Abstract
The involvement of viruses and SARS-CoV-2 in autoimmune diseases is well known. The recent demonstration that ChAdOx1 nCoV-19 Covid-19 (AstraZeneca) vaccine (ChA) favors the production of anti-platelet factor 4 (anti-PF4) antibodies, blood clots, and thrombocytopenia raises the question of whether other anti-CoViD-19 vaccines favor the same patterns of events.
We assessed the frequency of severe adverse events (SAEs) documented in the EudraVigilance European database up to April 16, 2021 related to thrombocytopenia, bleeding, and blood clots in recipients of ChA compared to that of recipients of the BNT162b2 Covid-19 (Pfizer/BioNTech) vaccine (BNT).
ChA administration was associated with a much higher frequency of SAEs in each AE Reaction Group as compared with that elicited by BNT. When considering AEs caused by thrombocytopenia, bleeding and blood clots, we observed 33 and 151 SAEs/1 million doses in BNT and ChA recipients, respectively. When considering patients with AEs related to cerebral/splanchnic venous thrombosis, and/or thrombocytopenia, we documented 4 and 30 SAEs and 0.4 and 4.8 deaths/1 million doses for BNT and ChA recipients, respectively. The highest risk following ChA vaccination is in young people and, likely, women of reproductive age, as suggested by hypothesized scenarios.
In conclusion, the immune reaction promoted by ChA vaccine may lead to not only thrombocytopenia and cerebral/splanchnic venous thrombosis but also other thrombotic and thromboembolic SAEs. These events are not favored by BNT vaccine. Our study may help in the evaluation of the benefit/risk profile of the ChA vaccine considering the epidemic curve present in a country.
Keywords: Anti-CoViD-19 vaccines, Severe adverse events, Venous thrombosis, Thrombocytopenia, Risk factors
1. Introduction
The involvement of viruses in autoimmune diseases has been known for a long time [1]. Indeed, viruses carry amino acid sequences similar to those of human self-tissues (molecular mimicry), resulting in the production of cross-reactive antibodies [2,3]. Severe acute respiratory syndrome coronavirus (SARS-CoV)-2 also has been demonstrated to promote dysregulation of humoral immunity and the production of autoantibodies [[4], [5], [6], [7], [8], [9], [10]]. Among the SARS-CoV-2 proteins, the level of molecular mimicry of Spike protein with human proteins is very high [11].
Anti-SARS-CoV-2 vaccines determining an immune response against the Spike protein of SARS-CoV-2 are the primary method to fight the pandemic. Real-world studies have described the efficacy of vaccines in preventing coronavirus disease 2019 (CoViD-19) and severe CoViD-19 disease to be similar [[12], [13], [14]]. On the contrary, the long-term safety profile of vaccines, particularly concerning rare auto-immune response, has not been evaluated. In March 2021, ChAdOx1 nCoV-19 Covid-19 (ChA) vaccine made by AstraZeneca has been associated with blood clots in unusual sites and bleeding events [15]. Subsequently, Greinacher and colleagues demonstrated that thrombocyte aggregation is observed in the presence of anti-platelet factor-4 antibodies (anti-PF4) [16] and Kowarz and colleagues suggest in a preliminary version of a manuscript that has not completed peer review at a journal that a spliced Spike soluble protein derived from the codon-optimized DNA present in ChA and Ad26.COV2·S (AdC) (manufactured by Jansen) vaccines binds to ACE2 and promotes antibody-dependent cell cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) of endothelial cells [17]. In a further document, European Medicine Agency (EMA) stated that ChA causes cerebral venous sinus thrombosis (CVST) and/or splanchnic venous thrombosis (SVT), in the presence of thrombocytopenia in about 10 ChA recipients out of one million doses (OMD) [18]. Though some co-factors responsible for antibody production have been hypothesized [19], we evaluated whether the vaccine-derived Spike glycoprotein is responsible, at least in part, for promoting pro-thrombotic events.
Therefore, we compared the frequency of severe AEs (SAEs) and non-SAEs reported in the EudraVigilance European database [20] following the administration of either ChA or the BNT162b2 Covid-19 (BNT) vaccine (manufactured by Pfizer/BioNTech). Both vaccines promote the expression of Spike glycoprotein, but BNT is based on nanoparticle mRNA-based technology, unlike the ChA vaccine, which is viral-vector based [21,22]. Therefore, in theory, each of them has factors that may synergize with the unwanted effects of the Spike glycoprotein, such as RNA and lipids (BNT) [23] and viral proteins different from the Spike protein (ChA). In particular, we aimed to investigate: i) whether the frequency of SAEs is different in ChA and BNT recipients; ii) whether the risk is limited to that described by the regulatory agencies; iii) whether age and sex represent a risk factor; iv) which is the risk in each age group.
2. Material and methods
2.1. Data source
Data regarding reported AEs after administration of ChAdOx1 nCoV-19 [reported as “COVID-19 VACCINE ASTRAZENECA (CHADOX1 NCOV-19)] or BNT162b2 [reported as “COVID-19 MRNA VACCINE PFIZER-BIONTECH (TOZINAMERAN)”] vaccines were obtained from EudraVigilance [20] using the in-site tools “Number of individual cases”, and “Number of Individual Cases By Reaction Groups – By Seriousness”. For in-dept analyses of severe adverse events (SAEs), a list of single case reports was obtained using the “Line Listing” in-site tool using the parameters specified below. The AEs single case reports were downloaded from EudraVigilance on April 20, 2021 and refer to SAEs added to data bank until April 16, 2021.
Data regarding the total number of ChAdOx1 nCoV-19 and BNT162b2 vaccine doses administered were downloaded from European Centre for Disease Prevention and Control (ECDC) database COVID-19 Vaccine Tracker tool [24] on April 16, 2021. They were equal to 20,869,192 (ChAdOx1 nCoV-19) and 71,210,981 (BNT162b2).
Vaccine-administration data in different age ranges were downloaded from the ECDC database and refer to “Vaccine rollout overview week 14th, 2021” (www.ecdc.europa.eu/en/covid-19/vaccine-roll-out-overview).
Data regarding the sex-based administration of ChAdOx1 nCoV-19 and BNT162b2 vaccines are available from the national websites of some European countries or from reports provided by the national medical agencies (countries and agencies are reported in Table S1).
2.2. Parameters for download of SAEs single case reports
A specific search was undertaken using the “Line Listing” in-site tool of EudraVigilance using the following parameters:
-
•
Seriousness: serious
-
•
Geographic Origin: all selected
-
•
Reporter Group: all selected
-
•
Sex: all selected
-
•
Age Group: all selected
-
•
Reaction Groups: all selected
-
•
Reported Suspected Reaction: keywords specified below
-
•
Gateway Date: 2021
The keywords we used for AE case reports download are reported in the Supplementary material.
The output of the “Line Listing” tool is an Excel™ (Microsoft, Redmond, WA, USA) table in which were reported AEs single case reports specifying: “EU Local Number”, “Patient Age Group”, “Patient Sex“, “Reaction List PT (Duration – Outcome - Seriousness Criteria)”, “Suspect/interacting Drug List (Drug Char – Indication PT – Action taken – [Duration – Dose – Route])”, and the link for download of the original single case report form.
2.3. Analyses of AE Reaction Groups
We used the in-site tool “Number of Individual Cases By Reaction Groups – By Seriousness” of EudraVigilance to assess the absolute number of non-SAEs and SAEs for both vaccines. Data were organized by the tool in 27 “Reaction Groups”. We excluded from further analyses the Reaction Groups with <1000 events reported in total between the two vaccines. Thus, 19 Reaction Groups were considered for further analyses. Excluded Reaction Groups were: “Congenital, familial and genetic disorders – 14 events”, “Endocrine disorders – 267 events”, “Hepatobiliary disorders – 462 events”, “Neoplasm benign, malignant and unspecified (incl cysts and polyps) – 187 events”, “Pregnancy, puerperium and perinatal conditions – 210 events”, “Product issues – 62 events”, “Social circumstances – 802 events”, and “Surgical and medical procedures – 434 events”.
2.4. Data normalization
2.4.1. Data normalization using the total number of administered doses
The total number of administered doses was downloaded from ECDC database using the “COVID-19 Vaccine Tracker” tool for both vaccines on April 16, 2021. Therefore, the number of events (people with AEs, SAEs, or death)/million doses was calculated using the following formula:
2.4.2. Normalization of AE data of administered doses by age ranges
For both vaccines, the number of administered doses in different age ranges (18–24, 25–49, 50–59, 60–69, 70–79, ≥80 years) was taken from the data of “COVID-19 Vaccine rollout overview week 14th, 2021” provided by the ECDC database for the available countries (21 out 30) (Table S2). Not all European countries provided these data. Hence, we created six simulations to extend the available data to all of Europe. One of these simulations, “European mean”, takes into account the data of all 21 countries for calculation of the percentage of administration in each age range. Each of the other five simulations considers the data of 5 of the 21 available countries chosen in a stochastic manner. For selection of the countries used in each of the five simulations, all 21 European countries were numbered using the “ = RAND()” formula provided by Excel. Therefore, we selected for calculation of the percentage of administration in each age range only the five countries with the highest numbers. The countries selected in the five simulations were: Austria, Finland, Latvia, Slovenia, and Sweden for Simulation 1; Czechia, Iceland, Italy, Lithuania, and Slovenia for Simulation 2; Austria, Czechia, Greece, Ireland, and Luxembourg for Simulation 3; Austria, Bulgaria, Estonia, Greece, and Lithuania for Simulation 4; Czechia, Hungary, Italy, Portugal, and Sweden for Simulation 5. For both vaccines, the overall number of doses administered was distributed in the age ranges using the fraction of administered doses in each age range obtained from the six simulations employing the following formula:
Consequently, the number of doses administered to each age range was obtained by multiplying the total number of administered vaccines according to the fraction of administered doses.
Data regarding the SAE distribution in each age range were obtained directly from the table generated by the “Line Listing” tool of EudraVigilance when the age ranges needed for analyses were 18–64 years and ≥65 years, and from the AEs single case report forms if the exact age was needed.
Each datum that concerned AE frequency normalized for different age ranges was calculated six times using the percentage of the administered dose obtained in the six simulations, and was reported as the mean (with SD or 95%CI) of obtained results.
2.4.3. Normalization of AE data for doses administered according to sex
Data regarding the sex-based distribution of vaccine administration are available from national websites or reports provided by the national medical agencies of eight European countries (Table S1). However, data detailed the sex of vaccinated people but did not state the sex of people vaccinated with each vaccine. All of these countries showed a similar sex-based distribution of vaccine administration, with a mean of 60.35% (95% CI = 56.91%–63.79%) of doses delivered to females, and a mean of 39.65% (95% CI = 36.21%–43.09%) of doses delivered to males. Moreover, the overall sex-based distribution of administration, calculated on the sum of doses administered in all of these countries to each sex, showed 59.78% of doses delivered to females and 40.22% of doses delivered to males. To extend the available data to all of Europe, we considered it likely that all European countries showed a similar distribution by sex and, based on this hypothesis, we defined a scenario in which the female:male ratio for vaccine administration was 3:2. We also hypothesized that in certain countries, for a specific vaccine and for a specific age range, the female:male ratio might be significantly different from 3:2. Hence, we also defined two other scenarios in which the ratio of vaccine administration for females:males was 2:2 and 4:2, respectively.
Each datum that concerned AE frequency normalized by sex was reported using these three hypothesized scenarios.
2.5. Statistical analyses
Statistical analyses were conducted using Prism v.8.0.1 (GraphPad, San Diego, CA, USA). We wished to evaluate differences between distribution of age ranges of thrombocytopenic and blood clots, SAEs, and death events following vaccination with ChAdOx1 nCoV-19 or BNT162b2 in different age ranges. Hence, significance was calculated using ordinary two-way ANOVA and Sidak's multiple comparisons post-hoc analysis, and multiplicity-adjusted P-values are reported for each comparison.
If comparing SAEs in age ranges, the Kolmogorov–Smirnov normality test was used on data from each group before statistical evaluation. The unpaired t-test with Welch's correction were used if the Kolmogorov–Smirnov normality test was passed, and the Mann–Whitney test was used if the Kolmogorov–Smirnov normality test failed.
3. Results
3.1. The frequency of AEs and SAEs is higher in ChA than BNT recipients
We first evaluated the number of people who suffered AEs as reported by the European EudraVigilance databank and normalized it with the number of doses of each vaccine administered in European countries. In those who received the BNT and ChA vaccines, we detected 2031 and 8117 AEs/OMDs, respectively, meaning a frequency 4 times higher in those who received ChA than BNT. This difference may have been due to: (i) a higher number of AEs at the injection site; (ii) self-resolving systemic AEs due to the response of the immune system to the vaccine; (iii) the different ages, comorbidities, and sex of vaccinated people.
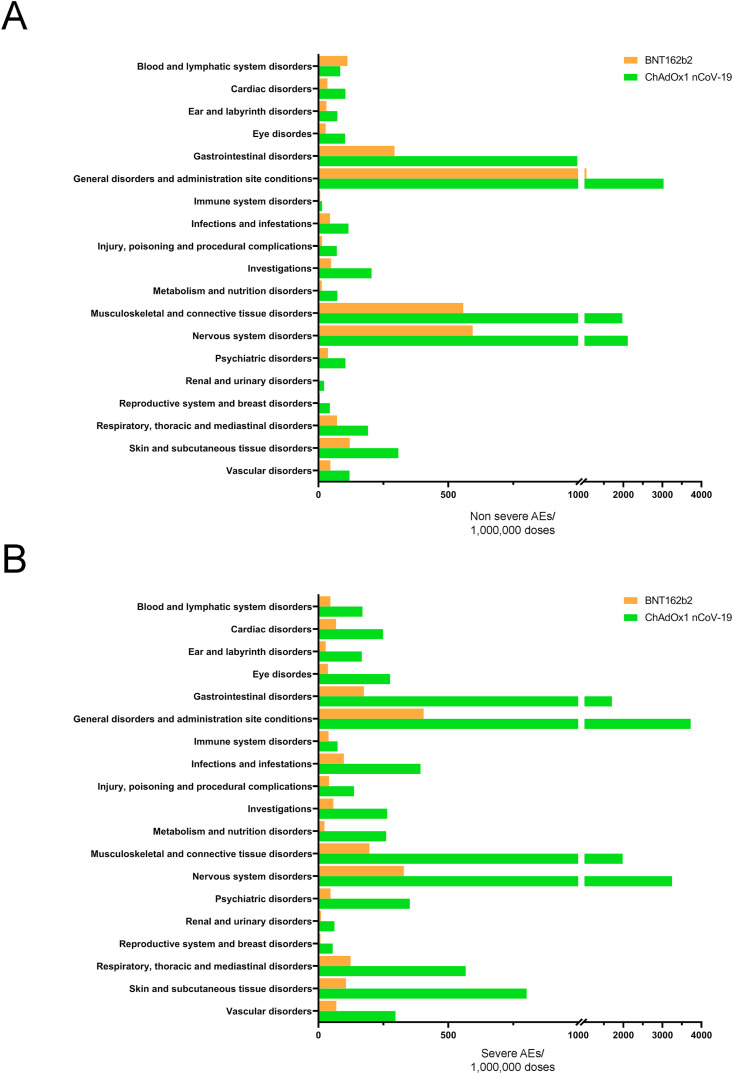
Therefore, we evaluated SAEs and non-SAEs affecting different target organs and other physiological systems (19 AE Reaction Groups), as reported by EudraVigilance (Table S3) and following normalization with the number of doses of each vaccine administered in European countries (Fig. 1 A and B). Both non-SAEs and SAEs were more frequent in ChA than in BNT recipients. The frequency was much higher concerning SAE (mean: 6.4-fold) than non-SAEs (mean: 3.6-fold).
Fig. 1.
Frequency of individual cases with AEs divided into Reaction Groups following vaccination by BNT or ChAdOx1 nCoV-19. The frequency of individual cases of AEs organized by Reaction Groups, as reported in the EudraVigilance database, was calculated by normalization of the number of individual cases with the doses of vaccines administered in Europe. Non-SAEs (A) and SAEs (B) are reported. Reaction Groups with <1000 individual cases (absolute number) with AEs are not reported.
The higher frequency of “general disorder and administration site condition” SAEs may be explained by a higher frequency of SAEs related to the site of vaccine injection which are painful but do not affect the health status of the subjects. In addition to the aforementioned Reaction Group, SAE's of the three specific Reaction Groups “nervous system disorders”, “gastrointestinal disorders” and “musculoskeletal and connective tissue disorders” occurred at a 9-fold increase in the ChA as compared with the BNT vaccine recipients and were very frequent in ChA recipients (>1500 SAEs/OMDs). In particular, the highest frequency was observed in “nervous system disorders” (~3 SAEs out of 1000 ChA recipients). Such differences may have been due to the different ages, comorbidities, or sex of vaccinated individuals (Tables S1, S2, S4, and S5). However, it seems unlikely that vaccination of a different population caused these differences. Indeed: i) the percentage of people aged ≥80 years vaccinated with BNT was much higher than that vaccinated with ChA; ii) despite the different policies in Europe, more people were vaccinated with BNT than ChA if they had comorbidity. For these reasons, we might have predicted that the SAE frequency is higher in BNT recipients.
In conclusion, even in the hypothesis that BNT does not cause any event included in the three Reaction Groups mentioned above (e.g., the number of SAEs in BNT recipients is equal to that observed in the untreated population), the frequency of SAEs in each aforementioned Reaction Group following ChA is > 1000 events per million people determining hospitalization, risk of patient's life, permanent lesions or death. The high frequency of AEs following ChA vaccination in the Reaction Groups “nervous system disorders”, “gastrointestinal disorders”, and “musculoskeletal and connective tissue disorders” could have been caused by the thrombocytopenia/bleeding and blood clot events observed in ChA recipients [16,18]. Therefore, we focused our study on SAEs related to such events.
3.2. The frequency of SAEs related to thrombocytopenia and blood clots is higher in ChA than in BNT recipients
Out of OMDs administered, 35.5 and 151.4 SAEs related to thrombocytopenia and blood clots were reported for recipients of BNT or ChA, respectively (Table S6). If considering that the event frequency observed in BNT recipients was equal to the frequency of the same event in the untreated population, then 1 SAE out of ~9000 ChA recipients was observed (HR > 4). Moreover, following OMD administration of BNT or ChA, respectively, 4.4 and 13.1 deaths possibly related to thrombocytopenia/bleeding and blood clots were reported, respectively, representing an excess of ~9 deaths/OMDs (HR = 3) in ChA recipients. Our data disagree with the data reported by the EMA (1 SAE per ~100,000 doses concerning thrombocytopenia and blood clots) [18].
3.3. In ChA recipients, the frequency of SAEs related to cerebral venous thrombosis, splanchnic venous thrombosis, and thrombocytopenia is lower than the frequency of SAEs related to thrombocytopenia and blood clots
To understand the reason for the discrepancy between EMA data and our results, we evaluated the SAEs due to cerebral venous sinus thrombosis together with cerebral venous thrombosis (CVT), splanchnic venous thrombosis (SVT), and thrombocytopenia. We did not evaluate SAEs in patients with CVT/SVT in the presence of thrombocytopenia but instead evaluated SAEs in patients with CVT/SVT and/or thrombocytopenia. Our choice was determined by the following reasoning: i) physicians may not investigate the platelet level in a patient with thrombosis; ii) physicians may not investigate the presence of thrombosis in a patient with thrombocytopenia and bleeding; iii) data relative to thrombocytopenia may not be available to the physician while completing an AE report; iv) physicians may not refer to all the events observed in patients while completing an AE report; v) clinically relevant thrombosis and thrombocytopenia may not be evident at the same time. In fact, a recent EMA document recommended monitoring ChA recipients with thrombocytopenia for thrombosis and vice versa [25].
Following OMD administration of BNT or ChA, respectively, 0.8 and 10 SAEs caused by CVT, 0.2 and 2.9 SAEs by SVT, and 3.1 and 23.3 SAEs by thrombocytopenia, respectively, were reported. Following OMD administration of BNT or ChA, respectively, the risk of death for CVT was 0.1 and 2.5/OMDs, for SVT was 0.0 and 0.4/OMDs and for thrombocytopenia was 0.3 and 3.9/OMDs.
Some of the SAEs and deaths reported in the analysis of CVT, SVT, and thrombocytopenia may be reported in more than one AE category. Therefore, to evaluate the overall risk to vaccine recipients, we undertook the same analysis considering individual reports with one or more of the above-mentioned AEs. Following OMD administration of BNT or ChA, 4 and 30 SAEs, respectively, were reported. The risk of death was 0.4 and 4.8 with the administration of BNT or ChA, respectively.
3.4. In ChA recipients, CVT, SVT, and thrombocytopenia are more frequent in young
It is known that the number of SAEs and deaths in ChA-vaccinated individuals was higher in younger (<60 years) than older (≥60 years) people (Tables S7 and S8) [18]. However, the finding may have been because more vaccines were delivered to younger people. Therefore, we tried to normalize data with the number of specific vaccines administered to each age range.
Not all European countries report to the ECDC the number of a specific vaccine administered to individuals within an age range (Figure S1) so that we know the age of individuals for less than half of the administered doses (Table S2). Therefore, we inferred the number of people vaccinated in each age range with ChA or BNT by knowing the number of doses administered to the whole European population (with/without indication of the age range) and the percentage of vaccinated people in a particular age range (Table S5), calculated based on the information from the above-mentioned countries.
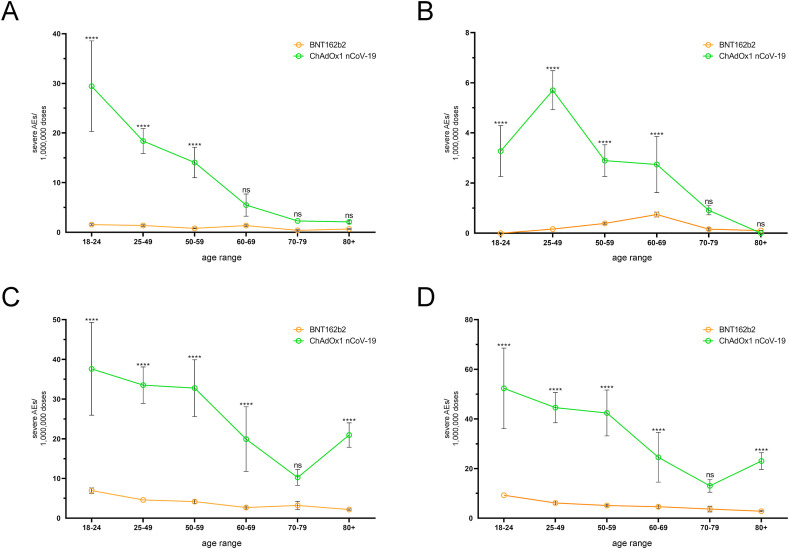
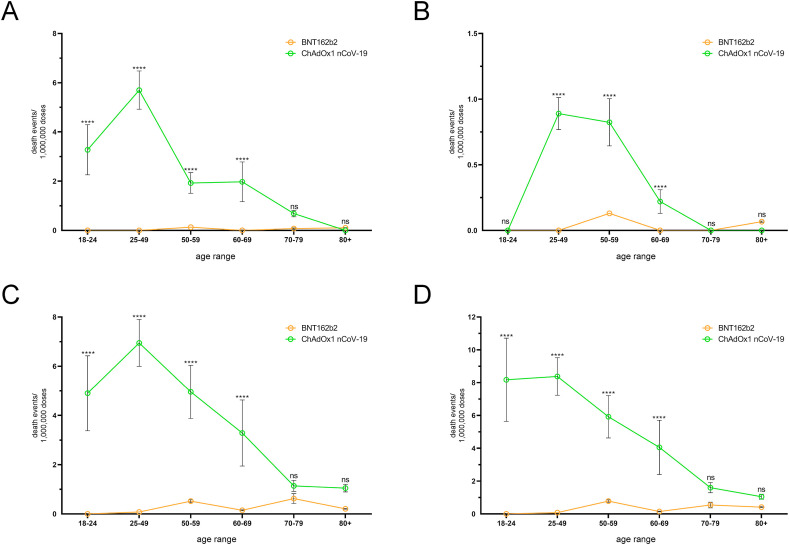
In individuals treated with ChA or BNT, the CVT SAE frequency was higher in younger than in older people and was much higher in ChA than in BNT recipients (Fig. 2 A and Table S9). In BNT recipients, the mortality rate due to CVT was <0.2 deaths/OMDs in each age range and was lower than that in ChA recipients, which show a peak of ~6 deaths/OMDs in the age range 25–49 years (Fig. 3 A and Table S10).
Fig. 2.
Frequency of individual cases with specific AEs among BNT and ChA recipients divided into age groups. The frequency of individual cases with specific AEs divided into age ranges was obtained by normalization of the number of individual cases (Table S7) with the doses supposedly administered to each age range in Europe at week-14 (Table S2). To evaluate the doses supposedly administered to each age range in Europe we considered the doses administered by the European countries providing data regarding administration of each vaccine to age ranges (Figure S1) and set up a method to evaluate the variance of the doses (see the Method sections for details). The frequency (mean ± SD) of individual cases with severe AEs consisting of cerebral venous thrombosis (A), splanchnic venous thrombosis (B), thrombocytopenia (C), and cerebral venous thrombosis and/or splanchnic venous thrombosis and/or thrombocytopenia (D) are reported. The frequency of AEs reported in panel D is lower than the sum of AE frequency reported in panels A, B, and C because one individual case may suffer from 2 or 3 AEs simultaneously. The ordinary two-way ANOVA (Sidak) test was used to assess the difference between ChA and BNT, and “multiplicity adjusted p-values” are reported. ns = p > 0.05, and **** = p < 0.0001.
Fig. 3.
Frequency of death due to specific AEs among BNT and ChA recipients divided into age groups. The frequency of death divided into age ranges was obtained by the normalization of death (Table S8) with the doses supposedly administered to each age range in Europe. To evaluate the doses supposedly administered to each age range in Europe, we considered the doses administered by the European countries providing data regarding administration of each vaccine to age ranges (Figure S1) and set up a method to evaluate dose variance (see the Material and methods section for details). The frequency (mean ± SD) of deaths due to cerebral venous thrombosis (A), splanchnic venous thrombosis (B), thrombocytopenia (C), and cerebral venous thrombosis and/or splanchnic venous thrombosis and/or thrombocytopenia (D) are reported. The frequency of deaths reported in panel D is lower than the sum of the frequency of death reported in panels A, B, and C because some patients died due to 2 or 3 AEs. The ordinary two-way ANOVA (Sidak) test was used to assess the difference between ChA and BNT, and “multiplicity adjusted p-values” are reported. ns = p > 0.05, and **** = p < 0.0001.
The SAE frequency of SVT and thrombocytopenia according to age are shown in Fig. 2B and C and Tables S11 and S12, respectively, and the mortality rate due to these SAEs is shown in Fig. 3B and C and Tables S13 and S14, respectively. An analysis of merged SAEs and death events when considering age is reported in Fig. 2, Fig. 3D and Tables S7, S8, S15, and S16. Overall, SAE frequency was higher in younger than in older people and was much higher in ChA than in BNT recipients. The SAE risk was highest in the age range 18–24 years for people vaccinated with ChA (~50 SAEs/OMDs in merged SAEs), was decreasing in the age ranges 25–49 years and 50–59 years, and lower in the >60-year people. However, even in this age range, the AE frequency in people treated with ChA was higher than that observed following vaccination with BNT. In each age range of BNT recipients, <10 SAEs/OMDs were documented in merged SAEs with a slightly higher risk in young than in old people. The mortality risk in ChA recipients was high in the age range 18–49 years, moderate in the age range 50–70 years, and low in the age range ≥70 years (in the latter, mortality was similar to that observed in BNT recipients).
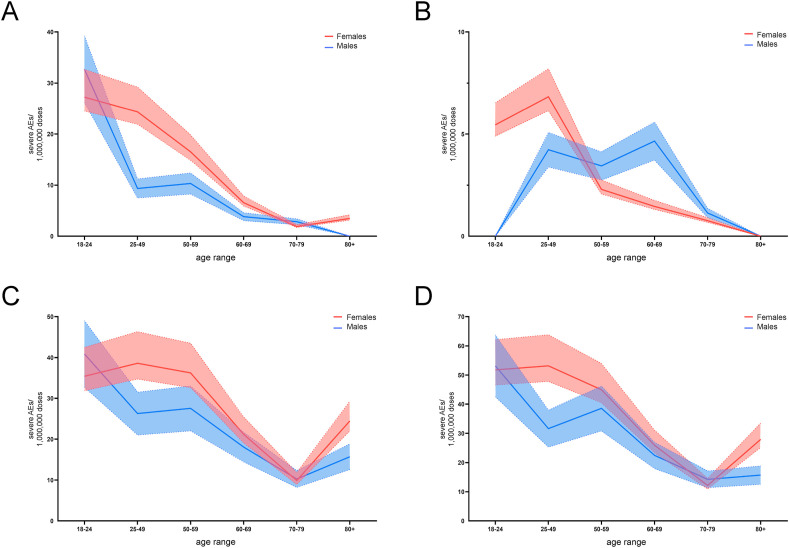
3.5. In ChA recipients, CVT, SVT, and thrombocytopenia frequency are similar in young females and males and more frequent in females in fertile age than males of the same age
It is known that the number of SAEs and deaths in ChA recipients was higher in females than in males (Tables S7 and S8). These observations may be dependent upon a higher number of vaccines administered to females but, in the ECDC database, the number of females and males to which a vaccine has been administered is not reported. However, some European countries specify the sex of vaccinated people (Table S1). Hence, in those countries, we know that more females were administered a vaccine than males (59.78 vs. 40.22). Therefore, by a coarse generalization, we hypothesized that each vaccine was given in a female:male ratio of 3:2. We also hypothesized that the female:male ratio was 2:2 and 4:2. Fig. 4 A, B and 4C shows the frequency of CVT, SVT and thrombocytopenia following administration of BNT or ChA in these three scenarios. An analysis of merged SAEs when considering age and sex is reported in Fig. 4D.
Fig. 4.
Frequency of individual cases with specific AEs among BNT and ChA recipients divided into age groups and sex. The frequency of individual cases with specific AEs divided into age ranges was obtained by normalization of the number of individual cases (Table S7) with the doses supposedly administered to each age range in Europe. To evaluate the doses supposedly administered to each age range in Europe, we considered the doses administered by the European countries providing data regarding administration of each vaccine to age ranges (Figure S1) and supposed that the same distribution was present in Europe. For the sex distribution of vaccine administration, we hypothesized three scenarios: the scenario with female (red):male (blue) = 3:2 is reported with solid lines; the scenarios where the female:male ratio is 4:2 and 2:2 are reported as dotted lines. The mean frequency of individual cases with SAEs consisting of cerebral venous thrombosis (A), splanchnic venous thrombosis (B), thrombocytopenia (C), and cerebral venous thrombosis and/or splanchnic venous thrombosis and/or thrombocytopenia (D) are reported. The frequency of AEs reported in panel D is lower than the sum of the AE frequency reported in panels A, B, and C because one individual case may suffer from 2 or 3 AEs simultaneously. Statistical analyses were not conducted because we could not establish which vaccine administration ratio for females:males was the “true” one, and it may be different at different ages. Therefore, the figure can suggest about the different frequency of AEs, particularly when the red area does not overlap with the blue area (mainly at the age range 25–49 years).
Overall, with regard to each SAE and the merged analysis, the SAE risk was highest in females and males in the age range 18–24 years for people vaccinated with ChA (~50 SAEs/OMDs). In the age ranges 25–49 years and 50–59 years, the SAE risk in females remained high, and that in males of the same age decreased. Similar SAE risk was observed between females and males aged >59 years treated with ChA (about 15–25 SAEs/OMDs).
3.6. ChA vaccine favors not only CVT, SVT, and thrombocytopenia but also stroke, lung thromboembolism, and other thrombotic and hemorrhagic events
In our analysis, the SAE frequency reporting CVT, SVT, or thrombocytopenia in ChA recipients was higher than that declared by the EMA. This is rational because we investigated AEs due to “blood clots and/or thrombocytopenia” and not “blood clots and thrombocytopenia”. However, the SAE frequency included in these three categories was 20–25% of the 151.4 SAEs/OMDs observed when considering reporting thrombocytopenia/bleeding and blood clot events. Therefore, we next evaluated the SAE frequency in young people and adults (18–64 years) and older (≥65 years) people in 10 categories describing thrombocytopenia/bleeding and blood clot SAEs using the same keywords employed to detect 151.4 SAEs/OMDs in ChA recipients but grouped for category events. The SAE frequency due to each category in ChA recipients was higher than that in BNT recipients concerning young people/adults and older people (Table 1 and Table S17). In the AE categories “other bleeding events” (more frequent in younger people), “other venous thrombosis” and “pulmonary thromboembolism” (similar frequency in younger and older individuals), and “ischemic stroke” (more frequent in older people) the SAE frequency in ChA recipients exceeded 10 events/OMDs that observed in BNT recipients. These AE categories were likely responsible for most of the 151.4 SAEs in ChA recipients due to thrombocytopenia and blood clots.
Table 1.
Differences in AE frequency between young/adults and old vaccine recipients and between ChA and BNT in young/adults and old people. The rate of individual cases is reported in Table S17.
| increase of SAE rate in 18–64 year subjects vs > 64 year subjects |
increase of SAE rate in ChA vs BNT in 18–64 year subjects |
increase of SAE rate in ChA vs BNT in >64 year subjects |
|||||
|---|---|---|---|---|---|---|---|
| BNT mean (95% CI) p value | ChAdOx1 nCoV-19 mean (95% CI) p value | AE rate of ChA over the AE rate of BNT mean (95%CI) | fold risk mean (95% CI) p value | AE rate of ChA over the AE rate of BNT mean (95%CI) |
fold risk mean (95% CI) p value |
||
| Severe AEs more frequent in younger (>2.0) | cerebral venous thrombosis | 2.0 (1.7–2.3) p < 0.01a | 5.7 (5.1–6.2) p < 0.0001 | 12.7 (12.3–13.2) | 12.4 (11.2–13.6) p < 0.01a | 1.9 (1.8–2.0) | 4.4 (4.0–4.7) p < 0.0001 |
| splanchnic vein thrombosis | 2.1 (1.8–2.4) p < 0.0001 | 4.0 (3.7–4.3) p < 0.0001 | 3.6 (3.5–3.7) | 12.4 (11.2–13.5) p < 0.0001 | 0.8 (0.8–0.9) | 6.5 (6.0–6.9) p < 0.0001 | |
| thrombocytopenia | 1.9 (1.6–2.2) p < 0.01a | 2.5 (2.3–2.7) p < 0.0001 | 23.6 (22.6–24.6) | 6.6 (6.0–7.2) p < 0.01a | 9.0 (8.5–9.6) | 5.0 (4.6–5.4) p < 0.0001 | |
| other bleeding events | 2.7 (2.3–3.1) p < 0.01a | 2.4 (2.3–2.6) p < 0.0001 | 11.6 (10.7–12.7) | 2.5 (2.3–2.8) p < 0.01a | 5.1 (4.7–5.5) | 2.8 (2.6–3.0) p < 0.0001 | |
| Severe AEs with similar rate in younger and older (2.0–0.6) | gastrointestinal bleeding | 1.6 (1.3–1.8) p < 0.001 | 1.6 (1.5–1.8) p < 0.0001 | 4.4 (4.0–4.8) | 2.4 (2.1–2.6) p < 0.0001 | 2.6 (2.3–2.8) | 2.2 (2.1–2.4) p < 0.0001 |
| cerebral bleeding | 0.6 (0.6–0.7) p < 0.01a | 1.4 (1.3–1.5) p < 0.0001 | 9.2 (8.8–9.6) | 5.9 (5.4–6.5) p < 0.01a | 4.9 (4.5–5.3) | 2.7 (2.5–2.9) p < 0.01a | |
| other venous thrombosis | 1.2 (1.0–1.4) p < 0.01a | 1.2 (1.1–1.3) p < 0.0001 | 43.9 (41.9–45.9) | 5.9 (5.4–6.4) p < 0.01a | 38.0 (35.8–40.3) | 6.1 (5.7–6.5) p < 0.0001 | |
| disseminated intravascular coagulation | 0.9 (0.8–1.1) p > 0.05a | 1.0 (0.9–1.1) p > 0.05 | 1.4 (1.4–1.5) | 18.8 (17.0–20.6) p < 0.01a | 1.4 (1.3–1.5) | 17.0 (15.8–18.1) p < 0.01a | |
| pulmonary thromboembolism | 0.8 (0.6–0.9) p < 0.01a | 0.9 (0.8–1.0) p < 0.01 | 24.0 (22.8–25.2) | 4.7 (4.3–5.1) p < 0.01a | 25.1 (23.5–26.8) | 4.0 (3.7–4.2) p < 0.0001 | |
| Severe AEs more frequent in older (<0.6) | ischemic stroke | 0.6 (0.5–0.6) p < 0.01a | 0.6 (0.5–0.6) p < 0.0001 | 11.0 (10.3–11.7) | 3.4 (3.1–3.7) p < 0.01a | 19.1 (17.8–20.5) | 3.3 (3.1–3.6) p < 0.0001 |
KS normality test failed.
3.7. The basal frequency of thromboembolic events in untreated subjects is similar or higher than the frequency of events following BNT
The data suggest that more thromboembolic events are observed in ChA than in BNT recipients, suggesting that ChA favors thromboembolic events and BNT does not or that BNT inhibits thromboembolic events and ChA neither inhibits nor favors thromboembolic events. Considering that the BNT vaccine is not a long-term treatment, we believe the former hypothesis is more likely. However, a third hypothesis cannot be ruled out: BNT also favors thromboembolic events, albeit to a lesser extent than ChA. To investigate whether BNT favors thromboembolic events, we compared the frequency of certain thrombotic events in the general population and BNT recipients.
In BNT recipients, 0.8 CVT/OMDs were observed. Recently, 13.2–15.7 CVT cases/1 million persons per year in the general population have been described [26], a frequency that is similar to that observed in BNT recipients in our analyses. Indeed, if we consider an observation period of 0.5–1 months, the frequency of events in BNT recipients is 9.6–19.2 events/1 million persons per year, suggesting that the BNT vaccine does not lead to CVT. Moreover, in people aged less than 65 years and treated with BNT, we found 12.8–25.7 events/1 million persons per year, very similar to that found by Pottegård and colleagues in vaccine-untreated people aged less than 65 years living in Denmark and Norway (20 and 10 events/1 million persons per year, respectively) [27]. These data also confirm that the BNT vaccine does not favor CVT.
Surprisingly, in evaluating thromboembolic events (overall and some specific events other than CVT), we found that the frequency of events was lower in BNT recipients than in the vaccine-untreated population. For example, the estimated incidence of overall venous thromboembolism among European people ranges from 1040 to 1830 events/1 million persons per year [28], and Pottegård and colleagues found an incidence of overall venous thromboembolic events leading to hospitalization of 1260–1580/1 million persons per year in vaccine-untreated people aged less than 65 years [27]. In BNT recipients we found 35/OMDs (people of any age) and 34/OMDs (people aged less than 65 years) SAEs related to venous thromboembolic events. If we consider an observation period of 0.5–1 month, the frequency of events in BNT recipients is 420–840 events/1 million persons per year (people of any age) and 407–814/OMDs (people aged less than 65 years), that is lower than the above mentioned.
As an example of specific events, we considered SVT. In BNT recipients, 0.2 SVT/OMDs were observed and, if we consider an observation period of 0.5–1 months, the frequency of events in BNT recipients is 2.5–5 SAEs/1 million persons per year. In contrast, 55–270 SVT/1 million persons per year in the general population have been described, including portal, hepatic, and mesenteric vein thrombosis [[29], [30], [31]]. Pottegård and colleagues found a similar incidence of SVT in vaccine-untreated people under 65 years (40 and 60 events/1 million persons per year, respectively) [27]. Similar differences are present if we consider lung thromboembolism (290–780/1 million persons per year in the general population [27,28] vs. about 84–168 SAE/1 million persons per year in BNT recipients). The above-mentioned differences and differences concerning other specific venous thromboembolic events are reported in Table S18. In conclusion, it appears that vaccine-related thromboembolic SAEs are underestimated in BNT recipients.
4. Discussion
In March 2021, some SAEs associated with blood clots were described following ChA administration. In April 2021, the EMA declared that “The most serious side effects” of ChA “are very rare cases of unusual blood clots with low blood platelets, which are estimated to occur in 1 in 100,000 vaccinated people.” The above-mentioned events are likely due to anti-PF4 Abs production resembling those produced following heparin administration [16,32,33]. Anti-PF4 Abs production has been hypothesized to be due to the adenoviral vector used in several anti-CoViD-19 vaccines (including the one produced by AstraZeneca and Johnson & Johnson) [22,34,35] and other factors [19]. Therefore, we analyzed the AEs, SAEs, and deaths following the administration of ChA or BNT to define their safety profiles in general and related to thrombohemorrhagic events. More thrombohemorrhagic events labeled as “severe” in ChA recipients than in BNT recipients (151.4 vs. 35.5/OMDs) were documented. The much higher SAE frequency in ChA recipients than in BNT recipients in the three Reaction Groups “nervous system disorders”, “gastrointestinal disorders”, and “musculoskeletal and connective tissue disorders” may have been due, at least in part, to the higher frequency of thrombohemorrhagic events in ChA recipients. Among these, SAEs with regard to CVT, SVT, and thrombocytopenia accounted only for 20–25% of all thrombohemorrhagic events in ChA recipients. Therefore, thromboembolic SAEs different from the above-specified are present in ChA recipients and merit further investigation.
4.1. Does BNT favor thrombohemorrhagic events?
A high frequency of blood clots has been observed in CoViD-19 [36]. Despite healthcare organizations did not alert about an increased risk of thrombohemorrhagic events following the BNT vaccine, the hypothesis that the SARS-CoV-2 spike protein produced following vaccine administration favors blood clots cannot be excluded. After establishing that the ChA vaccine promotes more thrombohemorrhagic events than BNT, we tried to investigate if even the BNT vaccine favors thrombohemorrhagic events. Comparison between our data and the incidence of events found in the general population demonstrates that the BNT vaccine does not lead to an increased risk of thrombohemorrhagic events.
4.2. Why does BNT seem to decrease the risk of thrombohemorrhagic events?
Surprisingly, we found a relevant decreased risk of thrombohemorrhagic events in BNT recipients. Considering that it is unlikely protection by BNT vaccine from thrombohemorrhagic events, we hypothesized three factors leading to underevaluation: i) bias in the selection of people to be vaccinated, ii) AE underreporting, iii) different lifestyle of people during the period of CoViD-19 infection which has decreased, for example, the frequency of infectious diseases, leading to a lower number of infection-dependent thrombohemorrhagic events. About point (i), we hypothesize that people affected by a life-threatening disease with a short-term risk of death may be excluded from vaccination.
Underreporting (point ii) is a well know bias affecting the analysis of drug-dependent AEs [[37], [38]]. In the case of adverse events following the administration of the vaccine, the underreporting may be due to the fact that some thrombohemorrhagic events were not considered as AEs as they were observed several days after the vaccine or because the event in people with comorbidities may be attributed to comorbidities itself rather than the vaccine (and not reported for this reason). For example, in a cirrhotic patient, SVT is a relatively common occurrence and, even if it occurs after the vaccination, it may not be attributed to the vaccine.
Therefore, we believe that the best way to assess the frequency of adverse events after vaccine administration is to compare the frequency with that of another vaccine. This is the first time comparison between similar vaccine is possible due to the exceptional circumstances of the CoViD-19 pandemic and the preparation and use of more than one vaccine for one disease.
4.3. Time between vaccine administration and AE reporting leads to further underestimation of AE frequency following vaccines
In our study, another factor determines an underevaluation of AE frequency in BNT or ChA recipients. We obtained data concerning the number of vaccinated people and the number of AEs and SAEs on the same day. However, it is rational to suppose that AEs were recorded in the EudraVigilance database 2–3 weeks after vaccination. This is due not only to the time passed between observation of the AE and its documentation in the database, but also because thrombocytopenia/bleeding and blood clots due to ChA are observed 6–24 days after vaccination [33]. Calculations using the number of people vaccinated 2 weeks before harvesting of AE reports showed a substantial increase in AE and SAE frequency (Table S19 and Figure S2). Using this approach, the risk of thrombocytopenia/bleeding and blood-clot events/OMDs in ChA recipients was 230 SAEs/OMDs.
4.4. Comparison between the conclusions of Pottegård et al. and our conclusions
Recently, Pottegård et al. have evaluated the risk of venous thromboembolic events in ChA recipients [27]. They found a higher than expected rate of CVT with a risk ratio equal to 20 and an excess of 25 events/OMDs. Their results indicate a slightly higher risk level in ChA recipients as compared to that found by us (12 risk ratio and an excess of 13 SAEs as compared to BNT) (Table 1). However, Pottegård et al. reported a risk ratio of overall venous thromboembolic events equal to 2.0 and 110 excess events/OMDs. Our analysis demonstrates an overall venous thromboembolic SAEs risk ratio of 4.3 and 116 excess events/OMDs and, if we consider the number of people vaccinated 2 weeks before harvesting of AE reports, the SAEs risk ratio of 4.7 and 181 excess events/OMDs. Therefore, our data indicate a higher risk in ChA recipients concerning overall venous thromboembolic SAEs as compared to that found by Pottegård et al. The discrepancy may be due to underevaluation of overall and specific venous thromboembolic ChA-dependent SAEs by Pottegård et al. in analogy with what we have observed for the BNT-dependent SAEs (Table S18), as above discussed.
4.5. Why does ChA favor more thrombohemorrhagic events than BNT?
Our study demonstrates a higher frequency of thrombohemorrhagic events in ChA compared to BNT recipients. Four mechanisms may explain the observed difference: i) After vaccination, ChA favors the production of a higher quantity of Spike protein than BNT; ii) The number of viral proteins in ChA recipients is greater than in BNT recipients; iii) The DNA sequence encoding the Spike present in the ChA vaccine determines alternative splicing of mRNA, leading to aberrant Spike proteins; iv) non-viral factors present in the vaccine may favor the development of local inflammatory response.
The first hypothesis has never been investigated. Considering that the Spike protein has a very high level of molecular mimicry with human proteins [11] a higher level of Spike protein production may determine a higher level of immune/autoimmune response with higher levels of autoantibodies. However, it was suggested in a manuscript that has not been completed peer review at a journal that thrombohemorrhagic events are not provoked by antibodies directed against the Spike antigen [39].
The second hypothesis starts from the consideration that in the ChA vaccine, more than 2.5 × 108 adenovirus particles are present [40]. Moreover, following the injection of a virus-based vaccine, 28 kbp of adenovirus genes are delivered to the cell nucleus alongside the SARS-CoV-2 S glycoprotein gene [41]. A wild-type adenovirus genome encodes approximately 35 proteins [42]. In the ChA adenovirus genome fewer proteins are coded because E1/E3 regions are deleted and the E4 is modified [43] and ChA is replication-defective [41]. However, in a ChA infected cell line adenoviral gene expression has been detected at very low levels. Therefore, it is possible to hypothesize that not only SARS-CoV-2 Spike glycoprotein but also adenoviral protein with mimicry activity are expressed following the vaccine delivery. On the contrary, following the BNT vaccine just SARS-CoV-2 Spike glycoprotein is expressed.
The third hypothesis has been suggested by Kowarz and colleagues in a manuscript that has not been completed peer review at a journal [17]. The authors confirm a previous finding [41] demonstrating that a small amount of the DNA-derived mRNA coding SARS-CoV-2 Spike protein undergoes splicing. The mRNA splicings of the Spike protein may show a different open reading frame after the splicing junction (aberrant Spike protein), are unable to bind to the membrane of the infected cells, and are secreted. The aberrant Spike proteins bind to the human ACE-2 receptor expressed on vascular endothelial cells and, when the anti-Spike antibodies produced in response to the vaccine bind the ACE-2-bound Spike protein, a local inflammatory reaction is activated either by antibody-dependent cell-mediated cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC). In turn, inflammation may favor thrombosis.
Greinacher and colleagues demonstrated that following ChA vaccination, anti-PF4 antibodies are observed [16]. It is reasonable to suppose that promotion of anti-PF4 antibodies is due to adenoviral proteins and Spike protein. Moreover, in a very recent manuscript that has not been completed peer review at a journal, Greinacher and colleagues demonstrated that EDTA present in the vaccine increases microvascular permeability favoring the development of a local inflammatory reaction through which the production of anti-PF4 antibodies is promoted (hypothesis iv) [19].
In conclusion, the mechanisms by which ChA promotes thrombohemorrhagic events may be different, it is likely they synergize each other and it is possible to hypothesize that other still undiscovered mechanisms are elicited by the vaccine.
4.6. Is the high frequency of thrombohemorrhagic events similar in virus-based vaccines?
AdC is the other virus-based anti-CoViD-19 vaccine authorized by EMA and FDA. ChA and AdC share several properties, including the use of a replicating-defective adenovirus (see point ii of paragraph 4.5), the use of DNA (see point iii) to carry the information for the synthesis of the Spike protein (see point i). However, they differ also. The dose of virus in AdC is 3.3 fold higher than that of ChA [40,44], likely implying a higher burden of Spike protein and adenoviral proteins. Moreover, the kind of adenovirus is different (human Ad26 and chimpanzee adenovirus, in AdC and ChA, respectively). Some other vaccines, including the Ebola vaccine, are Ad26-based vaccines [45]. However, they were not used to vaccinate millions of people. It is known that in humans, particularly in sub-Saharan Africa and Southeast Asia, antibodies anti-Ad26 are present, meaning that Ad26-based vaccines might not work well for these people [46]. The use in ChA of chimpanzee adenovirus that does not infect humans may avoid the problem of pre-existing antibodies [43]. If the presence of anti-adenovirus antibodies changes the frequency of SAEs is not known. Finally EDTA si present in ChA vaccine but not in AdC vaccine (see point iv) [40,44].
The frequency of CVST appears to be lower following AdC than ChA [18,47], but no updated information is available. Therefore we are analyzing the frequency of thrombohemorrhagic events following AdC to evaluate if the above-mentioned differences lead to a different frequency of SAE following AdC and ChA.
4.7. Limitations of the study
Our study had three main limitations. First, when analyzing an AE database, underreporting of some events and overreporting of other events can be anticipated. The robust institutional efforts employed to organize the pharmacovigilance of anti-CoViD-19 vaccines may limit these biases, but such biases cannot be excluded with certainty. To reduce underreporting/overreporting biases, we used as control the AE reported for another vaccine assuming that the reporting bias was comparable. However, after the EMA declaration of thrombohemorrhagic events due to the ChA vaccine, ChA-related overreporting in the weeks following declaration cannot be ruled out. Therefore, we considered the percentage of deaths following SAEs in BNT and ChA recipients across age groups (Table S20). If overreporting had been present in ChA recipients (e.g. hospitalization for epistaxis), the death rate would have been lower in them. In contrast, the death rate in ChA recipients was similar or even higher than that in BNT recipients, so overreporting appears not to be present.
Second, the AE rate in the age groups and sex may be approximate due to incomplete information regarding stratification of vaccinated people because: (i) The number of vaccinated people in each age range was inferred from data given from some European countries; (ii) We knew the sex of people suffering from an AE but did not know the number of females and males vaccinated with a specific vaccine in each age range; however, we believe that the true values fall within the three hypothesized scenarios; Third, we did not know the health status of vaccinated people; however, ≥70 years people have been vaccinated much more with BNT than ChA (Tables S2 and S5) and these people have a much higher incidence of co-morbidities and, reasonably, of events reported as SAEs and deaths. Therefore, a higher frequency of SAEs and death of BNT recipients as compared with ChA recipients might be expected. On the contrary, we found the opposite.
5. Conclusions
Differences among humans (genetics, epigenetics, sex, age) make some people much more prone to autoimmune reactions and more sensitive to rare AEs [48]. We here demonstrate a higher rate of thrombohemorrhagic events in ChA than BNT recipients. Specifically, CVT, SVT, and thrombocytopenia were more frequent in young people (18–24 years) and adult females (25–60 years). In BNT recipients, the frequency of thrombohemorrhagic events, including CVT, SVT, and thrombocytopenia, was not increased compared to that in the general population. Thus, vaccine-dependent production of the Spike protein may be a cofactor that favors serious thrombohemorrhagic adverse events, but it is not the only reason.
Our data may aid the evaluation of the risk-benefit ratio of the ChA vaccine taking into account the age and sex of the vaccinee, Covid-19 risk, and the depth of the pandemic in a particular country. The risk-benefit ratio of vaccines has to be evaluated more carefully in countries with low infection rate levels and when herd immunity is almost reached.
Author statement
Luigi Cari: Conceptualization, Data download, Formal analysis, Visualization. Paolo Fiore: Data download, Formal analysis, Visualization. Mahdieh Naghavi Alhosseini: Data download, Formal analysis, Visualization. Gianni Sava: Conceptualization, Writing – original draft. Giuseppe Nocentini: Conceptualization, Project administration, Funding acquisition, Writing – original draft
Funding
This research was funded by Ministero dell’Università e della Ricerca (MUR) under grants number 2017MLC3NF_005, and FISR2020IP_03103(granted to G.N.).
Declaration of competing interest
The authors have no relevant affiliation or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript. All this includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, and royalties.
Acknowledgments
We thank the EMA for having made public the data relating to the adverse events of vaccines and the European countries for having made public the number of individuals of different age groups and the sex to whom a specific vaccine has been administered.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaut.2021.102685.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Smatti M.K., Cyprian F.S., Nasrallah G.K., Al Thani A.A., Almishal R.O., Yassine H.M. Viruses and autoimmunity: a review on the potential interaction and molecular mechanisms. Viruses. 2019;11:762. doi: 10.3390/v11080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molina V., Shoenfeld Y. Infection, vaccines and other environmental triggers of autoimmunity. Autoimmunity. 2005;38:235–245. doi: 10.1080/08916930500050277. [DOI] [PubMed] [Google Scholar]
- 3.Saeki Y., Ishihara K. Infection–immunity liaison: pathogen-driven autoimmune-mimicry (PDAIM) Autoimmun. Rev. 2014;13:1064–1069. doi: 10.1016/j.autrev.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 4.Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.-H., Zhang Y., Dorgham K., Philippot Q., Rosain J., Béziat V., Manry J., Shaw E., Haljasmägi L., Peterson P., Lorenzo L., Bizien L., Trouillet-Assant S., Dobbs K., de Jesus A.A., Belot A., Kallaste A., Catherinot E., Tandjaoui-Lambiotte Y., Le Pen J., Kerner G., Bigio B., Seeleuthner Y., Yang R., Bolze A., Spaan A.N., Delmonte O.M., Abers M.S., Aiuti A., Casari G., Lampasona V., Piemonti L., Ciceri F., Bilguvar K., Lifton R.P., Vasse M., Smadja D.M., Migaud M., Hadjadj J., Terrier B., Duffy D., Quintana-Murci L., van de Beek D., Roussel L., Vinh D.C., Tangye S.G., Haerynck F., Dalmau D., Martinez-Picado J., Brodin P., Nussenzweig M.C., Boisson-Dupuis S., Rodríguez-Gallego C., Vogt G., Mogensen T.H., Oler A.J., Gu J., Burbelo P.D., Cohen J.I., Biondi A., Bettini L.R., D'Angio M., Bonfanti P., Rossignol P., Mayaux J., Rieux-Laucat F., Husebye E.S., Fusco F., Ursini M.V., Imberti L., Sottini A., Paghera S., Quiros-Roldan E., Rossi C., Castagnoli R., Montagna D., Licari A., Marseglia G.L., Duval X., Ghosn J., Tsang J.S., Goldbach-Mansky R., Kisand K., Lionakis M.S., Puel A., Zhang S.-Y., Holland S.M., Gorochov G., Jouanguy E., Rice C.M., Cobat A., Notarangelo L.D., Abel L., Su H.C., Casanova J.-L. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;80–:370. doi: 10.1126/science.abd4585. eabd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuo Y., Estes S.K., Ali R.A., Gandhi A.A., Yalavarthi S., Shi H., Sule G., Gockman K., Madison J.A., Zuo M., Yadav V., Wang J., Woodard W., Lezak S.P., Lugogo N.L., Smith S.A., Morrissey J.H., Kanthi Y., Knight J.S. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.abd3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodruff M.C., Ramonell R.P., Lee F.E.-H., Sanz I. Broadly-targeted autoreactivity is common in severe SARS-CoV-2 Infection. MedRxiv Prepr. Serv. Heal. Sci. 2020 doi: 10.1101/2020.10.21.20216192. [DOI] [Google Scholar]
- 7.Zhou Y., Han T., Chen J., Hou C., Hua L., He S., Guo Y., Zhang S., Wang Y., Yuan J., Zhao C., Zhang J., Jia Q., Zuo X., Li J., Wang L., Cao Q., Jia E. Clinical and autoimmune characteristics of severe and critical cases of COVID‐19. Clin. Transl. Sci. 2020;13:1077–1086. doi: 10.1111/cts.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caso F., Costa L., Ruscitti P., Navarini L., Del Puente A., Giacomelli R., Scarpa R. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun. Rev. 2020;19:102524. doi: 10.1016/j.autrev.2020.102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dotan A., Muller S., Kanduc D., David P., Halpert G., Shoenfeld Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun. Rev. 2021;20:102792. doi: 10.1016/j.autrev.2021.102792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang E.Y., Mao T., Klein J., Dai Y., Huck J.D., Jaycox J.R., Liu F., Zhou T., Israelow B., Wong P., Coppi A., Lucas C., Silva J., Oh J.E., Song E., Perotti E.S., Zheng N.S., Fischer S., Campbell M., Fournier J.B., Wyllie A.L., Vogels C.B.F., Ott I.M., Kalinich C.C., Petrone M.E., Watkins A.E., Dela Cruz C., Farhadian S.F., Schulz W.L., Ma S., Grubaugh N.D., Ko A.I., Iwasaki A., Ring A.M. Diverse functional autoantibodies in patients with COVID-19. Nature. 2021 doi: 10.1038/s41586-021-03631-y. [DOI] [PubMed] [Google Scholar]
- 11.Kanduc D., Shoenfeld Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: implications for the vaccine. Immunol. Res. 2020;68:310–313. doi: 10.1007/s12026-020-09152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasileiou E., Simpson C.R., Robertson C., Shi T., Kerr S., Agrawal U., Akbari A., Bedston S., Beggs J., Bradley D., Chuter A., de Lusignan S., Docherty A., Ford D., Hobbs R., Joy M., Katikireddi S.V., Marple J., McCowan C., McGagh D., McMenamin J., Moore E., Murray J.-L., Pan J., Ritchie L., Shah S.A., Stock S., Torabi F., Tsang R.S.M., Wood R., Woolhouse M., Sheikh A. Effectiveness of first dose of COVID-19 vaccines against hospital admissions in scotland: national prospective cohort study of 5.4 million people. SSRN Electron. J. 2021 doi: 10.2139/ssrn.3789264. [DOI] [Google Scholar]
- 13.Bernal J.L., Andrews N., Gower C., Stowe J., Robertson C., Tessier E., Simmons R., Cottrell S., Roberts R., O'Doherty M., Brown K., Cameron C., Stockton D., McMenamin J., Ramsay M. Early effectiveness of COVID-19 vaccination with BNT162b2 mRNA vaccine and ChAdOx1 adenovirus vector vaccine on symptomatic disease, hospitalisations and mortality in older adults in England. MedRxiv. 2021 doi: 10.1101/2021.03.01.21252652. 2021.03.01.21252652. [DOI] [Google Scholar]
- 14.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., Hernán M.A., Lipsitch M., Reis B., Balicer R.D. BNT162b2 mRNA covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.EMA - European Medicines Agency . 2021. COVID-19 Vaccine Safety Update for Vaxzevria (Previously COVID-19 Vaccine AstraZeneca): 29 March 2021.https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccine-safety-update-vaxzevria-previously-covid-19-vaccine-astrazeneca-29-march-2021_en.pdf [Google Scholar]
- 16.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2104840. NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kowarz E., Krutzke L., Reis J., Bracharz S., Kochanek S., Marschalek R. “Vaccine-Induced Covid-19 Mimicry” Syndrome: splice reactions within the SARS-CoV-2 Spike open reading frame result in Spike protein variants that may cause thromboembolic events in patients immunized with vector-based vaccines. Res. Sq. 2021 doi: 10.21203/rs.3.rs-558954/v1. [DOI] [Google Scholar]
- 18.EMA - European Medicines Agency . 2021. AstraZeneca's COVID-19 Vaccine: Benefits and Risks in Context.https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-benefits-risks-context [Google Scholar]
- 19.Greinacher A., Selleng K., Wesche J., Handtke S., Palankar R., Aurich K., Lalk M., Methling K., Völker U., Hentschker C., Michalik S., Steil L., Schönborn L., Beer M., Franzke K., Rangaswamy C., Mailer R.K., Thiele T., Kochanek S., Krutzke L., Siegerist F., Endlich N., Warkentin T.E., Renné T. Towards understanding ChAdOx1 nCov-19 vaccine-induced immune thrombotic thrombocytopenia (VITT) Res. Sq. 2021 doi: 10.21203/rs.3.rs-440461/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.EMA - European Medicines Agency EudraVigilance – european database of suspected adverse drug reaction reports. http://www.adrreports.eu/en/index.html (n.d.)
- 21.Mulligan M.J., Lyke K.E., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Raabe V., Bailey R., Swanson K.A., Li P., Koury K., Kalina W., Cooper D., Fontes-Garfias C., Shi P.-Y., Türeci Ö., Tompkins K.R., Walsh E.E., Frenck R., Falsey A.R., Dormitzer P.R., Gruber W.C., Şahin U., Jansen K.U. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 22.Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., Voysey M., Aley P.K., Angus B., Babbage G., Belij-Rammerstorfer S., Berry L., Bibi S., Bittaye M., Cathie K., Chappell H., Charlton S., Cicconi P., Clutterbuck E.A., Colin-Jones R., Dold C., Emary K.R.W., Fedosyuk S., Fuskova M., Gbesemete D., Green C., Hallis B., Hou M.M., Jenkin D., Joe C.C.D., Kelly E.J., Kerridge S., Lawrie A.M., Lelliott A., Lwin M.N., Makinson R., Marchevsky N.G., Mujadidi Y., Munro A.P.S., Pacurar M., Plested E., Rand J., Rawlinson T., Rhead S., Robinson H., Ritchie A.J., Ross-Russell A.L., Saich S., Singh N., Smith C.C., Snape M.D., Song R., Tarrant R., Themistocleous Y., Thomas K.M., Villafana T.L., Warren S.C., Watson M.E.E., Douglas A.D., Hill A.V.S., Lambe T., Gilbert S.C., Faust S.N., Pollard A.J., Aboagye J., Adams K., Ali A., Allen E.R., Allen L., Allison J.L., Andritsou F., Anslow R., Arbe-Barnes E.H., Baker M., Baker N., Baker P., Baleanu I., Barker D., Barnes E., Barrett J.R., Barrett K., Bates L., Batten A., Beadon K., Beckley R., Bellamy D., Berg A., Bermejo L., Berrie E., Beveridge A., Bewley K., Bijker E.M., Birch G., Blackwell L., Bletchly H., Blundell C.L., Blundell S.R., Bolam E., Boland E., Bormans D., Borthwick N., Boukas K., Bower T., Bowring F., Boyd A., Brenner T., Brown P., Brown-O’Sullivan C., Bruce S., Brunt E., Burbage J., Burgoyne J., Buttigieg K.R., Byard N., Cabera Puig I., Camara S., Cao M., Cappuccini F., Carr M., Carroll M.W., Cashen P., Cavey A., Chadwick J., Challis R., Chapman D., Charles D., Chelysheva I., Cho J.-S., Cifuentes L., Clark E., Collins S., Conlon C.P., Coombes N.S., Cooper R., Cooper C., Crocker W.E.M., Crosbie S., Cullen D., Cunningham C., Cuthbertson F., Datoo B.E., Dando L., Datoo M.S., Datta C., Davies H., Davies S., Davis E.J., Davis J., Dearlove D., Demissie T., Di Marco S., Di Maso C., DiTirro D., Docksey C., Dong T., Donnellan F.R., Douglas N., Downing C., Drake J., Drake-Brockman R., Drury R.E., Dunachie S.J., Edwards C.J., Edwards N.J., El Muhanna O., Elias S.C., Elliott R.S., Elmore M.J., English M.R., Felle S., Feng S., Ferreira Da Silva C., Field S., Fisher R., Fixmer C., Ford K.J., Fowler J., Francis E., Frater J., Furze J., Galian-Rubio P., Galloway C., Garlant H., Gavrila M., Gibbons F., Gibbons K., Gilbride C., Gill H., Godwin K., Gordon-Quayle K., Gorini G., Goulston L., Grabau C., Gracie L., Graham N., Greenwood N., Griffiths O., Gupta G., Hamilton E., Hanumunthadu B., Harris S.A., Harris T., Harrison D., Hart T.C., Hartnell B., Haskell L., Hawkins S., Henry J.A., Hermosin Herrera M., Hill D., Hill J., Hodges G., Hodgson S.H.C., Horton K.L., Howe E., Howell N., Howes J., Huang B., Humphreys J., Humphries H.E., Iveson P., Jackson F., Jackson S., Jauregui S., Jeffers H., Jones B., Jones C.E., Jones E., Jones K., Joshi A., Kailath R., Keen J., Kelly D.M., Kelly S., Kelly D., Kerr D., Khan L., Khozoee B., Killen A., Kinch J., King L.D.W., King T.B., Kingham L., Klenerman P., Knight J.C., Knott D., Koleva S., Lang G., Larkworthy C.W., Larwood J.P.J., Law R., Lee A., Lee K.Y.N., Lees E.A., Leung S., Li Y., Lias A.M., Linder A., Lipworth S., Liu S., Liu X., Lloyd S., Loew L., Lopez Ramon R., Madhavan M., Mainwaring D.O., Mallett G., Mansatta K., Marinou S., Marius P., Marlow E., Marriott P., Marshall J.L., Martin J., Masters S., McEwan J., McGlashan J.L., McInroy L., McRobert N., Megson C., Mentzer A.J., Mirtorabi N., Mitton C., Moore M., Moran M., Morey E., Morgans R., Morris S.J., Morrison H.M., Morshead G., Morter R., Moya N.A., Mukhopadhyay E., Muller J., Munro C., Murphy S., Mweu P., Noé A., Nugent F.L., O'Brien K., O'Connor D., Oguti B., Olchawski V., Oliveira C., O'Reilly P.J., Osborne P., Owen L., Owino N., Papageorgiou P., Parracho H., Parsons K., Patel B., Patrick-Smith M., Peng Y., Penn E.J., Peralta-Alvarez M.P., Perring J., Petropoulos C., Phillips D.J., Pipini D., Pollard S., Poulton I., Pratt D., Presland L., Proud P.C., Provstgaard-Morys S., Pueschel S., Pulido D., Rabara R., Radia K., Rajapaska D., Ramos Lopez F., Ratcliffe H., Rayhan S., Rees B., Reyes Pabon E., Roberts H., Robertson I., Roche S., Rollier C.S., Romani R., Rose Z., Rudiansyah I., Sabheha S., Salvador S., Sanders H., Sanders K., Satti I., Sayce C., Schmid A.B., Schofield E., Screaton G., Sedik C., Seddiqi S., Segireddy R.R., Selby B., Shaik I., Sharpe H.R., Shaw R., Shea A., Silk S., Silva-Reyes L., Skelly D.T., Smith D.J., Smith D.C., Smith N., Spencer A.J., Spoors L., Stafford E., Stamford I., Stockdale L., Stockley D., Stockwell L.V., Stokes M., Strickland L.H., Stuart A., Sulaiman S., Summerton E., Swash Z., Szigeti A., Tahiri-Alaoui A., Tanner R., Taylor I., Taylor K., Taylor U., te Water Naude R., Themistocleous A., Thomas M., Thomas T.M., Thompson A., Thompson K., Thornton-Jones V., Tinh L., Tomic A., Tonks S., Towner J., Tran N., Tree J.A., Truby A., Turner C., Turner R., Ulaszewska M., Varughese R., Verbart D., Verheul M.K., Vichos I., Walker L., Wand M.E., Watkins B., Welch J., West A.J., White C., White R., Williams P., Woodyer M., Worth A.T., Wright D., Wrin T., Yao X.L., Zbarcea D.-A., Zizi D. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verbeke R., Lentacker I., De Smedt S.C., Dewitte H. Three decades of messenger RNA vaccine development. Nano Today. 2019;28:100766. doi: 10.1016/j.nantod.2019.100766. [DOI] [Google Scholar]
- 24.ECDC - European Centre for Disease Prevention and Control ECDC's COVID-19 vaccine tracker. https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#uptake-tab (n.d.)
- 25.EMA - European Medicines Agency . 2021. COVID-19 Vaccine Safety Update for Vaxzevria (Previously COVID-19 Vaccine AstraZeneca): 21 May 2021.https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccine-safety-update-vaxzevria-previously-covid-19-vaccine-astrazeneca-21-may-2021_en.pdf [Google Scholar]
- 26.Riva N., Ageno W. Cerebral and splanchnic vein thrombosis: advances, challenges, and unanswered questions. J. Clin. Med. 2020;9:743. doi: 10.3390/jcm9030743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pottegård A., Lund L.C., Karlstad Ø., Dahl J., Andersen M., Hallas J., Lidegaard Ø., Tapia G., Gulseth H.L., Ruiz P.L.-D., Watle S.V., Mikkelsen A.P., Pedersen L., Sørensen H.T., Thomsen R.W., Hviid A. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 2021:n1114. doi: 10.1136/bmj.n1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heit J.A., Spencer F.A., White R.H. The epidemiology of venous thromboembolism. J. Thromb. Thrombolysis. 2016;41:3–14. doi: 10.1007/s11239-015-1311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ageno W., Dentali F., Pomero F., Fenoglio L., Squizzato A., Pagani G., Re R., Bonzini M. Incidence rates and case fatality rates of portal vein thrombosis and Budd-Chiari Syndrome. Thromb. Haemostasis. 2017;117:794–800. doi: 10.1160/TH16-10-0781. [DOI] [PubMed] [Google Scholar]
- 30.Acosta S., Alhadad A., Svensson P., Ekberg O. Epidemiology, risk and prognostic factors in mesenteric venous thrombosis. Br. J. Surg. 2008;95:1245–1251. doi: 10.1002/bjs.6319. [DOI] [PubMed] [Google Scholar]
- 31.Søgaard K.K., Darvalics B., Horváth–Puhó E., Sørensen H.T. Survival after splanchnic vein thrombosis: a 20-year nationwide cohort study. Thromb. Res. 2016;141:1–7. doi: 10.1016/j.thromres.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 32.Schultz N.H., Sørvoll I.H., Michelsen A.E., Munthe L.A., Lund-Johansen F., Ahlen M.T., Wiedmann M., Aamodt A.-H., Skattør T.H., Tjønnfjord G.E., Holme P.A. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2104882. NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scully M., Singh D., Lown R., Poles A., Solomon T., Levi M., Goldblatt D., Kotoucek P., Thomas W., Lester W. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2105385. NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadoff J., Le Gars M., Shukarev G., Heerwegh D., Truyers C., de Groot A.M., Stoop J., Tete S., Van Damme W., Leroux-Roels I., Berghmans P.-J., Kimmel M., Van Damme P., de Hoon J., Smith W., Stephenson K.E., De Rosa S.C., Cohen K.W., McElrath M.J., Cormier E., Scheper G., Barouch D.H., Hendriks J., Struyf F., Douoguih M., Van Hoof J., Schuitemaker H. Interim results of a phase 1–2a trial of Ad26.COV2.S covid-19 vaccine. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2034201. NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Logunov D.Y., V Dolzhikova I., V Shcheblyakov D., Tukhvatulin A.I., V Zubkova O., Dzharullaeva A.S., V Kovyrshina A., Lubenets N.L., Grousova D.M., Erokhova A.S., Botikov A.G., Izhaeva F.M., Popova O., Ozharovskaya T.A., Esmagambetov I.B., Favorskaya I.A., Zrelkin D.I., V Voronina D., Shcherbinin D.N., Semikhin A.S., V Simakova Y., Tokarskaya E.A., Egorova D.A., Shmarov M.M., Nikitenko N.A., Gushchin V.A., Smolyarchuk E.A., Zyryanov S.K., V Borisevich S., Naroditsky B.S., Gintsburg A.L. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dalager-Pedersen M., Lund L.C., Mariager T., Winther R., Hellfritzsch M., Larsen T.B., Thomsen R.W., Johansen N.B., Søgaard O.S., Nielsen S.L., Omland L., Lundbo L.F., Israelsen S.B., Harboe Z.B., Pottegård A., Nielsen H., Bodilsen J. Venous thromboembolism and major bleeding in patients with COVID-19: a nationwide population-based cohort study. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hazell L., Shakir S.A.W. Under-reporting of adverse drug reactions. Drug Saf. 2006;29:385–396. doi: 10.2165/00002018-200629050-00003. [DOI] [PubMed] [Google Scholar]
- 38.Lopez-Gonzalez E., Herdeiro M.T., Figueiras A. Determinants of under-reporting of adverse drug reactions. Drug Saf. 2009;32:19–31. doi: 10.2165/00002018-200932010-00002. [DOI] [PubMed] [Google Scholar]
- 39.Greinacher A., Selleng K., Mayerle J., Palankar R., Wesche J., Reiche S., Aebischer A., Warkentin T.E., Muenchhoff M., Hellmuth J.C., Keppler O.T., Duerschmied D., Lother A., Rieg S., Gawaz M.P., Mueller K.A.L., Scheer C.S., Napp M., Hahnenkamp K., Lucchese G., Vogelgesang A., Flöel A., Lovreglio P., Stufano A., Marschalek R., Thiele T. Anti-SARS-CoV-2 spike protein and anti-platelet factor 4 antibody responses induced by COVID-19 disease and ChAdOx1 nCov-19 vaccination. Res. Sq. 2021 doi: 10.21203/rs.3.rs-404769/v1. [DOI] [Google Scholar]
- 40.EMA - European Medicines Agency . 2021. Vaxzevria (Previously COVID-19 Vaccine AstraZeneca) : EPAR - Product Information.https://www.ema.europa.eu/en/documents/product-information/vaxzevria-previously-covid-19-vaccine-astrazeneca-epar-product-information_en.pdf [Google Scholar]
- 41.Almuqrin A., Davidson A.D., Williamson M.K., Lewis P.A., Heesom K.J., Morris S., Gilbert S.C., Matthews D.A. SARS-CoV-2 vaccine ChAdOx1 nCoV-19 infection of human cell lines reveals low levels of viral backbone gene transcription alongside very high levels of SARS-CoV-2 S glycoprotein gene transcription. Genome Med. 2021;13:43. doi: 10.1186/s13073-021-00859-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wold W.S.M., Toth K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr. Gene Ther. 2013;13:421–433. doi: 10.2174/1566523213666131125095046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dicks M.D.J., Spencer A.J., Edwards N.J., Wadell G., Bojang K., Gilbert S.C., Hill A.V.S., Cottingham M.G. A novel chimpanzee adenovirus vector with low human seroprevalence: improved systems for vector derivation and comparative immunogenicity. PloS One. 2012;7 doi: 10.1371/journal.pone.0040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.EMA - European Medicines Agency . 2021. COVID-19 Vaccine Janssen: EPAR - Product Information.https://www.ema.europa.eu/en/documents/product-information/covid-19-vaccine-janssen-epar-product-information_en.pdf [Google Scholar]
- 45.Pollard A.J., Launay O., Lelievre J.-D., Lacabaratz C., Grande S., Goldstein N., Robinson C., Gaddah A., Bockstal V., Wiedemann A., Leyssen M., Luhn K., Richert L., Bétard C., Gibani M.M., Clutterbuck E.A., Snape M.D., Levy Y., Douoguih M., Thiebaut R., McShane C., Callendret B., Dincq S., Ferrault C., Chai S.P., Gyselen M.P., van Looveren M., van Ballert S., de Cnodder T., Roza L., Forcheh C., Stevens K., Mastrandrea C., de Ridder S., Gundluru R., Swales N., Errijegers V., Willems W., Roorda V., Orzabal N., Assenberg M., Vialatte K., Remblier F., Porcar E., Ottavi A., Destandau E., Schwimmer C., Moinot L., Wallet C., Allais F., Savel H., Nedjaai N., Maugard A., Lenzi N., Loulergue P., Bahuaud M., Lainé F., Laviolle B., Boissel N., Thébault E., Vallée D., Nicolas J.-F., Gilbert S., Dahel K., Sagorny K., Lucht F., Paul S., Haccourt Chanavat A., Charra F., Mutter C., Lambour M., Muller C., Hutt-Clauss A., Aranda O., Bernard L., Gissot V., Hallouin-Bernard M.-C., Goudeau A., Suzzoni S., Auostin E., Brick L., Lopez-Zaragoza J.-L., Melic G., Carvalho M., Chesnel C., Hocini H., Wiedemann A., Hanot L., Rieux V., Puri A., Adeloye T., Boyce M., Dennison J., Loewenstein I., Sahgal O., van den Berg F., Calvert W., Faldon M., McClain B., Newell M.-L., Molenberghs G. Safety and immunogenicity of a two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in adults in Europe (EBOVAC2): a randomised, observer-blind, participant-blind, placebo-controlled, phase 2 trial. Lancet Infect. Dis. 2021;21:493–506. doi: 10.1016/S1473-3099(20)30476-X. [DOI] [PubMed] [Google Scholar]
- 46.Barouch D.H., V Kik S., Weverling G.J., Dilan R., King S.L., Maxfield L.F., Clark S., Ng’ang’a D., Brandariz K.L., Abbink P., Sinangil F., de Bruyn G., Gray G.E., Roux S., Bekker L.-G., Dilraj A., Kibuuka H., Robb M.L., Michael N.L., Anzala O., Amornkul P.N., Gilmour J., Hural J., Buchbinder S.P., Seaman M.S., Dolin R., Baden L.R., Carville A., Mansfield K.G., Pau M.G., Goudsmit J. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine. 2011;29:5203–5209. doi: 10.1016/j.vaccine.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.EMA - European Medicines Agency . 2021. COVID-19 Vaccine Safety Update for COVID-19 Vaccine Janssen: 22 April 2021.https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccine-safety-update-covid-19-vaccine-janssen-22-april-2021_en.pdf [Google Scholar]
- 48.DeMerle K., Angus D.C., Seymour C.W. Precision medicine for COVID-19. J. Am. Med. Assoc. 2021 doi: 10.1001/jama.2021.5248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.