Abstract
Background
Convalescent plasma (CP) containing antibodies derived from coronavirus disease 2019 (COVID-19) survivors has been proposed as a promising therapeutic option for severe COVID-19.
Methods
In our intensive care unit (ICU), 55 patients (46 male, median age 61 years) with PCR-confirmed COVID-19 (35 = 63.6% on mechanical ventilation, 7 = 14.5% on high-flow nasal oxygen, 12 = 20% on non-invasive ventilation, 1 = 1.8% without respiratory support) were treated with high-titre CP (200 mL per dose, range 1–6 doses, median 3 doses per patient, minimum titre > 1:100, Wantai test). 139 COVID-19 patients treated in the same ICU who did not receive CP served as control group. In 27 patients, the effect of CP on the individual levels of SARS-CoV-2 IgG antibodies was assessed by ELISA in serum sample pairs collected before and after CP transfusion.
Results
The first CP dose was administered at a median of 8 days after symptom onset. 13 patients in the plasma cohort died (28-day mortality 24.1%), compared to 42 (30.2%) in the cohort who did not receive CP (p = 0.5, Pearson Chi-squared test). Out of the 27 individuals investigated for the presence of IgG antibodies, 8 did not have detectable IgG levels before the first CP transfusion. In this subpopulation, 3 patients (37.5%) died. Not a single confirmed adverse reaction to CP was noted.
Conclusions
While adjunctive treatment with CP for severe and life-threatening COVID-19 was a very safe intervention, we did not observe any effect on mortality.
Keywords: Convalescent plasma, severe COVID-19, SARS-CoV-2, intensive care medicine
Introduction
As of early 2021, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the pathogen responsible for coronavirus disease 2019 (COVID-19), is continuing to ravage societies and economies everywhere around the globe, causing hundreds of thousands of premature deaths.
In contrast to astonishing achievements as far as testing strategies [1] and vaccinations [2–4] are concerned, for the significant minority of patients who develop severe or critical illness [5,6], a safe and effective treatment is still desperately needed.
As anti-SARS-CoV-2 antibodies have been proposed as supportive factors both in protecting patients from running a severe disease course in COVID-29 and from re-infection [7], human convalescent plasma (CP) collected from donors who have recovered from COVID-19 has been proposed as a potential therapeutic option.
One of the most prominent examples of CP administration was in outbreaks of Ebola viral disease (EVD), mostly in nonrandomized, comparative studies [8,9], failing to show a consistently positive effect. Some authors have even claimed that antibody dependent enhancement of EVD may contribute to its dire prognosis [10]. However, these findings for a highly virulent pathogen with exorbitantly high viremic levels cannot necessarily be extrapolated for a respiratory disease.
While in COVID-19 RNA viremia is not frequently encountered, it is associated with a worse prognosis [11]. According to a systematic review and meta-analysis, several publications have indicated that treatment with convalescent plasma may confer a mortality benefit in respiratory virus infections [12].
In a previous pandemic, the H1N1 influenza outbreak in 2009, a prospective cohort study showed that CP reduced viral loads, cytokine release and mortality [13].
Moreover, in a study performed in Hongkong in 2003 [14], patients with severe acute respiratory syndrome (SARS) who deteriorated despite high dose methylprednisolone and ribavirin treatment and received CP as a salvage therapy had a higher hospital discharge rate at day 22.
The results of clinical studies investigating the effect of CP transfusion on the outcome of moderate or severe and life-threatening COVID-19 have been rather heterogenous and sometimes difficult to interpret [15–24].
In our highly specialized department of infectious diseases, we have been studying several antiviral treatment alternatives in clinical studies during the COVID-19 pandemic outbreak. Herein, we describe the characteristics and outcomes of the 55 first COVID-19 patients treated in our department with convalescent plasma.
Material and methods
In this single-centre study, we administered convalescent plasma to 55 consecutive patients with PCR-confirmed COVID-19 who were treated in our intensive care unit in an open-label approach on a named patient basis. Additionally, all participants received standard-of-care treatment according to the best available evidence at any given time point.
CP had been collected at the Blood Centre for Vienna, Lower Austria and Burgenland of the Red Cross from voluntary donors at least 3 weeks after their recovery from COVID-19. Apheresis or whole blood plasma units from 2 to 5 individual donors were pooled and psoralen-UV-A treated.
Convalescent plasma was standardized for antibody concentrations by testing and pooling to reach virus neutralization test values of >1:320 in the end product. Donors were screened with neutralization test or a substitute (Euroimmun IgG ELISA or Wantai in dilution series in concordance with the package insert).
One CP dose consisted of 200 mL of convalescent plasma, given over 30 min. Although administration of three doses on three consecutive days was defined as standard treatment, the treating physicians could individually make adaptations.
The decision to treat a patient with CP was at the discretion of the treating physicians’ team after obtaining informed consent from the patient. Criteria for selection for CP treatment included a preferably short delay between symptom onset and ICU admission (and therefore, CP administration) and the absence of a do-not-resuscitate or do-not-intubate order or other circumstances preventing maximum intensive care or a curative aim. In the light of new evidence casting doubt on the benefit associated with CP transfusion, only one more patient with chronic lymphatic leukaemia and consecutive global immunoglobulin deficiency was treated with CP after November 2020.
CP was delivered as a frozen blood product and transfused according to international guidelines.
The primary clinical endpoint of this study was 28-day mortality. Secondary endpoints were conversion to a negative SARS-CoV-2 PCR at 72 h post-transfusion of the last dose of convalescent plasma and a change in the level of respiratory support and in the general clinical status rated by the 10 point WHO ordinal symptom score (https://www.who.int/docs/defaultsource/documents/emergencies/minimalcoreoutcomemeasure.pdf).
The patients had been enrolled in the Austrian CoronaVirus Adaptive Clinical trial ACOVACT (clinicaltrials.gov).
This study was performed in accordance with the Declaration of Helsinki, and the retrospective analysis of clinical data was approval by the Ethics Committee of the City of Vienna (EK 20-079).
To assess the level of anti-SARS-CoV-2 IgG antibodies immediately before and 30 min after transfusion of CP, the SARS-CoV-2 IgG ELISA (Euroimmun, Lübeck, Germany) was used, which has been shown to offer 100% sensitivity by the 11th day of illness and 98% specificity [25]. For an accurate quantification, serum samples were tested in serial dilutions of twofold steps starting at 1:5–1:1280 using dilution buffer, instead of diluting samples 1:101 as described in the package insert.
In a previous study, we demonstrated that dilution titres assessed with this ELISA strongly correlated with titres of neutralizing antibodies [26].
In a subgroup of 27 patients, the titre was measured with Euroimmun IgG Elisa in dilution series before and after each convalescent plasma transfusion.
To prove the concept of distribution and testing SARS-CoV-2 antibodies after transfusion of convalescent plasma a model for the calculation of SARS-CoV-2 antibody increment in vivo was established. On the basis of data from intravenous immunoglobulin G several assumptions were made [27,28]. A rapid IgG extravasation process was expected, driven by: 1. convective transport into the lymphatic system, 2. transcytosis through vascular epithelial cells (pinocytosis) and 3. passive diffusion (least relevant because of molecule size). The central compartment/plasma volume was calculated with a normal (fictive) haematocrit of 40% and the ideal body weight. The overall volume of distribution has been described in the literature with a range of 6–20 L [29]. To calculate the overall compartment in this model the plasma volume was multiplied by 2.5. A steady state with a 0.1 ratio of extravascular compartment to intravascular compartment concentration was assumed at least 12 h after transfusion. This is explained by a flow of anti-SARS-CoV-2 antibodies (and other IgG) from the lymphatic system back to the venous system and an FcRn-receptor -mediated transport to the intravascular space. The following formulae were used: Plasma volume patient (PV) in mL = ideal BW × 0.07 × 0.6 × 1000 (70 mL blood per kg BW); overall compartment patient in mL (OC) = PV × 2.5; increase of titre after 30 min = CPV × titreCPV/OC (CPV = convalescent plasma volume); increase of titre after 24 h = (CPV × titreCP) ×0.9/PV. To predict the titre after transfusion, the calculated increase was added to the measured titre of the patient. This model is suitable for patients before seroconversion. After seroconversion the autologously produced antibodies of the patient cannot be distinguished anymore from the transfused antibodies.
Statistical analyses
Descriptive statistics of variables of interest were computed and are presented as absolute and relative frequencies for categorical and mean, median, inter-quartile range, minimum, maximum and standard deviation for continuous variables. The primary endpoint was 28-day mortality, and the primary objective was to investigate the effect of CP on this endpoint. To assess the primary objective, a Chi-Square Test using a significance level of 0.05 was used. Further exploratory analyses were conducted to investigate the influence of CP on other exploratory endpoints such as time on mechanical ventilation and duration of stay in the ICU. Statistical methods used for these analyses are described alongside the results in the following section. For exploratory analyses, p-values <.05 were considered statistically significant, however as these p-values serve only descriptive purposes, no multiplicity correction was required. Statistical analyses were conducted using R 3.6.3 (R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.).
Results
Study cohort and control cohort
The cohort of patients who received CP consisted of 46 men (83.6%) and 9 women (16.4%) with a median age of 61 years (range 25–86, IQR 15 years).
Thus, it was well matched with the control cohort consisting of 142 patients (101 men, 71.1%, median age 63 years, range 20–87, IQR 20.2 years) who were not treated with CP.
Comorbidities in the CP vs. the no CP cohort included hypertension (33 = 60% vs. 90 = 60.3%), obesity, defined as body mass index > 30 (31 = 56.4% vs. 58 = 41.1%), diabetes (17 = 30.9% vs. 49 = 34.5%), coronary artery disease (9 = 16.4% vs. 31 = 21.8%), chronic heart failure (0 vs. 3 = 2.4%), chronic renal failure (4 = 7.3% vs. 12 = 8.5%), immunosuppression (2 = 3.6% vs. 12 = 8.5%), COPD (3 = 5.4% vs. 18 = 12.7%), asthma (1 = 1.8% vs. 7 = 4.9%), current active smoking (1 = 2% vs. 13 = 10.7%), active cancer (3 = 5.4% vs. 4 = 2.9%), active lymphoma (0 vs. 1 = 0.8%), chronic lymphatic leukaemia (1 = 1.8% vs. 3 = 2.1%).
All patients in the CP cohort received optimal supportive care, immunomodulation (prednisolone with or without tocilizumab or dexamethasone) and most of them also other antiviral therapies including remdesivir (38 = 70.4%), hydroxychloroquine (1 = 1.8%), lopinavir/ritonavir (8 = 14.8%) or camostat (3 = 5.4%).
At the time of administration of the first CP dose, 35 patients (63.6%) were mechanically ventilated, while 7 patients (12.7%) were on high-flow nasal oxygen, 12 (21.8%) on non-invasive ventilation and one patient (1.8%) did not receive any respiratory support. In comparison, in the control group of 139 patients, one day after admission to the ICU (which was the median time of administration of CP in the intervention group) 37 (26.6%) were on invasive mechanical ventilation, 55 (40%) on non-invasive ventilation, 35 (25.2%) on high-flow nasal oxygen, and 12 (8.6%) patients on low-flow oxygen.
Administration of CP
Three patients (5.5%) received only one dose of CP – one patient because of premature death, one because any further intervention was deemed futile due to advanced age, unresponsive hypoxaemia and a do-not-intubate-order, and one patient because of metabolic acidosis, tachycardia and hyperthermia hours after CP transfusion. Later, the treating physicians deemed this adverse reaction unrelated to CP but rather caused by sevoflurane as an inhalational add-on anaesthetic introduced just half an hour before the onset of clinical deterioration.
Two patients (3.6%) received two doses and forty-nine patients (89.1%) a total of three doses on three consecutive days, according to our standard protocol. One patient (1.8%) was treated with a prolonged course of CP consisting of six doses due to end-stage liver cirrhosis resulting in absolute paucity of immunoglobulins and extremely high viral loads (>1010 copies/mL) in nasopharyngeal swabs and tracheal secretions.
CP was administered at a mean of 8 (IQR 5) days after symptom onset and at a mean of 1 (IQR 1) day after admission to the intensive care unit.
Antibody kinetics
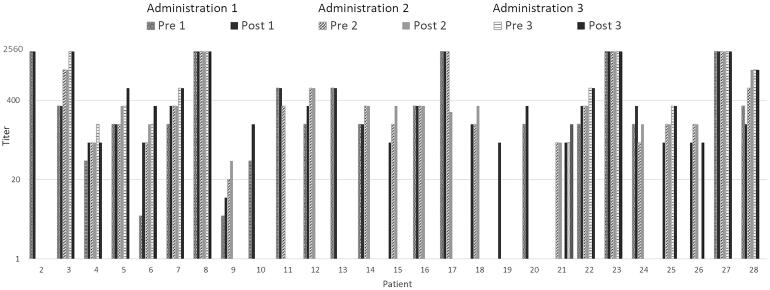
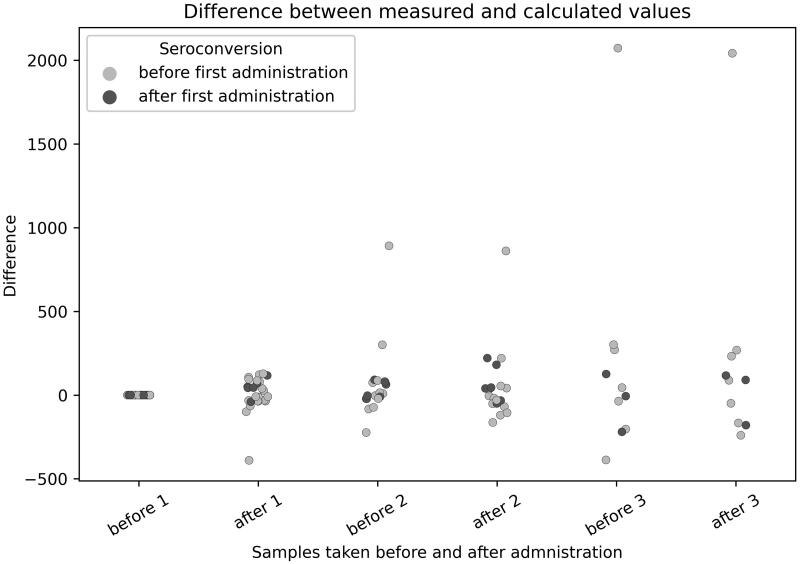
For 27 patients, quantitative measurements of anti-SARS-CoV-2 IgG antibodies before and after CP transfusion were performed. 8 of these patients had not yet seroconverted, while 19 patients already had measurable IgG before receiving the first CP dose. Median time from symptom onset to seroconversion was 9 days. The measured titres of these 27 patients are shown in comparison to the calculated titres to prove the concept of transferring SARS-CoV-2 specific antibodies through convalescent plasma transfusion (Figures 1 and 2).
Figure 1.
Euroimmun IgG ELISA titres of patients 2–28.
Figure 2.
Differences of calculated and measured titre values.
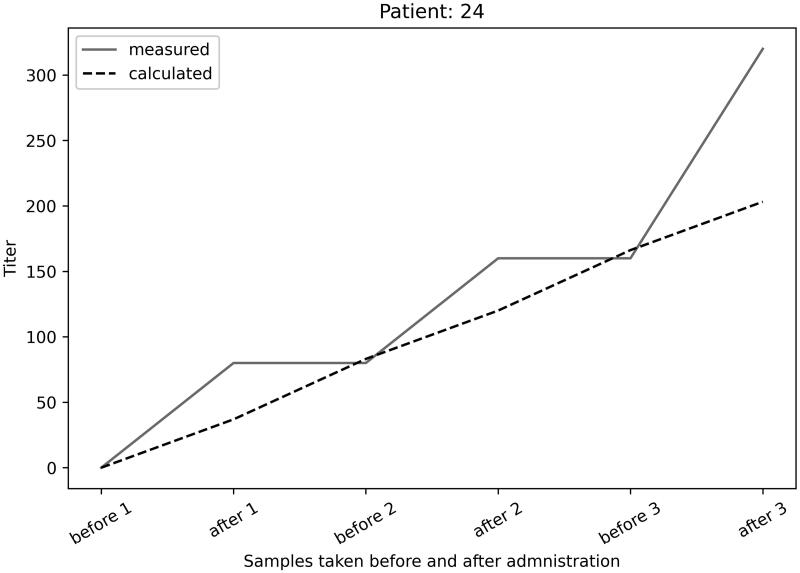
Furthermore, for 1 exemplary individual the calculated vs. measured antibody titres are shown in Figure 3.
Figure 3.
Measured vs. calculated antibody titres in patient 24.
The comparison between measured and calculated titres and their overlap proves the concept of passively transferring antibodies from donors to patients and their intravascular circulation. Despite the wide range (0.5–0.9 ratio extravascular/intravascular compartment) of antibody distribution in vivo described in the literature the assumption that 90% of transfused SARS-CoV-2 antibodies can be found intravascularly was shown with the titre prediction model and antibody testing for patients before seroconversion. After seroconversion the autologous antibodies overlap transfused antibodies in ELISA testing, especially in dilutions above 1:160. The limitation is that we cannot predict how much specific antibodies reach the infected (target) tissue of the patients.
Outcome
The primary endpoint of this study was mortality at day 28 after ICU admission. 13 patients in the plasma cohort died (28-day mortality 24.1%), compared to 49 (30.2%) out of 139 patients in the cohort who did not receive CP and for whom this endpoint was available at the time of writing of this manuscript. This difference did not reach statistical significance (p = .50). The Kaplan Meier plot for survival probability is shown in Figure 4.
Figure 4.
Kaplan Meier plot of the overall survival probability with and without plasma therapy.
Patients on mechanical ventilation had the highest probability of death: 9 out of 35 patients (25.7%) with CP therapy vs. 29 out of 64 (45.3%) patients without plasma transfusion.
However, this observation has to be interpreted with caution because this subgroup of patients without plasma transfusion included, among others, 2 individuals admitted to the ICU under cardiopulmonary resuscitation, one who died minutes after intubation due to unknown reasons and 2 with a very long delay between the onset of COVID-19 symptoms and ICU admission who died because of ischaemic strokes. Owing to this selection bias, we did not deem it appropriate to calculate a p-value for this comparison.
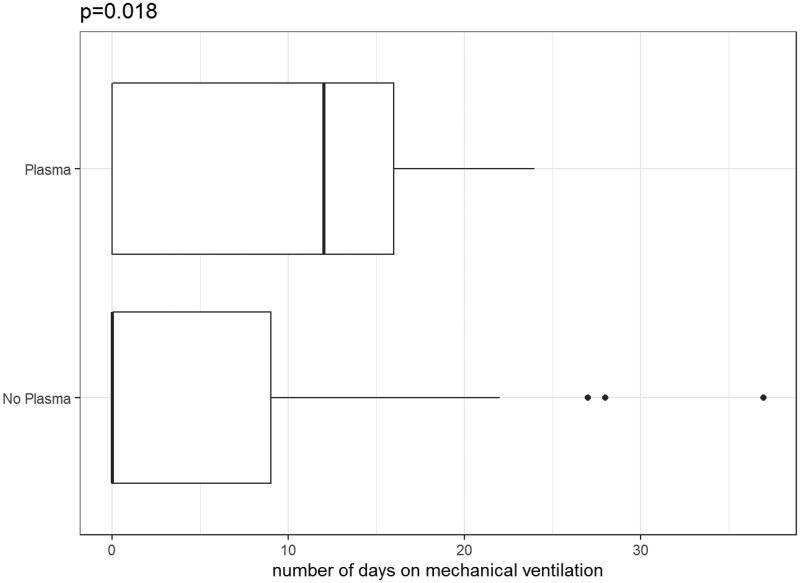
Moreover, in our cohort, observed time on mechanical ventilation was longer for patients with CP therapy (median 17 days, range 3–29, IQR 9 days) vs. without CP therapy (median 14 days, range 1–67, IQR 9.5 days) (p = .368, Wilcoxon test) (Figure 5).
Figure 5.
Time on mechanical ventilation with plasma therapy vs. without plasma therapy.
6 of the 55 patients (10.9%) who had received CP therapy went on to require extracorporeal membrane oxygenation (ECMO), compared to 6 of 139 patients (4.3%) without CP treatment.
As a secondary endpoint, we evaluated clinical status on the WHO 10-point ordinal symptom scale 6 days after the last CP transfusion. 16 (29.1%) patients showed the same clinical status 6 days after the last CP transfusion, 26 (47.3%) worsened, and 13 (23.6%) improved.
Moreover, we did not observe a clear trend towards viral clearance in the upper respiratory tract 3 days after the last CP transfusion: there was no SARS-CoV-2 PCR conversion (from positive to negative or to < 500 copies/mL) in 41 (85.4%) patients, while in 7 patients (14.6%) PCR turned negative, and for 7 patients (12.3%) this data was missing.
Finally, we observed a longer length of stay in the ICU for patients in the CP (median 18.5, range 4–110, IQR 20.2 days) vs. the no CP (median 9, range 1–119, IQR 15.2 days) cohort (p < .01, Wilcoxon Test).
The same held true for total time spent in our hospital in the CP (median 31, range 8–67, IQR 22.5 days) vs. the no CP cohort (median 19, range 1–99, IQR 14.5 days) (p = .065, Wilcoxon test).
In the subpopulation of patients without prior seroconversion for whom this analysis was available, 3 (37.5%) out of 8 died, compared to 3 (15%) out of 19 patients who already had seroconverted before the first CP administration.
Discussion
Since we admitted our first patient with COVID-19 to our ICU on the 27th of February 2020 [30], our experience with handling this complex disease has been steadily increasing.
However, like the rest of the world, we are still desperately waiting for an effective and safe treatment for severe and life-threatening cases of COVID-19.
The severity of clinical illness is still not well predictable in spite of the identification of several risk factors for an unfavourable prognosis, such as advanced age [31],but may be positively influenced by optimal antiviral and supportive therapeutic approaches.
Although the antiviral agent remdesivir has shown some promising early results [32], it has so far failed to yield a consistent proof of mortality reduction [33]. On the other hand, the benefits of corticosteroids can be substantial in the phase of massive inflammatory response [34]. One study demonstrated that dexamethasone lowers case fatality rates in patients in need of supplemental oxygen or higher respiratory support [35]. As it has been postulated that corticosteroids may delay viral clearance [36], adding a directly acting antiviral compound seems a biologically plausible approach.
While there have been early promising reports of favourable clinical outcomes in patients with life-threatening COVID-19 treated with CP [15,16], the small sample sizes do not allow any reliable conclusion as far as efficacy is concerned.
As was elegantly put forward in an editorial [37], it may be challenging to perform randomized clinical trials in the midst of a pandemic, aggravating some treating physicians’ fears of depriving patients of a potentially life-saving therapy. But it is still the only way to deliver meaningful clinical research. However, the sum of observational data can also contribute to finding means to improve the clinical outcome in this devastating most severe form of COVID-19.
The study of convalescent plasma is no exception to this rule, and CP may even be more seductive both to doctors and to patients as the idea of transferring ‘healing properties’ from a survivor to an acutely ill patient seems particularly appealing.
In our small cohort, where the majority of patients also received other antiviral and immunomodulatory (100% of patients) substances, CP turned out to be a very safe treatment.
Due to the small sample size, a clear survival benefit cannot be deduced from our data. 13 out of 55 patients (23.6%) treated with CP who had reached the primary endpoint died, which roughly corresponds to the overall mortality in our entire ICU cohort (56 out of 194 patients = 28.9%), or 42 out of 139 (30.2%) patients who did not receive CP.
Although we observed a signal towards decreased 28-day mortality in patients on mechanical ventilation with CP therapy versus patients without (25.7 vs. 45.3%), these numbers may simply reflect the fact that CP therapy was at our institution preferably given to patients deemed to have a good chance of survival. This fact might also serve as an explanation for the significantly longer length of ICU stay of patients receiving CP (median 21 vs. 8 days), because the full spectrum of intensive care medicine might have been judged as justified by the team of treating physicians for a longer timer period in patients with better initial health conditions. The same reasoning may hold true for the fact that more patients after CP therapy than without went on to receive ECMO therapy (10.9 vs. 4.3%).
In a small study performed in China [17], all 6 patients tested negative for SARS-CoV-2 at 72 h after receiving CP which was administered after a median of 21.5 days after the first positive PCR. Contrary to this cohort the majority of our patients did not show enhanced viral clearance from upper or lower respiratory tract specimens after 3 days (administered after a median of 8 days after symptom onset). However, the clinical outcome in our study was more favourable, with an overall mortality of 23.6% as opposed to 83% (5/6 patients) in the Chinese cohort.
Possible reasons for the lower mortality in our cohort may be optimal intensive care, including high-dose prophylactic anticoagulation, a late intubation strategy making use of awake proning and the use of immunomodulatory agents (dexamethasone or prednisolone or tocilizumab plus prednisolone) in all patients.
As mentioned above, in our cohort, convalescent plasma was used earlier than in the Chinese study, in which it was administered at a mean of 21.5 days after first detection of viral shedding. As with most other antimicrobial treatment strategies, administering the right dose to the right patient at the right time point – preferably as early as possible – is key to success. In a study published on a pre-print server involving more than 35,000 patients [18], a statistically significant trend towards improved survival was demonstrated if CP was transfused within 3 days after diagnosis of COVID-19 versus 4 days or later. It is therefore not surprising that in one of the first published randomized clinical trials published [19], no mortality benefit could be detected, as patients were randomized to CP at a median of 30 days after symptom onset.
In a randomized, double-blind, placebo-controlled trial performed in Argentina the early (within 72 h after symptom onset) administration of CP to patients 75 years or older or between 65 and 74 years old with at least one pre-existing medical condition resulted in a statistically significant relative risk reduction of 48% for severe respiratory disease [20]. The study had to be terminated prematurely because of a marked decline in COVID-19 cases, making the enrolment of further patients virtually impossible.
Consequently, age might be another factor influencing the degree of benefit derived from CP therapy. While there was a trend towards improved outcomes in elderly patients (65 years or older), otherwise no benefit regarding mortality or hospital discharge could be observed for patients treated with CP in a matched cohort study [21].
Similarly, a double-blind Argentinian trial in which patients were randomized in a 2:1 ratio to CP or placebo failed to show any benefit in mortality or clinical status at day 30 for hospitalized patients [22]. The median time from onset of clinical symptoms to enrolment was 8 days, which may have been too late to produce a positive effect on survival.
In a large Indian trial randomizing 235 patients to plasma transfusion plus standard of care treatment (intervention arm) and 229 to standard of care only, a trend towards resolution of shortness of breath and fatigue was noted at day 7 in the intervention arm, but the primary composite endpoint consisting in progression to severe disease and all-cause mortality at day 28 post-enrolment was not met [23].
Recent studies with monoclonal antibodies [38–40] contribute to the growing body of evidence that early passive antibody transfer conveys a meaningful benefit in COVID-19 treatment.
Moreover, not only the timing for CP administration seems to play a critical role, but also the quantity of antibodies transfused. This concept is not only biologically plausible, but can be inferred from a large retrospective U.S. registry-based analysis on passive antibody transfer in more than 3,000 hospitalized COVID-19 patients: Mortality was highest in the low-titre group and lowest in the high-titre group [41].
In our cohort, plasma transfusions were more stringently standardized and did not display a pronounced range in antibody titres. What is singular about our study is that most patients (52/55, 94.5%) received more than one unit of convalescent plasma, thus markedly raising the cumulative dose. However, it is uncertain if a larger total dose of convalescent plasma can display a markedly more potent antiviral effect.
Generally speaking, several limitations to the applicability and feasibility of treatment of COVID-19 with convalescent plasma need to be addressed: First, CP is not an unlimited resource, and is, secondly, associated with possible side effects such as the transmission of infectious diseases, transfusion-related lung injury, volume overload, and, most importantly, allergic reactions [24]. However, in our department, treatment with CP turned out to be a very safe procedure, with only one possible, albeit rather unlikely, adverse event reported in 55 patients.
Unfortunately, it is still unclear how important the humoral response of the innate immune system is in natural infection with COVID-19. Some authors have pointed out that the T cell response may have the leading role in clearing infection [42]. Although passive antibody transfer may accelerate viral clearance, it is not capable of reversing the lung damage caused by an abundant immune response in severe COVID-19 cases [43].
The major limitation of this study is its small sample size, making it underpowered to show a significant difference between the groups.
Moreover, it was not performed according to the gold standard of clinical research, which is a placebo-controlled, randomized controlled design. Also, CP was an add-on treatment administered in an open-label fashion, and thus it is impossible to differentiate the effect of CP from the protective effect of the antiviral and immunomodulatory drugs administered.
Further, the cohorts differed as far as respiratory support was concerned, with more patients on mechanical ventilation in the group treated with convalescent plasma; thus, a small effect on survival with CP treatment in this more severely ill patient cohort cannot be definitely excluded.
In conclusion, treatment of severe COVID-19 with human convalescent plasma is a very safe procedure and should be administered with the highest possible antibody titre and at the earliest possible time point. However, our study supports the evolving view that convalescent plasma may be an unsuitable treatment option for COVID-19 patients late in their disease course, as we did not observe any effect on mortality.
Glossary
Abbreviations
- COVID-19
coronavirus disease 2019
- CP
convalescent plasma
- CPV
convalescent plasma volume
- ECMO
extracorporeal membrane oxygenation
- FcRn
neonatal Fc receptor
- HFNC
high-flow nasal cannula
- ICU
intensive care unit
- Ig
Immunoglobulin
- OC
overall volume patient
- PC
plasma volume patient
- RT-PCR
real time polymerase chain reaction
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Mina MJ, Parker R, Larremore DB.. Rethinking Covid-19 test sensitivity – a strategy for containment. N Engl J Med. 2020;383:e120. [DOI] [PubMed] [Google Scholar]
- 2.Polack FP, Thomas SJ, Kitchin N, et al. . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dagan N, Barda N, Kepten E, et al. . BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baden LR, El Sahly HM, Essink B, et al. . Efficacy and safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Z, McGoogan JM.. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for disease control and prevention. JAMA. 2020;323(13):1239–1242. [DOI] [PubMed] [Google Scholar]
- 6.Hou W, Zhang W, Jin R, et al. . Risk factors for disease progression in hospitalized patients with COVID-19: a retrospective cohort study. Infectious Diseases. 2020;52(7):498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bentivegna E, Sentimentale A, Luciani M, et al. . New IgM seroconversion and positive RT‐PCR test after exposure to the virus in recovered COVID‐19 patient. J Med Virol. 2021;93(1):97–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown JF, Dye JM, Tozay S, et al. . Anti-Ebola virus antibody levels in convalescent plasma and viral load after plasma infusion in patients with ebola virus disease. J Infect Dis. 2018;218(4):555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mupapa K, Massamba M, Kibadi K, et al. . Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. J Infect Dis. 1999;179(s1):S18–S23. [DOI] [PubMed] [Google Scholar]
- 10.Takada A, Feldmann H, Ksiazek TG, et al. . Antibody-dependent enhancement of Ebola virus infection. J Virol. 2003;77(13):7539–7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu D, Zhou F, Sun W, et al. . Relationship between serum severe acute respiratory syndrome Coronavirus 2 Nucleic acid and organ damage in Coronavirus 2019 patients: a cohort study. Clin Infect Dis. 2020;2020:ciaa1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mair-Jenkins J, Saavedra-Campos M, Baillie JK, et al. . The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211(1):80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hung IF, To KK, Lee CK, et al. . Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52(4):447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soo YOY, Cheng Y, Wong R, et al. . Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect. 2004;10(7):676–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen C, Wang Z, Zhao F, et al. . Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. Jama. 2020;323(16):1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duan K, Liu B, Li C, et al. . Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci USA. 2020;117(17):9490–9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng Q-L, Yu Z-J, Gou J-J, et al. . Effect of convalescent plasma therapy on viral shedding and survival in patients with Coronavirus Disease 2019. J Infect Dis. 2020;222(1):38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joyner MJ, Senefeld JW, Klassen SA, et al. . Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience. Cold Spring Harbor Laboratory. 2020. [Google Scholar]
- 19.Li L, Zhang W, Hu Y, et al. . Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19. Jama. 2020;324(5):460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Libster R, Pérez Marc G, Wappner D, et al. . Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N Engl J Med. 2021;384(7):610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogers R, Shehadeh F, Mylona EK, et al. . Convalescent plasma for patients with severe COVID-19: a matched cohort study. Clin Infect Dis. 2020;2020:ciaa1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simonovich VA, Burgos Pratx LD, Scibona P, et al. . A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2021;384(7):619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agarwal A, Mukherjee A, Kumar G, et al. . Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). Bmj. 2020;:m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roback JD, Guarner J.. Convalescent plasma to treat COVID-19: possibilities and challenges. Jama. 2020;323(16):1561–1562. [DOI] [PubMed] [Google Scholar]
- 25.Traugott M, Aberle SW, Aberle JH, et al. . Performance of severe acute respiratory syndrome Coronavirus 2 antibody assays in different stages of infection: comparison of commercial enzyme-linked immunosorbent assays and rapid tests. J Infect Dis. 2020;222(3):362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weidner L, Gänsdorfer S, Unterweger S, et al. . Quantification of SARS-CoV-2 antibodies with eight commercially available immunoassays. J Clin Virol. 2020;129:104540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lobo ED, Hansen RJ, Balthasar JP.. Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci. 2004;93(11):2645–2668. [DOI] [PubMed] [Google Scholar]
- 28.Ryman JT, Meibohm B.. Pharmacokinetics of monoclonal antibodies. CPT Pharmacometrics Syst Pharmacol. 2017;6(9):576–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dirks NL, Meibohm B.. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:633–659. [DOI] [PubMed] [Google Scholar]
- 30.Seitz T, Hoepler W, Weseslindtner L, et al. . Successful management of the first reported case in Austria of COVID-19 with ARDS. Infection. 2020;48(4):647–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bentivegna E, Luciani M, Spuntarelli V, et al. . Extremely severe case of COVID-19 pneumonia recovered despite bad prognostic indicators: a didactic report. SN Compr Clin Med. 2020;2(8):1204–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beigel JH, Tomashek KM, Dodd LE, et al. . Remdesivir for the treatment of Covid-19 – final report. N Engl J Med. 2020;383(19):1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO Solidarity Trial Consortium . Repurposed antiviral drugs for Covid-19 – interim WHO solidarity trial results. New Eng J Med. 2020;384(6):497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pennica A, Conforti G, Falangone F, et al. . Clinical management of adult Coronavirus Infection Disease 2019 (COVID-19) positive in the setting of low and medium intensity of care: a short practical review. SN Compr Clin Med. 2020;2(6):694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with Covid-19 – preliminary report. New Eng J Med. 2020;384(8):693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanders JM, Monogue ML, Jodlowski TZ, et al. . Pharmacologic treatments for Coronavirus Disease 2019 (COVID-19). JAMA. 2020;323(18):1824–1836. [DOI] [PubMed] [Google Scholar]
- 37.Lane HC, Fauci AS.. Research in the context of a pandemic. New Eng J Med. 2020;384:755–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.ACTIV-3/TICO LY-CoV555 Study Group . A neutralizing monoclonal antibody for hospitalized patients with Covid-19. New Eng J Med. 2020;384:905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen P, Nirula A, Heller B, et al. . SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinreich DM, Sivapalasingam S, Norton T, et al. . REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384(3):238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joyner MJ, Carter RE, Senefeld JW, et al. . Convalescent plasma antibody levels and the risk of death from Covid-19. N Engl J Med. 2021;384(11):1015–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koblischke M, Traugott MT, Medits I, et al. . Dynamics of CD4 T cell and antibody responses in COVID-19 patients with different disease severity. Front Med. 2020;7:592629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jenkins MM, McCaw TR, Goepfert PA.. Mechanistic inferences from clinical reports of SARS-CoV-2. Infect Dis. 2020;52(8):527–537. [DOI] [PMC free article] [PubMed] [Google Scholar]