Abstract
Objectives
Gout, characterised by hyperuricaemia with monosodium urate crystal formation and inflammation, is the most common inflammatory arthritis in adults. Recent studies have found that elevated uric acid levels are related to the occurrence of dementia. We conducted a study to investigate the association between dementia and gout or hyperuricaemia.
Design
Systematic review and meta-analysis of cohort studies.
Data sources
Studies were screened from inception to 28 June 2019 by searching Medline, Embase and the Cochrane Library databases.
Eligibility criteria
Cohort studies comparing the risk of dementia in patients with gout and hyperuricaemia versus non-gout and non-hyperuricaemia controls were enrolled.
Data extraction and analysis
Two reviewers separately selected studies and extracted data using the Medical Subject Headings without restriction on languages or countries. The adjusted HRs were pooled using the DerSimonian and Laird random effects model. Sensitivity analyses were conducted to evaluate the stability of the results. Publication bias was evaluated using Egger’s and Begg’s tests. Quality assessment was performed according to the Newcastle-Ottawa Scale.
Results
Four cohort studies that met the inclusion criteria were included in our meta-analysis. We found that gout and hyperuricaemia did not increase the risk of dementia, with a pooled HR of 0.94 (95% CI 0.69 to 1.28), but might decrease the risk of Alzheimer’s disease (AD), with a pooled HR of 0.78 (95% CI 0.64 to 0.95). There was little evidence of publication bias. Quality assessment of the included studies was high (range: 6–8 points).
Conclusions
Our study shows that gout and hyperuricaemia do not increase the risk of dementia. However, gout and hyperuricaemia might have a protective effect against AD. Due to the limited number of research articles, more investigations are needed to demonstrate the potential relationship between dementia and gout or hyperuricaemia.
Keywords: internal medicine, dementia, stroke, neurology, rheumatology
Strengths and limitations of this study.
We conducted a meta-analysis of cohort studies to compare the risk of dementia in patients with gout and hyperuricaemia.
As there are few meta-analyses of cohort studies on dementia and gout, our research provides a basis for future research.
The results of our meta-analysis should be interpreted with caution due to the small number and high heterogeneity of the included studies.
Differences in countries, environmental factors, and clinical features, and lack of uniformity in study designs, inclusion criteria and follow-up durations were the main sources of heterogeneity in our study.
Introduction
With the ageing of the population and the improvement in living standards, the incidence of ageing-related cerebrovascular diseases and metabolic diseases is increasing. Dementia is a common age-dependent disorder characterised by progressive deterioration of cognitive ability and function, which can be caused by primary neurological, neuropsychiatric and other conditions, such as traumatic brain injury and tumours. Most senile dementias are caused by neurodegeneration. Common diseases include Alzheimer’s disease (AD), vascular dementia (VD), Lewy body dementia, frontotemporal degeneration and Parkinson’s disease.1 Notably, the number of patients with dementia worldwide is expected to increase to 115 million by 2050.2 Therefore, it is essential to study the pathogenesis of dementia to improve the prevention and treatment of this condition.
With changes in diet and increases in obesity, the incidence of hyperuricaemia also continues to rise. Hyperuricaemia is caused by increased production of uric acid in and/or decreased excretion of uric acid from the body.3 Hyperuricaemia may not cause obvious clinical symptoms for a long time, but with a persistent increase in blood uric acid levels, urate deposition may occur, which can damage tissues and internal organs. In fact, hyperuricaemia causes gout and is an independent risk factor for cardiovascular disease and chronic kidney disease.4 Gout is the most common inflammatory arthritis in adults. Common hallmarks of gout include hyperuricaemia, monosodium urate crystal formation and gouty arthritis.
Moreover, several epidemiological studies have reported an association between chronic inflammation and dementia. In recent years, blood uric acid levels have been considered to be closely related to neurodegenerative changes in Parkinson’s disease and AD.5 Many studies have evaluated the relationship between uric acid and cognitive impairment disorders.6–10 The findings of Lu et al8 supported the potential neuroprotective function of uric acid. In contrast, Schretlen et al11 found that elevated blood uric acid levels increased the risk of cognitive impairment. Thus, whether uric acid predisposes to or protects against dementia remains unclear.
Thus, we here performed a systematic review and meta-analysis of the data reported by cohort studies on the association between dementia and gout or hyperuricaemia.
Methods
A comprehensive literature search was conducted to identify all relevant articles in the Medline (Ovid), Embase (Ovid) and Cochrane Library databases from their respective inception dates until 28 June 2019. Two independent reviewers searched the databases systemically and extensively to obtain studies about the association between dementia and gout or hyperuricaemia, without any language or country restrictions. The Medical Subject Headings terms ‘gout’, ‘hyperuricemia’ and ‘dementia’ were used in the search strategy (see online supplemental file 1).
bmjopen-2020-041680supp001.pdf (85.4KB, pdf)
Data abstraction was performed by two independent reviewers using a unified form. From each primary study, we extracted the following information: the first author’s last name, year of publication, country where the study was performed, sample size, mean age, proportion of men, diagnostic criteria, subtype of dementia, average duration of follow-up years, adjusted covariates, quality of assessment and adjusted HR and 95% CI.
The quality of the studies was scored according to the Newcastle-Ottawa Scale, where a higher score (≥5 points) represents better quality. Three major components were scored: selection (0–4 points), comparability (0–2 points) and outcome (0–3 points).
Inclusion and exclusion criteria
Two investigators read the titles and abstracts of all identified papers and evaluated the literature eligibility independently. Then, we obtained full reports from the articles that appeared eligible, and two investigators again independently assessed whether the papers met the following inclusion criteria: (1) cohort study; (2) original, peer-reviewed study; (3) risk estimates of dementia morbidity in patients with gout or hyperuricaemia compared with non-gout or non-hyperuricaemia controls, with dementia occurring after gout or hyperuricaemia; (4) adjusted HR can be extracted or calculated; and (5) clearly stated dementia (any type, including VD and AD), gout and hyperuricaemia diagnostic criteria; and (6) if multiple studies were reported from the same centre and using the same patient population, we selected the study with the largest sample size or with the most comprehensive data for the meta-analysis. The following reports were excluded: (1) conference articles, review articles, editorials, commentaries, hypothesis papers, case reports and letters; (2) multiple publications from the same population; (3) studies without a control group; (4) studies without clear definition of hyperuricaemia, gout or dementia; and (5) studies with dementia occurring before the onset of gout or hyperuricaemia.
Statistical analysis
Review Manager V.5.3 software was used to calculate the pooled effect size estimate. The adjusted HRs were pooled using the DerSimonian and Laird random effects model, and the weights were equal to the inverse variance of each study’s effect estimation. Forest plots were produced for visual assessment of the HRs and corresponding 95% CIs across studies. Heterogeneity of HRs across studies was evaluated using the Cochrane Q statistic and the I2 statistic (values of 25%, 50% and 75% were considered to represent low, medium and high heterogeneity, respectively).12 13 Sensitivity analysis was conducted to evaluate the stability of the results. Publication bias was evaluated using Egger’s and Begg’s tests.
Results
A flow diagram of the selection process is presented in figure 1. A total of 740 potentially relevant articles were identified by keyword search from the electronic databases. After the initial screening based on titles and abstracts, 665 articles were excluded. Full-text evaluation was conducted in the remaining 75 articles, and 71 articles were excluded due to irrelevant topics or because of being case–control, review or meta-analysis reports, or because of not fulfilling our inclusion criteria. Ultimately, four cohort studies were selected for the meta-analysis.8–10 14
Figure 1.
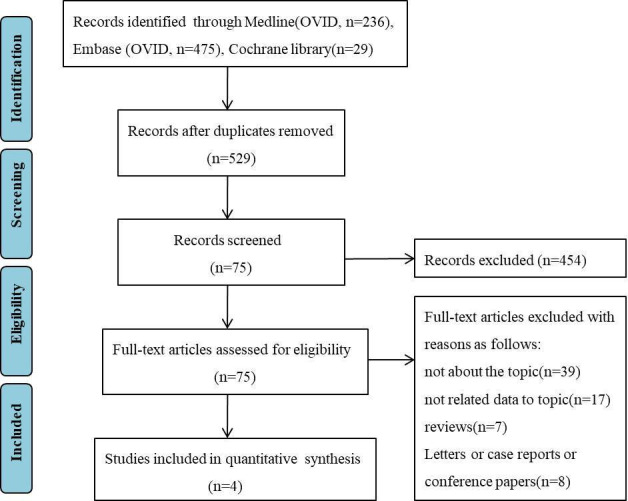
Flow diagram showing the identification and selection of cohort studies included in the review.
The characteristics of the included studies are shown in table 1. All four studies included both men and women, and the average age of participants was ≥60 years. These studies were conducted primarily in the UK, USA, Taiwan, China and France. The study samples ranged from 406 to 1 712 821, and the follow-up durations ranged from 2.3 to 12 years. We used the Newcastle-Ottawa Scale to assess the quality of individual studies. In short, a maximum of 9 points was assigned to each study: 4 for selection, 2 for comparability and 3 for outcomes. A final score >5 points was regarded as high quality. The quality of studies was good, ranging from 6 to 8 points.
Table 1.
Characteristics of included studies in the meta-analysis
| Lu et al8 | Singh and Cleveland et al14 | Hong et al10 | Latourte et al9 | |
| Country | UK | USA | Taiwan, China | France |
| Study design | Cohort study | Cohort study | Cohort study | Cohort study |
| Year | 2016 | 2018 | 2015 | 2018 |
| Age, mean years | 65 | 75.2 | 63.5 | 72.9 |
| Male (%) | 71 | 42.6 | 63 | 38.7 |
| Diagnosis of gout or hyperuricaemia |
Diagnostic code using the Read classification |
ICD-9-CM codes | ICD-9-CM codes | Hyperuricaemia: ≥360 µmol/L for men, ≥300 µmol/L for women |
| Diagnosis of dementia | AD diagnostic codes | ICD-9-CM codes | ICD-9-CM codes | DSM-IV NINCDS-ADRDA |
| Subtype of dementia | AD | All subtypes of dementia | All subtypes of dementia AD, VD |
All subtypes of dementia AD |
| Number of dementias with controls | 309/238 805 | 106 346/1 416 173 | 5905/114 742 AD: 102/114 742 VD: 210/114 742 |
78/1192 AD: 55/1192 |
| Number of dementias with cases | 1942/59 224 | 5310/296 648 | 1214/28 769 AD: 542/28 769 VD: 991/28 769 |
32/406 AD: 21/406 |
| Adjusted confounders | Age, gender, entry time, BMI, smoking, alcohol use, physician visits, social deprivation index, comorbidities and medication use | Age, gender, race, medical comorbidities, common medications for cardiac diseases and gout | Age, gender, relevant comorbidities | Age, gender, tobacco and alcohol consumption, cholesterol, medical comorbidities, medication use |
| Follow-up years (mean or mean±SD) | 5 | 2.3 (1.7) | 4.3 (2.1) | 12 |
| Quality grading | 7 points | 6 points | 7 points | 8 points |
AD, Alzheimer’s disease; BMI, body mass index; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Version IV; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; NINCDS-ADRDA, National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association; VD, vascular dementia.
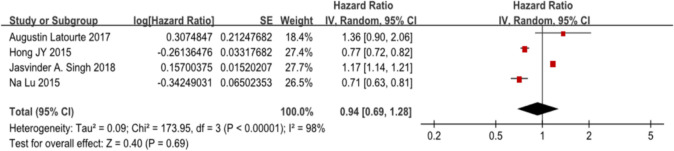
We used Egger’s (p =0.41) and Begg’s (p =1.00) tests to test the publication bias, and there was no statistical evidence of publication bias among the studies. We performed a sensitivity analysis by changing the analysis model from a random effects model to a fixed effects model, and found that the results were unstable for different analysis models, giving different results. When using the random effects model, we found that gout or hyperuricaemia did not increase the risk of dementia, with a pooled HR of 0.94 (95% CI 0.69 to 1.28). Using the fixed effects model, we found that gout or hyperuricaemia increased the risk of dementia, with a pooled HR of 1.07 (95% CI 1.04 to 1.10). In consideration of the high heterogeneity of the included articles, we preferred the results given by the random effects model.
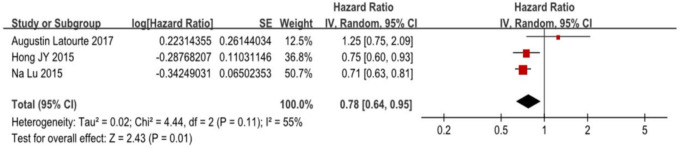
In our meta-analysis, we found no increased risk of dementia among patients with gout or hyperuricaemia, with a pooled HR of 0.94 (95% CI 0.69 to 1.28), with an I2 of 98%, p<0.001 (figure 2). However, there was a decreased risk of AD in patients with gout or hyperuricaemia, with a pooled HR of 0.78 (95% CI 0.64 to 0.95), with an I2 of 55%, p=0.01 (figure 3).
Figure 2.
Forest plot showing an association between dementia and patients with gout and hyperuricaemia.
Figure 3.
Forest plot showing an association between Alzheimer’s disease and patients with gout and hyperuricaemia.
Lu et al8 and Latourte et al9 analysed the patients with dementia in European populations, Singh and Cleveland14 analysed the patients in North America and Hong et al10 investigated the data of Asian populations. Lu et al used AD diagnostic codes, which were positively predicted at 83% in a UK-based GPRD-based validation study. Singh and Cleveland and Hong et al used the International Classification of Diseases, Ninth Revision, Clinical Modification as the diagnostic criterion for dementia. Latourte et al used the Diagnostic and Statistical Manual of Mental Disorders, Version IV criteria for the diagnosis of dementia and the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association as the diagnostic standard for AD. We consider that the reasons for the high degree of heterogeneity are related to the differences between races, diets, climate factors, clinical characteristics between different countries, lack of standardised design, inclusion criteria and uniform follow-up time, which may affect the final result universality.
Discussion
This meta-analysis was based on four articles of cohort studies performed in different countries, thus providing a comprehensive evaluation of the association between dementia and gout or hyperuricaemia. The four cohort studies analysed 121 136 cases of dementia, of which Lu et al included only patients with AD, while the other three analysed data on all types of dementia. In addition, of the four articles included in our meta-analysis, only one described a lower risk of developing VD in patients with gout.10 All studies were assessed as being of high quality based on the Newcastle-Ottawa Scale. The included studies had adjustments for other risk factors. Our meta-analysis showed no statistically significant risk of dementia in patients with gout or hyperuricaemia compared with the non-gout or non-hyperuricaemia controls. In contrast, gout or hyperuricaemia was negatively correlated with the risk of AD. The existing reports hold different views on the pathophysiological mechanism of the relationship between gout and dementia. Whether a high uric acid level has predisposed to or protects against dementia has not been uniformly summarised. Several studies have indicated that inflammatory pathways may play an important role in increasing the risk of dementia.15 16 Hyperuricaemia causes persistent systemic inflammation of gout and can also affect the inflammatory capacity of immune cells.17 However, there has been no unified conclusion about the relationship between dementia and gout or hyperuricaemia, which prompted our meta-analysis.
To date, it has been controversial whether gout or hyperuricaemia and dementia are related. There have been several speculations about the mechanism by which gout could affect the onset of dementia. Some reports propose that gout or hyperuricaemia may increase the risk of dementia9 14 because chronic inflammation from gout has some effects on the brain. Some studies have suggested that high inflammatory cytokine levels in brains with neurodegeneration play important roles in the pathogenesis of dementia.18–20 Others have suggested that gout is associated with oxidative stress, which may play a key role in the pathogenesis of dementia.21 22 Elevated inflammatory cytokines can damage endothelial cells, activate inflammatory cells and cause oxidative stress, which promotes the progression of atherosclerosis.19 23 Atherosclerotic cardiovascular diseases are well-established risk factors for dementia.24 Additionally, there has been some evidence that an association of hyperuricaemia with cardiovascular disease is due to concomitant oxidative stress.25 Taken together, chronic inflammation and oxidative stress may be the final pathways in dementia and gout or hyperuricaemia, which may explain the high risk of dementia reported by some studies in elderly people with gout or hyperuricaemia.
In contrast, some studies have confirmed that gout or hyperuricaemia may decrease the risk of dementia.8 10 Uric acid has been proposed to have both a pro-oxidant effect26 and an antioxidant effect.27 28 It can have some pro-oxidant effects in its oxidised state, but there is relatively little evidence that this is an important mechanism for circulating uric acid, in comparison to its antioxidant role. Uric acid is well established as an important circulating antioxidant and free radical scavenger. It is an antioxidant and metal chelator in vitro.29 30 It can effectively clear peroxynitrite and hydroxyl radicals27 31 32 and reduce oxidative stress as an antioxidant, and may exert possible neuroprotective effects. Moreover, it has been reported that the pathogenesis of AD is related to mitochondrial dysfunction. Some studies have shown that uric acid might preserve mitochondrial function and suppress oxyradical accumulation,27 thereby inhibiting the cytotoxic activity of lactoperoxidase33 and repairing free radical-induced DNA damage.34 Scheepers et al35 reported that a lower level of serum urate may lead to a higher risk of dementia since antioxidant capacity might be reduced with the decreasing of the urate concentration among middle-aged women. They surveyed and followed up 1462 women aged 38–60 years over 44 years and found that a higher serum urate may have a protective effect on dementia including AD and VD.35 Euser et al investigated that high serum urate levels are associated with a reduced risk of dementia and further improved cognitive function.36 In a recent study, Liu et al came to the same conclusion that a lower level of uric acid is associated with AD in Chinese.37 In our study, we analysed the AD subgroup of dementia and found that gout can reduce the risk of AD. This may also confirm the neuroprotective role of uric acid. Unlike asymptomatic hyperuricaemia, patients with gout receive anti-inflammatory or urate-lowering therapies, and it cannot be excluded that those drugs will affect the risk of dementia. Pandey et al reported that a urate-lowering drug benzbromarone can antagonise oxidative stress and improve the function of vascular endothelial cells, which may be related to the reduced probability of dementia in patients with gout.38 However, it is not yet fully clear whether antigout drugs can reduce the incidence of dementia and more research will enable us to understand the effects of antigout drugs on dementia.
Our study found no correlation between dementia and gout or hyperuricaemia. Dementia involves variable clinical symptoms, multiple subtypes and complex pathogenesis, which may explain the contradictory results of studies on gout or hyperuricaemia. More research is needed to support this viewpoint. The risk of AD is inversely proportional to gout, and the neuroprotective mechanism of uric acid may play a dominant role in the pathogenesis of AD.
However, there were some potential limitations to our study. First, there were only four studies in our meta-analysis, whose numbers of articles included were relatively small. Moreover, the cohort studies were mostly medical registry-based studies, which would raise concerns over coding inaccuracy and misclassification. Second, although studies included had been adjusted for other risk factors, the statistical heterogeneity is still high, which might be attributed to differences in demographics, dietary factors, environmental factors and clinical characteristics in different countries, or lack of standardised design, inclusion criteria and follow-up time. Third, this was a pooled analysis of observational studies, which could only demonstrate an association, but not causality. Therefore, we cannot determine the impact of gout, hyperuricaemia or other unknown confounding factors on the outcome of dementia risk. Moreover, in the sensitivity analysis, we found that the results were unstable when using different analysis models. Considering the high heterogeneity of the included articles, we preferred the results given by the random effects model. Consequently, caution is needed when interpreting the present meta-analysis findings.
In conclusion, our meta-analysis of cohort studies suggested that there is no risk of dementia among patients with gout and hyperuricaemia. However, high uric acid levels have a protective effect on AD. More investigations are needed to demonstrate the potential relationship between dementia and patients with gout and hyperuricaemia.
Supplementary Material
Footnotes
Contributors: Conceptualisation: YL. Methodology: SYP and RJC. Formal analysis: ZJX. Data curation: QPZ. Writing–original draft preparation: SYP and RJC. Writing–review and editing: YL. Approval of final manuscript: all authors.
Funding: The present work was supported by the National Key Research and Development Program of China (project number 2016YFC0906201) to YL, and the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (project number ZYGD18015) to YL.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Gale SA, Acar D, Daffner KR. Dementia. Am J Med 2018;131:1161–9. 10.1016/j.amjmed.2018.01.022 [DOI] [PubMed] [Google Scholar]
- 2.Prince M, Bryce R, Albanese E, et al. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 2013;9:63–75. 10.1016/j.jalz.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 3.Li L, Zhang Y, Zeng C. Update on the epidemiology, genetics, and therapeutic options of hyperuricemia. Am J Transl Res 2020;12:3167–81. [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta MK, Singh JA. Cardiovascular Disease in Gout and the Protective Effect of Treatments Including Urate-Lowering Therapy. Drugs 2019;79:531–41. 10.1007/s40265-019-01081-5 [DOI] [PubMed] [Google Scholar]
- 5.Mollenhauer B, Zimmermann J, Sixel-Döring F, et al. Baseline predictors for progression 4 years after Parkinson's disease diagnosis in the de novo Parkinson cohort (DeNoPa). Mov Disord 2019;34:67–77. 10.1002/mds.27492 [DOI] [PubMed] [Google Scholar]
- 6.Lai S-W, Lin C-L, Liao K-F. No association between gout and Alzheimer's disease: results of a case-control study in older people in Taiwan. Int J Geriatr Psychiatry 2013;28:1205–6. 10.1002/gps.3963 [DOI] [PubMed] [Google Scholar]
- 7.Engel B, Gomm W, Broich K, et al. Hyperuricemia and dementia - a case-control study. BMC Neurol 2018;18:131. 10.1186/s12883-018-1136-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu N, Dubreuil M, Zhang Y, et al. Gout and the risk of Alzheimer's disease: a population-based, BMI-matched cohort study. Ann Rheum Dis 2016;75:547–51. 10.1136/annrheumdis-2014-206917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Latourte A, Soumaré A, Bardin T, et al. Uric acid and incident dementia over 12 years of follow-up: a population-based cohort study. Ann Rheum Dis 2018;77:328–35. 10.1136/annrheumdis-2016-210767 [DOI] [PubMed] [Google Scholar]
- 10.Hong J-Y, Lan T-Y, Tang G-J, et al. Gout and the risk of dementia: a nationwide population-based cohort study. Arthritis Res Ther 2015;17:139. 10.1186/s13075-015-0642-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schretlen DJ, Inscore AB, Jinnah HA, et al. Serum uric acid and cognitive function in community-dwelling older adults. Neuropsychology 2007;21:136–40. 10.1037/0894-4105.21.1.136 [DOI] [PubMed] [Google Scholar]
- 12.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 13.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh JA, Cleveland JD. Gout and dementia in the elderly: a cohort study of Medicare claims. BMC Geriatr 2018;18:281. 10.1186/s12877-018-0975-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallin K, Solomon A, Kåreholt I, et al. Midlife rheumatoid arthritis increases the risk of cognitive impairment two decades later: a population-based study. J Alzheimers Dis 2012;31:669–76. 10.3233/JAD-2012-111736 [DOI] [PubMed] [Google Scholar]
- 16.Dregan A, Chowienczyk P, Gulliford MC. Are Inflammation and Related Therapy Associated with All-Cause Dementia in a Primary Care Population? J Alzheimer Dis 2015;46:1039–47. 10.3233/JAD-150171 [DOI] [PubMed] [Google Scholar]
- 17.Cabău G, Crișan TO, Klück V, et al. Urate-induced immune programming: consequences for gouty arthritis and hyperuricemia. Immunol Rev 2020;294:92–105. 10.1111/imr.12833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell IL, Stalder AK, Chiang C-S, et al. Transgenic models to assess the pathogenic actions of cytokines in the central nervous system. Mol Psychiatry 1997;2:125–9. 10.1038/sj.mp.4000225 [DOI] [PubMed] [Google Scholar]
- 19.Rafnsson SB, Deary IJ, Smith FB, et al. Cognitive decline and markers of inflammation and hemostasis: the Edinburgh artery study. J Am Geriatr Soc 2007;55:700–7. 10.1111/j.1532-5415.2007.01158.x [DOI] [PubMed] [Google Scholar]
- 20.Schram MT, Euser SM, de Craen AJM, et al. Systemic markers of inflammation and cognitive decline in old age. J Am Geriatr Soc 2007;55:708–16. 10.1111/j.1532-5415.2007.01159.x [DOI] [PubMed] [Google Scholar]
- 21.Praticò D, Uryu K, Leight S, et al. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci 2001;21:4183–7. 10.1523/JNEUROSCI.21-12-04183.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006;443:787–95. 10.1038/nature05292 [DOI] [PubMed] [Google Scholar]
- 23.Libby P. Inflammation in atherosclerosis. Nature 2002;420:868–74. 10.1038/nature01323 [DOI] [PubMed] [Google Scholar]
- 24.Dufouil C, Seshadri S, Chêne G. Cardiovascular risk profile in women and dementia. J Alzheimer Dis 2014;42:S353–63. 10.3233/JAD-141629 [DOI] [PubMed] [Google Scholar]
- 25.Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993;262:689–95. 10.1126/science.7901908 [DOI] [PubMed] [Google Scholar]
- 26.Kanellis J, Kang D-H. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin Nephrol 2005;25:39–42. 10.1016/j.semnephrol.2004.09.007 [DOI] [PubMed] [Google Scholar]
- 27.Yu ZF, Bruce-Keller AJ, Goodman Y, et al. Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J Neurosci Res 1998;53:613–25. [DOI] [PubMed] [Google Scholar]
- 28.Squadrito GL, Cueto R, Splenser AE, et al. Reaction of uric acid with peroxynitrite and implications for the mechanism of neuroprotection by uric acid. Arch Biochem Biophys 2000;376:333–7. 10.1006/abbi.2000.1721 [DOI] [PubMed] [Google Scholar]
- 29.Ames BN, Cathcart R, Schwiers E, et al. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A 1981;78:6858–62. 10.1073/pnas.78.11.6858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davies KJ, Sevanian A, Muakkassah-Kelly SF, et al. Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem J 1986;235:747–54. 10.1042/bj2350747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hooper DC, Spitsin S, Kean RB, et al. Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci U S A 1998;95:675–80. 10.1073/pnas.95.2.675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waring WS, Webb DJ, Maxwell SR. Systemic uric acid administration increases serum antioxidant capacity in healthy volunteers. J Cardiovasc Pharmacol 2001;38:365–71. 10.1097/00005344-200109000-00005 [DOI] [PubMed] [Google Scholar]
- 33.Everse J, Coates PW. The cytotoxic activity of lactoperoxidase: enhancement and inhibition by neuroactive compounds. Free Radic Biol Med 2004;37:839–49. 10.1016/j.freeradbiomed.2004.06.017 [DOI] [PubMed] [Google Scholar]
- 34.Anderson RF, Harris TA. Dopamine and uric acid act as antioxidants in the repair of DNA radicals: implications in Parkinson's disease. Free Radic Res 2003;37:1131–6. 10.1080/10715760310001604134 [DOI] [PubMed] [Google Scholar]
- 35.Scheepers LEJM, Jacobsson LTH, Kern S, et al. Urate and risk of Alzheimer's disease and vascular dementia: a population-based study. Alzheimers Dement 2019;15:754–63. 10.1016/j.jalz.2019.01.014 [DOI] [PubMed] [Google Scholar]
- 36.Euser SM, Hofman A, Westendorp RGJ, et al. Serum uric acid and cognitive function and dementia. Brain 2009;132:377–82. 10.1093/brain/awn316 [DOI] [PubMed] [Google Scholar]
- 37.Liu H, Reynolds GP, Wei X. Uric acid and high-density lipoprotein cholesterol are differently associated with Alzheimer's disease and vascular dementia. J Alzheimers Dis 2020;73:1125–31. 10.3233/JAD-191111 [DOI] [PubMed] [Google Scholar]
- 38.Pandey RN, Wang TS, Tadjuidje E, et al. Structure-activity relationships of benzbromarone metabolites and derivatives as EYA inhibitory anti-angiogenic agents. PLoS One 2013;8:e84582. 10.1371/journal.pone.0084582 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-041680supp001.pdf (85.4KB, pdf)
Data Availability Statement
No data are available.