Abstract
Significance
Cellular growth arrest, associated with ‘senescence’, helps to safeguard against the accumulation of DNA damage which is often recognized as the underlying mechanism of a wide variety of age-related pathologies including cancer. Cellular senescence has also been described as a ‘double-edged sword’. In cancer, for example, the creation of an immune-suppressive milieu by senescent tumor cells through the senescence-associated secretory phenotype contributes toward carcinogenesis and cancer progression.
Recent advances
The potential for cellular senescence to confer multi-faceted effects on tissue fate has led to a rejuvenated interest in its landscape and targeting. Interestingly, redox pathways have been described as both triggers and propagators of cellular senescence, leading to intricate cross-links between both pathways.
Critical issues
In this review, we describe the mechanisms driving cellular senescence, the interface with cellular redox metabolism as well as the role that chemotherapy-induced senescence plays in secondary carcinogenesis. Notably, the role that anti-apoptotic proteins of the Bcl-2 family play in inducing drug resistance via mechanisms that involve senescence induction.
Future directions
Though the therapeutic targeting of senescent cells as cancer therapy remains in its infancy, we summarize the current development of senotherapeutics, including recognized senotherapies, as well as the repurposing of drugs as senomorphic/senolytic candidates.
Keywords: Senescence, SASP, ROS, Cancer therapy, Senolytics
List of abbreviations
- 20(S)-Rg3
20(S)-ginsenoside Rg3
- AKT
Protein kinase B
- AMPK
AMP-activated protein kinase
- ATM
Ataxia-telangiectasia mutated
- ATR
Ataxia-telangiectasia and Rad3-related
- Bcl-2
B-cell lymphoma 2
- Bcl-xL
B-cell lymphoma-extra large
- BETi
Bromodomain and extra-terminal inhibition
- C/EBP-β
CCAAT/enhancer-binding protein beta
- CCL5
Chemokine ligand 5
- CDK2
Cyclin dependent kinase 2
- CDK4
Cyclin dependent kinase 4
- CDKN2B
Cyclin Dependent Kinase Inhibitor 2B
- cGAS/STING cyclic GMP–AMP -
Stimulator of interferon genes
- COPD
Chronic obstructive pulmonary disease
- CRB1
Crumbs homolog 1
- CRBN
Cereblon
- CXCL-5
C-X-C motif chemokine 5
- DDR
DNA damage response
- DLBCL
Diffuse large B-cell lymphoma
- DNA-PK
DNA-dependent protein kinase
- DQ
Dasatinib and quercetin
- DSBs
Double-strand breaks
- DUSP4
Dual-specificity phosphatase 4
- DUSP7
Dual-specificity phosphatase 7
- ECM
Extracellular matrix
- EdU
5-Ethynyl-2′-deoxyuridine
- ERK1/2
Extracellular signal-regulated kinase 1/2
- FOXO3a
Forkhead box O3
- FRTA
Free radical theory of aging
- GSTp1
Glutathione S-transferase pi
- H2O2
Hydrogen peroxide
- HCC
Hepatocellular carcinoma
- HDAC
Histone deacetylase
- HUVECs
Human umbilical vein endothelial cells
- IL-1β
Interleukin-1β
- IL-1α
Interleukin-1α
- IL-6
Interleukin-6
- IL-8
Interleukin-8
- IPF
Idiopathic pulmonary fibrosis
- JAK1/2
Janus kinase 1/2
- JNK
c-Jun N-terminal kinase
- KCNE2
Potassium voltage gated channel subfamily E regulatory subunit 2
- LPL
Lipoprotein lipase
- MAP
Mitogen-activated protein
- MAPKAPK2
Mitogen-activated protein kinase-activated protein kinase 2
- Mcl1
Myeloid cell leukemia-1
- MDM2
Mouse double minute 2 homolog
- MFRTA
Mitochondrial free radical theory of aging
- mTOR
Mammalian target of rapamycin
- NAC
N-acetylcysteine
- NADPH
Nicotinamide adenine dinucleotide phosphate
- Nav-Gal
Galacto-conjugated navitoclax
- NF-κB
Nuclear factor kappa B
- NF1
Neurofibromatosis 1
- NHEJ
Non-homologous end joining
- Nox4
NADPH oxidase 4
- Nox1
NADPH oxidase 1
- Nrf2
Nuclear factor erythroid 2-related factor 2
- NSCLC
Non-small cell lung cancer
- O2·-
Superoxide
- OAI
Osteoarthritis initiative
- OIS
Oncogene-induced senescence
- OXR1
Oxidation resistance 1
- p14ARF
ARF tumor suppressor
- p15INK4B
Cyclin Dependent Kinase Inhibitor 2B
- p16INK4A
Cyclin-dependent kinase inhibitor 2A
- PAI-1
Plasminogen activator inhibitor-1
- PARP1
Poly-ADP ribose polymerase 1
- PDGF-AA
Platelet-derived growth factor-AA
- PI3K
Phosphoinositide 3-kinase
- PML
Promyelocytic leukemia protein
- PPAR
Peroxisome proliferator-activated receptor
- PROTAC
Proteolysis-targeting chimera
- PTEN
Phosphatase and tensin homolog
- Rb
Retinoblastoma
- ROS
Reactive oxygen species
- SA-β-gal
Senescence-associated beta-galactosidase
- SAMD
Senescence-associated mitochondrial dysfunction
- SAS
Senescence-associated secretory
- SASP
Senescence-associated secretory phenotype
- SIPS
Stress-induced premature senescence
- SiRNA
Small interfering RNA
- SIRT3/5
Sirtuin 3/5
- SRC
Proto-oncogene tyrosine-protein kinase
- SSBs
Single-strand breaks
- STAT3
Signal transducer and activator of transcription 3
- TAK1
Mitogen-activated protein kinase 7
- TGF-β
Transforming growth factor beta
- TIS
Therapy-induced senescence
- TNBC
Triple-negative breast cancer
- TNF-α
Tumor necrosis factor-alpha
- TRAF3IP2
TRAF3 interacting protein 2
- TRAF6
TNF receptor associated factor 6
- VEGF
Vascular endothelial growth factor
- Zscan1
Zinc finger and SCAN domain-containing protein 1
- γH2AX
gamma-H2A histone family member X
1. Senescence and senescence associated secretory phenotype
Leonard Hayflick demonstrated in the 1960s that primary human fibroblasts obtained from embryonic lung tissues failed to proliferate after multiple cell divisions [1]. As such, a cell population can undergo a limited number of cell divisions before entering a state of permanent growth arrest termed as senescence.
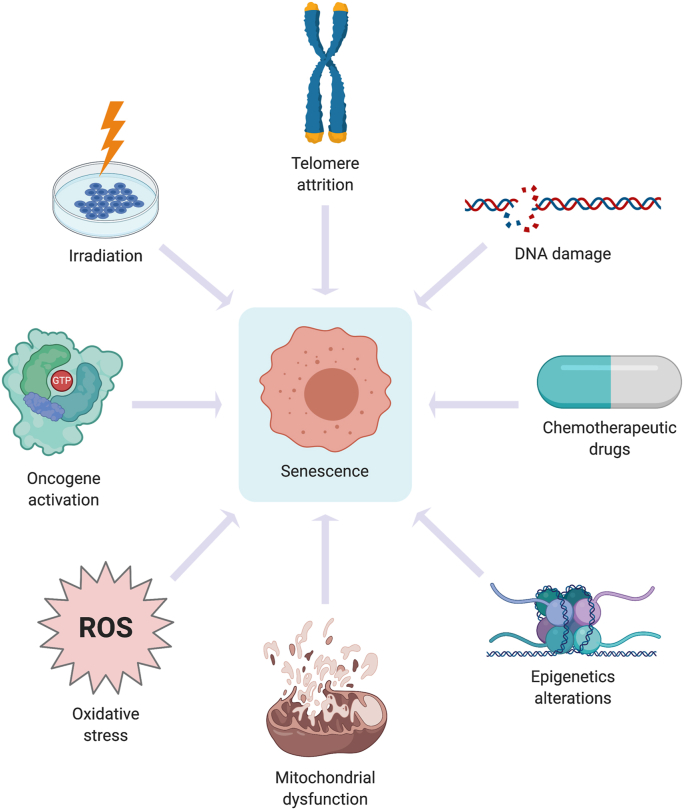
Originally attributed to telomere attrition due to multiple rounds of DNA replication as the cell ages [2], the establishment of the senescent state has also been shown following cells’ exposure to other triggers contributing to DNA damage. These triggers include oncogene activation, deregulated reactive oxygen species (ROS) production, exposure to chemotherapeutic agents, irradiation [3] and others (Fig. 1). Upon detection of single strand breaks (SSBs) and double strands breaks (DSBs) by Ataxia telangiectasia and RAD3-related (ATR) or Ataxia telangiectasia mutated (ATM) and DNA-dependent protein kinase (DNA-PK), tumor suppressor p53 is activated to trigger cell cycle arrest (senescence) and DNA repair. In the event of extensive and irreparable DNA damage, apoptosis-inducing transcriptional targets of p53 ensure apoptotic execution [[4], [5], [6]]. The induction of cell death or acquisition of senescence in response to stimuli that trigger DNA damage is necessary to prevent unwanted proliferation and maintain genomic stability [7,8]. However, apart from preventing unwanted cell proliferation, the acquisition of cellular senescence also serves important physiological function(s) such as tissue remodeling/repair during the course of embryonic development as well as in the maintenance of tissue homeostasis [9,10] (Fig. 2). These studies demonstrated that such instructive or programmed senescence is dependent on p21cip1 and coupled to macrophage-mediated clearance [9,10]. Furthermore, Demaria et al. demonstrated that endothelial and mesenchymal cells undergo senescence in response to cutaneous injury to accelerate wound healing via the secretion of a senescence-associated secretory (SAS) factor known as platelet-derived growth factor-AA (PDGF-AA) [11]. Similarly, the secretion of another SAS factor, interleukin-6 (IL-6), seems to be required for in vivo reprogramming in the context of tissue repair after skeletal muscle injury; selective elimination of senescent cells which had accumulated after skeletal muscle injury reduced reprogramming efficiency, indicating a beneficial paracrine effect of injury-induced senescence on tissue regeneration [12].
Fig. 1.
Triggers of senescence. Myriad triggers may initiate cellular senescence, such as telomere erosion, oncogene activation, deregulated ROS production, chemotherapy exposure and irradiation. All of these induce senescence through the activation of DNA damage response (DDR) pathway in the presence of DNA SSBs and DSBs. Also, stress-induced DNA damage can also result in the development of senescence that is caused by sub-lethal oxidative stress. During senescence, DDR activation can result in senescence-associated mitochondrial dysfunction (SAMD), which can be characterized by an increase in ROS production. c-Jun N-terminal kinase (JNK) is activated by mitochondrial ROS during senescence, thereby induces ROS production to sustain senescence.
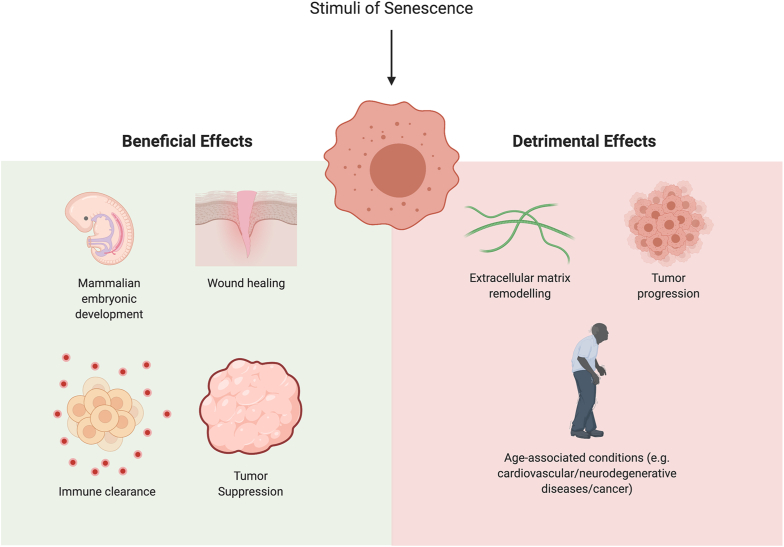
Fig. 2.
Senescence as a double-edged sword. Senescence has been reported by multiple studies to act as a double-edged sword. Programmed cellular senescence plays an essential role during physiological mammalian embryonic development. Such senescence is dependent on p21 and is coupled with macrophage-mediated clearance, which is important for promoting tissue homeostasis as well as remodeling during embryonic development. Also, in response to a cutaneous wound, endothelial and mesenchymal cells undergo senescence to accelerate wound healing via the secretion of PDGF-AA, a SASP component. Cell cycle arrest during senescence functions to suppress tumor growth and proliferation. During senescence, chemokines and cytokines are secreted to direct macrophages to M1 differentiation or recruits immune cells for tumor suppression or clearance of damaged tumor cells. On the flip side, senescence may also be detrimental. Continuous accumulation of senescent cells over time may eventually lead to the development of age-related pathologies such as metabolic disorders (obesity and diabetes), neurodegenerative disorders (Alzheimer's Disease and Parkinson's Disease), cardiovascular pathologies and cancer. Senescence promotes tumor progression due to the pro-inflammatory microenvironment around the senescent cells promoted by SASP factors. Lastly, the release of extracellular matrix proteins which are found in SASP remodel the extracellular matrix surrounding the senescent cells, which could promote invasion and migration.
Notwithstanding these physiological roles, cellular senescence may also be detrimental to tissues, organs or the whole organism, as aberrant or continuous accumulation of senescent cells over time may eventually lead to the development of age-related pathologies, a hallmark of aging (Fig. 2). Such age-related pathologies include metabolic diseases such as obesity and diabetes, as well as neurodegenerative disorders such as Alzheimer's disease or Parkinson's disease [13], and cancer. It is plausible that the accumulation of senescent cells arises from an increased generation of senescent cells from various stress stimuli, gradual accumulation of senescent cells with age, and/or a defective mechanism of senescent cell clearance [[14], [15], [16]].
Senescent cells display numerous distinctive changes in gene expression associated with growth arrest, such as the upregulation of p21cip1 and p16INK4A, which are involved in cell cycle regulation [17]. One physiologically critical feature of senescent cells is a phenotypic change into a secretory state characterized by the release of various inflammatory cytokines, growth factors, enzymes and extracellular matrix proteins known as the senescence-associated secretory phenotype (SASP) [18]. Components of the SASP can be categorized into the following sub-classes: soluble signaling factors (chemokines, interleukins and growth factors), secreted proteases, and secreted insoluble proteins/extracellular matrix (ECM) components [19]. In particular, IL-6 and interleukin-8 (IL-8) are two predominant cytokines responsible for promoting inflammation during senescence [20].
Secretory factors released upon the acquisition of SASP function in a paracrine manner to disturb tissue homeostasis by affecting the function of neighboring cells, which could promote pro-inflammatory pathologies and age-related disorders [20]. Interestingly, the inflammatory mediators in SASP also have the ability to induce growth arrest during senescence through autocrine signaling [21,22]. Moreover, SASP can suppress tumor growth by triggering immune responses that activate the ‘adaptive’ immune clearance of cancer cells [23]. In contrast, the chronic inflammatory state affected by the accumulation of senescent cells has also been shown to accelerate tumor metastases and other age-related pathologies. Similarly, in vascular endothelial cells, SAS factor secretion during senescence can lower the regenerative properties of these cells, thus resulting in the development of pro-inflammatory associated conditions [24]. This is supported by studies demonstrating that aging and vascular cell senescence compromised the integrity of endothelial cells, thereby playing a critical role in the development of age-associated cardiovascular conditions such as atherosclerosis [25]. These observations support the critical role that vascular endothelial cells might play in the maintenance of vascular integrity [26].
Interestingly, the development of SASP is dynamic during senescence. There are multiple levels of regulation of SAS factors at the transcriptional, translational, mRNA stability and secretory phases. Also, SASP strongly depends on positive-feedback loops, both autocrine and paracrine, to amplify its signals. Despite the occurrence of senescent growth arrest within 24 hours after induction, SAS factors are robustly expressed 4–7 days following the induction of senescence [18]. Notably, not all SAS factors are secreted concurrently. Despite a broad understanding of the triggers of SASP, the intracellular signaling network(s) initiating and sustaining SASP during senescence remains poorly understood. SASP activation is not solely dependent on p53 or p16INK4A, suggesting the presence of a separate, independent regulatory networks in senescence that promotes its development [27,28]. To that end, CCAAT/enhancer-binding protein-β (C/EBP-β), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), H2A histone family member X (γH2AX), ATM, macroH2A1 histone variants, janus kinases 1/2 (JAK1/2), p38, mitogen-activated protein kinase (MAPK), mammalian target of rapamycin (mTOR), transforming growth factor beta (TGF-β), IL-1α, sirtuin 3/5 (SIRT3/5) [18,22,[28], [29], [30], [31], [32], [33], [34]] and cyclic GMP–AMP (cGAS)/ stimulator of interferon genes (STING) [35,36] pathways have been shown to regulate SASP. Most of these signaling pathways converge at NF-κB and C/EBP-β activation. Interestingly, pharmacological targeting of mTOR using rapamycin has been shown to suppress the secretion of inflammatory cytokines, especially IL-6, and also reduces translation of membrane-bound IL-1α in senescent cells [37]. This has downstream effects in reducing NF-κB transcription, potentially limiting SASP acquisition [37].
It must be pointed out that a number of these mediators of SASP are involved in the setting of inflammation, thus highlighting the critical interplay between cellular senescence and chronic inflammation from the standpoint of tissue damage and organ dysfunction. In this regard, a remarkable level of coordinated interplay between the transcriptional activities of NF-κB and C/EBP-β enables the establishment and maintenance of SASP; both transcription factors bind to the promoter regions and induce the expression of SAS factors, IL-6 and IL-8 [31]. Two other transcription factors are worth mentioning for their ability to promote the senescent phenotype, namely, zinc finger and SCAN domain containing 1 (Zscan1), a zinc finger transcription factor, induced in response to DNA damage response (DDR) and involving the ATM-TNF receptor-associated factor 6 (TRAF6)-TGFβ-activated kinase 1 (TAK1) axis [38] and GATA4, which triggers NF-κB activation through IL-1α and TRAF3 interacting protein 2 (TRAF3IP2) to induce SASP, independent of the DDR, p53 and p16INK4A [39].
2. Onco-suppressor and oncogenic facets of senescence
Cellular senescence has been implicated in both the suppression and promotion of oncogenesis. As a mechanism of tumor suppression, senescence has been viewed as a process that limits aberrant cell proliferation and prevents neoplastic transformation by permanently arresting cell cycle. Following enforced cell cycle arrest, senescent cells produce SAS factors that allow immune clearance of damaged cells [14,15,[40], [41], [42], [43]]. Even though it is commonly known that oncogene activation invariably promotes the initiation and development of cancer, it can also serve as a stressor to the genome, thereby inducing tumor growth arrest [44]. Of note, senescence induction has been associated with oncogene activation (OIS); activation of Ras promotes senescence in IMR-90, a human lung fibroblast cell line [45]. Similarly, in mammary epithelial cells, mutant Ras promotes senescence and the activation of pathways involved in tumor suppression [46]. In addition, activation of other oncoproteins such as BRAF, protein kinase B (AKT), E2F transcription factor 1 (E2F1), cyclin E, as well as inhibition of tumor suppressors, phosphatase and tensin homolog (PTEN) and neurofibromin (NF1), contribute to OIS [47].
The INK/alternative reading frame (ARF) locus transcribed genes p14ARF, p15INK4B and p16INK4A are also important mediators of cell cycle arrest and senescence. Proteins p15INK4B and p16INK4A inhibit cyclin dependent kinases (CDKs) and keep retinoblastoma protein (Rb) in its hypo-phosphorylated state for Gap 1 (G1) cell cycle arrest. In models of peritoneal inflammation, tdTomato (tdTom)+ macrophages harboring activation of the p16INK4A promoter exhibited a phenotype with features of senescence, such as reduced cell proliferation and senescence-associated beta-galactosidase (SA β-Gal) activation [48,49]. In addition, p14ARF could also stabilize p53 by inhibiting mouse double minute 2 (MDM2), thereby increasing p53-dependent gene transcription [50]. In BRCA-deficient cells, where DNA strand breaks accumulate, p14ARF enables p53 to transcribe DUSP4 and DUSP7, encoding two phosphatases that inactivate extracellular signal‑regulated protein kinase 1/2 (ERK1/2), thus limiting cell proliferation and inducing senescence [51].
The dichotomous effects of senescence on the cancer phenotype is attributed to some of the SAS factors [11,12,42,43]. In this regard, IL-6 [52], IL-8 [18,53], vascular endothelial growth factor (VEGF) [54], osteopontin [55], chemerin [56], matrix metalloproteinase [57], C-X-C motif chemokine 11 (CXCL11) [58] are associated with tumor growth, vascularization, invasion, migration as well as metastasis [59]. For instance, MCF-7 and MDA-MB-231 breast cancer cells exhibit increased proliferation index, migratory and invasive capacities when cultured in conditioned media from senescent mesenchymal stromal or fibroblast cells, effects attributed to IL-6 and/or IL-8 and signal transducer and activator of transcription 3 (STAT3) activation [52,53]. In addition, cells that undergo senescence typically do not undergo cell death, and the possibility of exiting senescence to proliferate with an enhanced tumorigenic potential has also been demonstrated [60]. This is also supported by recent findings in hematologic cancers demonstrating senescence-associated reprogramming that drives cancer stemness as well as spontaneous exit from senescence or cell cycle re-entry [61]. Using genetically switchable models of senescence to mimic spontaneous escape from the arrested state, the study demonstrated that therapy-induced senescence (TIS) may generate cancer cells with enhanced WNT-dependent tumor growth upon forced release or spontaneous escape from senescence. Although, a permanent senescent cell-cycle block is per se incompatible with self-renewal, the authors argued that senescence is, in principle, reversible when essential senescence maintenance genes are no longer expressed [61]. Interestingly, IMR-90 normal human lung cells that undergo replicative senescence display a DNA methylome similar to that of a transformed cell. These cells that bypass senescence retain their methylation landscape, suggesting that this epigenome may promote malignancy if cells exit senescence or re-enter the cell cycle [62]. These findings strongly suggest the need to eliminate senescent cells in order to prevent cancer relapse.
TIS is a critical step in limiting proliferation of cancer cells and involves tumor suppressor proteins such as p53. For example, treatment with chemotherapeutic agents such as doxorubicin increases SA-β-gal in p53WT cells but not in mutant p53 or p53null cells [63,64]. Interestingly, overexpression of oncoprotein AKT or depletion of its negative regulator PTEN were shown to induce senescence via mTOR kinase-mediated phosphorylation-dependent activation of p53 at serine-15 [65]. Stabilized p53 activates transcription of senescence associated genes such as p21cip1, E2F7, PML, PAI-1 and DEC-1 as well as miR-34 [8]. In addition, p53-driven senescence has been shown to induce cytokine and chemokine secretion, which directs macrophages to M1 differentiation or recruits immune cells for tumor suppression or clearance [40,66,67].
Notably, there is experimental evidence to demonstrate that senescent tumor cells in TIS do not necessarily have a permanent cell cycle arrest [[68], [69], [70], [71]]. Such findings have been termed “pseudo-senescence” to differentiate between tumor cells that have regained proliferative capacity from those that are still in a state of permanent growth arrest. In fact, senescence is one of the potential mechanisms by which tumor cells escape the impact of chemotherapy-induced cytotoxic stress, thereby allowing the cells to stay in a dormant state, only to be able to exit dormancy and recover proliferative ability to contribute to tumor recurrence. What triggers that escape to allow re-entry into the cell cycle and recover proliferative capacity remains poorly understood. Tumor cells that escape TIS are further characterized by increased stemness and aggressiveness; 1% of A549 non-small cell human lung adenocarcinoma that undergoes TIS express CD34 and CD117, which are markers of cancer cell stemness [60]. Similarly, another study reported that tumor cells undergoing TIS had stem-like features such as CD24+ and Nanog homeobox (NANOG) [72]. Furthermore, cells escaping TIS have been observed to be more aggressive, form fast-growing colonies in vitro and highly-malignant tumors in vivo [61]. Similarly, evidence of a higher fraction of cells positive for nuclear β-catenin with increased expression of WNT-target genes in recurrent diffuse large B-cell lymphoma (DLBCL) patients, previously exposed to chemotherapy, has been demonstrated [61]. Others have also documented re-entry into the cell cycle of fibroblasts cells, induced into senescent arrest by telomere dysfunction [73]. Here, cells harboring a lower p16INK4A level were able to resume growth via the inactivation of p53, suggesting that senescence arrest was maintained by p53 and reversed by its inactivation [73].
It is noteworthy that cellular mechanisms that drive senescence and tumor dormancy could also contribute to inflammation and immune evasion, thereby creating a conducive milieu for potential cell cycle re-entry and escape from senescence. Notwithstanding that, as the acquisition of TIS or pseudo-senescence appear to form the niche from which subsequent proliferative (re-entry into cell cycle) populations emerge to give rise to secondary malignancies, one might dare to conjecture that targeting the initial mechanisms that induce senescence could still serve to prevent the subsequent escape. Alternatively, strategies targeting senescence in combination with anti-biologicals or conventional therapeutics could be envisioned. Taken together, as the beneficial and detrimental senescence outcomes are seemingly context-dependent, it is imperative to distinguish and understand these different contexts in order to take advantage of senescence for therapeutic purposes.
3. Redox perspective on senescence
One of the critical underlying cellular perturbations commonly shared between signaling networks that drive the acquisition and/or maintenance of cellular senescence is a significant ‘pro-oxidant’ shift in the cellular micro-environment. This could be a factor of either an increase in cellular generation of oxidants and/or depletion or inefficiency of the anti-oxidant defense systems. The resultant low to moderate level of sustained oxidative stress is a critical factor in macromolecular alterations/damage such as lipid peroxidation, post-translational modification(s) of proteins and damage to nucleic acids [74]. These modifications serve to alter signaling networks underlying a host of cellular processes, thereby culminating in DDR activation, changes in transcription factor activity, promoting inflammation and altering cell fate and state decisions. Collectively, these changes have a profound effect on cell cycle progression, in particular the acquisition and maintenance of the senescent phenotype (Fig. 3). As accumulation of senescent cells with time is associated with cellular and organismal aging, the age-dependent accumulation of oxidant-induced macromolecular changes forms the basis for the damage-based theory of aging [75,76] and aging-related disorders such as diabetes, neurodegenerative diseases and cancer [[77], [78], [79]]. Of note, mitochondria function as an important intracellular source of ROS and there is compelling experimental evidence to suggest that senescence-associated mitochondrial dysfunction (SAMD) promotes reduced respiratory coupling and an increase in ROS production [80,81]. As a matter of fact, SAMD-induced ROS production is essential and sufficient for the activation of NF-κB during senescence [82].
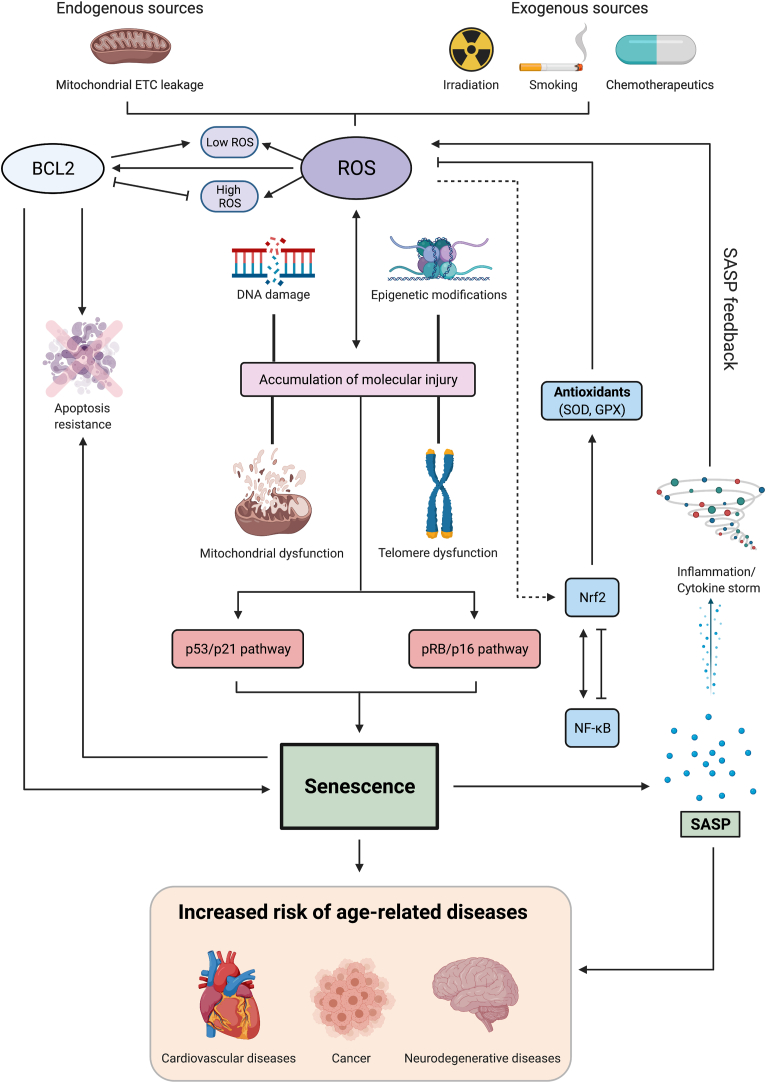
Fig. 3.
Interplay between ROS and senescence. Numerous factors, both exogenous and endogenous, can trigger the production of ROS which can eventually lead to the development of senescence. Continuous accumulation of senescent cells over time may eventually lead to the development of age-related pathologies. Accumulation of dysfunctional mitochondria can induce ROS production which is essential for maintaining the senescent phenotype. Genotoxic stress from irradiation, chemotherapy and smoking, telomere uncapping, overexpression of activated oncogenes contribute to increased ROS production, which induce DNA damage resulting in the activation of DDR and the subsequent activation of the p53/p21 or/and pRB/p16 pathway, which signal for cell cycle arrest and senescence acquisition. ROS also interplay with other factors such as epigenetic modifications, mitochondrial dysfunction and telomere attrition to trigger DNA damage and DDR. One important characteristic of senescence is the secretion of SASP. ROS have been linked to the activation of NF-κB, one of the major transcription factors that can promote the transcription of SASP components such as IL-6 and IL-8. Also, Nrf2, a transcription factor essential for cellular antioxidant response, encodes for proteins involved in antioxidant and glutathione synthesis to reduce ROS production. Depending on the cell type and context, at the transcriptional level, NF-κB can activate Nrf2 expression due to the presence of several binding sites in the promoter region of the NFE2L2 gene. However, NF-κB attenuates Nrf2 activity via mechanisms that are Keap-1 (Nrf2 regulator) dependent or independent, such as competition between p65 and Nrf2 for co-activator CBP-p300 complex. Notably, crosstalk between cellular redox milieu and pro-survival activity of the anti-apoptotic protein Bcl-2 has also been demonstrated. Numerous findings also suggest that increased expression Bcl-2 and Bcl-xL could direct cells into senescence by preventing apoptosis. Pro-survival Bcl-2 phosphorylation could also impair the G1/S cell cycle transition hence potentially promoting senescence induction.
The link between a change in cellular redox status and the senescent phenotype has been demonstrated in the various cellular processes involved in senescence acquisition such as OIS, stress-induced premature senescence (SIPS) by chemotherapeutic drugs, irradiation and replicative senescence. Firstly, oncogenic Ras activation reportedly upregulates NADPH oxidases such as Nox1 or Nox4 [83], which promotes hyperproliferation, thereby resulting in replicative stress, DDR activation and senescence [84]. Whilst these observations provide a rationale for the judicious use of strategies to alleviate oxidative stress in Ras driven cancers, it also provides food for thought for exploiting this redox vulnerability through pharmacological hyperactivation of Ras to induce redox catastrophe and selective execution of mutant Ras expressing cancer cells [85]. Secondly, senescent fibroblasts harbor higher levels of cytosolic and mitochondrial superoxide (O2•-) compared to their younger counterparts [2,86,87]. These oxidants are responsible for causing damage/shortening of telomeres [88], which results in DDR activation and the subsequent induction of replicative senescence [89,90]. Further evidence is provided by recent observations that replication stress-induced DNA damage triggers cellular senescence phenotype via intermediary sub-lethal oxidative stress [91]. Notably, activation of downstream effectors of DDR during senescence results in a significant increase in ROS generation; genotoxic stress, telomere uncapping, oncogene activation as well as induction of cell cycle checkpoints such as p53 and its downstream targets p21cip1 and p16 contribute to increased ROS production [81,[92], [93], [94]]. Despite these pieces of evidence, the temporal and spatial relationship between factors that trigger cellular senescence and those that alter cellular redox milieu and their crosstalk still remain less well characterized.
Whilst on the subject of cellular redox status and senescence in the context of carcinogenesis, its rather disappointing that the use of general anti-oxidant approaches has not had the expected effects. This does not rule out the critical involvement of an altered redox state in the various processes driving carcinogenesis and its progression, including senescence, but strongly suggests a closer look at the underlying redox biology. To that end, an important aspect of the redox biology of cell fate and state determination is the somewhat generalized use of the terms ROS and oxidative stress. As ROS and reactive nitrogen species (RNS) comprise of various chemical entities with different reactivities and target selectivity, it is highly desirable and prudent to identify the specific form of ROS/RNS while attributing a particular biological activity. This has been elegantly articulated by Jones and Sies by way of unraveling the complexity of the ‘redox code’ as well as highlighting the need for the identification of the specific species responsible for altering what they describe as the ‘oxidative eustress’ [[95], [96], [97]]. Along similar lines, our past and recent works have attempted to decipher the diverse effects of the different ROS/RNS species from the standpoint of carcinogenesis and its progression [85,[98], [99], [100], [101], [102], [103], [104]]. Corroborating this, sub-lethal concentrations of chemotherapeutic drugs or hydrogen peroxide (H2O2) trigger early onset of DNA damage and inhibition of DNA synthesis in various in vitro models [[105], [106], [107]], thereby inducing premature senescence, which could be rescued by catalase and N-acetylcysteine (NAC) [108,109]. On the other hand, a significant increase in mitochondrial O2•- production was observed in MCF-7 breast carcinoma cells upon a short treatment with doxorubicin, while U87 glioma cells produced cytosolic O2•-, peroxynitrite (ONOO-) and hydroxyl radical upon treatment with 20(S)-ginsenoside Rg3 [20(S)-Rg3] [110]. Importantly, treatment before or concurrently with nicotinamide or NAC blunted the accumulation of oxidants, thus preventing the development of senescence [111]. Collectively, these studies not only establish the link between ROS-induced DNA damage and activation of DDR and senescence acquisition, but more importantly also appear to differentiate O2•--mediated signaling from H2O2-driven networks, thus presenting a scenario whereby the cellular balance between SODs and iNOS might be a critical factor in determining the readout of the specific micro-environment. As redox modulation critically impacts cell cycle progression and its regulation as well as cellular response to survival and death stimuli and deregulation of cell cycle drives carcinogenesis, it is imperative to fine-tune oxidant-mediated signals and their ramifications to enable more selective and specific design of intervention strategies.
Interestingly, phosphorylation-induced stability of the anti-apoptotic protein Bcl-2 could also impair the G1/S cell cycle transition [112]. Relevantly, constitutively active Rac1L61 mutant was shown to be capable of inducing senescence, in part due to the increase in intracellular ROS levels [113]. A different constitutively active Rac1 mutant, Rac1V12, was also found to increase intracellular ROS levels, which promoted Bcl-2 phosphorylation [114]. These interesting findings suggest that active Rac1-induced senescence could be in part due to ROS-mediated Bcl-2 phosphorylation. Furthermore, our recent work demonstrates that ROS-mediated Serine 70 phosphorylation of Bcl-2 acts as a protective signal for cancer cell survival by modulating ROS-induced DNA damage [104]. As Bcl-2 phosphorylation impairs G1/S cell cycle transition [112], it is likely that an increase in intracellular ROS could induce Bcl-2 phosphorylation to retard cell cycle progression to avoid further ROS-mediated DNA damage, particularly at the DNA replicative S phase, and subsequent cellular cytotoxicity. Collectively, as the overexpression of anti-apoptotic Bcl-2 family members as well as their phosphorylation (such as that of Bcl-2) promote tumorigenesis or chemoresistance, their functions in switching to senescence or impairing cell cycle progression could be a viable ‘way-out’ to avoid drug-induced cytotoxicity or cell death.
4. Senescence targeting in the treatment of cancer and other diseases
4.1. Senescence: consequence of chemotherapy and cause of chemoresistance
Traditionally, chemotherapeutic agents induce high levels of DNA damage in tumor cells to promote cell death [115]. Notably, a subpopulation of the drug-exposed tumor cells are able to enter senescence [116,117], which could be in response to persistent DDR within the tumor and the surrounding stroma [[4], [5], [6]]. In addition, tumor heterogeneity prevents the complete elimination of cancerous cells, thus allowing the small percentage of surviving cells to trigger disease relapse(s). Such heterogeneity might also determine if the small fraction that survives drug-induced execution enters a permanent senescence state or a temporary proliferative arrest state [118]. There is also experimental evidence that the surviving fraction could evade or escape senescence and begin proliferating, thereby resulting in tumor regrowth and metastasis [115]. To that end, senescence evasion has been demonstrated as a mechanism of breast cancer relapse after chemotherapy [115,119,120].
The existence and persistence of senescent tumor cells pose a number of issues and therapeutic challenges [121]. Firstly, SAS factors released by the senescent tumor cells promote cell growth, angiogenesis and immune evasion [11,12,42,43,54,59]. Secondly, senescent cells are also found in non-tumor regions, and since most therapeutic interventions are administered through systemic routes [122], if these non-tumor senescent cells are not removed, their accumulation could promote the onset and/or accelerate the progression of a variety of age-related metabolic disorders [123]. Also, the genomic landscape of cancer cells is dynamic and unstable, which promotes senescence evasion to restore proliferation, as discussed above.
Importantly, senescent cells are resistant to apoptosis due to the upregulation of anti-apoptotic mechanisms [124,125]. In this regard, the anti-apoptotic members of the Bcl-2 family have been documented in senescence induction [126]. Interestingly, various findings suggest that increased expression of anti-apoptotic proteins such as Bcl-2 and Bcl-xL direct cells into senescence by preventing apoptosis. These experiments were typically done through overexpression or knockdown of these anti-apoptotic proteins. For example, inducible expression of Bcl-2 in HeLa cervical cancer cells not only inhibited apoptotic morphology following treatment with aphidicolin, a DNA replication inhibitor, but also prevented colony formation, thereby suggesting the possibility of Bcl-2 in regulating the senescence process [127]. In another study, inducible expression of Bcl-2 in the non-small cell lung cancer cell line H1299 led to G1 cell cycle arrest, cell growth inhibition, SA-β-Gal induction and upregulation of cell cycle arrest markers such as p27. An increase in p27 and Cdk2 interactions and the inactivation of Cdk2 via reduced phosphorylation and activity were observed, which were further confirmed to be downstream of Bcl-2 in inducing senescence [128]. Similarly, Bcl-xL upregulation was also observed in senescent colon cancer HCT166 cell line or triple negative breast cancer (TNBC) cells. In these different cancer models, Bcl-xL seems to be capable of switching cancer cell fate from apoptosis to senescence following drug treatment [129,130]. As such, TNBC cells that failed to undergo senescence had lower Bcl-xL levels following treatment with bromodomain and extra-terminal inhibition (BETi). Consistent with this, knockdown of Bcl-xL pushes cells to undergo apoptosis rather than senescence, while overexpression of Bcl-xL prevents apoptosis [130]. Since most chemotherapeutic agents trigger apoptotic execution of cancer cells and overexpression of anti-apoptotic Bcl-2 proteins not only promotes drug resistance but also contributes to the acquisition of cellular senescence, a novel way to approach cancer treatment would be to eliminate therapy-induced senescent cells to prevent tumor relapse and limit any detrimental side effects to non-tumor cells. As such, a combination approach using conventional therapeutics together with modalities that specifically target the senescent population(s), is envisioned as a more effective strategy.
4.2. Senomorphic and senolytic approaches
The prominent role of cellular senescence in tumorigenesis, tumor persistence and progression has led to the emergence of strategies targeting senescence in cancer therapy. By eliminating therapy-induced senescent cancer cells which have potential to re-enter the cell cycle and cause disease relapse, or by suppressing the SAS factors and their cancer promoting effects, therapeutic targeting of senescence holds promise against disease relapse [131]. Moreover, targeting non-tumoral senescent cells may also contribute to the reversal of pathology and treatment of undesirable side effects of chemotherapy or radiotherapy on bystander cells in normal tissues.
Pioneering efforts to control the acquisition of cellular senescence have led to the identification of senolytic and senomorphic agents involved in the control of either the senescent cells accumulation or mitigate the effect of SAS factor, respectively, thus opening up exciting new opportunities for therapeutic intervention [132]. Senolytics are molecules that preferentially eliminate senescent cells [133,134] by disabling relevant pro-survival pathways, thus promoting senescent cells to die by apoptosis. In cancer therapy, senolytics have been proposed to hold theoretical benefits to supplement existing treatments. Conversely, senotherapeutic molecules termed ‘senomorphics’ or ‘senostatics’ are preferentially modulating the function(s) and morphology of senescent cells or targeting the undesirable effects of SAS factors [135]. Furthermore, considering the remarkable interplay between cellular redox status and senescence in the various processes driving carcinogenesis, it is tempting to hypothesize that aberrant redox signaling serves as a stimulus for the cell cycle re-entry of senescent cells to promote secondary carcinogenesis and hence presents as an attractive target for intervention.
4.2.1. Naturally occurring senolytics and senomorphics
-
(i)
Quercetin
Several naturally occurring compounds have emerged as senolytics in recent years. One of the first senolytic compounds identified was quercetin, a naturally occurring flavonoid polyphenol [136] found in red wine, tea and vegetables. Quercetin has described roles in the modulation of several intracellular signaling pathways such as PI3K-AKT-mTOR, estrogen receptor signaling and NF-κB [137]. In vivo studies on the effect of quercetin in colorectal cancer have demonstrated protective effect on carcinogenesis [138]. An ongoing study investigating quercetin as a chemopreventive against squamous cell carcinoma in patients with known Fanconi anemia is currently recruiting (NCT03476330). Quercetin's senolytic activity has been linked to multiple mechanisms, including inhibition of PI3Kδ [139], the potassium voltage gated channel subfamily E regulatory subunit 2 (KCNE2) [140], and lipoprotein lipase (LPL) [136]. In TNBC, quercetin increased transcription of forkhead box O3 (FOXO3a) and induced its nuclear translocation [141], resulting in increased apoptosis in senescent cells. The therapeutic applicability of quercetin in humans is actively being studied. Recently, a phase I clinical trial of quercetin in chronic obstructive pulmonary disease (COPD) patients has reported results regarding in-human safety [142]. Patients with mild-to-severe COPD received increasing doses of quercetin for a duration of 1 week. Quercetin was tolerated up to 2000 mg/day by patients with no severe drug-related adverse events reported [142]. It's worth pointing out that quercetin has antioxidant properties [135], which provides further support that a common denominator between small molecules that alter or target the senescence phenotype could be the modulation of cellular redox metabolism. Further corroborating this are observations that quercetin interacts with and stabilizes nuclear factor erythroid 2-related factor 2 (Nrf2), a redox responsive transcription factor [143]. Also, studies have described a reduction of Nrf2 protein levels and reduced ROS in breast cancer cells upon exposure to quercetin and vitamin C [143,144].
-
(ii)
Quercetin-Dasatinib Combination Therapy
The combination of dasatinib, a tyrosine kinase inhibitor targeting c-KIT, SRC and ephrin receptors, and quercetin has been well studied and shown to eliminate a wide range of senescent cells [139]. While dasatinib was able to eliminate senescent human preadipocytes, quercetin appeared to eliminate human endothelial cells more effectively [139]. The combination of dasatinib and quercetin (DQ) reduced senescent cell burden in naturally-aged and progeroid mice, improved cardiovascular function and carotid vascular reactivity in aged mice after just a single dose, and also reduced markers of senescence in mouse limbs that had undergone radiation [139]. Periodic administration of DQ improved health span in progeroid mouse models, delaying the onset of age-related pathologies such as osteoporosis and degenerative disc disease [139]. In humans, a phase I study of patients with diabetic kidney disease treated with dasatinib 100 mg/day plus quercetin 1000 mg/day for 3 days sought to demonstrate the in-human senolytic pharmacodynamic effects of this combination. Paired adipose tissue, skin biopsies and blood were collected from patients prior to therapy and at 14 days after starting treatment. Interestingly, this study demonstrated reduction in senescent cells within adipose and skin tissue after this short DQ therapy, together with reductions in circulating SASP factors IL-1α, IL-6 and MMPs [145], confirming in-human senolytic activity of this combination.
In murine models of bleomycin-induced pulmonary fibrosis, DQ also eliminated senescent cells, thus improving lung function [146]. Similar results were seen in ex vivo mouse alveolar cells from fibrotic lungs which also have reduced markers of senescence and fibrosis noted [147]. This preclinical data provided the rationale for a phase I study combining intermittently dosed dasatinib 100 mg/day with quercetin 1250 mg/day, 3 days per week for a total of 3 weeks, for the treatment of idiopathic pulmonary fibrosis (IPF). Importantly, amongst 14 patients recruited, meaningful improvement in patients' physical function as measured by a 6-min walk distance, 4-m gait speed and chair-to-stand time was improved after the 3-week course of therapy. No changes in pulmonary function test or frailty index were observed [148]. Finally, in preclinical studies of Alzheimer's dementia, neuritic plaque-associated oligodendrocyte progenitor cells showed a senescence-like phenotype with upregulation of senescence-associated markers [149]. Treatment of transgenic mice expressing mutant human amyloid precursor protein (APP) and presenilin-1 (PS1), which cause early onset familial Alzheimer's dementia, with the DQ senolytic cocktail led to the selective elimination of senescent cells and reduced neuroinflammation, while improving cognitive deficits [149]. Currently, a clinical trial investigating DQ as a senolytic therapy to moderate Alzheimer's disease progression is recruiting (NCT04063124). In this context, combinatorial strategies of senolytics from different classes could be explored to synergistically target senescence pathways in a variety of senescence-related conditions [150]. This may also require lower doses of each agent, potentially improving upon side effect profiles. Despite these highly encouraging results, heterogeneity in senolytic activity amongst various cell types has been reported with DQ, suggesting that the potential beneficial effects may be cell-type specific [150]. For example, DQ at maximal cytostatic doses lacked efficacy in clearing doxorubicin-induced senescent cells in HepG2 and Huh-7 hepatocellular carcinoma (HCC) cells [151].
-
(iii)
Fisetin
Fisetin is another member of the flavonoid family. Naturally, fisetin is found in fruits and vegetables such as strawberries, onions, cucumbers and grapes [152]. Data have indicated the anti-tumor activity of fisetin to occur through its effects in targeting the Bcl-2 superfamily, and by inactivation of oncogenic drivers such as ERK1/2. Similar to quercetin, the role of fisetin in regulating the PI3K/AKT, NF-κB, peroxisome proliferator-activated receptor (PPAR), poly(ADP-ribose) polymerase 1 (PARP1) axes has been described. These diverse effects on various replicative pathways have led to the observation of anti-proliferative and pro-apoptotic activity in a variety of cancer cell lines [153,154]. In colorectal, hepatic and pancreatic cancer cell lines, fisetin induced apoptosis and growth inhibition [155]. Gene expression analysis revealed this anti-tumor effect to be mediated through modulation of topoisomerases and CDKs [155]. In non-small cell lung cancer (NSCLC) NCI-H460 cells, fisetin displayed dose-dependent cytotoxicity, and induced apoptosis by reducing Bcl-2 expression, increasing Bcl-xL and activating downstream caspase-3 and -9 [156]. Similarly, in MCF-7 breast cancer cell lines, fisetin promoted caspase -7, -8 and -9 activity, indicating increased apoptosis in tumor cells compared to normal cells [157]. Fisetin also showed increased apoptosis induction in bladder cancer models in vitro and in vivo, by up-regulating p53 while downregulating NF-κB activity [158]. Fisetin also reduced the incidence of bladder cancers induced by intravesical N-methyl-N-nitrosourea by modulating NF-κB pathways and was well tolerated by mice with no toxicity [158].
Similar to quercetin, fisetin has been described to have senolytic and potent antioxidant properties [150,159]. Amongst 10 different flavonoid compounds tested, fisetin was the most potent in reducing markers of senescence in aged mice, restoring tissue homeostasis, and reducing the development of age-related pathologies [159]. However, similar to quercetin, cell-type specific senolysis has been observed. In humans, various clinical trials investigating the senolytic potential for fisetin to improve skeletal health (NCT04313634, NCT04210986) and the frail elderly syndrome (NCT03430037) are recruiting. No studies for fisetin in cancer therapy have been registered to date.
-
(iv)
Piperlongumine and related analogues
Another naturally-occurring senolytic identified is piperlongumine, an alkaloid found in peppers and medicinal plants [160,161]. Its anti-tumoral properties in NSCLC xenografts have been described [160,161]. Combined with doxorubicin, piperlongumine resulted in a synergistic pro-apoptotic effect on DU-145 prostate cancer cells [162]. The senolytic activity of piperlongumine has been attributed to its effects targeting oxidation resistance 1 (OXR1), which is a sensor of oxidative stress and regulates expression of downstream anti-oxidant enzymes such as glutathione S-transferase Pi 1 (GSTp1) and crumbs cell polarity complex component 1 (CRB1) [160,163]. Piperlongumine was able to induce ubiquitin-proteasome mediated degradation of oxidation resistance protein 1 (OXR1) selectively in senescent cells [164]. Other potential targets of piperlongumine include the ERK1/2 and RAF1 pathways [160], which relate to its ability to suppress proliferation and tumor progression. Piperlongumine has also been suggested to modulate NF-κB pathway and caspase-mediated apoptosis [165]. Preferential activity against senescent WI-38 fibroblasts induced by oncogenic Ras expression or ionizing radiation has also been reported for piperlongumine [163]. Notably, senolytic synergy for piperlongumine with ABT-263 (navitoclax), a Bcl-2/Bcl-xL inhibitor, has been recently demonstrated [163].
-
(v)
Curcumin analogues
EF24 is a novel curcumin analog with senolytic activity with broad action against a range of cell types including human fibroblasts, renal epithelial cells as well as human umbilical vein endothelial cells (HUVECs) [166]. EF24 has been shown to reduce cell viability in senescent cells induced by ionizing radiation, extensive replication or Ras oncogene transfection [166]. The mechanism of action for its senolytic activity has been described as due to apoptosis induction by both ROS production-dependent and -independent mechanisms. One ROS-independent mechanism described is through the reduction of the anti-apoptotic members of the Bcl-2 superfamily, such as Mcl-1 and Bcl-xL, in senescent cells, inducing selective apoptosis [166]. No preclinical data exists regarding the efficacy of EF24 as a senolytic in the context of cancer therapy.
-
(vi)
Cardiac glycosides
Cardiac glycosides such as digoxin and ouabain, originally from the foxglove plant, were identified as broad-spectrum senolytic molecules [167,168]. This senolytic action is related to their inhibition of the Na+/K+-ATPase pump, which leads to imbalance in the electrochemical cellular gradient. Senescent cells are particularly vulnerable to this approach due to the presence of an already acidified cellular environment as well as a slightly depolarized plasma membrane. To evaluate the antitumor activity of this senolytic effect, immunodeficient nude mice were subcutaneously injected with A549 lung cancer cells, treated with gemcitabine, digoxin (2 mg/kg) or the combination of these two drugs. Although gemcitabine and digoxin alone both showed a slight antitumor effect compared to controls, the combination of the two led to a robust antitumor effect and several complete responses were noted in vivo. Immunohistochemistry performed on tumors treated with gemcitabine alone led to increased markers of senescence. However, tumors treated with gemcitabine and digoxin had only very few SA-β-Gal positive cells. Interestingly, a crosstalk between the senolytic activity of digoxin and modulation of redox homeostasis may exist. In gemcitabine-resistant pancreatic cancer cell lines, digoxin was shown to regulate and inhibit Nrf2 at the transcriptional level, which reversed gemcitabine resistance [169]. Furthermore, treatment with digoxin led to markedly decreased expression of Nrf2 target genes encoding antioxidant, glutathione synthesis, drug transport and drug metabolism enzymes in gemcitabine-resistant pancreatic cells [169]. In patient-derived xenografts (PDXs) of breast cancer treated with doxorubicin, elevated senescence markers were noted, indicative of TIS. Treating these PDXs with the combination of doxorubicin and digoxin led to improved tumor outcomes as well as reduced SA-β-Gal expression [168]. Similarly, ouabain treatment preferentially killed RAS-driven senescent cells [167], as well as TIS cells that had history of prior exposure to common cancer therapies such as etoposide, doxorubicin or palbociclib [167]. In models of bleomycin-induced pulmonary fibrosis, digoxin also showed increased elimination of senescent cells, suggesting thatt the senolytic activity of cardiac glycosides could be a potential means of ameliorating this feared side effect of cancer therapy [168].
4.2.2. Bcl-2 inhibitors
Small molecule inhibitors targeting mitochondrial intrinsic apoptotic pathways have been shown to hold senolytic activity [[170], [171], [172]]. One notable category of senolytic drugs is the BH3 mimetics, in particular the triple Bcl-2, Bcl-xL and Bcl-w inhibitor, navitoclax (formerly ABT-263), which was designed as an orally bioavailable drug for clinical testing to promote apoptotic cell death as well as ABT-737, and A1331852 and A1155463 which are selective for Bcl-xL [[171], [172], [173]]. The senolytic activity of navitoclax appears to be primarily due to its inhibition of Bcl-w and Bcl-xL [[170], [171], [172]]. In human mesenchymal stromal cells treated with navitoclax, a significant but moderate senolytic effect was reported along with a reduction in SA-β-Gal staining. However, no rejuvenation of mesenchymal stromal cells by measure of improved telomere length or epigenetic senescence signature was noted from navitoclax treatment [174]. In vivo, treating mice who harbored TIS from prior chemotherapy with navitoclax led to clearance of senescent tumor cells, potentially reducing the risk for subsequent tumor recurrence [134,170].
Notably, similar to other described senolytics, navitoclax appears to be active in selective senescent cell-types. Zhu and colleagues described reduced viability of human lung fibroblast cells and senescent HUVECs, but not preadipocytes. Combined siRNA targeting of Bcl-2, Bcl-xL and Mcl-1 failed to induce senolysis, whereas similar combination siRNA targeting of Bcl-2, Bcl-xL and Bcl-w was able to induce senolysis in HUVECs and IMR-90 cells [172]. Importantly, the efficacy of navitoclax in the context of cancer cells remains to be fully established. In mouse models, navitoclax eliminated both TIS tumor and non-tumoral stromal cells after chemotherapy, thus reducing cancer recurrence. In other models of melanoma and NSCLC, navitoclax was able to selectively kill senescent tumor cells [175]. In TP53-wild type breast cancer cells, navitoclax had no effect on proliferating cells, but selectively induced apoptosis in a subset of cells that had been pre-treated with chemotherapy. Low Noxa (a pro-apoptotic BH3 protein) expression was associated with resistance to navitoclax in some cells, which could be overcome with Mcl-1 inhibition. These senescent breast cancer cells were shown to rely on Bcl-xL and/or Mcl-1 for survival [176]. In vivo breast cancer models showed that the addition of navitoclax after chemotherapy improved tumor regression, and mouse survival, with increased apoptosis noted [176]. These findings suggest successful elimination of chemotherapy-induced senescent cells by BH3 mimetics, potentially reducing residual disease burden after chemotherapy [176]. However, in prostate cancer cells, navitoclax failed to show senolytic efficacy in androgen-sensitive senescent prostate cancer cells which had been treated with androgen deprivation therapy [177]. Finally, the toxicity profile of navitoclax is a concern. In clinical studies of navitoclax, dose-limiting thrombocytopenia and neutropenia have been reported [178], which may limit its translational applicability at cytotoxic doses in cancer patients who may already have diminished marrow reserves after chemotherapy. This may necessitate the evaluation of lower doses of navitoclax for senolysis, or potentially in combination with other less toxic senolytics.
Since navitoclax was first noted to be a senolytic, few studies have attempted to improve navitoclax toxicity profile by reducing platelet toxicity. Gualda et al. recently showed that the use of galactose-encapsulated nanoparticles or more specifically a pro-drug galacto-conjugated navitoclax (Nav-Gal) that can be preferentially activated by SA-β-Gal activity found in senescent cells [179]. Senescent cells with increased lysosomal and galactosidase activity allow hydrolysis of a cleavable galactose, thereby facilitating the targeted release of navitoclax to senescent cells. In addition, Nav-Gal was shown to be more specific at inducing cell death in senescent cells while protecting non-senescent cells compared to navitoclax. Importantly, Nav-Gal induced less thrombocytopenia compared to navitoclax in ex vivo human and murine blood samples as well as in vivo murine models; the latter at therapeutic relevant doses [179]. A different group similarly converted navitoclax to a Bcl-xL proteolysis-targeting chimera (PROTAC), known as PZ15227, which targets Bcl-xL to the cereblon (CRBN) E3 ligase for degradation. As CRBN is poorly expressed in platelets, this makes PZ15227 less toxic towards platelets. Importantly, PZ15227 effectively clears senescent cells without severe thrombocytopenia and rejuvenates tissue stem and progenitor cells in aged mice [180]. Collectively, the improved versions of navitoclax, Nav-Gal and PZ15227, are promising compounds that could be further developed into drugs that are eventually clinically safe and active at targeting senescent cancer cells and even in some cases rejuvenating aged or injured tissues plagued with senescent cells.
Finally, other BH3 mimetics targeting Bcl-w and Bcl-xL and the discovery of selective Bcl-xL inhibitors A1331852 and A1155463 hold senolytic potential as well. Bcl-xL has been shown to be essential for the survival of senescent HUVECs. The selective Bcl-xL inhibitors A1331852 and A1155463 have described senolytic activity in senescent HUVECs and IMR-90 lung fibroblasts, but not in preadipocytes [150]. When combined with standard chemotherapy in vivo models of breast, ovarian and NSCLC, Bcl-xL inhibition improved chemotherapy efficacy with tolerable toxicity [181].
Interestingly, a senomorphic effect has also been demonstrated for navitoclax by way of suppressing SASP expression in several pre-clinical models for senescence. In mouse lung models, reduction in cyclin dependent kinase inhibitor 2A (CDKN2A), TNF-α and C-C motif chemokine ligand 5 (CCL5) were observed [170], and in bone marrow stromal cell cultures from aged mice, navitoclax both reduced levels of SASP components as well as eliminated senescent cells [182]. Collectively, its rather remarkable that a large number of compounds with senolytic activity are mechanistically redox modulators, thus strongly suggesting a cause-effect relationship. Perhaps, a closer evaluation of other redox modulating compounds will provide novel senotherapeutics.
4.3. Repurposing of drugs as senotherapies
-
(i)
Rapamycin
Rapamycin is a natural macrolide mTOR kinase inhibitor isolated from Streptomyces strains, and was initially developed as an immunosuppressive agent [183]. Increased understanding of the role of mTORC1 in regulating SASP [139] led to several groups exploiting the potential senomorphic or senostatic role of rapamycin in suppressing the SASP effects in cancer. This has been described to be affected through Nrf2-dependent and -independent mechanisms [175]. mTOR interacts with the MAP kinase pathway, and ultimately leads to increased mitogen-activated protein kinase-activated protein kinase 2 (MAPKAPK2) translation [184], and nuclear translocation of NF-κB. Rapamycin led to downregulation of inflammatory cytokines including IL-6, IL-8, and reduction of transcription of IL-1α and IL-1β [37] as well as STAT3 pathway activation. Rapamycin also reduced production of mitochondrial ROS in vitro and in vivo [185]. In prostate cancer mouse models, introduction of rapamycin suppressed SAS factors in senescent stromal fibroblasts and their effects on prostatic tumor growth [186]. Therefore, rapamycin and other mTOR inhibitors may have a potential preventative role in suppressing tumor growth by blocking the deleterious effects of the SAS factors [187]. Other mTOR pathway inhibitors such as everolimus, temsirolimus and deforolimus are being explored as senomorphics in cancer therapy as well.
-
(ii)
Metformin
Metformin is commonly used for the treatment of type II diabetes mellitus, which holds potential for repurposing in senotherapy. Metformin has several mechanisms of action and has been identified as an inhibitor of mitochondrial oxidative phosphorylation through its inhibition of complex I [188]. In senescent cells, metformin has been identified to reduce ROS production and resultant DNA damage, while maintaining cell cycle arrest [189], thereby suggesting its potential as a senomorphic agent. Additional effects in modulating SAS factors have also been described through its action in reducing translocation of NF-κB into the cellular nucleus [190]. The known effect of metformin in inhibiting AMP-activated protein kinase (AMPK) activation is also expected to downregulate the mTORC pathway, which may additionally contribute to its senomorphic activity [191]. Ras-mutant IMR-90 fibroblasts treated with metformin also showed reduced levels of CXCL-5, IL-6, IL-8 and IL-1β, which are known inflammatory cytokines found in SASP. Similarly, in prostate cancer cells, metformin reduced the pro-tumorigenic inflammatory effects of SAS factors [190].
-
(iii)
Histone deacetylase (HDAC) inhibitors
Panobinostat is a HDAC inhibitor that has also been repurposed as a senolytic due to the links between senescence and histone deacetylation. Senolytic activity has been demonstrated for panobinostat in NSCLC, as well as head and neck squamous cell carcinoma [192], producing dose-dependent synergy when combined with paclitaxel chemotherapy in the respective cell lines. The use of panobinostat was shown to be more efficacious compared to repeat dosing with paclitaxel or cisplatin, and the authors suggested that panobinostat was able to selectively target persistent senescent cells that accumulate after chemotherapy treatment [192]. Treatment with panobinostat led to increased apoptosis in treatment-induced senescent cells, with increased caspase-3 and -7 activity noted, as well as increased histone acetylation and reduced Bcl-xL expression. This suggests that panobinostat may be useful as a senolytic agent in the post-chemotherapy setting.
-
(iv)
Inhibitors of bromodomain and extra-terminal proteins (BETi)
BETi have known anti-tumor activity through its targeting of oncogenic pathways, including c-MYC, Bcl-2 and CDK4 [193]. More recently, bromodomain-containing protein 4 (BRD4), which is also targeted by BETi, was shown to regulate SASP expression and immune surveillance during OIS [194]. In this light, BETi have been purported to induce senolysis through attenuation of non-homologous end joining (NHEJ) and by increasing cellular autophagy. Tasdemir and colleagues treated senescent hepatocytes with the BETi I-BET2762, which blunted SASP and suppressed the progression to HCC in mouse models [194]. In xenografts, eliminating chemotherapy-induced senescent cells using BETi increased overall chemotherapy efficacy [195]. In TNBC, BETi have been reported to induce mitotic catastrophe which may result in apoptosis or senescence. One study investigating these two cell fates [130] described that this terminal response is determined by intrinsic levels of Bcl-xL. Cell lines that underwent senescence rather than apoptosis after BETi therapy, showed higher levels of Bcl-xL, and forced overexpression of Bcl-xL diverted cells from apoptosis to senescence instead. Inhibition of Bcl-xL in cells that were senescent after BETi treatment, further induced cell death [130].
-
(v)
Macrolide Antibiotics
Azithromycin and roxithromycin were initially developed as macrolide antibiotics. Recent studies have shed light on their senolytic properties in human fibroblasts. Azithromycin treatment of human fibroblasts was able to remove approximately 97% of senescent cells, potentially through the induction of aerobic glycolysis and autophagy [196]. Interestingly, the parent antibiotic, erythromycin, showed no senolytic activity. No preclinical data exists thus far for the repurposing of macrolide antibiotics for senolysis in the context of cancer therapy.
-
(vi)
Fibrates
Fenofibrate, a drug used in dyslipidemia therapy, has also been touted to have pro-autophagic activity, and was able to reduce the number of senescent human chondrocyte cells via increased autophagic flux and apoptosis [197]. The group also highlighted retrospective data from the Osteoarthritis Initiative cohort, describing reductions in clinical osteoarthritis in patients who had received fibrate therapy.
The aforementioned examples of drugs that were developed for other indications but have potential for use as senomorphic/senostatic/senolytic, also seem to impact cellular/mitochondrial redox metabolism. The emerging theme underlying the ability of these drugs to target the senescence phenotype appears to be related directly or indirectly to their redox modulating properties.
5. Concluding remarks
Myriad stress stimuli trigger the acquisition of senescence and/or its maintenance, which in addition to promoting tissue repair and remodeling also functions as an effector mechanism driving age-related pathologies. The functional dichotomy of senescence is visibly manifested in regulating signaling networks that suppress or promote the process of carcinogenesis and its progression. These biological responses are a function of the slew of cytokines and chemokines secreted by cells upon acquiring SASP. Despite the current advancement in the understanding of various stimuli and signaling networks upstream and downstream of SASP, there is relative lack of clarity with respect to the temporo-spatial factors/events that govern the switch from the good (onco-suppressor) to the bad (oncogenic). Furthermore, the well-recognized pathogenic role of senescent cells has also drawn attention to the study of post-mitotic cells and the relevance of senescence in these to tissue physiology and ageing [198,199]. To date, evidence surrounding the role of post-mitotic cell senescence in health and disease remains scarce and this is an area that urgently needs to be addressed.
Importantly, the intricate crosstalk between senescence and cellular redox metabolism has potential therapeutic implications. To that end, it's worth pointing out that a majority of small molecule compounds with senomorphic and/or senolytic activities also elicit redox regulatory effects (Fig. 4). The challenge obviously would be to untangle the inherent complexity of the redox-senescence interplay, which will inform the appropriate clinical utility of these strategies as well as selective repurposing of other drugs. Notwithstanding the tremendous advances in the search for potential senotherapeutics, the double-edged nature of senescence-targeting means that caution must be taken in moving these therapies from bench to bedside. To date, human cell culture has provided most of the preliminary information on senescence targeting; however, given that senolysis may have beneficial or detrimental effects, improved pre-clinical models that may better mimic human disease in the context of an intact immune system are needed to validate the potential benefits of such therapies. Importantly, such models may also help us to narrow down the senescence signaling pathways which are most relevant to the disease pathology of interest, as well as to prioritize disease conditions most likely to benefit from this approach, ultimately culminating in appropriately-prioritized clinical trials.
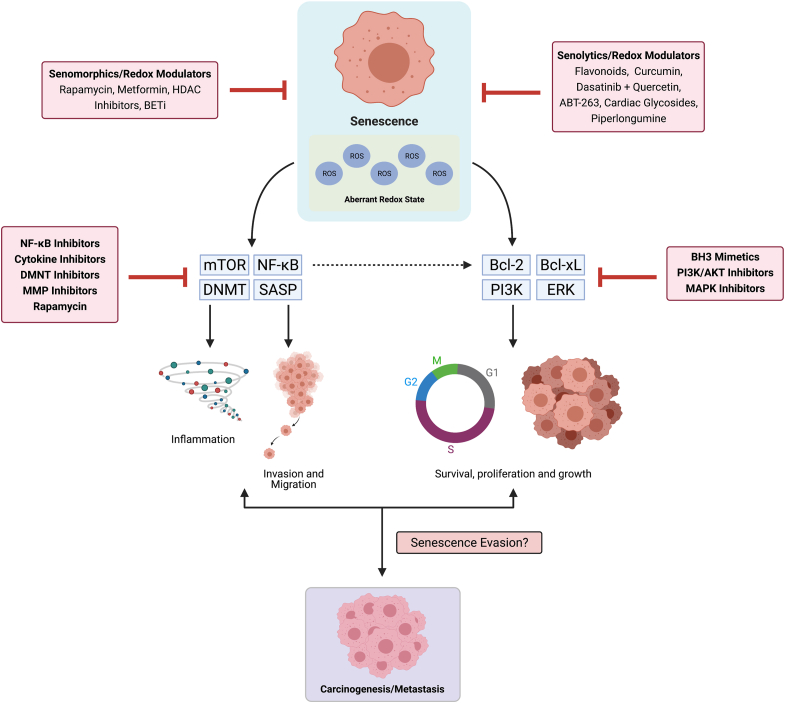
Fig. 4.
Targeting senescence as a therapeutic strategy. While an increase in ROS signals for senescence acquisition, there is also evidence that senescent cells exhibit aberrant redox signaling; a significant ‘pro-oxidant’ shift in the cellular environment. Redox modulators have shown promise as senomorphic or senolytic agents, such as the flavonoids, rapamycin, metformin and other repurposing approaches. In addition, senescent cells are refractory to death stimuli, and the possibility of exiting senescence to proliferate with an enhanced tumorigenic potential has also been demonstrated by multiple studies. SASP factors such as IL-6, IL-8, VEGF, osteopontin, chemerin, matrix metalloproteinase (MMP), CXCL11 are associated with tumor growth, vascularization, invasion, migration as well as metastasis. Also, CXCL-5, IL-6, IL-8 and IL-1β are known inflammatory cytokines found in SASP that promote inflammation and this can be inhibited through targeting with MMP inhibitors or cytokine inhibitors. mTOR pathway has been shown to regulate SASP through NF-κB activation, and their pharmacological targeting can potentially inhibit or limit SASP acquisition, as shown with Rapamycin or NF-κB inhibitors. Interestingly, senescent cells that bypass senescence retain the methylation landscape of a transformed cell, suggesting that this epigenome may promote malignancy if cells exit senescence or re-enter the cell cycle. As such, DNA methyltransferase (DNMT) inhibitors could be an effective senomorphic approach. Also, AKT overexpression or depletion of its negative regulator PTEN were also observed to induce senescence via mTOR kinase-mediated phosphorylation-dependent activation of p53 at serine-15. Also, p14ARF enables p53 to transcribe DUSP4 and DUSP7 that inactivate ERK1/2, thus limiting cell proliferation and inducing senescence. Various findings also suggest that increased expression of anti-apoptotic proteins such as Bcl-2 and Bcl-xL direct cells into senescence by preventing apoptosis. As such, PI3K/AKT inhibitors and BH3 mimetics could be used as senolytics to eliminate senescent cells.
Could regulation of cellular redox status be the common denominator in senolytic and senomorphic strategies? In this regard, aside from the deleterious effects on bio-molecules, aberrant redox signaling, downstream of DNA damage response activation, could be critical in the maintenance of senescence, and as such restoring redox homeostasis could have the dual advantage of blocking the acquisition as well as maintenance of the senescent phenotype [200]. Hence, one might dare to conjecture that, in addition to accumulating oxidant-mediated damage over time, ageing involves a further role for an aberrant redox micro-environment in promoting cellular senescence.
Author disclosure statement
Natalie YL Ngoi: Honoraria: Janssen, AstraZeneca, Thermofisher, Angeline QX Liew: No competing financial interests exist., Stephen JF Chong: No competing financial interests exist. Matthew S Davids: Consultancy/Advisory Boards at AbbVie, Adaptive, Ascentage, Astra-Zeneca, Beigene, Eli Lilly, Genentech, Merck, Novartis, Pharmacyclics, Janssen, MEI Pharma, Verastem, and Zentalis; and has received research funding from Ascentage, Genentech, Pharmacyclics, TG Therapeutics, BMS, Surface Oncology, MEI Pharma, Verastem, Novartis, and Astra-Zeneca. Marie-Veronique Clement: No competing financial interests exist. Shazib Pervaiz: No competing financial interests exist.
Acknowledgements
The authors wish to acknowledge the contributions of those whose work might have been inadvertently overlooked. Shazib Pervaiz (SP) is supported by National Medical Research Council of Singapore (NMRC CIRG/1433/2015 and OFIRG/0041/2017) and a USPC-NUS 2021 award. Marie-Veronique Clement (MVC) is supported by a USPC-NUS 2018 award. SP and MVC are supported by a grant under the Healthy Longevity Catalyst Award 2020 (HLCA20Jan-0098).
References
- 1.Hayflick L., Moorhead P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 2.Harley C.B., Futcher A.B., Greider C.W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 3.Petrova N.V., Velichko A.K., Razin S.V., Kantidze O.L. Small molecule compounds that induce cellular senescence. Aging Cell. 2016;15:999–1017. doi: 10.1111/acel.12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirama T., Koeffler H.P. Role of the cyclin-dependent kinase inhibitors in the development of cancer. Blood. 1995;86:841–854. [PubMed] [Google Scholar]
- 5.Xiong Y., Hannon G.J., Zhang H., Casso D., Kobayashi R., Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 6.Harper J.W., Adami G.R., Wei N., Keyomarsi K., Elledge S.J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 7.Qian Y., Chen X. Senescence regulation by the p53 protein family. Methods Mol. Biol. 2013;965:37–61. doi: 10.1007/978-1-62703-239-1_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qian Y., Chen X. Tumor suppression by p53: making cells senescent. Histol. Histopathol. 2010;25:515–526. doi: 10.14670/hh-25.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munoz-Espin D., Canamero M., Maraver A., Gomez-Lopez G., Contreras J., Murillo-Cuesta S., Rodriguez-Baeza A., Varela-Nieto I., Ruberte J., Collado M. Programmed cell senescence during mammalian embryonic development. Cell. 2013;155:1104–1118. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Storer M., Mas A., Robert-Moreno A., Pecoraro M., Ortells M.C., Di Giacomo V., Yosef R., Pilpel N., Krizhanovsky V., Sharpe J. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell. 2013;155:1119–1130. doi: 10.1016/j.cell.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 11.Demaria M., Ohtani N., Youssef S.A., Rodier F., Toussaint W., Mitchell J.R., Laberge R.M., Vijg J., Van Steeg H., Dolle M.E. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell. 2014;31:722–733. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiche A., Le Roux I., von Joest M., Sakai H., Aguin S.B., Cazin C., Salam R., Fiette L., Alegria O., Flamant P. Injury-induced senescence enables in vivo reprogramming in skeletal muscle. Cell Stem Cell. 2017;20:407–414 e404. doi: 10.1016/j.stem.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Cue C., Rueda N. Cellular senescence in neurodegenerative diseases. Front. Cell. Neurosci. 2020;14:16. doi: 10.3389/fncel.2020.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker D.J., Wijshake T., Tchkonia T., LeBrasseur N.K., Childs B.G., van de Sluis B., Kirkland J.L., van Deursen J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song P., An J., Zou M.H. Immune clearance of senescent cells to combat ageing and chronic diseases. Cells. 2020;9 doi: 10.3390/cells9030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karin O., Agrawal A., Porat Z., Krizhanovsky V., Alon U. Senescent cell turnover slows with age providing an explanation for the Gompertz law. Nat. Commun. 2019;10:5495. doi: 10.1038/s41467-019-13192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H. Molecular signaling and genetic pathways of senescence: its role in tumorigenesis and aging. J. Cell. Physiol. 2007;210:567–574. doi: 10.1002/jcp.20919. [DOI] [PubMed] [Google Scholar]
- 18.Coppe J.P., Patil C.K., Rodier F., Sun Y., Munoz D.P., Goldstein J., Nelson P.S., Desprez P.Y., Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coppe J.P., Desprez P.Y., Krtolica A., Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wajapeyee N., Serra R.W., Zhu X., Mahalingam M., Green M.R. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell. 2008;132:363–374. doi: 10.1016/j.cell.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acosta J.C., Banito A., Wuestefeld T., Georgilis A., Janich P., Morton J.P., Athineos D., Kang T.W., Lasitschka F., Andrulis M. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013;15:978–990. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eggert T., Wolter K., Ji J., Ma C., Yevsa T., Klotz S., Medina-Echeverz J., Longerich T., Forgues M., Reisinger F. Distinct functions of senescence-associated immune responses in liver tumor surveillance and tumor progression. Canc. Cell. 2016;30:533–547. doi: 10.1016/j.ccell.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olivieri F., Lazzarini R., Recchioni R., Marcheselli F., Rippo M.R., Di Nuzzo S., Albertini M.C., Graciotti L., Babini L., Mariotti S. MiR-146a as marker of senescence-associated pro-inflammatory status in cells involved in vascular remodelling. Age (Dordr) 2013;35:1157–1172. doi: 10.1007/s11357-012-9440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayashi T., Matsui-Hirai H., Miyazaki-Akita A., Fukatsu A., Funami J., Ding Q.F., Kamalanathan S., Hattori Y., Ignarro L.J., Iguchi A. Endothelial cellular senescence is inhibited by nitric oxide: implications in atherosclerosis associated with menopause and diabetes. Proc. Natl. Acad. Sci. U. S. A. 2006;103:17018–17023. doi: 10.1073/pnas.0607873103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behrendt D., Ganz P. Endothelial function. From vascular biology to clinical applications. Am. J. Cardiol. 2002;90:40L–48L. doi: 10.1016/s0002-9149(02)02963-6. [DOI] [PubMed] [Google Scholar]
- 27.Coppe J.P., Rodier F., Patil C.K., Freund A., Desprez P.Y., Campisi J. Tumor suppressor and aging biomarker p16(INK4a) induces cellular senescence without the associated inflammatory secretory phenotype. J. Biol. Chem. 2011;286:36396–36403. doi: 10.1074/jbc.M111.257071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodier F., Coppe J.P., Patil C.K., Hoeijmakers W.A., Munoz D.P., Raza S.R., Freund A., Campeau E., Davalos A.R., Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freund A., Patil C.K., Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30:1536–1548. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodier F., Munoz D.P., Teachenor R., Chu V., Le O., Bhaumik D., Coppe J.P., Campeau E., Beausejour C.M., Kim S.H. DNA-SCARS: distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J. Cell Sci. 2011;124:68–81. doi: 10.1242/jcs.071340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuilman T., Michaloglou C., Vredeveld L.C., Douma S., van Doorn R., Desmet C.J., Aarden L.A., Mooi W.J., Peeper D.S. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 32.Chen H., Ruiz P.D., McKimpson W.M., Novikov L., Kitsis R.N., Gamble M.J. MacroH2A1 and ATM play opposing roles in paracrine senescence and the senescence-associated secretory phenotype. Mol. Cell. 2015;59:719–731. doi: 10.1016/j.molcel.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu M., Tchkonia T., Ding H., Ogrodnik M., Lubbers E.R., Pirtskhalava T., White T.A., Johnson K.O., Stout M.B., Mezera V. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E6301–E6310. doi: 10.1073/pnas.1515386112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiley C.D., Velarde M.C., Lecot P., Liu S., Sarnoski E.A., Freund A., Shirakawa K., Lim H.W., Davis S.S., Ramanathan A. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metabol. 2016;23:303–314. doi: 10.1016/j.cmet.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dou Z., Ghosh K., Vizioli M.G., Zhu J., Sen P., Wangensteen K.J., Simithy J., Lan Y., Lin Y., Zhou Z. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature. 2017;550:402–406. doi: 10.1038/nature24050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang H., Wang H., Ren J., Chen Q., Chen Z.J. cGAS is essential for cellular senescence. Proc. Natl. Acad. Sci. U. S. A. 2017;114:E4612–E4620. doi: 10.1073/pnas.1705499114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laberge R.M., Sun Y., Orjalo A.V., Patil C.K., Freund A., Zhou L., Curran S.C., Davalos A.R., Wilson-Edell K.A., Liu S. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Biol. 2015;17:1049–1061. doi: 10.1038/ncb3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang B., Fu D., Xu Q., Cong X., Wu C., Zhong X., Ma Y., Lv Z., Chen F., Han L. The senescence-associated secretory phenotype is potentiated by feedforward regulatory mechanisms involving Zscan4 and TAK1. Nat. Commun. 2018;9:1723. doi: 10.1038/s41467-018-04010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang C., Xu Q., Martin T.D., Li M.Z., Demaria M., Aron L., Lu T., Yankner B.A., Campisi J., Elledge S.J. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science. 2015;349:aaa5612. doi: 10.1126/science.aaa5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xue W., Zender L., Miething C., Dickins R.A., Hernando E., Krizhanovsky V., Cordon-Cardo C., Lowe S.W. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pereira B.I., Devine O.P., Vukmanovic-Stejic M., Chambers E.S., Subramanian P., Patel N., Virasami A., Sebire N.J., Kinsler V., Valdovinos A. Senescent cells evade immune clearance via HLA-E-mediated NK and CD8(+) T cell inhibition. Nat. Commun. 2019;10:2387. doi: 10.1038/s41467-019-10335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faget D.V., Ren Q., Stewart S.A. Unmasking senescence: context-dependent effects of SASP in cancer. Nat. Rev. Canc. 2019;19:439–453. doi: 10.1038/s41568-019-0156-2. [DOI] [PubMed] [Google Scholar]
- 43.Rao S.G., Jackson J.G. SASP: tumor suppressor or promoter? Yes! Trends Canc. 2016;2:676–687. doi: 10.1016/j.trecan.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Saretzki G. Cellular senescence in the development and treatment of cancer. Curr. Pharmaceut. Des. 2010;16:79–100. doi: 10.2174/138161210789941874. [DOI] [PubMed] [Google Scholar]
- 45.Serrano M., Lin A.W., McCurrach M.E., Beach D., Lowe S.W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 46.Sarkisian C.J., Keister B.A., Stairs D.B., Boxer R.B., Moody S.E., Chodosh L.A. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat. Cell Biol. 2007;9:493–505. doi: 10.1038/ncb1567. [DOI] [PubMed] [Google Scholar]
- 47.Courtois-Cox S., Jones S.L., Cichowski K. Many roads lead to oncogene-induced senescence. Oncogene. 2008;27:2801–2809. doi: 10.1038/sj.onc.1210950. [DOI] [PubMed] [Google Scholar]
- 48.Liu J.Y., Souroullas G.P., Diekman B.O., Krishnamurthy J., Hall B.M., Sorrentino J.A., Parker J.S., Sessions G.A., Gudkov A.V., Sharpless N.E. Cells exhibiting strong p16 (INK4a) promoter activation in vivo display features of senescence. Proc. Natl. Acad. Sci. U. S. A. 2019;116:2603–2611. doi: 10.1073/pnas.1818313116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monasor A., Murga M., Lopez-Contreras A.J., Navas C., Gomez G., Pisano D.G., Fernandez-Capetillo O. INK4a/ARF limits the expansion of cells suffering from replication stress. Cell Cycle. 2013;12:1948–1954. doi: 10.4161/cc.25017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y., Xiong Y., Yarbrough W.G. ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell. 1998;92:725–734. doi: 10.1016/s0092-8674(00)81401-4. [DOI] [PubMed] [Google Scholar]
- 51.Carlos A.R., Escandell J.M., Kotsantis P., Suwaki N., Bouwman P., Badie S., Folio C., Benitez J., Gomez-Lopez G., Pisano D.G. ARF triggers senescence in Brca2-deficient cells by altering the spectrum of p53 transcriptional targets. Nat. Commun. 2013;4:2697. doi: 10.1038/ncomms3697. [DOI] [PubMed] [Google Scholar]
- 52.Di G.H., Liu Y., Lu Y., Liu J., Wu C., Duan H.F. IL-6 secreted from senescent mesenchymal stem cells promotes proliferation and migration of breast cancer cells. PloS One. 2014;9 doi: 10.1371/journal.pone.0113572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ortiz-Montero P., Londono-Vallejo A., Vernot J.P. Senescence-associated IL-6 and IL-8 cytokines induce a self- and cross-reinforced senescence/inflammatory milieu strengthening tumorigenic capabilities in the MCF-7 breast cancer cell line. Cell Commun. Signal. 2017;15:17. doi: 10.1186/s12964-017-0172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coppe J.P., Kauser K., Campisi J., Beausejour C.M. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J. Biol. Chem. 2006;281:29568–29574. doi: 10.1074/jbc.M603307200. [DOI] [PubMed] [Google Scholar]
- 55.Pazolli E., Luo X., Brehm S., Carbery K., Chung J.J., Prior J.L., Doherty J., Demehri S., Salavaggione L., Piwnica-Worms D. Senescent stromal-derived osteopontin promotes preneoplastic cell growth. Canc. Res. 2009;69:1230–1239. doi: 10.1158/0008-5472.CAN-08-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farsam V., Basu A., Gatzka M., Treiber N., Schneider L.A., Mulaw M.A., Lucas T., Kochanek S., Dummer R., Levesque M.P. Senescent fibroblast-derived Chemerin promotes squamous cell carcinoma migration. Oncotarget. 2016;7:83554–83569. doi: 10.18632/oncotarget.13446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu D., Hornsby P.J. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Canc. Res. 2007;67:3117–3126. doi: 10.1158/0008-5472.CAN-06-3452. [DOI] [PubMed] [Google Scholar]
- 58.Hwang H.J., Lee Y.R., Kang D., Lee H.C., Seo H.R., Ryu J.K., Kim Y.N., Ko Y.G., Park H.J., Lee J.S. Endothelial cells under therapy-induced senescence secrete CXCL11, which increases aggressiveness of breast cancer cells. Canc. Lett. 2020;490:100–110. doi: 10.1016/j.canlet.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 59.Tato-Costa J., Casimiro S., Pacheco T., Pires R., Fernandes A., Alho I., Pereira P., Costa P., Castelo H.B., Ferreira J. Therapy-induced cellular senescence induces epithelial-to-mesenchymal transition and increases invasiveness in rectal cancer. Clin. Colorectal Canc. 2016;15:170–178 e173. doi: 10.1016/j.clcc.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 60.Sabisz M., Skladanowski A. Cancer stem cells and escape from drug-induced premature senescence in human lung tumor cells: implications for drug resistance and in vitro drug screening models. Cell Cycle. 2009;8:3208–3217. doi: 10.4161/cc.8.19.9758. [DOI] [PubMed] [Google Scholar]
- 61.Milanovic M., Fan D.N.Y., Belenki D., Dabritz J.H.M., Zhao Z., Yu Y., Dorr J.R., Dimitrova L., Lenze D., Monteiro Barbosa I.A. Senescence-associated reprogramming promotes cancer stemness. Nature. 2018;553:96–100. doi: 10.1038/nature25167. [DOI] [PubMed] [Google Scholar]
- 62.Cruickshanks H.A., McBryan T., Nelson D.M., Vanderkraats N.D., Shah P.P., van Tuyn J., Singh Rai T., Brock C., Donahue G., Dunican D.S. Senescent cells harbour features of the cancer epigenome. Nat. Cell Biol. 2013;15:1495–1506. doi: 10.1038/ncb2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang B.D., Xuan Y., Broude E.V., Zhu H., Schott B., Fang J., Roninson I.B. Role of p53 and p21waf1/cip1 in senescence-like terminal proliferation arrest induced in human tumor cells by chemotherapeutic drugs. Oncogene. 1999;18:4808–4818. doi: 10.1038/sj.onc.1203078. [DOI] [PubMed] [Google Scholar]
- 64.Chang B.D., Broude E.V., Dokmanovic M., Zhu H., Ruth A., Xuan Y., Kandel E.S., Lausch E., Christov K., Roninson I.B. A senescence-like phenotype distinguishes tumor cells that undergo terminal proliferation arrest after exposure to anticancer agents. Canc. Res. 1999;59:3761–3767. [PubMed] [Google Scholar]
- 65.Jung S.H., Hwang H.J., Kang D., Park H.A., Lee H.C., Jeong D., Lee K., Park H.J., Ko Y.-G., Lee J.-S. mTOR kinase leads to PTEN-loss-induced cellular senescence by phosphorylating p53. Oncogene. 2019;38:1639–1650. doi: 10.1038/s41388-018-0521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iannello A., Thompson T.W., Ardolino M., Lowe S.W., Raulet D.H. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J. Exp. Med. 2013;210:2057–2069. doi: 10.1084/jem.20130783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lujambio A., Akkari L., Simon J., Grace D., Tschaharganeh D.F., Bolden J.E., Zhao Z., Thapar V., Joyce J.A., Krizhanovsky V. Non-cell-autonomous tumor suppression by p53. Cell. 2013;153:449–460. doi: 10.1016/j.cell.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saleh T., Tyutyunyk-Massey L., Gewirtz D.A. Tumor cell escape from therapy-induced senescence as a model of disease recurrence after dormancy. Canc. Res. 2019;79:1044–1046. doi: 10.1158/0008-5472.CAN-18-3437. [DOI] [PubMed] [Google Scholar]
- 69.Saleh T., Tyutyunyk-Massey L., Murray G.F., Alotaibi M.R., Kawale A.S., Elsayed Z., Henderson S.C., Yakovlev V., Elmore L.W., Toor A. Tumor cell escape from therapy-induced senescence. Biochem. Pharmacol. 2019;162:202–212. doi: 10.1016/j.bcp.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 70.Laud P.R., Multani A.S., Bailey S.M., Wu L., Ma J., Kingsley C., Lebel M., Pathak S., DePinho R.A., Chang S. Elevated telomere-telomere recombination in WRN-deficient, telomere dysfunctional cells promotes escape from senescence and engagement of the ALT pathway. Genes Dev. 2005;19:2560–2570. doi: 10.1101/gad.1321305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dirac A.M., Bernards R. Reversal of senescence in mouse fibroblasts through lentiviral suppression of p53. J. Biol. Chem. 2003;278:11731–11734. doi: 10.1074/jbc.C300023200. [DOI] [PubMed] [Google Scholar]
- 72.Was H., Barszcz K., Czarnecka J., Kowalczyk A., Bernas T., Uzarowska E., Koza P., Klejman A., Piwocka K., Kaminska B. Bafilomycin A1 triggers proliferative potential of senescent cancer cells in vitro and in NOD/SCID mice. Oncotarget. 2017;8:9303–9322. doi: 10.18632/oncotarget.14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beausejour C.M., Krtolica A., Galimi F., Narita M., Lowe S.W., Yaswen P., Campisi J. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–4222. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sies H. Hydroperoxides and thiol oxidants in the study of oxidative stress in intact cells and organs. Oxid. Stress. 1985;1:73–90. [Google Scholar]
- 75.Cui M.S., Wang X.L., Tang D.W., Zhang J., Liu Y., Zeng S.M. Acetylation of H4K12 in porcine oocytes during in vitro aging: potential role of ooplasmic reactive oxygen species. Theriogenology. 2011;75:638–646. doi: 10.1016/j.theriogenology.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 76.Sergiev P.V., Dontsova O.A., Berezkin G.V. Theories of aging: an ever-evolving field. Acta Naturae. 2015;7:9–18. [PMC free article] [PubMed] [Google Scholar]
- 77.Sawada M., Carlson J.C. Changes in superoxide radical and lipid peroxide formation in the brain, heart and liver during the lifetime of the rat. Mech. Ageing Dev. 1987;41:125–137. doi: 10.1016/0047-6374(87)90057-1. [DOI] [PubMed] [Google Scholar]
- 78.Chung H.Y., Song S.H., Kim H.J., Ikeno Y., Yu B.P. Modulation of renal xanthine oxidoreductase in aging: gene expression and reactive oxygen species generation. J. Nutr. Health Aging. 1999;3:19–23. [PubMed] [Google Scholar]
- 79.Liguori I., Russo G., Curcio F., Bulli G., Aran L., Della-Morte D., Gargiulo G., Testa G., Cacciatore F., Bonaduce D. Oxidative stress, aging, and diseases. Clin. Interv. Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Korolchuk V.I., Miwa S., Carroll B., von Zglinicki T. Mitochondria in cell senescence: is mitophagy the weakest link? EBioMedicine. 2017;21:7–13. doi: 10.1016/j.ebiom.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Passos J.F., Nelson G., Wang C., Richter T., Simillion C., Proctor C.J., Miwa S., Olijslagers S., Hallinan J., Wipat A. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol. Syst. Biol. 2010;6:347. doi: 10.1038/msb.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nelson G., Kucheryavenko O., Wordsworth J., von Zglinicki T. The senescent bystander effect is caused by ROS-activated NF-kappaB signalling. Mech. Ageing Dev. 2018;170:30–36. doi: 10.1016/j.mad.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kodama R., Kato M., Furuta S., Ueno S., Zhang Y., Matsuno K., Yabe-Nishimura C., Tanaka E., Kamata T. ROS-generating oxidases Nox1 and Nox4 contribute to oncogenic Ras-induced premature senescence. Gene Cell. 2013;18:32–41. doi: 10.1111/gtc.12015. [DOI] [PubMed] [Google Scholar]
- 84.Ogrunc M., Di Micco R., Liontos M., Bombardelli L., Mione M., Fumagalli M., Gorgoulis V.G., d'Adda di Fagagna F. Oncogene-induced reactive oxygen species fuel hyperproliferation and DNA damage response activation. Cell Death Differ. 2014;21:998–1012. doi: 10.1038/cdd.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iskandar K., Rezlan M., Yadav S.K., Foo C.H., Sethi G., Qiang Y., Bellot G.L., Pervaiz S. Synthetic lethality of a novel small molecule against mutant KRAS-expressing cancer cells involves AKT-dependent ROS production. Antioxidants Redox Signal. 2016;24:781–794. doi: 10.1089/ars.2015.6362. [DOI] [PubMed] [Google Scholar]
- 86.Allsopp R.C., Vaziri H., Patterson C., Goldstein S., Younglai E.V., Futcher A.B., Greider C.W., Harley C.B. Telomere length predicts replicative capacity of human fibroblasts. Proc. Natl. Acad. Sci. U. S. A. 1992;89:10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hutter E., Unterluggauer H., Uberall F., Schramek H., Jansen-Durr P. Replicative senescence of human fibroblasts: the role of Ras-dependent signaling and oxidative stress. Exp. Gerontol. 2002;37:1165–1174. doi: 10.1016/s0531-5565(02)00136-5. [DOI] [PubMed] [Google Scholar]
- 88.Saretzki G., Von Zglinicki T. Replicative aging, telomeres, and oxidative stress. Ann. N. Y. Acad. Sci. 2002;959:24–29. doi: 10.1111/j.1749-6632.2002.tb02079.x. [DOI] [PubMed] [Google Scholar]
- 89.Di Micco R., Cicalese A., Fumagalli M., Dobreva M., Verrecchia A., Pelicci P.G., di Fagagna F. DNA damage response activation in mouse embryonic fibroblasts undergoing replicative senescence and following spontaneous immortalization. Cell Cycle. 2008;7:3601–3606. doi: 10.4161/cc.7.22.7152. [DOI] [PubMed] [Google Scholar]
- 90.d'Adda di Fagagna F., Reaper P.M., Clay-Farrace L., Fiegler H., Carr P., Von Zglinicki T., Saretzki G., Carter N.P., Jackson S.P. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 91.Venkatachalam G., Surana U., Clement M.V. Replication stress-induced endogenous DNA damage drives cellular senescence induced by a sub-lethal oxidative stress. Nucleic Acids Res. 2017;45:10564–10582. doi: 10.1093/nar/gkx684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Macip S., Igarashi M., Berggren P., Yu J., Lee S.W., Aaronson S.A. Influence of induced reactive oxygen species in p53-mediated cell fate decisions. Mol. Cell Biol. 2003;23:8576–8585. doi: 10.1128/MCB.23.23.8576-8585.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Macip S., Igarashi M., Fang L., Chen A., Pan Z.Q., Lee S.W., Aaronson S.A. Inhibition of p21-mediated ROS accumulation can rescue p21-induced senescence. EMBO J. 2002;21:2180–2188. doi: 10.1093/emboj/21.9.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takahashi A., Ohtani N., Yamakoshi K., Iida S., Tahara H., Nakayama K., Nakayama K.I., Ide T., Saya H., Hara E. Mitogenic signalling and the p16INK4a-Rb pathway cooperate to enforce irreversible cellular senescence. Nat. Cell Biol. 2006;8:1291–1297. doi: 10.1038/ncb1491. [DOI] [PubMed] [Google Scholar]
- 95.Sies H., Jones D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020;21:363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 96.Sies H., Berndt C., Jones D.P. Oxidative stress. Annu. Rev. Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 97.Jones D.P., Sies H. The redox code. Antioxidants Redox Signal. 2015;23:734–746. doi: 10.1089/ars.2015.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pervaiz S., Cao J., Chao O.S.P., Chin Y.Y., Clément M.-V. Activation of the RacGTPase inhibits apoptosis in human tumor cells. Oncogene. 2001;20:6263–6268. doi: 10.1038/sj.onc.1204840. [DOI] [PubMed] [Google Scholar]
- 99.Clément M.V., Hirpara J.L., Pervaiz S. Decrease in intracellular superoxide sensitizes Bcl-2-overexpressing tumor cells to receptor and drug-induced apoptosis independent of the mitochondria. Cell Death Differ. 2003;10:1273–1285. doi: 10.1038/sj.cdd.4401302. [DOI] [PubMed] [Google Scholar]
- 100.Chen Z.X., Pervaiz S. Bcl-2 induces pro-oxidant state by engaging mitochondrial respiration in tumor cells. Cell Death Differ. 2007;14:1617–1627. doi: 10.1038/sj.cdd.4402165. [DOI] [PubMed] [Google Scholar]
- 101.Tochhawng L., Deng S., Pugalenthi G., Kumar A.P., Lim K.H., Tan T.Z., Yang H., Hooi S.C., Goh Y.C., Maciver S.K. Gelsolin-Cu/ZnSOD interaction alters intracellular reactive oxygen species levels to promote cancer cell invasion. Oncotarget. 2016;7:52832–52848. doi: 10.18632/oncotarget.10451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Raman D., Chong S.J.F., Iskandar K., Hirpara J.L., Pervaiz S. Peroxynitrite promotes serine-62 phosphorylation-dependent stabilization of the oncoprotein c-Myc. Redox Biol. 2020;34 doi: 10.1016/j.redox.2020.101587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hirpara J.L., Subramaniam K., Bellot G., Qu J., Seah S., Loh T., Tucker-Kellogg L., Clement M.V., Pervaiz S. Superoxide induced inhibition of death receptor signaling is mediated via induced expression of apoptosis inhibitory protein cFLIP. Redox Biol. 2020;30 doi: 10.1016/j.redox.2019.101403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chong S.J.F., Iskandar K., Lai J.X.H., Qu J., Raman D., Valentin R., Herbaux C., Collins M., Low I.C.C., Loh T. Serine-70 phosphorylated Bcl-2 prevents oxidative stress-induced DNA damage by modulating the mitochondrial redox metabolism. Nucleic Acids Res. 2020;48:12727–12745. doi: 10.1093/nar/gkaa1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Furukawa A., Tada-Oikawa S., Kawanishi S., Oikawa S. H2O2 accelerates cellular senescence by accumulation of acetylated p53 via decrease in the function of SIRT1 by NAD+ depletion. Cell. Physiol. Biochem. 2007;20 doi: 10.1159/000104152. [DOI] [PubMed] [Google Scholar]
- 106.Marazita M.C., Dugour A., Marquioni-Ramella M.D., Figueroa J.M., Suburo A.M. Oxidative stress-induced premature senescence dysregulates VEGF and CFH expression in retinal pigment epithelial cells: implications for Age-related Macular Degeneration. Redox Biol. 2016;7:78–87. doi: 10.1016/j.redox.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen Q., Ames B.N. Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells. Proc. Natl. Acad. Sci. U. S. A. 1994;91:4130–4134. doi: 10.1073/pnas.91.10.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marazita M.C., Dugour A., Marquioni-Ramella M.D., Figueroa J.M., Suburo A.M. Oxidative stress-induced premature senescence dysregulates VEGF and CFH expression in retinal pigment epithelial cells: implications for age-related macular degeneration. Redox Biol. 2016;7:78–87. doi: 10.1016/j.redox.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen Q., Ames B.N. Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells. Proc. Natl. Acad. Sci. Unit. States Am. 1994;91:4130–4134. doi: 10.1073/pnas.91.10.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sin S., Kim S.Y., Kim S.S. Chronic treatment with ginsenoside Rg3 induces Akt-dependent senescence in human glioma cells. Int. J. Oncol. 2012;41:1669–1674. doi: 10.3892/ijo.2012.1604. [DOI] [PubMed] [Google Scholar]
- 111.Kwak J.Y., Ham H.J., Kim C.M., Hwang E.S. Nicotinamide exerts antioxidative effects on senescent cells. Mol. Cell. 2015;38:229. doi: 10.14348/molcells.2015.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Deng X., Gao F., May W.S., Jr. Bcl2 retards G1/S cell cycle transition by regulating intracellular ROS. Blood. 2003;102:3179–3185. doi: 10.1182/blood-2003-04-1027. [DOI] [PubMed] [Google Scholar]
- 113.Debidda M., Williams D.A., Zheng Y. Rac1 GTPase regulates cell genomic stability and senescence. J. Biol. Chem. 2006;281:38519–38528. doi: 10.1074/jbc.M604607200. [DOI] [PubMed] [Google Scholar]
- 114.Chong S.J.F., Lai J.X.H., Qu J., Hirpara J., Kang J., Swaminathan K., Loh T., Kumar A., Vali S., Abbasi T. A feedforward relationship between active Rac1 and phosphorylated Bcl-2 is critical for sustaining Bcl-2 phosphorylation and promoting cancer progression. Canc. Lett. 2019;457:151–167. doi: 10.1016/j.canlet.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 115.Ashraf H.M., Moser J., Spencer S.L. Senescence evasion in chemotherapy: a sweet spot for p21. Cell. 2019;178:267–269. doi: 10.1016/j.cell.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Roninson I.B. Tumor cell senescence in cancer treatment. Canc. Res. 2003;63:2705–2715. [PubMed] [Google Scholar]
- 117.Roninson I.B., Broude E.V., Chang B.-D. If not apoptosis, then what? Treatment-induced senescence and mitotic catastrophe in tumor cells. Drug Resist. Updates. 2001;4:303–313. doi: 10.1054/drup.2001.0213. [DOI] [PubMed] [Google Scholar]
- 118.Guillon J., Petit C., Toutain B., Guette C., Lelievre E., Coqueret O. Chemotherapy-induced senescence, an adaptive mechanism driving resistance and tumor heterogeneity. Cell Cycle. 2019;18:2385–2397. doi: 10.1080/15384101.2019.1652047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Achuthan S., Santhoshkumar T.R., Prabhakar J., Nair S.A., Pillai M.R. Drug-induced senescence generates chemoresistant stemlike cells with low reactive oxygen species. J. Biol. Chem. 2011;286:37813–37829. doi: 10.1074/jbc.M110.200675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Elmore L.W., Di X., Dumur C., Holt S.E., Gewirtz D.A. Evasion of a single-step, chemotherapy-induced senescence in breast cancer cells: implications for treatment response. Clin. Canc. Res. 2005;11:2637–2643. doi: 10.1158/1078-0432.CCR-04-1462. [DOI] [PubMed] [Google Scholar]
- 121.Collado M., Gil J., Efeyan A., Guerra C., Schuhmacher A.J., Barradas M., Benguría A., Zaballos A., Flores J.M., Barbacid M. Senescence in premalignant tumours. Nature. 2005;436 doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 122.Ewald J.A., Desotelle J.A., Wilding G., Jarrard D.F. Therapy-induced senescence in cancer. JNCI: J. Natl. Cancer Inst. 2010;102:1536–1546. doi: 10.1093/jnci/djq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Campisi J., Andersen J.K., Kapahi P., Melov S. vol. 21. Elsevier; 2011. pp. 354–359. (Seminars in Cancer Biology). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Beauséjour C.M., Krtolica A., Galimi F., Narita M., Lowe S.W., Yaswen P., Campisi J. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–4222. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yu J., Wang Z., Kinzler K.W., Vogelstein B., Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc. Natl. Acad. Sci. Unit. States Am. 2003;100:1931–1936. doi: 10.1073/pnas.2627984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chong S.J.F., Marchi S., Petroni G., Kroemer G., Galluzzi L., Pervaiz S. Noncanonical cell fate regulation by bcl-2 proteins. Trends Cell Biol. 2020;30:537–555. doi: 10.1016/j.tcb.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 127.Yin D.X., Schimke R.T. BCL-2 expression delays drug-induced apoptosis but does not increase clonogenic survival after drug treatment in HeLa cells. Canc. Res. 1995;55:4922–4928. [PubMed] [Google Scholar]
- 128.Crescenzi E., Palumbo G., Brady H.J. Bcl-2 activates a programme of premature senescence in human carcinoma cells. Biochem. J. 2003;375:263–274. doi: 10.1042/BJ20030868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hayward R.L., Macpherson J.S., Cummings J., Monia B.P., Smyth J.F., Jodrell D.I. Antisense Bcl-xl down-regulation switches the response to topoisomerase I inhibition from senescence to apoptosis in colorectal cancer cells, enhancing global cytotoxicity. Clin. Canc. Res. 2003;9:2856–2865. [PubMed] [Google Scholar]
- 130.Gayle S.S., Sahni J.M., Webb B.M., Weber-Bonk K.L., Shively M.S., Spina R., Bar E.E., Summers M.K., Keri R.A. Targeting BCL-xL improves the efficacy of bromodomain and extra-terminal protein inhibitors in triple-negative breast cancer by eliciting the death of senescent cells. J. Biol. Chem. 2019;294:875–886. doi: 10.1074/jbc.RA118.004712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pluquet O., Abbadie C., Coqueret O. Connecting cancer relapse with senescence. Canc. Lett. 2019;463:50–58. doi: 10.1016/j.canlet.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 132.Kim E.C., Kim J.R. Senotherapeutics: emerging strategy for healthy aging and age-related disease. BMB Rep. 2019;52:47–55. doi: 10.5483/BMBRep.2019.52.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Childs B.G., Gluscevic M., Baker D.J., Laberge R.M., Marquess D., Dananberg J., van Deursen J.M. Senescent cells: an emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017;16:718–735. doi: 10.1038/nrd.2017.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Demaria M., O'Leary M.N., Chang J., Shao L., Liu S., Alimirah F., Koenig K., Le C., Mitin N., Deal A.M. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Canc. Discov. 2017;7:165–176. doi: 10.1158/2159-8290.CD-16-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Myrianthopoulos V., Evangelou K., Vasileiou P.V.S., Cooks T., Vassilakopoulos T.P., Pangalis G.A., Kouloukoussa M., Kittas C., Georgakilas A.G., Gorgoulis V.G. Senescence and senotherapeutics: a new field in cancer therapy. Pharmacol. Ther. 2019;193:31–49. doi: 10.1016/j.pharmthera.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 136.Kim J.K., Park S.U. Quercetin and its role in biological functions: an updated review. EXCLI J. 2018;17:856–863. doi: 10.17179/excli2018-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Feitelson M.A., Arzumanyan A., Kulathinal R.J., Blain S.W., Holcombe R.F., Mahajna J., Marino M., Martinez-Chantar M.L., Nawroth R., Sanchez-Garcia I. Sustained proliferation in cancer: mechanisms and novel therapeutic targets. Semin. Canc. Biol. 2015;35:S25–S54. doi: 10.1016/j.semcancer.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Murakami A., Ashida H., Terao J. Multitargeted cancer prevention by quercetin. Canc. Lett. 2008;269:315–325. doi: 10.1016/j.canlet.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 139.Zhu Y., Tchkonia T., Pirtskhalava T., Gower A.C., Ding H., Giorgadze N., Palmer A.K., Ikeno Y., Hubbard G.B., Lenburg M. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhu Y., Teng T., Wang H., Guo H., Du L., Yang B., Yin X., Sun Y. Quercetin inhibits renal cyst growth in vitro and via parenteral injection in a polycystic kidney disease mouse model. Food Funct. 2018;9:389–396. doi: 10.1039/c7fo01253e. [DOI] [PubMed] [Google Scholar]
- 141.Nguyen L.T., Lee Y.H., Sharma A.R., Park J.B., Jagga S., Sharma G., Lee S.S., Nam J.S. Quercetin induces apoptosis and cell cycle arrest in triple-negative breast cancer cells through modulation of Foxo3a activity. KOREAN J. PHYSIOL. PHARMACOL. 2017;21:205–213. doi: 10.4196/kjpp.2017.21.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Han M.K., Barreto T.A., Martinez F.J., Comstock A.T., Sajjan U.S. Randomised clinical trial to determine the safety of quercetin supplementation in patients with chronic obstructive pulmonary disease. BMJ Open Respir. Res. 2020;7 doi: 10.1136/bmjresp-2018-000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kerimi A., Williamson G. Differential impact of flavonoids on redox modulation, bioenergetics, and cell signaling in normal and tumor cells: a comprehensive review. Antioxidants Redox Signal. 2018;29:1633–1659. doi: 10.1089/ars.2017.7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mostafavi-Pour Z., Ramezani F., Keshavarzi F., Samadi N. The role of quercetin and vitamin C in Nrf2-dependent oxidative stress production in breast cancer cells. Oncol. Lett. 2017;13:1965–1973. doi: 10.3892/ol.2017.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hickson L.J., Langhi Prata L.G.P., Bobart S.A., Evans T.K., Giorgadze N., Hashmi S.K., Herrmann S.M., Jensen M.D., Jia Q., Jordan K.L. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine. 2019;47:446–456. doi: 10.1016/j.ebiom.2019.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schafer M.J., White T.A., Iijima K., Haak A.J., Ligresti G., Atkinson E.J., Oberg A.L., Birch J., Salmonowicz H., Zhu Y. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017;8:14532. doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lehmann M., Korfei M., Mutze K., Klee S., Skronska-Wasek W., Alsafadi H.N., Ota C., Costa R., Schiller H.B., Lindner M. Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur. Respir. J. 2017;50 doi: 10.1183/13993003.02367-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Justice J.N., Nambiar A.M., Tchkonia T., LeBrasseur N.K., Pascual R., Hashmi S.K., Prata L., Masternak M.M., Kritchevsky S.B., Musi N. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–563. doi: 10.1016/j.ebiom.2018.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang P., Kishimoto Y., Grammatikakis I., Gottimukkala K., Cutler R.G., Zhang S., Abdelmohsen K., Bohr V.A., Misra Sen J., Gorospe M. Senolytic therapy alleviates Abeta-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer's disease model. Nat. Neurosci. 2019;22:719–728. doi: 10.1038/s41593-019-0372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhu Y., Doornebal E.J., Pirtskhalava T., Giorgadze N., Wentworth M., Fuhrmann-Stroissnigg H., Niedernhofer L.J., Robbins P.D., Tchkonia T., Kirkland J.L. New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging (Albany NY) 2017;9:955–963. doi: 10.18632/aging.101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kovacovicova K., Skolnaja M., Heinmaa M., Mistrik M., Pata P., Pata I., Bartek J., Vinciguerra M. Senolytic cocktail Dasatinib+Quercetin (D+Q) does not enhance the efficacy of senescence-inducing chemotherapy in liver cancer. Front. Oncol. 2018;8:459. doi: 10.3389/fonc.2018.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Arai Y., Watanabe S., Kimira M., Shimoi K., Mochizuki R., Kinae N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J. Nutr. 2000;130:2243–2250. doi: 10.1093/jn/130.9.2243. [DOI] [PubMed] [Google Scholar]
- 153.Kashyap D., Mittal S., Sak K., Singhal P., Tuli H.S. Molecular mechanisms of action of quercetin in cancer: recent advances. Tumour Biol. 2016;37:12927–12939. doi: 10.1007/s13277-016-5184-x. [DOI] [PubMed] [Google Scholar]
- 154.Lall R.K., Adhami V.M., Mukhtar H. Dietary flavonoid fisetin for cancer prevention and treatment. Mol. Nutr. Food Res. 2016;60:1396–1405. doi: 10.1002/mnfr.201600025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Youns M., Abdel Halim Hegazy W. The natural flavonoid fisetin inhibits cellular proliferation of hepatic, colorectal, and pancreatic cancer cells through modulation of multiple signaling pathways. PloS One. 2017;12 doi: 10.1371/journal.pone.0169335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kang K.A., Piao M.J., Kim K.C., Cha J.W., Zheng J., Yao C.W., Chae S., Hyun J.W. Fisetin attenuates hydrogen peroxide-induced cell damage by scavenging reactive oxygen species and activating protective functions of cellular glutathione system. In Vitro Cell. Dev. Biol. Anim. 2014;50:66–74. doi: 10.1007/s11626-013-9681-6. [DOI] [PubMed] [Google Scholar]
- 157.Yang P.M., Tseng H.H., Peng C.W., Chen W.S., Chiu S.J. Dietary flavonoid fisetin targets caspase-3-deficient human breast cancer MCF-7 cells by induction of caspase-7-associated apoptosis and inhibition of autophagy. Int. J. Oncol. 2012;40:469–478. doi: 10.3892/ijo.2011.1203. [DOI] [PubMed] [Google Scholar]
- 158.Li J., Qu W., Cheng Y., Sun Y., Jiang Y., Zou T., Wang Z., Xu Y., Zhao H. The inhibitory effect of intravesical fisetin against bladder cancer by induction of p53 and down-regulation of NF-kappa B pathways in a rat bladder carcinogenesis model. Basic Clin. Pharmacol. Toxicol. 2014;115:321–329. doi: 10.1111/bcpt.12229. [DOI] [PubMed] [Google Scholar]
- 159.Yousefzadeh M.J., Zhu Y., McGowan S.J., Angelini L., Fuhrmann-Stroissnigg H., Xu M., Ling Y.Y., Melos K.I., Pirtskhalava T., Inman C.L. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. 2018;36:18–28. doi: 10.1016/j.ebiom.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bezerra D.P., Pessoa C., de Moraes M.O., Saker-Neto N., Silveira E.R., Costa-Lotufo L.V. Overview of the therapeutic potential of piplartine (piperlongumine) Eur. J. Pharmaceut. Sci. 2013;48:453–463. doi: 10.1016/j.ejps.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 161.Piska K., Gunia-Krzyzak A., Koczurkiewicz P., Wojcik-Pszczola K., Pekala E. Piperlongumine (piplartine) as a lead compound for anticancer agents - synthesis and properties of analogues: a mini-review. Eur. J. Med. Chem. 2018;156:13–20. doi: 10.1016/j.ejmech.2018.06.057. [DOI] [PubMed] [Google Scholar]
- 162.Piska K., Koczurkiewicz P., Wnuk D., Karnas E., Bucki A., Wojcik-Pszczola K., Jamrozik M., Michalik M., Kolaczkowski M., Pekala E. Synergistic anticancer activity of doxorubicin and piperlongumine on DU-145 prostate cancer cells - the involvement of carbonyl reductase 1 inhibition. Chem. Biol. Interact. 2019;300:40–48. doi: 10.1016/j.cbi.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 163.Wang Y., Chang J., Liu X., Zhang X., Zhang S., Zhang X., Zhou D., Zheng G. Discovery of piperlongumine as a potential novel lead for the development of senolytic agents. Aging (Albany NY) 2016;8:2915–2926. doi: 10.18632/aging.101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang X., Zhang S., Liu X., Wang Y., Chang J., Zhang X., Mackintosh S.G., Tackett A.J., He Y., Lv D. Oxidation resistance 1 is a novel senolytic target. Aging Cell. 2018;17 doi: 10.1111/acel.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zheng J., Son D.J., Gu S.M., Woo J.R., Ham Y.W., Lee H.P., Kim W.J., Jung J.K., Hong J.T. Piperlongumine inhibits lung tumor growth via inhibition of nuclear factor kappa B signaling pathway. Sci. Rep. 2016;6:26357. doi: 10.1038/srep26357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li W., He Y., Zhang R., Zheng G., Zhou D. The curcumin analog EF24 is a novel senolytic agent. Aging (Albany NY) 2019;11:771–782. doi: 10.18632/aging.101787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Guerrero A., Herranz N., Sun B., Wagner V., Gallage S., Guiho R., Wolter K., Pombo J., Irvine E.E., Innes A.J. Cardiac glycosides are broad-spectrum senolytics. Nat. Metab. 2019;1:1074–1088. doi: 10.1038/s42255-019-0122-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Triana-Martinez F., Picallos-Rabina P., Da Silva-Alvarez S., Pietrocola F., Llanos S., Rodilla V., Soprano E., Pedrosa P., Ferreiros A., Barradas M. Identification and characterization of Cardiac Glycosides as senolytic compounds. Nat. Commun. 2019;10:4731. doi: 10.1038/s41467-019-12888-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhou Y., Zhou Y., Yang M., Wang K., Liu Y., Zhang M., Yang Y., Jin C., Wang R., Hu R. Digoxin sensitizes gemcitabine-resistant pancreatic cancer cells to gemcitabine via inhibiting Nrf2 signaling pathway. Redox Biol. 2019;22 doi: 10.1016/j.redox.2019.101131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chang J., Wang Y., Shao L., Laberge R.M., Demaria M., Campisi J., Janakiraman K., Sharpless N.E., Ding S., Feng W. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 2016;22:78–83. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yosef R., Pilpel N., Tokarsky-Amiel R., Biran A., Ovadya Y., Cohen S., Vadai E., Dassa L., Shahar E., Condiotti R. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat. Commun. 2016;7:11190. doi: 10.1038/ncomms11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhu Y., Tchkonia T., Fuhrmann-Stroissnigg H., Dai H.M., Ling Y.Y., Stout M.B., Pirtskhalava T., Giorgadze N., Johnson K.O., Giles C.B. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell. 2016;15:428–435. doi: 10.1111/acel.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mavrogonatou E., Pratsinis H., Kletsas D. The role of senescence in cancer development. Semin. Canc. Biol. 2020;62:182–191. doi: 10.1016/j.semcancer.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 174.Grezella C., Fernandez-Rebollo E., Franzen J., Ventura Ferreira M.S., Beier F., Wagner W. Effects of senolytic drugs on human mesenchymal stromal cells. Stem Cell Res. Ther. 2018;9:108. doi: 10.1186/s13287-018-0857-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang L., Leite de Oliveira R., Wang C., Fernandes Neto J.M., Mainardi S., Evers B., Lieftink C., Morris B., Jochems F., Willemsen L. High-throughput functional genetic and compound screens identify targets for senescence induction in cancer. Cell Rep. 2017;21:773–783. doi: 10.1016/j.celrep.2017.09.085. [DOI] [PubMed] [Google Scholar]
- 176.Shahbandi A., Rao S.G., Anderson A.Y., Frey W.D., Olayiwola J.O., Ungerleider N.A., Jackson J.G. BH3 mimetics selectively eliminate chemotherapy-induced senescent cells and improve response in TP53 wild-type breast cancer. Cell Death Differ. 2020;27:3097–3116. doi: 10.1038/s41418-020-0564-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pungsrinont T., Sutter M.F., Ertingshausen M., Lakshmana G., Kokal M., Khan A.S., Baniahmad A. Senolytic compounds control a distinct fate of androgen receptor agonist- and antagonist-induced cellular senescent LNCaP prostate cancer cells. Cell Biosci. 2020;10:59. doi: 10.1186/s13578-020-00422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hantel A., Wynne J., Lacayo N., Khaw S.L., Rubnitz J., Mullighan C., Schmidt M., Zhou Y., Ross J.A., Rosenwinkel L. Safety and efficacy of the BCL inhibitors venetoclax and navitoclax in combination with chemotherapy in patients with relapsed/refractory acute lymphoblastic leukemia and lymphoblastic lymphoma. Clin. Lymphoma, Myeloma & Leukemia. 2018;18:S184–S185. [Google Scholar]
- 179.Gonzalez-Gualda E., Paez-Ribes M., Lozano-Torres B., Macias D., Wilson J.R., 3rd, Gonzalez-Lopez C., Ou H.L., Miron-Barroso S., Zhang Z., Lerida-Viso A. Galacto-conjugation of Navitoclax as an efficient strategy to increase senolytic specificity and reduce platelet toxicity. Aging Cell. 2020;19 doi: 10.1111/acel.13142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.He Y., Zhang X., Chang J., Kim H.-N., Zhang P., Wang Y., Khan S., Liu X., Zhang X., Lv D. Using proteolysis-targeting chimera technology to reduce navitoclax platelet toxicity and improve its senolytic activity. Nat. Commun. 2020;11:1996. doi: 10.1038/s41467-020-15838-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Leverson J.D., Phillips D.C., Mitten M.J., Boghaert E.R., Diaz D., Tahir S.K., Belmont L.D., Nimmer P., Xiao Y., Ma X.M. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.aaa4642. [DOI] [PubMed] [Google Scholar]
- 182.Jeon O.H., Kim C., Laberge R.M., Demaria M., Rathod S., Vasserot A.P., Chung J.W., Kim D.H., Poon Y., David N. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 2017;23:775–781. doi: 10.1038/nm.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Li J., Kim S.G., Blenis J. Rapamycin: one drug, many effects. Cell Metabol. 2014;19:373–379. doi: 10.1016/j.cmet.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Herranz N., Gallage S., Mellone M., Wuestefeld T., Klotz S., Hanley C.J., Raguz S., Acosta J.C., Innes A.J., Banito A. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat. Cell Biol. 2015;17:1205–1217. doi: 10.1038/ncb3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Correia-Melo C., Birch J., Fielder E., Rahmatika D., Taylor J., Chapman J., Lagnado A., Carroll B.M., Miwa S., Richardson G. Rapamycin improves healthspan but not inflammaging in nfkappab1(-/-) mice. Aging Cell. 2019;18 doi: 10.1111/acel.12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Imrali A., Mao X., Yeste-Velasco M., Shamash J., Lu Y. Rapamycin inhibits prostate cancer cell growth through cyclin D1 and enhances the cytotoxic efficacy of cisplatin. Am. J. Canc. Res. 2016;6:1772–1784. [PMC free article] [PubMed] [Google Scholar]
- 187.Walters H.E., Cox L.S. mTORC inhibitors as broad-spectrum therapeutics for age-related diseases. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19082325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ngoi N.Y.L., Eu J.Q., Hirpara J., Wang L., Lim J.S.J., Lee S.C., Lim Y.C., Pervaiz S., Goh B.C., Wong A.L.A. Targeting cell metabolism as cancer therapy. Antioxidants Redox Signal. 2020;32:285–308. doi: 10.1089/ars.2019.7947. [DOI] [PubMed] [Google Scholar]
- 189.Algire C., Moiseeva O., Deschenes-Simard X., Amrein L., Petruccelli L., Birman E., Viollet B., Ferbeyre G., Pollak M.N. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Canc. Prev. Res. (Phila) 2012;5:536–543. doi: 10.1158/1940-6207.CAPR-11-0536. [DOI] [PubMed] [Google Scholar]
- 190.Moiseeva O., Deschenes-Simard X., St-Germain E., Igelmann S., Huot G., Cadar A.E., Bourdeau V., Pollak M.N., Ferbeyre G. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-kappaB activation. Aging Cell. 2013;12:489–498. doi: 10.1111/acel.12075. [DOI] [PubMed] [Google Scholar]
- 191.Sinnett-Smith J., Kisfalvi K., Kui R., Rozengurt E. Metformin inhibition of mTORC1 activation, DNA synthesis and proliferation in pancreatic cancer cells: dependence on glucose concentration and role of AMPK. Biochem. Biophys. Res. Commun. 2013;430:352–357. doi: 10.1016/j.bbrc.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Samaraweera L., Adomako A., Rodriguez-Gabin A., McDaid H.M. A novel indication for panobinostat as a senolytic drug in NSCLC and HNSCC. Sci. Rep. 2017;7:1900. doi: 10.1038/s41598-017-01964-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Filippakopoulos P., Qi J., Picaud S., Shen Y., Smith W.B., Fedorov O., Morse E.M., Keates T., Hickman T.T., Felletar I. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tasdemir N., Banito A., Roe J.S., Alonso-Curbelo D., Camiolo M., Tschaharganeh D.F., Huang C.H., Aksoy O., Bolden J.E., Chen C.C. BRD4 connects enhancer remodeling to senescence immune surveillance. Canc. Discov. 2016;6:612–629. doi: 10.1158/2159-8290.CD-16-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wakita M., Takahashi A., Sano O., Loo T.M., Imai Y., Narukawa M., Iwata H., Matsudaira T., Kawamoto S., Ohtani N. A BET family protein degrader provokes senolysis by targeting NHEJ and autophagy in senescent cells. Nat. Commun. 2020;11:1935. doi: 10.1038/s41467-020-15719-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ozsvari B., Nuttall J.R., Sotgia F., Lisanti M.P. Azithromycin and Roxithromycin define a new family of "senolytic" drugs that target senescent human fibroblasts. Aging (Albany NY) 2018;10:3294–3307. doi: 10.18632/aging.101633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Nogueira-Recalde U., Lorenzo-Gomez I., Blanco F.J., Loza M.I., Grassi D., Shirinsky V., Shirinsky I., Lotz M., Robbins P.D., Dominguez E. Fibrates as drugs with senolytic and autophagic activity for osteoarthritis therapy. EBioMedicine. 2019;45:588–605. doi: 10.1016/j.ebiom.2019.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sapieha P., Mallette F.A. Cellular senescence in postmitotic cells: beyond growth arrest. Trends Cell Biol. 2018;28:595–607. doi: 10.1016/j.tcb.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 199.von Zglinicki T., Wan T., Miwa S. Senescence in post-mitotic cells: a driver of aging? Antioxidants Redox Signal. 2021;34:308–323. doi: 10.1089/ars.2020.8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Clement M.V., Luo L. Organismal aging and oxidants beyond macromolecules damage. Proteomics. 2020;20 doi: 10.1002/pmic.201800400. [DOI] [PubMed] [Google Scholar]