Highlights
-
•
CUAE and PUAE parameters were optimized by the maximization of the yield in TPC.
-
•
Compared to conventional methods, UAE offered a higher yield of antioxidants.
-
•
Gallic acid was the most abundant polyphenolic compound in carob pulp extracts.
-
•
Solvent concentration was the most influential processing parameter.
-
•
DPA-6S demonstrated the best ability to recuperate the target analytes.
Keywords: Polyphenols, Carobs, Polyphenol extraction, Ultrasound-assisted extraction, Response surface methodology
Abstract
Polyphenols in carobs have recently attracted great attention due to their wide range of biological and health promoting effects. A comprehensive study was conducted to find an optimum method for the extraction, purification and characterization of these valuable bioactive substances. Under this framework, the ultrasound-assisted extraction (UAE) of polyphenols from carob pulp was optimized by the maximization of the yield in total phenolics using response surface methodology. In particular, the effects of solid-solvent ratio, solvent concentration, extraction time, sonication amplitude, and sonication mode were investigated and optimized using a complete experimental design. In comparison to conventional extraction techniques, UAE offered a higher yield of antioxidants and a shorter processing time. Solid-phase extraction was evaluated as a clean-up strategy prior to the electrophoretic analysis of extracts. The results from the analysis of real samples revealed the predominance of gallic acid and highlighted the great influence of the ripening stage on carobs composition.
1. Introduction
The carob tree (Ceratonia siliqua L.) is an evergreen species belonging to the Leguminosae family, widely cultivated in the Mediterranean region usually in mild and dry places with poor soils [1]. The fruit of the carob tree, also referred to as carob pod or carob, consists of two major parts: the pulp (90%) and the seeds (10%) [2]. Due to its unique chemical composition, which strongly depends on the cultivar, the origin and the harvesting time, the carob pod has attracted great attention in recent years [2]. The carob pulp is mainly composed of sugars, fibres, amino acids and minerals, while the seeds are composed principally of galactomannans [3]. Carob pods also contain considerable amounts of polyphenols, which have been described to present antioxidant activity and a diversity of potential benefits in human health [4]. In this respect, the recent explosion of interest in carob’s polyphenolic composition is mainly attributed to their several biological properties, including antimicrobial, anti-carcinogenic, anti-inflammatory, antiviral, and anti-allergic activity, among others [5].
The extraction of the target analytes from plant matrices is a crucial step for both the identification and quantification process. As it has been proven, the efficiency and effectiveness of employed extraction techniques strongly influence the obtained polyphenolic patterns and compositions of carob extracts. Various conventional extraction methods including maceration, infusion, decoction, and Soxhlet extraction have, so far, been utilized for the recovery of polyphenols from carob pods and derived products [6], [7], [8], [9]. However, these classical techniques are often time-consuming, they require relatively large quantities of solvents, and the examined active components may degrade or oxidise due to the long processing times and high temperatures used [4], [10].
Some alternative methods of extraction are microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE), supercritical fluid extraction (SFE), pressurized liquid extraction (PLE), and many others. Among them, UAE offers a simple, innovative, inexpensive, environmentally friendly, and efficient way of extraction [11]. The enhancement of process efficiency by sonication is mainly attributed to the phenomenon of acoustic cavitation and its consequence thermal and mechanical effects [12]. Increased mass transfer and significant disruption of cell walls come as a result of these combined effects, offering higher extraction yields and significantly reduced processing times in comparison to the conventional techniques [13]. Moreover, UAE technique is achieved at lower temperatures, and hence, it is considered to be more suitable for the extraction of thermally unstable constituents [14]. Nowadays, this method of extraction has been successfully applied for the recovery of antioxidants from a variety of plant matrices. In regard to UAE of phenolic compounds from carob samples, some studies have analysed the phenolic profile of extracts derived by sonication. However, only in few cases, UAE has been optimized thoroughly and has been compared with conventional extraction techniques [4], [15], [16], [17].
The efficiency of UAE is greatly affected by several factors, including solvent type and concentration, solid-solvent ratio, particle size, extraction time and temperature, sonication amplitude, and sonication mode (continuous or pulsed). The optimization of these parameters is considered to be essential in order to maximize the yield of antioxidants and reach an accurate analysis. Classical single factor experiments and response surface methodology (RSM) are two types of optimization techniques that are commonly used during the optimization of an extraction process [18], [19]. Classical optimization studies use the one-factor-at-a-time approach, in which only one factor is varied at a time, while all others are kept constant [18]. Although popular, this method is time-consuming and laborious, and provides limited information on how different variables interact. This, in turn, gives rise to misleading conclusions [18]. RSM can overcome these difficulties, since it allows the evaluation of the effect of the independent variables as well as their possible interactions [20]. It is actually a collection of sophisticated mathematical and statistical techniques, useful for developing, improving, and optimizing processes by establishing empirical models [13], [18]. RSM is based on the fit of a polynomial equation to the experimental data, which, in turn, must describe the behaviour of the data set and make statistical previsions. The objective is the simultaneous optimization of process variables in order to attain the best overall extraction performance [21].
Although the complete polyphenolic profile of carob pulp extracts has already been published, literature data concerning the optimization of polyphenols recovery is very scarce. The optimal conditions for the recovery of carob’s polyphenols are not yet well established, resulting in significant differences and not comparable results among the literature. Therefore, there is undoubtedly a clear need to develop an optimum and appropriate method for the extraction, the purification as well as the characterization of carobs phenolics in order to achieve highly accurate results. Under this framework, the UAE of polyphenols from unripe carob pulp was optimized by the maximization of the yield in total phenolics using RSM. To the best of our knowledge, there is no previously published report regarding the optimization of pulsed UAE (PUAE) conditions for the recovery of carob’s polyphenols. In addition, despite its extensive use in phytochemicals analysis, capillary electrophoresis (CE) has not been previously applied for both the qualitative and quantitative determination of carob pulp phenolics. Although Roseiro et al. (2013) and Almanasrah et al. (2015) attempted to analyse carob pulp phenolics by use of a CE system, they failed to achieve a complete separation, identification and quantification of the target analytes [7], [22], [23].
2. Material and methods
2.1. Standards and reagents
The analytes of (+)-catechin (1), (±)-naringenin (2), catechol (3), trans-cinnamic acid (4), chlorogenic acid (5), gentisic acid (6), kaempferol (7), ferulic acid (8), quercetin (9), myricetin (10), caffeic acid (11) and gallic acid (12) were purchased for Sigma-Aldrich. Stock standard solutions, 1 mg mL−1, were prepared by dissolving in methanol (MeOH) the appropriate amount of substance and stored at −20 °C for not more than 3 months. Intermediate working solutions were prepared weekly from these stock standard solutions by appropriate dilution with MeOH.
HPLC grade MeOH and acetone were obtained from Supelco. Reagents for spectrophotometric assay, Folin-Ciocalteu (FC) reagent and sodium carbonate (Na2CO3), were purchased from Sigma-Aldrich. For background electrolyte (BGE) preparation, sodium tetraborate decahydrate (Na2B4O7·10H2O, borate) and β-cyclodextrin (β-CD) were obtained from Fluka and Sigma-Aldrich, respectively, while L-alanine tert butyl ester lactate (L-AlaC4Lac) was synthesized in the laboratory, based on a previously described procedure [24]. The pH of BGE was adjusted with sodium hydroxide (NaOH, SUPELCO) and acidification of extracts was performed using hydrochloric acid (HCl, Scharlau). All solutions were filtered through a 0.45-μm pore size syringe filter and degassed prior to use.
2.2. Plant material
Mature and immature carobs were collected in 2019 from the same tree cultivated in the region of Archimandrita (Cyprus, altitude: 436 m), at different dates according to their ripening stages (June: unripe pods and September: ripe pods). The moisture content of unripe and ripe carob pulp material, which was determined by gravimetric analysis according to the AOAC established procedure [25], was 71.7 ± 0.1% and 14.5 ± 0.2%, respectively. For polyphenols content determination, the fresh pods were washed; seeds were removed and pulps were lyophilized (LyoDry Compact Benchtop Freeze Dryer, MECHATECK SYSTEMS), ground to a fine powder (Thermomix® TM5, VORWERK), and passed through a 250-μm sieve (Endecotts) to obtain uniformly sized particles. The samples were vacuum-packed (4100050 Sealcom-V, J. P. SELECTA) and stored at −20 °C until extraction and analysis.
2.3. Extraction procedures
2.3.1. Ultrasound-assisted extraction
UAE of phenolics was performed by use of a 500-W power and a 20-kHz frequency ultrasonic probe system (CY-500, Optic Ivymen System®). For the extraction, 2 g of carob pulp powder were mixed with an appropriate volume of acetone solution, and the obtained suspension was exposed to acoustic waves under controlled UAE conditions (according to the experimental design). In particular, the extraction was carried out using different solid-solvent ratios and acetone concentrations for varying periods of time and sonication amplitudes. The tip of the probe was submerged 2 cm into the extraction solution and the sonication was conducted in both continuous (0s:0s) and pulsed (5s:5s) modes. After the ultrasound treatment, the obtained mixture was centrifuged at 4400 rpm for 20 min, and filtered through Whatman no.1 filter paper. The treated sample was then concentrated under vacuum using a rotary evaporator (RE300, stuart®), lyophilized, and vacuum-stored at −20 °C until further analysis. All extracts were prepared in triplicate.
2.3.2. Conventional methods of extraction
2.3.2.1. Agitation extraction
Briefly, 2 g of carob pulp powder were mixed with 50 mL of acetone solution (57%, v/v, acetone) and agitated at a moderate speed at room temperature for 14 min using a magnetic stirrer. On the completion of the extraction procedure, stirring was stopped; the extract was centrifuged at 4400 rpm for 20 min and filtered through Whatman no.1 filter paper in order to remove the insoluble particles. The resulting solution was evaporated to dryness using a rotary evaporator and a freeze dryer, vacuum-packed, and stored at −20 °C for subsequent analysis.
2.3.2.2. Soxhlet extraction
Carob pulp powder (6 g) was extracted with 150 mL of aqueous acetone solution (57%, v/v, acetone), refluxing in a Soxhlet apparatus for 6 h. The obtained extract solution was cooled to room temperature, the organic solvent was evaporated under vacuum using a rotary evaporator and the remaining water was removed using a lyophilizer. Dried-extracts were stored under vacuum at −20 °C until further use.
2.4. Determination of total phenolic content
The total phenolic content (TPC) of the extracts was determined according to the FC colorimetric method described by Singleton and Rossi (1965) [26]. Prior to the spectrophotometric analysis, the freeze-dried extracts were redissolved in the extraction solvent (20 mL) and filtered through a 0.45-μm membrane filter to remove any insoluble particle. Then, 2 mL of properly diluted in water extract solution were mixed with 10 mL of FC reagent, pre-diluted, 10 times, with distilled water. After standing for a period of 1–8 min at room temperature, 8 mL of a saturated solution of Na2CO3 (7.5%, w/v) were added into the reaction mixture. The absorbance of the samples was measured after 2 h of incubation at room temperature and in the dark at 765 nm using a UV–Vis spectrophotometer (UV-1601, Shimadzu). Gallic acid was used as the reference standard, and total phenolics were expressed as mg of gallic acid equivalents (GAE) per g of dried carob pulp and in some cases, for comparison studies, as mg GAE/g of dried carob pulp extract.
2.5. Experimental design and statistical analysis
Different experimental design methodologies were employed sequentially for both screening and optimization of UAE parameters. At first, a five-factor and two-level full factorial design (25) was employed to investigate the effect of solid-solvent ratio (A), solvent concentration (B), extraction time (C), sonication amplitude (D), and sonication mode (E) on polyphenols recovery. Based on the results obtained from the initial screening procedure, two different 24 full factorial designs were developed to further investigate the influence of solid-solvent ratio (A), solvent concentration (B), extraction time (C), and sonication amplitude (D) on TPC using both continuous and pulsed modes. On the basis of the preliminary screening designs, critical influencing factors for both extraction modes were confirmed and then optimized using RSM. Solvent concentration and extraction time were the two main independent variables, selected based on the preliminary experiments, whose optimum levels were determined, for each extraction mode, using RSM based on 32 full factorial designs. The design of the experiments, analysis of the results, and predictions of the responses were performed using RStudio statistical software (version 1.3.1073).
2.6. Solid-phase purification
2.6.1. Preliminary assessments-Selection of SPE cartridge
Three commercially available SPE cartridges, namely, Maxi-Clean™ C18 (900 mg, GRACE), Oasis® HLB (400 mg, Waters), and Discovery® DPA-6S (500 mg, SUPELCO), were compared in terms of polyphenols recovery from an acidic standard solution. The cartridges were selected due to their affinity to the target analytes. All SPE experiments were performed on a vacuum manifold system with a 12 positions rack (Visiprep™ SPE Vacuum Manifold, SUPELCO). Prior to extraction, sorbents were conditioned with MeOH (5 mL) and equilibrated with an acidified methanolic solution (5 mL), 20:80 (v/v) MeOH:H2O acidified to pH 2.0 with HCl. Then, approximately 2 mL of acidified standard mixture solution, containing 50 μg/mL of each analyte in 20:80 (v/v) MeOH:H2O (pH 2.0, HCl), were loaded on each type of sorbent (C18, HLB, DPA-6S). Acidification of standard mixture solution was deemed to be necessary in order to prevent ionization of polyphenolic compounds and minimize their loss during the purification step. The presence of MeOH in the standard mixture enhances the solubility of polyphenols and, therefore, their successful transfer to the respective SPE cartridges. Finally, the loaded cartridges were washed with 5 mL of acidified water (pH 2.0, HCl) in order to remove the co-extracted substances, and the retained polyphenolic compounds in the case of C18 and HLB sorbent materials were eluted using a mixture of MeOH:H2O 80:20 (v/v) (2 × 5 mL). In the case of polyamide cartridges (DPA-6S), the elution step was assessed with a mixture of acetone:H2O 80:20 (v/v) (2 × 5 mL), as recommended by cartridges manufacturers. The final fractions were collected and evaporated to dryness using a rotary evaporator (T < 35 °C) and a freeze dryer. The obtained residues were redissolved in a known volume of MeOH (1 mL), filtered through a 0.45-μm pore size membrane filter, and further analyzed by use of the proposed CE analytical method. The entire extraction procedure was repeated at least three times for each examined sorbent material. Discovery® DPA-6S sorbent material was found to be the most suitable for the analysis of the target compounds.
2.6.2. Extracts purification
Based on the preliminary results of SPE sorbent testing, a Discovery® DPA-6S sorbent material was employed for further purification of carob pulp extracts. As previously described, the polyamide SPE cartridges were preconditioned with 5 mL of MeOH and equilibrated with 5 mL of acidified methanolic solution. The sample mixture, which was prepared by dissolving the obtained freeze-dried extract in acidified methanolic solution (20 mL), was passed through the cartridge, which was then washed with 5 mL of acidified water, in order to remove the co-extracted substances (e.g. sugars). The bound phenolics were then eluted with an aqueous acetone mixture (2 × 5 mL), and the obtained purified extracts were evaporated to dryness. The residues were redissolved in a known volume of MeOH (1 mL), filtered through a 0.45-μm pore size membrane filter, properly diluted, and injected into the CE system.
2.7. Electrophoretic procedure
All CE experiments were performed on an Agilent G1600A Capillary Electrophoresis System equipped with a diode-array detector (DAD) set at 205 nm. ChemStation software was used for data acquisition, processing and storage. A fused-silica capillary (Polymicro TECHNOLOGIES) with an internal diameter of 50 μm and an effective length of 40 cm (total length 48.5 cm) was used for the separation. Prior to use, new capillaries were activated by flushing sequentially with H20 (30 min), 1 M NaOH (60 min), H2O (30 min), and finally with the BGE (30 min). At the beginning of each working session, the capillary was conditioned with H2O (5 min), 1 M NaOH (10 min), H2O (5 min), and then with the BGE (5 min). Between each analysis, the capillary was rinsed with the BGE for 3 min, which was refreshed after three consecutive runs. At the end of each session, the capillary was stored after rinsing with water.
The electrophoretic conditions for the separation of the target analytes were optimized in a previous investigation. Briefly, polyphenols analysis was performed by using a BGE consisting of 35 mM borate along with 15 mM β-CD and 3 mM L-AlaC4Lac ionic liquid (IL) as running buffer additives. For the separation, the capillary was thermostated at 25 °C and the separation voltage was set at 30 kV. Samples were loaded by pressure-assisted hydrodynamic injection by applying a pressure of 50 mbar for 6 s. Peak identification was carried out by comparing retention times and UV spectra with that of authentic standards as well as by the application of the standard addition approach (spiking method).
3. Results and discussion
3.1. Optimization of polyphenols recovery
3.1.1. Screening of variables influencing extraction efficiency
The factorial experimental design methodology was employed to estimate the influence of different independent variables on the extraction efficiency. In particular, a screening design was developed, in order to evaluate the influence of operating parameters on the extraction recovery of polyphenols from unripe carob pulp using UAE. Five potential independent variables, namely, solid-solvent ratio (A), solvent concentration (B), extraction time (C), sonication amplitude (D), and sonication mode (E) were investigated by a five-factor and two-level full factorial design (25) to identify the factors that exerted significant effects on the dependent variable, namely, TPC (Y). Each factor was examined at the two most promising levels with the natural and coded values of the selected independent variables demonstrated in Table 1. The choice of the limits of each independent variable in the experimental design was based on preliminary experiments and previous related investigations. The design matrix of the experimental outcome, as carried out above the specified levels of investigated process variables and expressed as TPC of dried carob pulp, can be found in the Supplementary Material (Table S1). It should be noted that for each condition, the extraction was conducted in triplicate, and the responses presented in Table S1 are the average values of the three replicates among with the variances expressed by standard deviation (SD). Under the established conditions, the experimental values for TPC varied from 70.14 to 129.33 mg GAE/g of carob pulp.
Table 1.
Natural and coded levels of independent variables used in two-level full factorial screening design.
| Factor | Symbol | Factor levels |
|
|---|---|---|---|
| Low (−1) | High (+1) | ||
| Solid-solvent ratio | A | 1:10 | 1:25 |
| Solvent concentration (% acetone, v/v) | B | 50 | 80 |
| Extraction time (min) | C | 10 | 20 |
| Sonication amplitude (%) | D | 50 | 75 |
| Sonication mode (pulse duration: pulse interval (sec)) | E | continuous (0:0) | pulsed (5:5) |
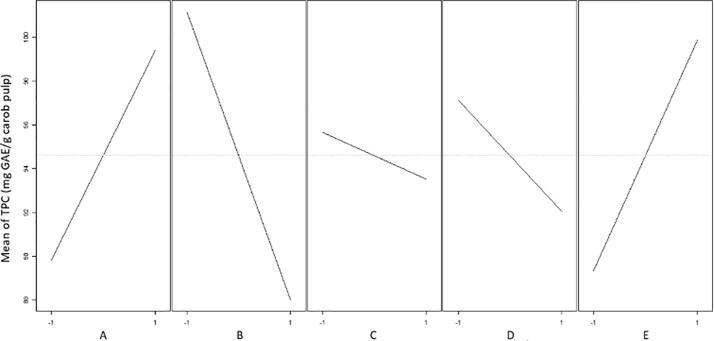
3.1.1.1. Main effects plot
A graphical analysis of the data from the experimental design was performed, and the main effects plot produced is demonstrated in Fig. 1. A main effects plot can be used to compare the relative strength of the effects of various process variables as well as to determine whether a factor has a positive or a negative effect on the response. The gradients of the graphs (Fig. 1), due to the solvent concentration (B), sonication mode (E), solid-solvent ratio (A), sonication amplitude (D), and extraction time (C) indicate that these variables had a significant effect on the response. Fig. 1 reveals that the solvent concentration (B) was the most influential parameter. From further analysis of the main effects plot, it is inferred that the solvent concentration (B), the sonication amplitude (D) and the extraction time (C) had a negative effect on the response, as a decrease of TPC was observed when each factor was changed from its lower to its higher level. For the solid-solvent ratio (A), the opposite behaviour was observed, indicating its positive effect on the response. In regard to the sonication mode (E), higher extraction yields were observed when UAE was performed in the pulsed mode. This indicates that PUAE may be a more efficient technique for the recovery of polyphenols.
Fig. 1.
Main effects plot for TPC.
3.1.1.2. Effect of process variables on the UAE performance
As already mentioned, the solvent concentration demonstrated a pronounced influence on the response. The addition of water to the organic solvent created a more polar medium, which, in turn, enhanced the extraction of bioactive compounds from the plant cells. Since the majority of phenolic compounds occur naturally as glycosides, and the sugar moiety makes the phenolic compounds more water-soluble, lower acetone concentrations proved to be more suitable for the effective recovery of polyphenols [27]. Furthermore, the addition of water into acetone enhances the swelling of plant material, and it increases the contact surface area between the solid plant matrix and the extraction solvent. As Prasad et al. (2012) stated, water acts as a swelling agent that enables better mass transfer of the bioactive compounds, and thus, improves the efficiency of extraction [28].
The yield of antioxidants in UAE process was also influenced by sonication mode. The highest TPC values were achieved during extraction in pulsed mode, in all examined procedures. As demonstrated in other studies, the continuous exposure of the plant cell to cavitation causes an increase in both temperature and pressure, which, in turn, decomposes the sensitive bioactive substances [29]. This finding is in accordance with previous studies which also reported the superiority of the pulsed mode over continuous extraction. Lower energy input, better temperature control, and a decrease of erosion of the ultrasound probe tip are some of the advantages that have already been mentioned for PUAE [30].
Solid-solvent ratio had also an important positive effect on the TPC. Briefly, an increase in the solid-solvent ratio results in better penetration of the solvent into the plant matrix, which, in turn, enhances the mass transfer of polyphenols and, consequently, the yield of extraction. On the contrary, the excessive amount of plant material, in the case of small ratios, causes the rise of solvent viscosity and thus, inhibits the diffusion of polyphenols through the extraction medium [31]. At a lower ratio, the solvent can also attain saturation soon, during extraction, and the extraction process cannot be completed [28]. The presence of a large amount of plant material into the extraction solvent may also contribute to the attenuation of the ultrasonic wave, which causes difficulties in cavitation and lower extraction yields [31]. Based on the above, it was concluded that a sufficient solvent volume is required to enable the effective solvation of the phytoconstituents from the extracting material in order to obtain high extraction recoveries.
In regard to the effect of sonication amplitude, the phenolic content was found to decrease upon increasing its level. Although the increase of sonication amplitude results in an increase of sonochemical effects, the use of excessively high ultrasonic amplitudes could degrade or decompose the phenolic components, adversely affecting the extraction yield [19], [32].
Lower extraction yields were also obtained by prolonging the extraction time. In general, plant cells are disrupted more by longer extraction time and, consequently, the release and diffusion of the polyphenols are enhanced. However, when the extraction time is longer than the optimum, the antioxidants might be degraded due to heat generation which results in the chemical decomposition of bioactive compounds and thereby decreases the extraction efficiency [32], [33]. In addition, the oxidation of the bioactive substances by extension of extraction time is probably another factor that might lower the total phenolic yield.
3.1.1.3. Statistical analysis for each extraction mode
Based on the preliminary study of screening design, all the individual factors were found to significantly affect the total phenolic yield: four numerical (solid-solvent ratio, solvent concentration, extraction time, sonication amplitude) and one categorical variable (sonication mode). Since the sonication mode, which is a qualitative variable, demonstrated a great influence on the extraction efficiency, separate statistical analyses for each extraction mode were performed in order to assay the factors that exert a significant effect on the extraction yield using Continuous UAE (CUAE) and PUAE, respectively. Therefore, experimental data from the previous investigation has been introduced to two different 24 full factorial designs to further investigate the influence of solid-solvent ratio (A), solvent concentration (B), extraction time (C), and sonication amplitude (D) on the polyphenols’ recovery using both continuous and pulsed modes.
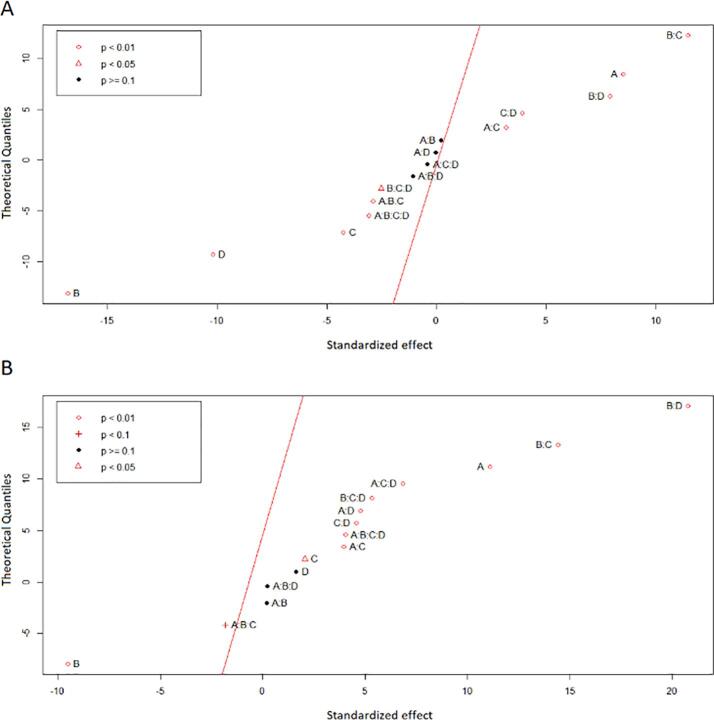
Normal probability plots (Fig. 2) were created to compare the magnitude and statistical significance of the independent variables as well as their interaction terms. Based on the analysis of CUAE, all of the main factors (B, D, A, C) and some of their interactions (B∙C, B∙D, C∙D, A∙C, A∙B∙C∙D, A∙B∙C, B∙C∙D) were found to be significant regarding the recovery of polyphenols. Based on the distance from the straight line, it was evident that the solvent concentration (B) was the most dominant variable that affects the efficiency of polyphenols extraction. Solvent concentration (B), sonication amplitude (D), extraction time (C), and some of their interaction terms (A∙B∙C∙D, A∙B∙C, B∙C∙D) were found to be located on the left side of the line; so, their contribution had a negative effect. At the same time, the remaining significant variables were considered to have a positive effect on the response, as they were distributed on the right side of the line. The normal probability plot, which resulted using the pulsed mode, indicated that the TPC was influenced by a smaller number of main factors (A, B, C) and a greater number of interaction terms (B∙D, B∙C, A∙C∙D, B∙C∙D, A∙D, C∙D, A∙B∙C∙D, A∙C). The solid-solvent ratio (A) and solvent concentration (B) demonstrated the greatest effect, as their points lied furthest from the straight line. In contrast to the continuous extraction, the recovery of polyphenols, using PUAE, was not affected by the amplitude of the ultrasonic wave, as its point was distributed along the straight line. In addition, the normal probability plot for PUAE reveals that, among the main factors, only the solvent concentration (B) had a negative effect on the response.
Fig. 2.
Normal probability plots for (A) CUAE and (B) PUAE.
3.1.2. Optimization of extraction parameters
After carrying out the detailed statistical analysis for both extraction modes, a final optimization of solvent concentration (B) and extraction time (C) was deemed to be necessary, in order to find the optimum extraction conditions and, consequently, to attain the best extraction yield. Therefore, the critical variables (B, C) were studied in detail (B: 50, 65, 80%, C: 10, 15, 20 min), while solid-solvent ratio and sonication amplitude were fixed at their previously determined optimum values, 1:25 solid-solvent ratio and 50% amplitude. The solid-solvent ratio, which demonstrated a significant effect on the response for both extraction modes, was excluded from further optimization of the extraction procedure, and it was fixed at its higher level, which resulted in a better extraction yield. As far as the sonication amplitude is concerned, it was excluded from the final optimization process, and it was fixed at its lower examined level, because it significantly affected the TPC only in the case of CUAE, and its smaller values resulted in better extraction yields and improved energy consumption. The final optimization procedure was based on an RSM approach, and the experimental design, along with the obtained results for continuous and pulsed modes are presented in Tables S2 and S3 (Supplementary Material), respectively.
3.1.2.1. Regression analysis and ANOVA for RSM approach
The TPC experimental data was initially introduced into a multiple regression analysis in order to generate the mathematical models that describe the empirical relationship between the two examined variables and the corresponding response. The empirical correlation was obtained by fitting the experimental results to second order polynomial models, and the acquired mathematical equations for both extraction modes are provided below (in terms of natural values).
| (1) |
| (2) |
The results obtained from the statistical analysis (ANOVA) of the generated regression models (Table 2) proved that the developed mathematical equations adequately explained the data variations and significantly represented the actual relationship between the examined variables and the corresponding response. The statistical parameters (F-values, p-values, R2, R2adj, R2pred) indicated that the resulted quadratic models have a very good predictability and they could be used in RSM in order to optimize the extraction procedure by maximizing the TPC. The performed statistical analysis also confirmed the significant terms of the obtained response surface quadratic models. It is concluded from Table 2 that the variables with the largest impact on the extraction yield using CUAE were the linear terms of solvent concentration (B) and extraction time (C), the quadratic term of solvent concentration (B2) as well as the interaction term between the two critical variables (B∙C). In the case of PUAE, the results indicated that first-order linear and second-order quadratic effects were significant for solvent concentration, whereas the extraction time had a significant effect on the response only in its quadratic form. Non-significant interaction was obtained between the two examined factors. Once again, the solvent, and more specifically the acetone concentration, was found to be the most critical factor affecting the recovery of these valuable bioactive compounds.
Table 2.
ANOVA report for the quadratic models of CUAE and PUAE.
| Source | Sum of squares (SS) | Degree of freedom (DF) | Mean square (MS) | F-value | p-value |
|---|---|---|---|---|---|
| CUAE | |||||
| Model | 110.10 | < 0.0001 (2.5·10-14) *** | |||
| B | 2948.1 | 1 | 2948.1 | 345.60 | < 0.0001 (1.6∙10-14) *** |
| C | 76.0 | 1 | 76.0 | 8.91 | 0.0071 ** |
| B2 | 1056.6 | 1 | 1056.6 | 123.86 | < 0.0001 (2.9∙10-10) *** |
| C2 | 12.9 | 1 | 12.9 | 1.51 | 0.2324 |
| B∙C | 601.2 | 1 | 601.2 | 70.47 | < 0.0001 (3.8 ∙10-8) *** |
| Residuals | 179.1 | 21 | 8.5 | ||
| R2 = 0.963, R2adj = 0.955, R2pred = 0.937 | |||||
| PUAE | |||||
| Model | 97.08 | < 0.0001 (8.8∙10-14) *** | |||
| B | 3595.5 | 1 | 3595.5 | 349.98 | < 0.0001 (1.4∙10-14) *** |
| C | 38.1 | 1 | 38.1 | 3.71 | 0.0678 |
| B2 | 1156.4 | 1 | 1156.4 | 112.56 | < 0.0001 (6.8∙10-10) *** |
| C2 | 172.4 | 1 | 172.4 | 16.78 | 0.0005 *** |
| B∙C | 24.4 | 1 | 24.4 | 2.38 | 0.1381 |
| Residuals | 215.7 | 21 | 10.3 | ||
| R2 = 0.959, R2adj = 0.949, R2pred = 0.929 | |||||
* Significant at p ≤ 0.05.
** Significant at p ≤ 0.01.
*** Significant at p ≤ 0.001.
3.1.2.2. Response surface analysis
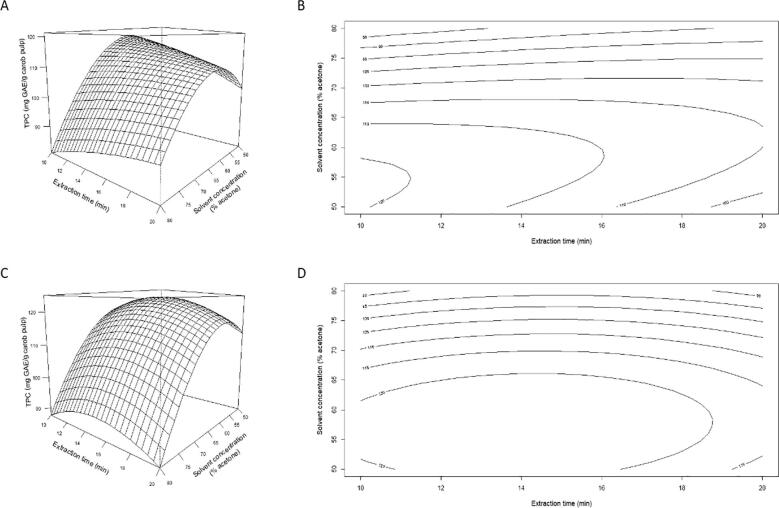
In the present study, in order to visualize the relationship between independent and dependent variables, response surface and contour plots were generated on the basis of the acquired polynomial equations (Eqs. (1), (2)). Fig. 3 illustrates the response surfaces plotted to represent the relationship between the TPC of unripe carob pulp and the examined variables, solvent concentration and extraction time, by use of both CUAE and PUAE.
Fig. 3.
Response surface and contour plots demonstrating the effects of solvent concentration and extraction time on TPC using (A, B) CUAE and (C, D) PUAE.
The features of the obtained graphs suggest that the recovery of polyphenols from unripe carob pulp can be optimized by proper selection of the experimental conditions. In the case of CUAE, TPC was initially increased upon the raise of solvent concentration, and reached a maximum value, after which it started to decrease with a further increase in solvent concentration. At the upper and medium region of acetone concentration, the recovery of polyphenols was enhanced markedly upon an increase of extraction time level, reached a peak value, and then, it was slightly reduced, probably due to the decomposition or oxidation of the sensitive phytochemical constituents. In regard to the influence of extraction time at the lower region of acetone concentration, the TPC was decreased gradually with the growth of ultrasonication processing time. In addition, it is obvious from Fig. 3B that the mutual interaction between solvent concentration and extraction time was highly significant due to the elliptical contour plot obtained. In particular, different shapes of contour plots indicate whether the mutual interactions between the studied variables are significant. Circular contour plots mean that the interaction between the corresponding variables are negligible, while elliptical contour plots suggest that the interactions have a significant effect on the response [34]. In the case of PUAE, the solvent concentration and extraction time have a positive influence on the extraction yield until a maximum value is reached at 57% (v/v) of acetone and 14 min of extraction time. Above these optimum values though, TPC is negatively affected by the growth of both acetone concentration and extraction time. The obtained response surface and contour plots for both extraction modes indicate that the optimum recovery of polyphenols occurs with a relatively low percentage of acetone in water as well as a short time of extraction.
3.1.2.3. Optimization and model validation
Numerical optimizations were performed in order to determine the optimum level of each independent variable. According to the regression model of CUAE, the optimum conditions for obtaining maximum total phenolic yield were as follows: an extraction solvent containing 54% (v/v) of acetone in water and an extraction time of 10 min, for a solid-solvent ratio and a sonication amplitude of 1:25 and 50%, respectively. In the case of PUAE, the optimal conditions were quite similar: 57% (v/v) of acetone in water and 14 min of extraction, again for a solid-solvent ratio of 1:25 and a sonication amplitude of 50%. Although the optimal conditions obtained for both extraction modes were almost the same, PUAE resulted in the most desirable response, indicating once again its superiority over continuous extraction.
To verify the reliability of the response surface models for quantitative predictions, experiments on estimated optimal conditions were performed. As observed, the experimental results (TPCCUAE = 116.56 ± 2.39 mg GAE/g carob pulp, TPCPUAE = 121.53 ± 0.82 mg GAE/g carob pulp) were in close agreement with the predicted values (TPCCUAE = 121.13 mg GAE/g carob pulp, TPCPUAE = 124.93 mg GAE/g carob pulp), with a percentage error of <3.92 and 2.80% for CUAE and PUAE, respectively. The good correlation between the experimental and predicted values confirms the effectiveness and validity of the response surface models to reflect the response values and, consequently, to determine the best extraction conditions.
3.2. Comparison of the optimized extraction procedure with conventional extraction techniques
A comparison study was performed between the proposed optimal extraction method (PUAE) and the classical extraction techniques in order to estimate and validate the efficiency of ultrasounds on antioxidants extraction. In the first stage of experiments, the specific impact of ultrasounds was established by comparing the polyphenols’ yields achieved, under the proposed optimized PUAE method (121.53 ± 0.82 mg GAE/g carob pulp), with recoveries obtained by use of a conventional procedure performed under the exact same experimental conditions, but without the presence of ultrasounds (stirring the solid plant material with 57%, v/v, of acetone for 14 min) (95.35 ± 5.84 mg GAE/g carob pulp). It is worth mentioning here that this conventional procedure (agitation extraction) was performed only for comparison studies and the parameters used were not optimized. It is possible to observe from the results obtained that the recovery of polyphenols was greatly enhanced by the use of ultrasonic waves. In particular, the UAE technique increased TPC by more than 25%. This is in agreement with results obtained in other studies that reported the greater efficiency of UAE technique over conventional extraction methods, in terms of both increased phenolic yields and considerably shortened extraction times [35], [36].
Soxhlet apparatus was also used as a conventional method of extraction in order to evaluate the behaviour of polyphenols in different extraction systems. Recoveries of total phenolics obtained by the Soxhlet approach (18.31 ± 0.67 mg GAE/g carob pulp), performed using the same solvent system (57%, v/v, of acetone) but for a considerably longer period of time (6 h), demonstrated the inadequacy of the method for extracting these bioactive substances. Polyphenolic compounds may be more easily degraded under the high temperatures of Soxhlet extraction [35]. Taking into consideration the massive use of solvents, the duration of the process and the extremely low levels of TPC obtained, it was concluded that Soxhlet extraction is not an appropriate method for polyphenols isolation. The results of the recent study confirm that UAE is undoubtedly more effective compared to classical extraction techniques.
3.3. Changes in phenolic content during maturation
During ripening, a series of complex biochemical reactions occur, including degradation or synthesis and accumulation of bioactive components. Thus, the levels of health-related phytochemicals are affected, and this, in turn, leads to the development of the final characteristics of the mature fruit [37], [38]. In the present study, it was proven that the ripening process has a remarkable effect on polyphenolic composition. In particular, a significant reduction of polyphenols, which are of exceptionally high biological activity and an important index of fruit quality, were recorded during the ripening period. The highest levels of TPC were reached at the unripe stage (121.53 ± 0.82 mg GAE/g carob pulp or 180.99 ± 0.77 mg GAE/g carob pulp extract), and were then gradually decreased to attain the lowest levels, at the end of the maturity (14.24 ± 0.17 mg GAE/g carob pulp or 21.57 ± 0.32 mg GAE/g carob pulp extract). The observed diminution in TPC, which reached the rate of 88%, could be attributed to the oxidation of phenolic compounds by polyphenol oxidase enzyme and/or to the conversion of soluble phenolic compounds into insoluble ones, which are bound to polysaccharides in the cell wall and are not free to dissolve into the solvent [37].
Based on the results, immature carobs should be preferred, as they are more enriched in polyphenols and are likely to exhibit stronger antioxidant, anti-inflammatory, antidiabetic, cardioprotective, and anticancer effects. The observed progressive pattern was in agreement with the findings reported by Benchikh et al. (2014), Ouzounidou et al. (2012), and Saci et al. (2020), who also mentioned that polyphenolic content of carobs declined gradually with ripening, revealing significant loss rates ranging from 83 to 93% [37], [38], [39], [40].
Data on carob pod total phenolic is scarce in literature, and, as it is proven, it varies according to several parameters, namely, extraction method, geographical origin, variety, conditions of cultivation, and degree of maturation [4]. This makes the quantitative comparison of results from different studies a very difficult task. The TPC recorded, in this study, for the unripe carob pulp was markedly higher than those reported by Sebai et al. (2013) (28.07 ± 0.99 mg GAE/kg carob pulp) and Qasem et al. (2018) (127.02 ± 7.18 mg GAE/g dry extract) for extracts of immature carobs, but, at the same time, lower than those found by Benchikh et al. (2014) (19.82 ± 0.48 g GAE/100 g carob dry weight) and Saci et al. (2020) (258.55 ± 2.57 mg GAE/g dry matter) [37], [38], [41], [42]. More recently, Ydjedd et al. (2017) reported the content of 162.55 ± 5.44 mg GAE/g dry extract for immature carobs grown in Algeria, a value that is closed to the one reported in the present study [43]. As already mentioned, TPC decreased gradually to attain the lowest levels, at the end of the ripening process. However, the obtained TPC (14.24 ± 0.17 mg GAE/g carob pulp or 21.57 ± 0.32 mg GAE/g carob pulp extract) remains higher than those reported by Papagiannopoulos et al. (2004) (8.31 g GAE/kg) and Makris and Kefalas (2004) (9.28 mg GAE/g) [44], [45]. Roseiro, Duarte et al. (2013) reported higher levels of polyphenols by use of supercritical fluid extraction (SFE) (27.1 ± 0.8 mg GAE/g extract), while the use of UAE yielded very similar results (20.4 ± 1.8 mg GAE/g extract) [4]. Although the amount of phenolics obtained from mature pods was found to be of the same order of magnitude as that previously reported in a number of related studies [37], [46], [47], it should be emphasized, once again, that the chemical composition of carob pulp depends on a variety of parameters.
3.4. Solid-phase purification-Recoveries of polyphenols for different adsorbent materials
Solid-phase extraction (SPE) methodology has been evaluated as a clean-up strategy prior to the electrophoretic analysis of phenolic compounds in carob pulp extracts. SPE procedure was developed in order to reduce the matrix effects resulting from the co-eluting residual matrix components, including sugars, pectins, proteins, and polyalcohols. All of these components are indeed known to react with the FC reagent due to their reducing character, a fact that explains the relatively high TPC obtained [48].
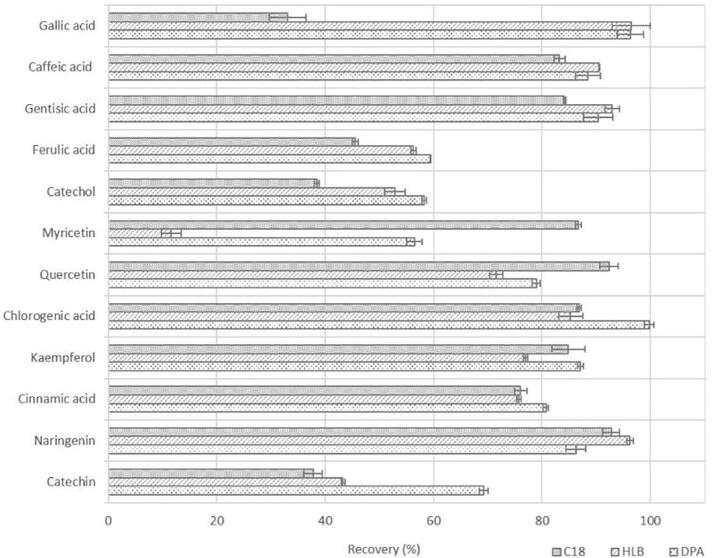
Preliminary studies were performed to select the most desirable sorbent system and sample pre-treatment. Three commercially available SPE sorbents with good retention characteristics toward the target analytes (Maxi-Clean™ C18, Oasis® HLB, Discovery® DPA-6S) were compared in terms of polyphenols recovery and elimination of interference from other endogenous constituents of carob pulp samples. Fig. 4 illustrates the values of process efficiency or recoveries, along with relative standard deviations (RSDs) obtained for the acidified standard solution submitted to different SPE procedures. At this point, it should be mentioned that the organic solvents utilized in the process of elution of the SPE cartridges were chosen after several trials with MeOH (for Maxi-Clean™ C18 and Oasis® HLB) and acetone (for Discovery® DPA-6S) or their mixtures with water. Given the acidic character of phenolic compounds, acidification of samples prior to SPE was deemed to be necessary in order to minimise their ionization and enhance the efficiency of the process. In the course of the optimisation, it was concluded that Discovery® DPA-6S cartridges were the most suitable for the analysis of the target compounds. Among the examined types of cartridges, Discovery® DPA-6S demonstrated the best ability to recuperate the target analytes with high recovery values (56.45–99.80%) and acceptable RSDs (lower than 2.65%). Polyamide resin sorbent (DPA-6S) is usually used to absorb polar components (mostly polyphenols) through hydrogen bonding between compound hydroxyl groups and amide groups of the resin.
Fig. 4.
Recoveries of examined polyphenolic compounds on different SPE sorbents.
On the other hand, polyphenols have a much lower affinity for Maxi-Clean™ C18 and Oasis® HLB sorbent systems. Although C18 sorbent material was widely used in polyphenolic compound separation, in the present study, it did not provide acceptable phenolic yields. As observed, some analytes, and specifically the more polar ones, exhibited extremely low affinity toward the C18 sorbent material. This kind of adsorbent material has a strong hydrophobicity, ideal to extract non-polar compounds via non-polar Van der Waals interactions (dispersion forces), demonstrating reduced yields for the highly polar constituents [49]. In the case of Oasis® HLB cartridges, the two monomers of their chemical structure (divinylbenzene and N-vinylpyrrolidone) confer superior lipophilic and hydrophilic retention capacities, resulting in better extraction yields in comparison to Maxi-Clean™ C18 [49]. Michalkiewicz et al. (2008) reported that the better performance of polymeric sorbents (HLB) is attributed to their aromatic structures that can sorb phenolic compounds via π-π interactions [50].
3.5. Electrophoretic analysis of ripe and unripe carob pulp extracts
Although spectrophotometric assays allow fast screening of different samples for the qualitative and quantitative determination of antioxidant components, they are usually prone to overestimate the phenolic content by cross-reactions with other reducing agents (like sugars) [44]. Therefore, the identification of the type and content of phenolic compounds present in carob pulp extracts by an electrophoretic method was considered to be essential.
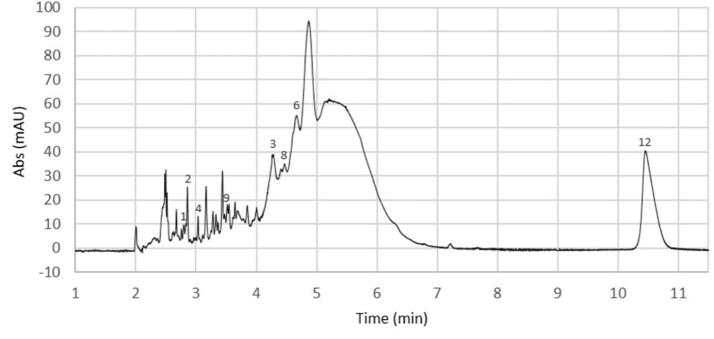
Identification and quantification of polyphenols in the optimised carob pulp extracts were carried out using CE-DAD instrumentation, in order to pinpoint the individual polyphenolic components that contribute to the TPC. The phenolic profile of ripe carob pulp extract, as obtained under the determined optimum extraction conditions, is demonstrated in Fig. 5. Carob pulp was found to contain a rich variety of phenolic antioxidants. Several peaks were observed, in both cases, due to the large number of different phytochemicals present in plant material. The developed electrokinetic chromatographic (EKC) method enabled the identification and quantification of eight phenolic compounds in mature carob pulp extracts. The substances identified comprise catechin, naringenin, cinnamic acid, quercetin, catechol, ferulic acid, gentisic acid, and gallic acid. Similarly, eight phenolic substances were also detected in the extract obtained from unripe carob pulp, but only seven polyphenols were quantified (chlorogenic acid, quercetin, myricetin, catechol, gentisic acid, caffeic acid, and gallic acid). Catechin was identified but not quantified due to its coelution with another component. Unfortunately, a number of other constituents present in carob pulp extracts could not be identified.
Fig. 5.
Electropherogram of ripe carob pulp extract obtained under the optimum separation conditions (35 mM borate, 15 mM β-CD, 3 mM L-AlaC4Lac, pH 9.5, 30 kV).
The quantitative data of the analysed compounds is reported in Table 3. As observed earlier in TPC analysis, the unripe carob pulp extract revealed a higher amount of phenolics (912.58 μg/g carob pulp) than the fraction obtained from ripe pods (283.13 μg/g carob pulp). According to Ydjedd et al. (2017), the high content of phenolics recorded at the unripe stage is a result of the effort of the plant to protect itself against biotic and/or abiotic environmental hazards during its growing [43]. Gallic acid was found to be the most abundant phenolic compound in both ripe and unripe carob pulp extracts. Gentisic acid, caffeic acid, chlorogenic acid, and quercetin demonstrated a significant contribution to the phenolic content of unripe pulp extract (21%, 18%, 15%, 13%, respectively). These substances though were found in small or even negligible amounts in extracts obtained from mature pods. With respect to ripe pulp extract, the phenolic profile was dominated by gallic acid (65%).
Table 3.
Quantitative data from the EKC-DAD analysis of ripe and unripe carob pulp extracts.
| Compound | Concentration (μg/g carob pulp) |
% Content |
||
|---|---|---|---|---|
| Ripe | Unripe | Ripe | Unripe | |
| Catechin | 4.63 ± 0.16 | n.q. | 2 | 0 |
| Naringenin | 12.79 ± 0.33 | n.d. | 5 | 0 |
| Cinnamic acid | 9.74 ± 0.20 | n.d. | 3 | 0 |
| Kaempferol | n.d. | n.d. | 0 | 0 |
| Chlorogenic acid | n.d. | 135.44 ± 1.89 | 0 | 15 |
| Quercetin | 19.84 ± 0.70 | 122.32 ± 1.81 | 7 | 13 |
| Myricetin | n.d. | 60.80 ± 1.58 | 0 | 7 |
| Catechol | 11.99 ± 0.62 | 33.86 ± 0.82 | 4 | 4 |
| Ferulic acid | 9.18 ± 0.79 | n.d. | 3 | 0 |
| Gentisic acid | 31.03 ± 1.03 | 190.85 ± 2.66 | 11 | 21 |
| Caffeic acid | n.d. | 164.20 ± 2.83 | 0 | 18 |
| Gallic acid | 183.92 ± 3.50 | 205.10 ± 1.77 | 65 | 22 |
| Total | 283.13 | 912.58 | 100 | 100 |
a) non-detected.
b) non-quantified.
In comparison with other investigations, remarkable differences were observed in terms of identified phenolics and respective quantification. Nonetheless, most of the studies performed highlighted the predominance of gallic acid in carob’s pods, leaves, bark, and derived products, which is in agreement with the present findings [5], [16], [17], [51], [52], [53], [54]. Different polyphenolic patterns were obtained during the analysis of ripe and unripe carob pulp extracts in a number of previous investigations [43], [55], [56], highlighting once again the great influence of the ripening stage on carobs polyphenolic composition. According to the literature, the phenolic profile of an extract could vary, not only due to the gender, cultivar, and geographical origin, but also due to the processes used for preparation, extraction and analysis [10]. Processing method (e.g. roasting and sugar removal) is also another factor that strongly influences the phenolic composition of carob-derived products [44], [57], [58], resulting in significant differences among the literature and also in non-comparable results.
4. Conclusions
In this work, a non-conventional extraction process, namely UAE, was successfully optimized for the extraction of natural antioxidants from the unripe carob pulp using RSM in conjunction with a full factorial design. The effects of solid-solvent ratio, solvent concentration, extraction time, sonication amplitude, and sonication mode (continuous or pulsed) were evaluated and optimized for the maximization of the yield in total phenolics. Among the examined variables, the solvent concentration was found to be the most influential parameter, significantly affecting the extraction efficiency. The optimal extraction conditions obtained were quite similar for both continuous and pulsed modes. In particular, the optimum conditions for obtaining maximum total phenolic yield using CUAE were as follows: an extraction solvent containing 54% (v/v) of acetone in water and an extraction time of 10 min, for a solid-solvent ratio and a sonication amplitude of 1:25 and 50%, respectively. In the case of PUAE, the optimal conditions were achieved using 57% (v/v) of acetone in water and 14 min of extraction, again for a solid-solvent ratio of 1:25 and a sonication amplitude of 50%. Additionally, when compared to conventional extraction techniques, PUAE offered significantly higher extraction yields and markedly reduced processing times. Consequently, this process can be considered a simple, economic, and efficient method for the extraction of the desired natural antioxidants form carob samples, as it allows simplified handling, time reduction, and improvement in the quantitation of the target components.
Prior to the electrophoretic analysis of the optimized carob pulp extracts, a SPE methodology was also evaluated as a clean-up strategy in order to reduce the matrix effects resulting from co-eluting residual matrix components. Among the examined types of cartridges, DPA-6S demonstrated the best ability to recuperate the target analytes with high recovery values and acceptable RSDs. Finally, the phenolic compositions of the optimally obtained and purified extracts were determined by use of the developed electrophoretic method. The results revealed the predominance of gallic acid in both ripe and unripe carob pulp extracts and highlighted the great influence of the ripening stage on carob’s polyphenolic composition. Extracts obtained from unripe carob pods were more enriched in polyphenols, indicating that unripe carobs can be considered a functional nutrient and an excellent source of natural antioxidants.
CRediT authorship contribution statement
Atalanti Christou: Investigation, Writing - original draft, Methodology, Validation. Ioannis J. Stavrou: Formal analysis, Conceptualization. Constantina P. Kapnissi-Christodoulou: Supervision, Project administration, Conceptualization, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was co-funded by the European Regional Development Fund and the Republic of Cyprus through the Research & Innovation Foundation, Cyprus (Project: BlackGold INTEGRATED /0916/0019).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2021.105630.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Ydjedd S., Bouriche S., López-Nicolás R., Sánchez-Moya T., Frontela-Saseta C., Ros-Berruezo G., Rezgui F., Louaileche H., Kati D.-E. Effect of in vitro gastrointestinal digestion on encapsulated and nonencapsulated phenolic compounds of carob (Ceratonia siliqua L.) pulp extracts and their antioxidant capacity. J. Agric. Food Chem. 2017;65(4):827–835. doi: 10.1021/acs.jafc.6b05103. [DOI] [PubMed] [Google Scholar]
- 2.van Rijs P., Fogliano V. Roasting carob flour decreases the capacity to bind glycoconjugates of bile acids. Food Funct. 2020;11(7):5924–5932. doi: 10.1039/D0FO01158D. [DOI] [PubMed] [Google Scholar]
- 3.Rico D., Martín-Diana A.B., Martínez-Villaluenga C., Aguirre L., Silván J.M., Dueñas M., De Luis D.A., Lasa A. In vitro approach for evaluation of carob by-products as source bioactive ingredients with potential to attenuate metabolic syndrome (MetS) Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e01175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roseiro L.B., Duarte L.C., Oliveira D.L., Roque R., Bernardo-Gil M.G., Martins A.I., Sepúlveda C., Almeida J., Meireles M., Gírio F.M., Rauter A.P. Supercritical, ultrasound and conventional extracts from carob (Ceratonia siliqua L.) biomass: effect on the phenolic profile and antiproliferative activity. Ind. Crops Prod. 2013;47:132–138. doi: 10.1016/j.indcrop.2013.02.026. [DOI] [Google Scholar]
- 5.Dhaouadi K., Belkhir M., Akinocho I., Raboudi F., Pamies D., Barrajón E., Estevan C., Fattouch S. Sucrose supplementation during traditional carob syrup processing affected its chemical characteristics and biological activities. LWT – Food Sci. Technol. 2014;57(1):1–8. doi: 10.1016/j.lwt.2014.01.025. [DOI] [Google Scholar]
- 6.Ghanemi F.Z., Belarbi M., Fluckiger A., Nani A., Dumont A., De Rosny C., Aboura I., Khan A.S., Murtaza B., Benammar C., Lahfa B.F., Patoli D., Delmas D., Rébé C., Apétoh L., Khan N.A., Ghringhelli F., Rialland M., Hichami A. Carob leaf polyphenols trigger intrinsic apoptotic pathway and induce cell cycle arrest in colon cancer cells. J. Funct. Foods. 2017;33:112–121. doi: 10.1016/j.jff.2017.03.032. [DOI] [Google Scholar]
- 7.Roseiro L.B., Tavares C.S., Roseiro J.C., Rauter A.P. Antioxidants from aqueous decoction of carob pods biomass (Ceretonia siliqua L.): optimisation using response surface methodology and phenolic profile by capillary electrophoresis. Ind. Crops Prod. 2013;44:119–126. doi: 10.1016/j.indcrop.2012.11.006. [DOI] [Google Scholar]
- 8.Custódio L., Fernandes E., Escapa A.L., Fajardo A., Aligué R., Alberício F., Neng N.R., Nogueira J.M.F., Romano A. Antioxidant and cytotoxic activities of carob tree fruit pulps are strongly influenced by gender and cultivar. J. Agric. Food Chem. 2011;59(13):7005–7012. doi: 10.1021/jf200838f. [DOI] [PubMed] [Google Scholar]
- 9.Huma Z.-E., Jayasena V., Nasar‐Abbas S.M., Imran M., Khan M.K. Process optimization of polyphenol extraction from carob (Ceratonia siliqua) kibbles using microwave-assisted technique. J. Food Process. Preserv. 2018;42(2):e13450. doi: 10.1111/jfpp.2018.42.issue-210.1111/jfpp.13450. [DOI] [Google Scholar]
- 10.Stavrou I.J., Christou A., Kapnissi-Christodoulou C.P. Polyphenols in carobs: a review on their composition, antioxidant capacity and cytotoxic effects, and health impact. Food Chem. 2018;269:355–374. doi: 10.1016/j.foodchem.2018.06.152. [DOI] [PubMed] [Google Scholar]
- 11.Wang J., Sun B., Cao Y., Tian Y., Li X. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008;106(2):804–810. doi: 10.1016/j.foodchem.2007.06.062. [DOI] [Google Scholar]
- 12.Ghitescu R.E., Volf I., Carausu C., Bühlmann A.M., Gilca I.A., Popa V.I. Optimization of ultrasound-assisted extraction of polyphenols from spruce wood bark. Ultrason. Sonochem. 2015;22:535–541. doi: 10.1016/j.ultsonch.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Ramić M., Vidović S., Zeković Z., Vladić J., Cvejin A., Pavlić B. Modeling and optimization of ultrasound-assisted extraction of polyphenolic compounds from Aronia melanocarpa by-products from filter-tea factory. Ultrason. Sonochem. 2015;23:360–368. doi: 10.1016/j.ultsonch.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Ajila C.M., Brar S.K., Verma M., Tyagi R.D., Godbout S., Valéro J.R. Extraction and analysis of polyphenols: recent trends. Crit. Rev. Biotechnol. 2011;31(3):227–249. doi: 10.3109/07388551.2010.513677. [DOI] [PubMed] [Google Scholar]
- 15.F. Saci, Y. Benchikh, H. Louaileche, M. Bachir, Received on 25, 42 (2018) 26–39.
- 16.Goulas V., Georgiou E. Utilization of carob fruit as sources of phenolic compounds with antioxidant potential: extraction optimization and application in food models. Foods. 2020;9(1):20. doi: 10.3390/foods9010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hadrich B., Dimitrov K., Kriaa K. Modelling investigation and parameters study of polyphenols extraction from carob (Ceratonia siliqua L.) using experimental factorial design. J. Food Process. Preserv. 2017;41(2):e12769. doi: 10.1111/jfpp.2017.41.issue-210.1111/jfpp.12769. [DOI] [Google Scholar]
- 18.Ciric A., Krajnc B., Heath D., Ogrinc N. Response surface methodology and artificial neural network approach for the optimization of ultrasound-assisted extraction of polyphenols from garlic. Food Chem. Toxicol. 2020;135:110976. doi: 10.1016/j.fct.2019.110976. [DOI] [PubMed] [Google Scholar]
- 19.Ismail B.B., Guo M., Pu Y., Wang W., Ye X., Liu D. Valorisation of baobab (Adansonia digitata) seeds by ultrasound assisted extraction of polyphenolics. Optimisation and comparison with conventional methods. Ultrason. Sonochem. 2019;52:257–267. doi: 10.1016/j.ultsonch.2018.11.023. [DOI] [PubMed] [Google Scholar]
- 20.Silva E., Rogez H., Larondelle Y. Optimization of extraction of phenolics from Inga edulis leaves using response surface methodology. Sep. Purif. Technol. 2007;55(3):381–387. doi: 10.1016/j.seppur.2007.01.008. [DOI] [Google Scholar]
- 21.Tabaraki R., Heidarizadi E., Benvidi A. Optimization of ultrasonic-assisted extraction of pomegranate (Punica granatum L.) peel antioxidants by response surface methodology. Sep. Purif. Technol. 2012;98:16–23. doi: 10.1016/j.seppur.2012.06.038. [DOI] [Google Scholar]
- 22.Almanasrah M., Roseiro L.B., Bogel-Lukasik R., Carvalheiro F., Brazinha C., Crespo J., Kallioinen M., Mänttäri M., Duarte L.C. Selective recovery of phenolic compounds and carbohydrates from carob kibbles using water-based extraction. Ind. Crops Prod. 2015;70:443–450. doi: 10.1016/j.indcrop.2015.02.051. [DOI] [Google Scholar]
- 23.Almanasrah M., Brazinha C., Kallioinen M., Duarte L.C., Roseiro L.B., Bogel-lukasik R., Carvalheiro F., Mänttäri M., Crespo J.G. Nanofiltration and reverse osmosis as a platform for production of natural botanic extracts: the case study of carob by-products. Sep. Purif. Technol. 2015;149:389–397. doi: 10.1016/j.seppur.2015.06.008. [DOI] [Google Scholar]
- 24.Stavrou I.J., Kapnissi-Christodoulou C.P. Use of chiral amino acid ester-based ionic liquids as chiral selectors in CE. Electrophoresis. 2013;34(4):524–530. doi: 10.1002/elps.v34.410.1002/elps.201200469. [DOI] [PubMed] [Google Scholar]
- 25.Horwitz W., Latimer G.W. 18th ed. AOAC International; Gaithersburg, MD: 2007. AOAC International 2007, Official methods of analysis of AOAC International; p. 2005. [Google Scholar]
- 26.Singleton V.L., Rossi J.A., Jr J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16:144–158. [Google Scholar]
- 27.Alberti A., Zielinski A.A.F., Zardo D.M., Demiate I.M., Nogueira A., Mafra L.I. Optimisation of the extraction of phenolic compounds from apples using response surface methodology. Food Chem. 2014;149:151–158. doi: 10.1016/j.foodchem.2013.10.086. [DOI] [PubMed] [Google Scholar]
- 28.Prasad K.N., Kong K.W., Ramanan R.N., Azlan A., Ismail A. Selection of experimental domain using two-level factorial design to determine extract yield, antioxidant capacity, phenolics, and flavonoids from Mangifera pajang Kosterm. Sep. Sci. Technol. 2012;47:2417–2423. doi: 10.1080/01496395.2012.672511. [DOI] [Google Scholar]
- 29.Mahindrakar K.V., Rathod V.K. Ultrasonic assisted aqueous extraction of catechin and gallic acid from Syzygium cumini seed kernel and evaluation of total phenolic, flavonoid contents and antioxidant activity. Chem. Eng. Process. – Process Intensif. 2020;149:107841. doi: 10.1016/j.cep.2020.107841. [DOI] [Google Scholar]
- 30.Brás T., Paulino A.F.C., Neves Luísa A., Crespo João G., Duarte M.F. Ultrasound assisted extraction of cynaropicrin from Cynara cardunculus leaves: optimization using the response surface methodology and the effect of pulse mode. Ind. Crops Prod. 2020;150:112395. doi: 10.1016/j.indcrop.2020.112395. [DOI] [Google Scholar]
- 31.Jovanović A.A., Đorđević V.B., Zdunić G.M., Pljevljakušić D.S., Šavikin K.P., Gođevac D.M., Bugarski B.M. Optimization of the extraction process of polyphenols from Thymus serpyllum L. herb using maceration, heat- and ultrasound-assisted techniques. Sep. Purif. Technol. 2017;179:369–380. doi: 10.1016/j.seppur.2017.01.055. [DOI] [Google Scholar]
- 32.Zhou Y., Zheng J., Gan R.Y., Zhou T., Xu D.P., Bin Li H. Optimization of ultrasound-assisted extraction of antioxidants from the mung bean coat. Molecules. 2017;22:1–13. doi: 10.3390/molecules22040638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chavan Y., Singhal R.S. Ultrasound-assisted extraction (UAE) of bioactives from arecanut (Areca catechu L.) and optimization study using response surface methodology. Innov. Food Sci. Emerg. Technol. 2013;17:106–113. doi: 10.1016/j.ifset.2012.10.001. [DOI] [Google Scholar]
- 34.Liu Y., Wei S., Liao M. Optimization of ultrasonic extraction of phenolic compounds from Euryale ferox seed shells using response surface methodology. Ind. Crop. Prod. 2013;49:837–843. doi: 10.1016/j.indcrop.2013.07.023. [DOI] [Google Scholar]
- 35.D.P. Xu, J. Zheng, Y. Zhou, Y. Li, S. Li, H. Bin Li, Ultrasound-assisted extraction of natural antioxidants from the flower of Limonium sinuatum: optimization and comparison with conventional methods, Food Chem. 217 (2017) 552–559. https://doi.org/10.1016/j.foodchem.2016.09.013. [DOI] [PubMed]
- 36.Luo X., Cui J., Zhang H., Duan Y., Zhang D., Cai M., Chen G. Ultrasound assisted extraction of polyphenolic compounds from red sorghum (Sorghum bicolor L.) bran and their biological activities and polyphenolic compositions. Ind. Crop. Prod. 2018;112:296–304. doi: 10.1016/j.indcrop.2017.12.019. [DOI] [Google Scholar]
- 37.Benchikh Y., Louaileche H., George B., Merlin A. Changes in bioactive phytochemical content and in vitro antioxidant activity of carob (Ceratonia siliqua L.) as influenced by fruit ripening. Ind. Crops Prod. 2014;60:298–303. doi: 10.1016/j.indcrop.2014.05.048. [DOI] [Google Scholar]
- 38.Saci F., Bachir bey M., Louaileche H., Gali L., Bensouici C. Changes in anticholinesterase, antioxidant activities and related bioactive compounds of carob pulp (Ceratonia siliqua L.) during ripening stages. J. Food Meas. Charact. 2020;14(2):937–945. doi: 10.1007/s11694-019-00344-9. [DOI] [Google Scholar]
- 39.Ouzounidou G., Vekiari S., Asfi M., Gork M.G., Sakcali M.S., Ozturk M. Photosynthetic characteristics of carob tree (Ceratonia siliqua L.) and chemical composition of its fruit on diurnal and seasonal basis. Pakistan J. Bot. 2012;44:1689–1695. [Google Scholar]
- 40.Benchikh Y., Louailèche H. Effects of extraction conditions on the recovery of phenolic compounds and in vitro antioxidant activity of carob (Ceratonia siliqua L.) pulp. Acta Bot. Gall. 2014;161(2):175–181. doi: 10.1080/12538078.2014.909325. [DOI] [Google Scholar]
- 41.Sebai H., Souli A., Chehimi L., Rtibi K., Amri M. In vitro and in vivo antioxidant properties of Tunisian carob (Ceratonia siliqua L.) J. Med. Plants Res. 2013;7:85–90. doi: 10.5897/JMPR12.915. [DOI] [Google Scholar]
- 42.M.A. Qasem, M.I. Noordin, A. Arya, A. Alsalahi, S.N. Jayash, Evaluation of the glycemic effect of Ceratonia siliqua pods (Carob) on a streptozotocin-nicotinamide induced diabetic rat model, PeerJ 2018 (2018) 1–27. https://doi.org/10.7717/peerj.4788. [DOI] [PMC free article] [PubMed]
- 43.Ydjedd S., Chaalal M., Richard G., Kati D.E., López-Nicolás R., Fauconnier M.L., Louaileche H. Assessment of antioxidant potential of phenolic compounds fractions of Algerian Ceratonia siliqua L. pods during ripening stages. Int. Food Res. J. 2017;24:2041–2049. [Google Scholar]
- 44.Papagiannopoulos M., Wollseifen H.R., Mellenthin A., Haber B., Galensa R. Identification and quantification of polyphenols in carob fruits (Ceratonia siliqua L.) and derived products by HPLC-UV-ESI/MSn. J. Agric. Food Chem. 2004;52:3784–3791. doi: 10.1021/jf030660y. [DOI] [PubMed] [Google Scholar]
- 45.Makris D.P., Kefalas P. Carob pods (Ceratonia siliqua L.) As a source of polyphenolic antioxidants. Food Technol. Biotechnol. 2004;42:105–108. [Google Scholar]
- 46.Avallone R., Plessi M., Baraldi M., Monzani A. Determination of chemical composition of carob (Ceratonia siliqua): protein, fat, carbohydrates, and tannins. J. Food Compos. Anal. 1997;10(2):166–172. doi: 10.1006/jfca.1997.0528. [DOI] [Google Scholar]
- 47.Makris D.P., Boskou G., Andrikopoulos N.K. Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts. J. Food Compos. Anal. 2007;20(2):125–132. doi: 10.1016/j.jfca.2006.04.010. [DOI] [Google Scholar]
- 48.Jerman Klen T., Mozetič Vodopivec B. Ultrasonic extraction of phenols from olive mill wastewater: comparison with conventional methods. J. Agric. Food Chem. 2011;59:12725–12731. doi: 10.1021/jf202800n. [DOI] [PubMed] [Google Scholar]
- 49.Pascual-Maté A., Osés S.M., Fernández-Muiño M.A., Sancho M.T. Analysis of polyphenols in honey: extraction, separation and quantification procedures. Sep. Purif. Rev. 2018;47(2):142–158. doi: 10.1080/15422119.2017.1354025. [DOI] [Google Scholar]
- 50.Michalkiewicz A., Biesaga M., Pyrzynska K. Solid-phase extraction procedure for determination of phenolic acids and some flavonols in honey. J. Chromatogr. A. 2008;1187(1-2):18–24. doi: 10.1016/j.chroma.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 51.Cavdarova M., Makris D.P. Extraction kinetics of phenolics from carob (Ceratonia siliqua L.) kibbles using environmentally benign solvents. Waste Biomass Valoriz. 2014;5(5):773–779. doi: 10.1007/s12649-014-9298-3. [DOI] [Google Scholar]
- 52.Chait Y.A., Gunenc A., Bendali F., Hosseinian F. Simulated gastrointestinal digestion and in vitro colonic fermentation of carob polyphenols: bioaccessibility and bioactivity. LWT. 2020;117:108623. doi: 10.1016/j.lwt.2019.108623. [DOI] [Google Scholar]
- 53.Corsi L., Avallone R., Cosenza F., Farina F., Baraldi C., Baraldi M. Antiproliferative effects of Ceratonia siliqua L. on mouse hepatocellular carcinoma cell line. Fitoterapia. 2002;73(7-8):674–684. doi: 10.1016/S0367-326X(02)00227-7. [DOI] [PubMed] [Google Scholar]
- 54.Quiles-Carrillo L., Mellinas C., Garrigos M.C., Balart R., Torres-Giner S. Optimization of microwave-assisted extraction of phenolic compounds with antioxidant activity from carob pods. Food Anal. Methods. 2019;12(11):2480–2490. doi: 10.1007/s12161-019-01596-3. [DOI] [Google Scholar]
- 55.Benchikh Y., Paris C., Louaileche H., Charbonnel M., Céline G., Chebil L. Comparative characterization of green and ripe carob (Ceratonia siliqua L.): physicochemical attributes and phenolic profile, SDRP. J. Food Sci. Technol. 2016;1:85–91. [Google Scholar]
- 56.Farag M.A., El-Kersh D.M., Ehrlich A., Choucry M.A., El-Seedi H., Frolov A., Wessjohann L.A. Variation in Ceratonia siliqua pod metabolome in context of its different geographical origin, ripening stage and roasting process. Food Chem. 2019;283:675–687. doi: 10.1016/j.foodchem.2018.12.118. [DOI] [PubMed] [Google Scholar]
- 57.Ortega Nàdia, Macià A., Romero M.-P., Trullols E., Morello J.-R., Anglès N., Motilva M.-J. Rapid determination of phenolic compounds and alkaloids of carob flour by improved liquid chromatography tandem mass spectrometry. J. Agric. Food Chem. 2009;57(16):7239–7244. doi: 10.1021/jf901635s. [DOI] [PubMed] [Google Scholar]
- 58.Owen R.W., Haubner R., Hull W.E., Erben G., Spiegelhalder B., Bartsch H., Haber B. Isolation and structure elucidation of the major individual polyphenols in carob fibre. Food Chem. Toxicol. 2003;41(12):1727–1738. doi: 10.1016/S0278-6915(03)00200-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.