in the actual population, and which factors and biomarkers are relevant for representing these clusters.[30, 47]
The recent progress in geriatric research indicates that complex geriatric conditions and functional impairments which cannot be coded as classical diseases, have a predictive value which extends beyond the effect of comorbidities.[48] These conditions, termed geriatric syndromes, include impairments such as walking difficulties, sarcopenia (muscle wasting), predisposition to falls, sensory impairments, urinary incontinence, cognitive impairment, chronic pain, and frailty.[39] These conditions further increase the diversity of the older part of the population, which makes the prediction process for older individuals extremely difficult.
The efforts to link these observations from population-based studies with the recent knowledge on the mechanisms of chronic diseases, have led to the emergence of a new concept of aging, termed inflamm-aging. The research group (Bologna, Italy) of Franceschi, et al.[49] has authorized the use of this name. This research group postulated that changes in the innate immune system, associated with low-grade, chronic inflammation, which occurs as a response to life-long exposure to external and internal antigenic stimuli, provides the biological background for the development of common aging diseases, including Alzheimer’s disease, atherosclerosis, osteoporosis, heart disease, type 2 diabetes mellitus, and cancer.[49] This theory has evolved over time and aims to incorporate the new evidence into an integrated view of the aging process. Throughout its several updates, this theory has pointed out that there is an interdependence between the neuro-endocrine, metabolic, and immune systems, which directs the course of aging, either successfully or unsuccessfully. The preservation of defense mechanisms is important for survival and the maintenance of good functioning.[49]
These authors introduced the concept of anti-inflamm-aging to explain the controversial results of studies performed on people who have reached the limits of the human lifespan (nonagenarians and centenarians) with a relatively good functional status, despite the presence of a substantial number of comorbidities. This concept states that preserving the network of anti-inflammatory mechanisms (which is to a great extent, genetically determined) can compensate for the deleterious effect of inflamm-aging and accumulated comorbidities.[50]
The recent findings highlight that the individuals who have reached the limits of the human lifespan are still a heterogeneous group and are not completely free from chronic conditions, however, these conditions are present at a lower rate than in their younger counterparts.[50] These new findings indicate that the appearance of chronic conditions may be delayed, even though the molecular and cellular mechanisms that drive their development are the same across the aging process. These new findings represent a step away from the strong dichotomy of either successful or unsuccessful aging, towards a more integrated view of the aging process and the development of age-related diseases. Accordingly, the original inflamm-aging theory has been complemented by the new integrated theory of aging.[51] This theory states that the pathways that can be characterized as “healthy aging” or “aging burdened with chronic diseases and functional impairments”, are merely the extremes in a continuum of aging trajectories.[51]
According to this line of thought, CVD should be considered to be part of the same inflamm-aging continuum as other aging conditions. That means that, in older individuals, the CV risk status evaluation should embrace the complexity imposed by the concomitant conditions.[44]
Changing CV Risk Factors with Aging
Despite the fact that CVD is concentrated in the older part of the population and the existence of a large amount of mechanistic knowledge about the development of CVD, clinical guidelines have very limited information on CV risk factors in this population group. In addition to reasons such as the need for a long-term follow-up (for estimation of the ten-year risk) and a greater dropout rate, compared to middle-aged individuals, an important reason for not including older individuals in clinical trials is the great variation in comorbidities, which makes predicting outcomes uncertain.[52]
However, some recent studies indicate that the association between the traditional CV risk factors and CVD is attenuated in elderly individuals, and that other age-related factors, indicating comorbid conditions, compete with traditional risk factors and should be taken into account when predicting CV risks. In an example of such a paper, an analysis was performed on the Atherosclerosis Risk in Communities study dataset.[53] It was shown that the strength of the associations between total cholesterol and low-density lipoprotein cholesterol, and CVD, accounts for differences in age and the level of inflammation (indicated by CRP values). This association was significant but weakened in younger, old individuals (< 65 years) if the CRP values were above 2 mg/L. In older, old individuals (> 65 years), this association was similar to that in the younger, old individuals, unless CRP was increased, in which case the association became non-significant. This example clearly indicates that chronological age may not be sufficient to determine CV risks, and that other variables, indicating pathophysiology disorders, should also be considered.
In a recent longitudinal study, changes in the impact of traditional CV risk factors on men during the aging process, were followed during a long period of four decades (from 50 to over 80 years of age).[54] The study showed that although the impact of these factors generally declined with aging, some of the factors remained significant, even at ages of over 80 years. Since this variation depends on the pathophysiology pathways reflected by the CV risk factors, the factors’ importance varies depending on the outcome measured (incident myocardial infarction, ischemic stroke or heart failure).
During the 6.5-year follow-up of a recent, large-scale study performed on individuals aged 70−78 years, it was shown that some traditional risk factors remained predictors of fatal and nonfatal CVD, while others did not, and that some new factors, such as polypharmacy and apathy, performed better as CV predictors.[22] In a study of participants from the Framingham Heart Study, which assessed the relative importance of measures of hypertension, including diastolic, systolic and pulse pressure, as predictors for CHD, it was demonstrated that after the age of 50 years, one particular single measurement might be superior to the other two. However, which measurement was important depended on the age and associated pathophysiology disorders.
In the study by Saaed, et al.,[55] performed on data from the Atherosclerosis Risk in Communities cohort and individuals aged 69−88 years, it was shown that using biomarkers of inflammation and cardiac pathways in CVD development, might only improve the prediction accuracy for those older people who are burdened with pathophysiology disorders but cannot make a distinction between them and those who are relatively healthy. The relative importance of specific CV risk factors accounted for differences in the outcome measures.
However, the studies cited require validation in other populations before their results can be generalized. Taken together, they highlight the fact that different kinds of factors should be used for assessing CV risks in older individuals, with respect to differences in age, the existing pathophysiology disorders, and the outcomes measured.
The recent achievements in understanding geriatric syndromes and their prognostic relevance, are expected to shed more light on CVD developmental pathways in older individuals.[56] In particular, the attention of researchers and clinicians has been attracted to the concept of frailty, the prognostic value of which has been confirmed for different health-related outcomes, in well-designed, large-scale studies.[57] Although it is not yet well-understood, this concept refers to the exhaustion of reserves in multiple physiological systems, as the final pathway in the accumulation of comorbid conditions during the aging process.[58] The frailty concept has been operationalized by Fried, et al.,[57] in the form of a simple score system, and is defined by a set of nonspecific features which indicate decreased muscle mass and strength, weakness, slow gait speed, subjective feelings of exhaustion, and low activity. A more comprehensive prediction system, known as the Cumulative Deficit Model, is based on counting medical, cognitive, psychological and functional deficits, as described by symptoms, functional performance tests, and laboratory abnormalities.[59]
The evidence suggests that there is a close association between CVD and frailty, meaning that frailty increases the risk for CVD, and that patients with CVD who are frail, have an increased risk of bad outcomes.[60] This link might be due to the high level of comorbidity that is known to accompany the presence of CVD, in particular, including diabetes mellitus and chronic kidney disease, which are also associated with frailty.[46] Although the concept of frailty may be wider than that of inflamm-aging, the evidence suggests a strong association between frailty and chronic inflammation.[61] Chronic inflammation has also been recognized as the mechanism which links CVD with frailty.
The global epidemic of obesity is also affecting the elderly population, further modifying the anthropometric changes which occur naturally with aging.[62] It has been found that severe obesity may provoke frailty, thus diminishing the observed beneficial effect from increased body weight on survival in older individuals.[63]
Although the evidence is still weak, there is mounting evidence to indicate that frailty, originally assumed to be the body shrinking, could explain the paradoxical phenomena that patients with CVD who are overweight or obese, show a better prognosis than those who are of normal weight (a phenomenon termed the obesity paradox).[63] Moreover, the overlap between frailty and other geriatric conditions associated with the body shrinking and muscle wastage, such as malnutrition and sarcopenia, might be those factors that influence the weakening of the association between traditional CV risk factors and CVD or mortality, as observed in older individuals.[64] There is an ongoing debate about whether the phenomena termed the risk factors paradox or reverse epidemiology, which states that increased rather than decreased values of traditional CV risk factors, such as systolic blood pressure, cholesterol, and BMI, are prognostically favorable, is simply a “statistical artifacts”, or whether it is the result of “selection bias”, or whether it is caused by the real changes in the body composition and the concomitant changes in physiological functions.[65, 66] The recent evidence suggests that the problem is less straightforward. The prevalence and importance of particular traditional CV risk factors may vary occasionally and be dependent on the end point.[22] However, the association between frailty and low blood pressure has been shown to be relatively stable across studies and populations, so that the current version of the European guidelines on the management of hypertension has called for special attention to the treatment of older hypertensive patients with frailty.[67]
Based on this discussion, and in order to clarify the role of CV risk factors in older individuals, the following questions arise: How do particular comorbidity patterns, including information on frailty and malnutrition, coexist in the particular population? How do these patterns correlate with the chronological age of the individuals in the population, the development of CVD, and the risk of death? Which biomarkers represent these patterns and are relevant for predicting the outcomes?
A NEED TO MOVE FROM THE REDUCTIONIST RESEARCH APPROACH TO RESEARCH IN COMPLEXITY
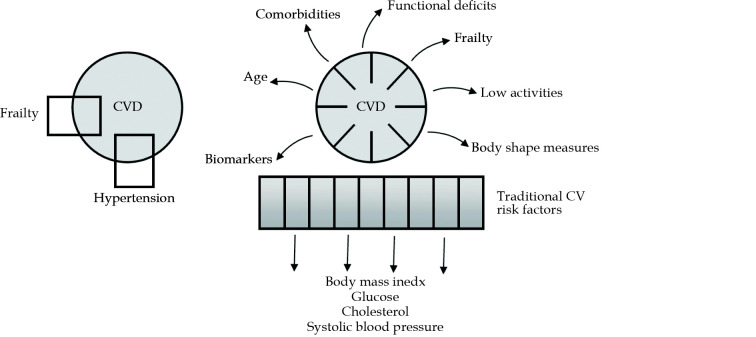
There is an increasing awareness that descriptions of the pathways leading to CVD events in older people, should comprise many variables of different kinds. These variables exhibit a high level of interdependence and cross-connection, interacting in complex and non-linear ways. The changes in variable values during the aging process are influenced by multiple factors, such as changes in body composition and body weight, the presence of comorbidities, and the development of conditions such as frailty, muscle wasting and malnutrition, as well as the effects of medication treatment (Figure 1).[68]
Figure 1.
Reductionist versus systems research approach.
In the reductionist view, the system’s behavior (outcome measure-CVD) is explained by the selected components (e.g., hypertension and frailty). A research question: is hypertension alone or hypertension in combination with frailty, a better predictor of CVD? In the systems view, the system’s behavior (outcome measure-CVD) is the result of the interactions between a multitude of components. A research question: which of the factors associated with hypertension and frailty (e.g., the body mass index category; the age category; the degree of decline in kidney function; the level of blood pressure) impact the association of the coexistence of hypertension and frailty with CVD? CV: cardiovascular; CVD: cardiovascular disease.
At this level of complexity, the theoretical and operative approaches currently prevailing in medical science, are no longer adequate to allow further progress. These approaches include the method of reasoning known as reductionist thinking, and the methodological framework that works within the limitations of classical statistical solutions. This methodological framework is characterized by its aim of using one regression equation to describe diverse patient subgroups, each of which has its own set of descriptors, rules of interaction, and dynamics of change. Therefore, in order to make further progress on CV risk factors in older individuals, and overcome the current gaps in knowledge and the methodological constraints, it is necessary to change both the way we think, by switching from a reductionist to a complex way of reasoning, and the research approach, by moving from classical statistical solutions to solutions using computer science and artificial intelligence research approaches.
In the reductionism paradigm, the way of thinking is based on the postulate that natural phenomena can be explained by a limited number of logical rules and static mathematical models. There is no place for contradictions or uncertainties in scientific reasoning. It relies on a strong hypothesis, which is expected to be provided by unambiguous answers. From the analytical perspective, the concept assumes that the system under investigation can be broken into its constituent components, which are then analyzed and interpreted independently of each other (Figure 1).
In contrast, the science of complexity assumes that biological systems behave as complex systems.[69] In a complex system, the system’s properties emerge from the interaction of its components. The research approach arising from this theory proposes that the system’s behavior can be understood by the simultaneous interpretation of multiple factors, which are viewed within the common context (Figure 1).[70] Elements of different theoretical and analytical approaches can be used and mixed during the analytical process, in order to arrive at the solution. Within the same paradigm, complex thinking is a way of human reasoning that results from the interactions of multiple mental processes, occurring either simultaneously or at different points of time, and which need to be activated when solving complex tasks.[71]
When viewed through the lens of the above discussion, it is possible to critically evaluate the current approaches to studying CV risk factors in people of older age. Firstly, the current clinical trials examine CV risk factors separately and without considering the clinical context, which can underestimate the complexity of the problem.[52] Elderly people are either excluded or grouped with younger ones.[70] The effect of gender is not adequately addressed, despite evidence suggesting the existence of different cardio-metabolic pathways in men and women.[72] The effect of frailty on the expression of CV risk factors, such as hypertension, cholesterol and glucose, is often neglected.[73] If this effect is taken into account, it is done straightforwardly, rather than by considering the wider clinical context, including the effects of the common determinants of frailty and CVD, such as age, renal function stages, and comorbidity patterns.[74]
In the example study, the impact of hypertension on mortality rates was examined, depending on whether or not hypertension was associated with frailty.[75] The reductionist research approach was used, indicated by the fact that all patients diagnosed with hypertension were grouped together, with no distinction being made on the basis of socio-demographic or clinical characteristics; this was the reason that no effect of frailty was found on the association between hypertension and mortality. This research approach, known as reductionism, in which the focus is on one characteristic of the system and all others are ignored, can lead to conflicting conclusions and the diversity of patient groups being overlooked (Figure 1). Following the same reasoning, in a meta-analysis evaluating the association of hypertension with frailty, this association was found in some studies and not in others, leaving the result of the whole analysis in doubt.[76]
The misapprehensions of the reductionist research approach came to light in constructs such as reverse epidemiology or the risk factors paradox, which were introduced in studies on aging and CV risk factors, to name the conflicting findings, as well as the inability of the analytical methods, used in the current studies, to cope with the health related diversity of older individuals.[77, 78] The typical examples are studies examining homocysteine (the sulfur amino-acid with strong oxidative properties) as a CV risk factor, which yielded the controversial results.[79] Homocysteine levels rise in chronic kidney disease and are associated with increased CV morbidity and mortality. The mechanistic studies proposed cause-effect relationships between increased homocysteine and CV pathology. However, some population studies have found that lower rather than higher, homocysteine levels predict mortality. In these associations, low homocysteine levels can be considered to be the confounding factor, due to the effect of a more advanced stage of chronic kidney disease and its clinical context, including a higher degree of frailty, on the development of CVD, without there being a causal effect on the development of CVD and the risk of mortality.[79] This observed contradiction may be due to non-linear associations between homocysteine levels and different stages of kidney function decline.[80]
A NEED TO RECONCILE THE CV RISK ASSESSMENT TOOLS AND PREVENTION RECOMMENDATIONS
It has become increasingly clear that if we want to overcome the gaps in the current CV risk assessment tools, and improve screening and treatment strategies for CVD prevention, it is necessary to expand the research methods.[52] There are two main issues of concern which need to be resolved: (1) how to cope with the diversity of CV risk, in particular, in relation to the heterogeneity of older people’s health status, and to enable as much individualized treatment as possible; and (2) how to address the temporal perspective of CV risk factors which change over time. To solve these issues, we propose using the emerging approaches of data analysis, in the areas of machine learning and the application of artificial intelligence application. Using machine learning methods makes it possible to extract hidden patterns from the data, and to generate new knowledge, outside the solutions of the classical research methods.[81]
Precision medicine is a requirement of modern clinical practice, in general, and CV medicine, in particular.[82] The term indicates using risk stratification and management strategies to target the specific characteristics of individuals or patient groups, in order to optimize treatment efficacy, and to avoid side-effects from unnecessary medication.[83] The term was originally coined in genomic and epigenetic medicine, in which machine learning methods are necessary for analyzing the massive volumes of data. Since these sophisticated laboratory methods are resource intensive and not widely available the current trend in CV risk assessment is to use data from electronic health records (eHRs), which indicates individuals’ socio-demographic and clinical characteristics; this is cost-effective and widely available for population assessments.[84]
In particular, in the general practice setting, eHRs allow the extensive and systematic collection of information on the natural history of chronic diseases, prescribed medication, and other aspects of care for elderly people. In many European countries, general practitioner eHRs are the central point in networking health data from different sources, which could increase the opportunities for research enormously.
The trend of using data from eHRs for predictive purposes, is reflected in the QRISK2 risk stratification method, which was developed in the United Kingdom using data from the thousands of general practitioner eHRs.[14] Although the QRISK2 assessment system is still maintained within the framework of the classical risk assessment methods, using eHRs as sources of data has enabled a much wider range of variables to be used in comparison to, for example, the European Systematic Coronary Risk Evaluation risk assessment system, in which data were collected in a prospective population survey. The QRISK2 system uses data indicating social deprivation issues, family history of CVD, hypertension treatment, BMI, and some comorbidities for which there is evidence indicating an association with CVD, such as atrial fibrillation, chronic kidney disease, diabetes mellitus, and rheumatoid arthritis. The added value of using machine learning research methods in CV risk assessment, is the opportunities it offers for making the maximum use of the data available in eHRs, by identifying latent phenotypes, and generating new concepts.[81] Moreover, this research approach enables the performance of analyses of the heterogeneous (native) population, without the need for selection or randomization procedures.[82] The data from eHRs is cost-effective and enables the modeling procedures to be repeated, as well as determining the CV risk factors for different, specifically defined, population groups (e.g., those older than 80 years, postmenopausal women, etc.), and for different CV-related and non-CV-related outcomes.
A more personalized approach to CV risk assessment would be important for clinical practice because the results of the risk stratification procedure dictate the treatment recommendations. If the CV risk assessment system could address the risk diversity of elderly people, it would be an important improvement, since the current CV risk assessment systems are unable to do so.[25, 85] The current risk score systems overestimate the risk in this population group, which could lead to overtreatment. The concern is that elderly people usually have multiple comorbidities and frailty, which increases their risk for adverse drug reactions and may compromise treatment.[40, 86]
With respect to therapy with statins (hypolipemic drugs) in primary prevention of CVD in elderly people, there is insufficient evidence on their benefits and safety to inform decisions.[87, 88] The result is that statins are prescribed less often for elderly than younger people, although there may be individuals in these age groups who would be likely to benefit from this therapy. Although, in general, the evidence supports the beneficial effects of pharmacological treatment for hypertension into advanced age, due to the enormous functional heterogeneity in this population group and the increased prevalence of frailty, the recent update of the European guidelines on the management of hypertension contains warning notes for the treatment of frail, older individuals.[89]
Another major issue of concern which has not yet been resolved by the current, risk-based, CV risk assessment strategies, relates to the measurement of CV risk factors at one point in time.[52, 90] This strategy fails to take into account these factors’ long-term effects on the circulatory system, which in turn, would be closer to the causal exposure paradigm, given the fact that atherosclerosis, as the precursor of vascular injury for CV events, shows a constant progression throughout life.[91] The inclusion of a time perspective in CV risk prediction strategies is a challenge, due to the close association between age and the end points in the absolute risk assessment (CVD events or CV-related death), and the interaction with CV risk factors. In addition, in older people, the CV-related end points compete with non-CV related mortality.[85]
The long-term (or even lifetime) view of the effects of CV risk factors on the development of CVD, has been considered relevant for communicating CV risk to younger individuals burdened with CV risk factors, who have a long time before the possible occurrence of the CV end point, or for better distinguishing between individuals with intermediate risks, a significant proportion of whom are perimenopausal and postmenopausal women.[90] Shorter periods of time (5−6 years) are only used to express the absolute CV risk in population studies of older people.
In recent years, researchers have become increasingly interested in the long-term patterns (pathways or trajectories) of traditional CV risk factors, and their effects on CV-related or other age-related outcomes. Such studies can be used to highlight health-related heterogeneity, and to identify trends in pathophysiology pathway generation. The study by Allen, et al.,[92] identified five distinct blood pressure trajectories in young adults, which were described as low stable, moderate stable, moderate increasing, elevated stable, and elevated increasing. It examined their association with the presence of coronary artery calcification (determined by an imaging test based on computed tomography), as a measure of subclinical atherosclerosis, after 25 years of follow-up and based on seven separate blood pressure measurements. The study by Strandberg, et al.,[93] assessed the effect of body weight trajectories, measured as BMI, on the incidence of disability, frailty, and mortality. In this study, it was possible to demystify the obesity paradox phenomenon by representing the heterogeneity of the body shape-related phenotypes. Namely, the increased mortality rates were shown to be associated with more than one body weight category (including weight loss and constant overweight), indicating the influence of hidden confounding factors.
Although these studies represent a step forward in coping with health-related heterogeneity, they still remain within the framework of classical statistical methods, such as latent mixture modelling. In this model, phenotypic groups are identified on the basis of “one CV risk factor labelling”, while a multitude of confounding factors, which may impact the association between the identified phenotypes and the outcome measure, remain unseen. In studies of aging, there have been examples of using composite measures to represent the health-risk stratification by trajectories, such as the chronic disease burden in the integrated theory of aging, or “the composite measure of the reduction in functional capacities of older people”, which is used by policy makers to represent the decline in the health status of older people.[94, 95] In the study by Vetrano, et al.,[96] trajectories of functional decline (expressed as time-dependent changes in walking speed and daily living activity) have been associated with the level of comorbidity of neuropsychiatric and CV diseases.
Members of the artificial intelligence and machine learning research community have contributed to the efforts to find solutions for coping with the heterogeneity of the health status in older individuals, and expressing the dynamics of non-linear changes in this status over time. There are many machine learning algorithms and techniques that can be used to perform research tasks associated with the correlation, prediction, classification, and clustering problems, that arise from multiple interacting factors.[81] The current trend is to use a large data set obtained from eHRs, prepare it for analysis using data pre-processing and dimension reduction methods, and then apply some of the machine learning technique algorithms to it.[97] In the example paper, Guo, et al.[98] used cluster analysis to explore clinical phenotypes in hypertensive patients. These authors identified four distinct hypertension groups, and found, as new knowledge, that the group with blood pressure reverse dipping might be of value for predicting coronary artery disease in hypertensive patients.
The aim is to resolve researchers and clinicians’ lack of trust in the performance accuracy of machine learning algorithms, by improving the validation methods, such as cross-validation and bootstrapping. By splitting the dataset into the training and the testing sets, these methods give the model the opportunity to train on multiple train-test splits, in order to predict how well it will perform on unseen data (an external validation).[99] Specifically, deep learning methods such as artificial neural networks, and other advanced machine learning techniques, have been used in research on complex chronic diseases, such as CVD, which are characterized by multiple overlapping comorbidities and functional impairments, to search for latent phenotypes in the data.[100] Algorithms for searching for temporal trends in data, or a combination of methods (hybrid methods) have been used to track the evolution of these phenotypes over time.[101] For example, in the paper by Zhao, et al.,[102] an advanced factorization method was applied on eHRs data in order to characterize the complexity of CVD. These authors identified fourteen temporal sub-phenotypes, among others, those indicating vitamin D deficiency and urinary infection, which was a novelty compared to the existing knowledge. However, many of these advanced methods need to achieve better performance accuracy before being implemented in clinical practice.
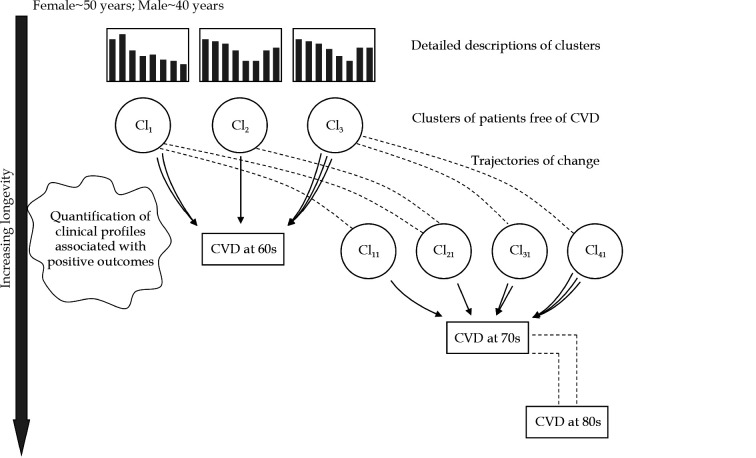
Based on the authors’ own research experience in using machine learning methods to solve tasks associated with chronic complex diseases, in this paper, we suggest a new research approach for CV risk assessment among older people.[103–105] This approach aims to use methods which have been proved to perform well, such as clustering methods (e.g., the latent class analysis, k-means, or the fuzzy c-means), in combination with trajectories of numerical variables change over time. The clustering methods are based on grouping patients according to the probability distribution of a range of factors, in order to enable relevant phenotypes to be identified without a prior assumption on classification criteria.[106, 107] Age, as a challenging variable in CV risk factor research among older people, will be linked to CVD as the outcome measure, in order to exclude its interactions with other variables and with the outcome CV-related measures (Figure 2).
Figure 2.
Proposed research methodology approach.
The follow-up of people in the general population is initiated at the age when CV risk factors begin to accumulate (women in perimenopause, men in their 40s). Clustering methods are used to automatically organize those individuals who are free from CVD into the groups. The clusters are created (modeled) based on a set of variables, including traditional CV risk factors, the degree of renal function decline, frailty grade, malnutrition grade, inflammation, scores achieved on psychological and functional examinations, socio-demographic indices, etc. The cluster descriptions are supplemented with information on comorbidities and the presence of other geriatric conditions. Individuals from these initial clusters will be followed up for the onset of CVD (outcomes) at the age of 60 years. Quantification of this transition will be indicated by the extent to which individuals from particular initial clusters (e.g., which proportion of those coming from cluster 1, and which proportion of those coming from cluster 2) have experienced the CV event. For a more specific quantification of contribution of particular clusters to the onset of CVD, changes in different numerical values (e.g., body mass index, cholesterol, glycated hemoglobin, C-reactive protein, serum albumin, urin albumin/creatinine ratio, etc.) can be used to supplement the quantification procedure. The quantification procedure will allow clinical profiles that are associated with the early development of CVD (people in their 60s), to be identified and quantified. The remaining part of the population (those free from CVD) are again divided into clusters, and the whole procedure is repeated, with the aim of identifying which CV risk factors are relevant for the onset of CVD in the 70s. The quantification procedure allows the CV risk factors at the next CVD development generation (at the ages > 70 years and at the ages > 80 years) to be compared with those at the start (at the age > 60 years). CV: cardiovascular; CVD: cardiovascular disease.
CONCLUSIONS
CVD is a leading cause of morbidity and mortality worldwide, with the highest incidence and prevalence in an older population (> 60 years). Ever since the traditional CV risk factors were identified in the Framingham Heart Study, at the end of the 20th century, they have been used as the basis of risk-based strategies for predicting CVD and initiating drug therapy in primary CVD prevention.
A number of predictive functions and score systems have been developed for CV risk assessment. Although there are some variations between the systems, most of them use the same limited set of variables, including age, sex, smoking, blood pressure and cholesterol, to predict the ten-year absolute risk for developing CVD, or CVD-related death.
The current CV risk assessment systems have several limitations, which limit their implementation in practice and their efficacy in reducing the burden of CVD. Most systems perform well in the population from which they were derived but not in other populations. It is necessary to recalibrate the prediction equation for application in other populations, to allow for different CVD mortality rates and risk factor distributions. Another limitation is these systems’ inability to represent the inter-individual variations in the CV risk accurately, so that a substantial proportion of individuals are wrongly classified, which can lead to either insufficient treatment or overtreatment. The variable age is the strongest CV predictor, and the current prediction models cannot distinguish between age and other risk factors.
Recent studies indicate that the effects of traditional CV risk factors attenuate among older individuals, and that other age-related factors, including comorbid conditions, become important for predicting CVD in older age. Based on the current knowledge, CVD develops concurrently with many comorbidities and other geriatric conditions, such as frailty, malnutrition, and sarcopenia, which share the common mechanisms and pathophysiology pathways as CVD. This is the reason why older people are very heterogeneous with respect to differences in their health status and functional performances, which makes CV risk prediction in older individuals complicated.
In the recent years, trajectories (regression models which take into account differences in time trends in a variable value) have been used to express variations in time trends among older individuals with respect to particular CV risk factors or functional disorders. The establishment of eHRs in many countries’ health care systems, and the recent developments in machine learning and deep learning methods, meet the prerequisites for using these methods to search for solutions for coping with the heterogeneity of older individuals with respect to their health status, and how to express the dynamics of changes in this status over time. Based on the authors’ own research experience, in this paper, we suggest a combination of clustering techniques and trajectories, which can be used to identify CV risk factors in older people who develop a particular type of CVD at some time during their lives, including in their 60s, 70s, and 80s.
ACKNOWLEDGMENTS
All authors had no conflicts of interest to disclose.
References
- 1.Winslow CE Public health at the crossroads. 1926. Am J Public Health. 1999;89:1645–1648. doi: 10.2105/AJPH.89.11.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.KAGAN A, DAWBER TR, KANNEL WB, et al The Framingham study: a prospective study of coronary heart disease. Fed Proc. 1962;21:52–57. [PubMed] [Google Scholar]
- 3.Graham IM The importance of total cardiovascular risk assessment in clinical practice. Eur J Gen Pract. 2006;12:148–155. doi: 10.1080/13814780600976282. [DOI] [PubMed] [Google Scholar]
- 4.von Eckardstein A Is there a need for novel cardiovascular risk factors? Nephrol Dial Transplant. 2004;19:761–765. doi: 10.1093/ndt/gfh111. [DOI] [PubMed] [Google Scholar]
- 5.Damen JA, Hooft L, Schuit E, et al Prediction models for cardiovascular disease risk in the general population: systematic review. BMJ. 2016;353:i2416. doi: 10.1136/bmj.i2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goff DC Jr, Lloyd-Jones DM, Bennett G, et al 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piepoli MF, Hoes AW, Agewall S, et al 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Studziński K, Tomasik T, Krzysztoń J, et al Effect of using cardiovascular risk scoring in routine risk assessment in primary prevention of cardiovascular disease: an overview of systematic reviews. BMC Cardiovasc Disord. 2019;19:11. doi: 10.1186/s12872-018-0990-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uchmanowicz I, Hoes A, Perk J, et al Optimising implementation of European guidelines on cardiovascular disease prevention in clinical practice: what is needed? Eur J Prev Cardiol. 2020;2:2047487320926776. doi: 10.1177/2047487320926776. [DOI] [PubMed] [Google Scholar]
- 10.Stewart J, Manmathan G, Wilkinson P Primary prevention of cardiovascular disease: a review of contemporary guidance and literature. JRSM Cardiovasc Dis. 2017;6:2048004016687211. doi: 10.1177/2048004016687211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leening MJG, Cook NR, Ridker PM Should we reconsider the role of age in treatment allocation for primary prevention of cardiovascular disease? Eur Heart J. 2017;38:1542–1547. doi: 10.1093/eurheartj/ehw287. [DOI] [PubMed] [Google Scholar]
- 12.Leening MJG, Ikram MA Primary prevention of cardiovascular disease: the past, present and future of blood pressure- and cholesterol-lowering treatments. PLoS Med. 2018;15:e1002539. doi: 10.1371/journal.pmed.1002539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Agostino RB Sr, Vasan RS, Pencina MJ, et al General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 14.Welcome to the QRISK®2-2017 Cardiovascular Disease Risk Calculator Home Page. https://qrisk.org/2017 (accessed Jan 1, 2021).
- 15.Jackson R, Lawes CM, Bennett DA, et al Treatment with drugs to lower blood pressure and blood cholesterol based on an individual’s absolute cardiovascular risk. Lancet. 2005;365:434–441. doi: 10.1016/S0140-6736(05)70240-3. [DOI] [PubMed] [Google Scholar]
- 16.Cooney MT, Dudina AL, Graham IM Value and limitations of existing scores for the assessment of cardiovascular risk: a review for clinicians. J Am Coll Cardiol. 2009;54:1209–1227. doi: 10.1016/j.jacc.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 17.Steyerberg EW, van der Ploeg T, Van Calster B Risk prediction with machine learning and regression methods. Biom J. 2014;56:601–606. doi: 10.1002/bimj.201300297. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery AA, Fahey T, Peters TJ, et al Evaluation of computer based clinical decision support system and risk chart for management of hypertension in primary care: randomized controlled trial. BMJ. 2000;320:686–690. doi: 10.1136/bmj.320.7236.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trtica-Majnarić L, Včev A. In Designing Computer-Based Clinical Guidelines Decision Support by a Clinician; Holzinger A, Pasi G, Eds. ; Human-Computer Interaction and Knowledge Discovery in Complex, Unstructured, Big Data. HCI-KDD 2013. Springer, Berlin, Heidelberg, 2013; Lecture Notes in Computer Science, 7947.
- 20.Wilson PW, Pencina M, Jacques P, et al C-reactive protein and reclassification of cardiovascular risk in the Framingham Heart Study. Circ Cardiovasc Qual Outcomes. 2008;1:92–97. doi: 10.1161/CIRCOUTCOMES.108.831198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conroy RM, Pyörälä K, Fitzgerald AP, et al Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. doi: 10.1016/S0195-668X(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 22.van Bussel EF, Richard E, Busschers WB, et al A cardiovascular risk prediction model for older people: development and validation in a primary care population. J Clin Hypertens (Greenwich) 2019;21:1145–1152. doi: 10.1111/jch.13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zethelius B, Berglund L, Sundström J, et al Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–2116. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 24.Anand SS, Islam S, Rosengren A, et al Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur Heart J. 2008;29:932–940. doi: 10.1093/eurheartj/ehn018. [DOI] [PubMed] [Google Scholar]
- 25.Cooney MT, Selmer R, Lindman A, et al Cardiovascular risk estimation in older persons: SCORE O. P. Eur J Prev Cardiol. 2016;23:1903–1103. doi: 10.1177/2047487316663546. [DOI] [PubMed] [Google Scholar]
- 26.Virani SS, Alonso A, Benjamin EJ, et al Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 27.Causes of death statistics-people over 65 Home Page. https://ec.europa.eu/eurostat/statistics (accessed Jan 1, 2021).
- 28.Mensah GA, Wei GS, Sorlie PD, et al Decline in cardiovascular mortality: possible causes and implications. Circ Res. 2017;120:366–380. doi: 10.1161/CIRCRESAHA.116.309115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prentice AM The emerging epidemic of obesity in developing countries. Int J Epidemiol. 2006;35:93–99. doi: 10.1093/ije/dyi272. [DOI] [PubMed] [Google Scholar]
- 30.North BJ, Sinclair DA The intersection between aging and cardiovascular disease. Circ Res. 2012;110:1097–1108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber C, Noels H Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 32.Tuttle CSL, Maier AB Towards a biological geriatric assessment. Exp Gerontol. 2018;107:102–107. doi: 10.1016/j.exger.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 33.Martín-Fernández B, Gredilla R Mitochondria and oxidative stress in heart aging. Age (Dordr) 2016;38:225–238. doi: 10.1007/s11357-016-9933-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakou ES, Parthenakis FI, Kallergis EM, et al Healthy aging and myocardium: a complicated process with various effects in cardiac structure and physiology. Int J Cardiol. 2016;209:167–175. doi: 10.1016/j.ijcard.2016.02.039. [DOI] [PubMed] [Google Scholar]
- 35.Mancuso P, Bouchard B The impact of aging on adipose function and adipokine synthesis. Front Endocrinol (Lausanne) 2019;10:137. doi: 10.3389/fendo.2019.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulligan AA, Lentjes MAH, Luben RN, et al Changes in waist circumference and risk of all-cause and CVD mortality: results from the European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) cohort study. BMC Cardiovasc Disord. 2019;19:238. doi: 10.1186/s12872-019-1223-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akintoye E, Briasoulis A, Afonso L Biochemical risk markers and ten-year incidence of atherosclerotic cardiovascular disease: independent predictors, improvement in pooled cohort equation, and risk reclassification. Am Heart J. 2017;193:95–103. doi: 10.1016/j.ahj.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Barnett K, Mercer SW, Norbury M, et al Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 39.Onder G, Palmer K, Navickas R, et al Time to face the challenge of multimorbidity. A European perspective from the joint action on chronic diseases and promoting healthy ageing across the life cycle (JA-CHRODIS) Eur J Intern Med. 2015;26:157–159. doi: 10.1016/j.ejim.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 40.Calderón-Larrañaga A, Vetrano DL, Ferrucci L, et al Multimorbidity and functional impairment-bidirectional interplay, synergistic effects and common pathways. J Intern Med. 2019;285:255–271. doi: 10.1111/joim.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marengoni A, Angleman S, Melis R, et al Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10:430–439. doi: 10.1016/j.arr.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Buddeke J, Bots ML, van Dis I, et al Comorbidity in patients with cardiovascular disease in primary care: a cohort study with routine healthcare data. Br J Gen Pract. 2019;69:e398–e406. doi: 10.3399/bjgp19X702725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willadsen TG, Siersma V, Nicolaisdóttir DR, et al Multimorbidity and mortality: a 15-year longitudinal registry-based nationwide Danish population study. J Comorb. 2018;8:2235042X18804063. doi: 10.1177/2235042X18804063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forman DE, Maurer MS, Boyd C, et al Multimorbidity in older adults with cardiovascular disease. J Am Coll Cardiol. 2018;71:2149–2161. doi: 10.1016/j.jacc.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu Y, Hajifathalian K, Ezzati M, et al Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet. 2014;383:970–983. doi: 10.1016/S0140-6736(13)61836-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdelhafiz AH, Brown SH, Bello A, et al Chronic kidney disease in older people: physiology, pathology or both? Nephron Clin Pract. 2010;116:c19–c24. doi: 10.1159/000314545. [DOI] [PubMed] [Google Scholar]
- 47.Marengoni A, Roso-Llorach A, Vetrano DL, et al Patterns of multimorbidity in a population-based cohort of older people: sociodemographic, lifestyle, clinical, and functional differences. J Gerontol A Biol Sci Med Sci. 2020;75:798–805. doi: 10.1093/gerona/glz137. [DOI] [PubMed] [Google Scholar]
- 48.Cigolle CT, Langa KM, Kabeto MU, et al Geriatric conditions and disability: the Health and Retirement Study. Ann Intern Med. 2007;147:156–164. doi: 10.7326/0003-4819-147-3-200708070-00004. [DOI] [PubMed] [Google Scholar]
- 49.Franceschi C, Bonafè M, Valensin S, et al Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x.. [DOI] [PubMed] [Google Scholar]
- 50.Clerencia-Sierra M, Ioakeim-Skoufa I, Poblador-Plou B, et al Do centenarians die healthier than younger elders? A comparative epidemiological study in Spain. J Clin Med. 2020;9:1563. doi: 10.3390/jcm9051563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Franceschi C, Garagnani P, Morsiani C, et al The continuum of aging and age-related diseases: common mechanisms but different rates. Front Med (Lausanne) 2018;5:61. doi: 10.3389/fmed.2018.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leening MJG, Ikram MA Primary prevention of cardiovascular disease: the past, present, and future of blood pressure-and cholesterol-lowering treatments. PLoS Med. 2018;15:e1002539. doi: 10.1371/journal.pmed.1002539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whelton SP, Roy P, Astor BC, et al Elevated high-sensitivity C-reactive protein as a risk marker of the attenuated relationship between serum cholesterol and cardiovascular events at older age. The ARIC Study. Am J Epidemiol. 2013;178:1076–1084. doi: 10.1093/aje/kwt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lind L, Sundström J, Ärnlöv J, et al Impact of aging on the strength of cardiovascular risk factors: a longitudinal study over 40 years. J Am Heart Assoc. 2018;7:e007061. doi: 10.1161/JAHA.117.007061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saeed A, Nambi V, Sun W, et al Short-term global cardiovascular disease risk prediction in older adults. J Am Coll Cardiol. 2018;71:2527–2536. doi: 10.1016/j.jacc.2018.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosso AL, Eaton CB, Wallace R, et al Combined impact of geriatric syndromes and cardiometabolic diseases on measures of functional impairment. J Gerontol A Biol Sci Med Sci. 2011;66:349–354. doi: 10.1093/gerona/glq230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fried LP, Tangen CM, Walston J, et al Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 58.Fried LP, Xue QL, Cappola AR, et al Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009;64:1049–1057. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cesari M, Calvani R, Marzetti E Frailty in older persons. Clin Geriatr Med. 2017;33:293–303. doi: 10.1016/j.cger.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 60.Frisoli A Jr, Ingham SJ, Paes ÂT, et al Frailty predictors and outcomes among older patients with cardiovascular disease: data from Fragicor. Arch Gerontol Geriatr. 2015;61:1–7. doi: 10.1016/j.archger.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 61.Soysal P, Stubbs B, Lucato P, et al Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev. 2016;31:1–8. doi: 10.1016/j.arr.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 62.Villareal DT, Apovian CM, Kushner RF, et al Obesity in older adults: technical review and position statement of the American Society for Nutrition and Naaso, the Obesity Society. Am J Clin Nutr. 2005;82:923–934. doi: 10.1093/ajcn/82.5.923. [DOI] [PubMed] [Google Scholar]
- 63.Elagizi A, Kachur S, Lavie CJ, et al An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis. 2018;61:142–150. doi: 10.1016/j.pcad.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 64.Calvani R, Picca A, Marini F, et al The “BIOmarkers associated with Sarcopenia and PHysical frailty in EldeRly pErsons” (BIOSPHERE) study: rationale, design and methods. Eur J Intern Med. 2018;56:19–25. doi: 10.1016/j.ejim.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Charnigo R, Guglin M Obesity paradox in heart failure: statistical artifact, or impetus to rethink clinical practice? Heart Fail Rev. 2017;22:13–23. doi: 10.1007/s10741-016-9577-0. [DOI] [PubMed] [Google Scholar]
- 66.Banack HR, Kaufman JS Does selection bias explain the obesity paradox among individuals with cardiovascular disease? Ann Epidemiol. 2015;25:342–349. doi: 10.1016/j.annepidem.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 67.Anker D, Santos-Eggimann B, Zwahlen M, et al Blood pressure in relation to frailty in older adults: a population-based study. J Clin Hypertens (Greenwich) 2019;21:1895–1904. doi: 10.1111/jch.13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Calderón-Larrañaga A, Vetrano DL, Ferrucci L, et al Multimorbidity and functional impairment-bidirectional interplay, synergistic effects and common pathways. J Intern Med. 2019;285:255–271. doi: 10.1111/joim.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goldberger AL, Peng CK, Lipsitz LA What is physiologic complexity and how does it change with aging and disease? Neurobiol Aging. 2002;23:23–26. doi: 10.1016/S0197-4580(01)00266-4. [DOI] [PubMed] [Google Scholar]
- 70.Cohen AA, Milot E, Li Q, et al Detection of a novel, integrative aging process suggests complex physiological integration. PloS One. 2015;10:e0116489. doi: 10.1371/journal.pone.0116489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Knauff M, Wolf AG Complex cognition: the science of human reasoning, problem-solving, and decision-making. Cogn Process. 2010;11:99–102. doi: 10.1007/s10339-010-0362-z. [DOI] [PubMed] [Google Scholar]
- 72.Raberin A, Connes P, Barthélémy JC, et al Role of gender and physical activity level on cardiovascular risk factors and biomarkers of oxidative stress in the elderly. Oxid Med Cell Longev. 2020;2020:1315471. doi: 10.1155/2020/1315471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liang Y, Vetrano DL, Qiu C The role of biological age in the management of hypertension in old age: does SPRINT tell the whole story? Int J Cardiol. 2016;222:699–700. doi: 10.1016/j.ijcard.2016.08.085. [DOI] [PubMed] [Google Scholar]
- 74.Orkaby AR, Onuma O, Qazi S, et al Preventing cardiovascular disease in older adults: one size does not fit all. Cleve Clin J Med. 2018;85:55–64. doi: 10.3949/ccjm.85a.16119. [DOI] [PubMed] [Google Scholar]
- 75.Todd OM, Wilkinson C, Hale M, et al Is the association between blood pressure and mortality in older adults different with frailty? A systematic review and meta-analysis. Age Ageing. 2019;48:627–635. doi: 10.1093/ageing/afz072. [DOI] [PubMed] [Google Scholar]
- 76.Vetrano DL, Rizzuto D, Calderón-Larrañaga A, et al Trajectories of functional decline in older adults with neuropsychiatric and cardiovascular multimorbidity: a Swedish cohort study. PLoS Med. 2018;15:e1002503. doi: 10.1371/journal.pmed.1002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dhana K, Koolhaas CM, van Rossum EF, et al Metabolically healthy obesity and the risk of cardiovascular disease in the elderly population. PloS One. 2016;11:e0154273. doi: 10.1371/journal.pone.0154273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Flegal KM, Ioannidis JPA The obesity paradox: a misleading term that should be abandoned. Obesity (Silver Spring) 2018;26:629–630. doi: 10.1002/oby.22140. [DOI] [PubMed] [Google Scholar]
- 79.Marcus J, Sarnak MJ, Menon V Homocysteine lowering and cardiovascular disease risk: lost in translation. Can J Cardiol. 2007;23:707–710. doi: 10.1016/S0828-282X(07)70814-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yıldırım P, Ljiljana MT, Bosnić Z, et al Complexity and non-linearity of cardiovascular risk factors in older patients with multimorbidity and reduced renal function. J Integr Cardiol Open Access. 2020;3:1–11. [Google Scholar]
- 81.Sidey-Gibbons JAM, Sidey-Gibbons CJ Machine learning in medicine: a practical introduction. BMC Med Res Methodol. 2019;19:64. doi: 10.1186/s12874-019-0681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seyhan AA, Carini C Are innovation and new technologies in precision medicine paving a new era in patients centric care? J Transl Med. 2019;17:114. doi: 10.1186/s12967-019-1864-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Karmali KN, Lloyd-Jones DM, van der Leeuw J, et al Blood pressure-lowering treatment strategies based on cardiovascular risk versus blood pressure: a meta-analysis of individual participant data. PLoS Med. 2018;15:e1002538. doi: 10.1371/journal.pmed.1002538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cowie MR, Blomster JI, Curtis LH, et al Electronic health records to facilitate clinical research. Clin Res Cardiol. 2017;106:1–9. doi: 10.1007/s00392-016-1025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leening MJG, Cook NR, Ridker PM Should we reconsider the role of age in treatment allocation for primary prevention of cardiovascular disease? Eur Heart J. 2017;38:1542–1547. doi: 10.1093/eurheartj/ehw287. [DOI] [PubMed] [Google Scholar]
- 86.Onder G, Vetrano DL, Marengoni A, et al Accounting for frailty when treating chronic diseases. Eur J Intern Med. 2018;56:49–52. doi: 10.1016/j.ejim.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 87.Finegold JA, Manisty CH, Goldacre B, et al What proportion of symptomatic side effects in patients taking statins are genuinely caused by the drug? Systematic review of randomized placebo-controlled trials to aid individual patient choice. Eur J Prev Cardiol. 2014;21:464–474. doi: 10.1177/2047487314525531. [DOI] [PubMed] [Google Scholar]
- 88.Giral P, Neumann A, Weill A, et al Cardiovascular effect of discontinuing statins for primary prevention at the age of 75 years: a nationwide population-based cohort study in France. Eur Heart J. 2019;40:3516–3525. doi: 10.1093/eurheartj/ehz458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Benetos A, Petrovic M, Strandberg T Hypertension management in older and frail older patients. Circ Res. 2019;124:1045–1060. doi: 10.1161/CIRCRESAHA.118.313236. [DOI] [PubMed] [Google Scholar]
- 90.Leening MJ, Berry JD, Allen NB Lifetime perspectives on primary prevention of atherosclerotic cardiovascular disease. JAMA. 2016;315:1449–1450. doi: 10.1001/jama.2016.1654. [DOI] [PubMed] [Google Scholar]
- 91.Sniderman AD, Toth PP, Thanassoulis G, et al Taking a longer term view of cardiovascular risk: the causal exposure paradigm. BMJ. 2014;348:g3047. doi: 10.1136/bmj.g3047. [DOI] [PubMed] [Google Scholar]
- 92.Allen NB, Siddique J, Wilkins JT, et al Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age. JAMA. 2014;311:490–497. doi: 10.1001/jama.2013.285122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Strandberg TE, Stenholm S, Strandberg AY, et al The “obesity paradox, ” frailty, disability, and mortality in older men: a prospective, longitudinal cohort study. Am J Epidemiol. 2013;178:1452–1460. doi: 10.1093/aje/kwt157. [DOI] [PubMed] [Google Scholar]
- 94.Franceschi C, Garagnani P, Morsiani C, et al The continuum of aging and age-related diseases: common mechanisms but different rates. Front Med (Lausanne) 2018;5:61. doi: 10.3389/fmed.2018.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beard JR, Officer A, de Carvalho IA, et al The world report on ageing and health: a policy framework for healthy ageing. Lancet. 2016;387:2145–2154. doi: 10.1016/S0140-6736(15)00516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vetrano DL, Palmer KM, Galluzzo L, et al Hypertension and frailty: a systematic review and meta-analysis. BMJ Open. 2018;8:e024406. doi: 10.1136/bmjopen-2018-024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boulton C, Wilkinson JM Use of public datasets in the examination of multimorbidity: opportunities and challenges. Mech Ageing Dev. 2020;190:111310. doi: 10.1016/j.mad.2020.111310. [DOI] [PubMed] [Google Scholar]
- 98.Guo Q, Lu X, Gao Y, et al Cluster analysis: a new approach for identification of underlying risk factors for coronary artery disease in essential hypertensive patients. Sci Rep. 2017;7:43965. doi: 10.1038/srep43965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peterson ED Machine learning, predictive analytics, and clinical practice: can the past inform the present? JAMA. 2019;322:2283–2284. doi: 10.1001/jama.2019.17831. [DOI] [PubMed] [Google Scholar]
- 100.Weng C, Shah NH, Hripcsak G Deep phenotyping: embracing complexity and temporality-towards scalability, portability, and interoperability. J Biomed Inform. 2020;105:103433. doi: 10.1016/j.jbi.2020.103433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hassaine A, Canoy D, Solares JRA, et al Learning multimorbidity patterns from electronic health records using non-negative matrix factorisation. J Biomed Inform. 2020;112:103606. doi: 10.1016/j.jbi.2020.103606. [DOI] [PubMed] [Google Scholar]
- 102.Zhao J, Zhang Y, Schlueter DJ, et al Detecting time-evolving phenotypic topics via tensor factorization on electronic health records: cardiovascular disease case study. J Biomed Inform. 2019;98:103270. doi: 10.1016/j.jbi.2019.103270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Majnarić-Trtica L, Vitale B, Martinis M Is it time for a new approach in cardiovascular risk assessment? Period Biol. 2008;1110:45–50. [Google Scholar]
- 104.Majnarić-Trtica L, Vitale B Systems biology as a conceptual framework for research in family medicine; use in predicting response to influenza vaccination. Prim Health Care Res. 2011;12:310–321. doi: 10.1017/S1463423611000089. [DOI] [PubMed] [Google Scholar]
- 105.Šabanović Š, Ljiljana MT, Babič F, et al Metabolic syndrome in hypertensive women in the age of menopause: a case study on data from general practice electronic health records. BMC Med Inform Decis Mak. 2018;18:24. doi: 10.1186/s12911-018-0601-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bekić S, Babič F, Filipčić I, et al Clustering of mental and physical comorbidity and the risk of frailty in patients aged 60 years or more in primary care. Med Sci Monit. 2019;25:6820–6835. doi: 10.12659/MSM.915063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Majnarić LT, Bekić S, Babič F, et al Cluster analysis of the associations among physical frailty, cognitive impairment and mental disorders. Med Sci Monit. 2020;26:e924281. doi: 10.12659/MSM.924281.. [DOI] [PMC free article] [PubMed] [Google Scholar]