Abstract
Background and aims
The risk of use of immune-mediated diarrhea and colitis (imDC) in patients with preexisting inflammatory bowel disease (IBD) is not fully understood. We report the incidence of imDC in these patients, and compare with a matched cohort of patients with cancer and without IBD.
Methods
Patients with IBD from a tertiary center cancer registry who underwent immune checkpoint inhibitor (ICI) therapy from 2011 to 2019 were identified. A 1:5 matched cohort of patients with and without a history of IBD was created, based on age, ICI therapy, and cancer type. Demographic data, clinical history of IBD, cancer, ICI agent, imDC events after ICI therapy, and overall survival were analyzed. Overall survival and time-to-imDC (TTimDC) were estimated by Kaplan-Meier and multivariate Cox proportional-hazards models.
Results
From a retrospective cohort of 3900 patients who received ICI therapy, 30 patients with IBD were matched with 150 patients without a history of IBD. Most patients received PD-1/PD-L1 inhibitor monotherapy (154/180, 85.6%). Individuals with preexisting IBD showed significantly shorter TTimDC than those in the non-IBD group (1-year imDC-free rate 67% vs 93%; HR 7.59, 95% CI 3.00 to 19.15, p<0.0001). Eleven (36%) from the IBD cohort experienced imDC events; none led to life-threatening conditions needing surgical interventions or death. Corticosteroids or biologics were needed in 8/11 (73%) patients, and discontinuation of therapy improved imDC in the remaining three. Half of patients required hospitalization. In contrast, no significant difference in overall survival was observed between IBD and non-IBD cohorts (HR 0.89, 95% CI 0.54 to 1.48). Both groups had overall comparable rates of other non-imDC immune-related adverse events.
Conclusion
Patients with preexisting IBD had worse time-to-imDC than non-IBD matched controls, yet did not exhibit worse overall survival. While close monitoring of patients with preexisting IBD is warranted while on immunotherapy, this comorbidity should not preclude ICI therapy if clinically required.
Keywords: immunotherapy, autoimmunity, CTLA-4 antigen, programmed cell death 1 receptor, immunity
Introduction
Immune checkpoint inhibitor (ICI) agents enhance T-cell activity against cancer cells by inhibiting negative regulatory components of the immune response. Antibodies against cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) and programmed cell death protein-1 and its ligand (PD-1/PD-L1) have dramatically expanded the arsenal of cancer therapy, although at the cost of unique side effects. This spectrum of novel autoimmune-like conditions has now been coined immune-related adverse events (irAEs). One of the most commonly encountered among irAEs are immune-mediated diarrhea and colitis (imDC), which range between 0.5% and 11% in the immunotherapy-treated population. Some studies report rates of diarrhea as high as 30%.1 Anti-CTLA4 therapy, mainly ipilimumab, results in more frequent gastrointestinal-related irAEs than anti-PD-1/PD-L1 therapy, and the combination of both anti-CTLA4 and anti-PD-1 agents exhibits the highest risk.2 3
Due to the immune pathophysiology of irAEs, patients with autoimmune disorders were in fact excluded from early clinical trials.4 5 Specific to patients with inflammatory bowel disease (IBD) was a concern that these patients would suffer an increased risk of adverse gastrointestinal (GI) complications. Shared features between IBD and imDC include aggressive T-cell responses and baseline gut dysbiosis, which are both pathophysiological responses contributing to ipilimumab-induced colitis.6 In fact, endoscopic and histologic findings between IBD colitis and imDC have been reported to frequently be indistinguishable,7–9 although more recent evidence suggests to varied expressions of PD-L1 and PD1 on inflammatory cells of the colonic mucosa between IBD and anti-PD-1-related colitis.10
Despite early concerns with the risk of treating patients with IBD, evidence has accumulated which suggest an acceptable, although higher, rate of complications.11–13 A recent multicenter, retrospective study of 102 patients with IBD receiving ICI therapy for various cancers detected a higher risk of developing GI-related adverse events as compared with patients without preexisting IBD (41% vs 11%, p<0.001).14 Still, multiple outstanding questions remain in the rapidly evolving field of GI irAE surrounding treatment of patients with IBD, in particular, if the introduction of immunotherapy in an IBD cohort harms overall survival (OS). In this retrospective study, we aimed to measure the true incidence of IBD flare in patients with preexisting IBD and understand the influence of an irAE-related flare on toxicity-related or cancer-related OS.
Methods
Patient population
An institutional review board approved this minimal risk research study. We conducted a retrospective study of 3900 patients who underwent ICI therapy for cancer in a tertiary center from January 2011 to December 2019. We included all patients who had received at least 1 dose of ICI therapy and queried for a baseline IBD diagnosis using ICD codes followed by chart review. Patients were assessed as having IBD based on clinical data which includes documentation of symptoms of IBD lasting 6 months with radiographic or endoscopic data diagnostic of IBD and pathological evidence of chronic inflammation. When proof of diagnosis was not available, such as patients with history of achieving remission after surgery or being on chronic IBD therapy, cases were adjudicated by an IBD specialist (JRP). We then performed a propensity score 5:1 matching by cancer type, cancer therapy, and age between patients with IBD and patients with cancer and without IBD history. In order to match combination therapies, we defined the start and stop dates of ICI therapy by determining an uninterrupted course of any ICI therapy that was administered, as long as no more than 5 weeks have passed between any two doses. Following matching, we extracted demographic data, clinical history of IBD, cancer, ICI treatment, all irAEs including colitis and diarrhea events after ICI therapy, and OS from the electronic health records. Due to the inability to properly distinguish an IBD flare from imDC, as was the same issue with other publications,15 16 all colitis events will be considered as imDC. ICI colitis was diagnosed based on a constellation of symptoms which include abdominal pain, rectal bleeding, and blood or mucus in stools. Both ICI colitis and ICI diarrhea were graded according to Modified Common Terminology Criteria for Adverse Events (CTCAE) version 5.17 Imaging, inflammatory laboratory testing, and colonoscopies were additive, but not all were required for each case’s diagnosis. Data that were relevant to IBD, cancer, or ICI therapy were extracted by manual chart review, whereas data regarding demographic variables such as gender, race, and smoking history were done by electronic coding extraction. Occurrence of other irAEs were based on manual extraction from oncologist, gastroenterologist, or other specialists’ assessments. Baseline IBD activity assessment was limited by the retrospective design of the study. IBD baseline disease activity was inferred from a combination of the following: physician assessment in the last office visit, Mayo Score Severity of Ulcerative Colitis index or Crohn’s Disease Activity Index and laboratory, symptomatic, imaging, endoscopic, or histologic evidence of active disease prior to ICI therapy initiation.
Statistical analysis
Patient characteristics and irAEs were summarized using frequencies and percentages. Age, time from diagnosis to ICI start, and ICI duration were summarized using medians and ranges. Baseline characteristic comparisons between IBD and control groups were carried out using Fisher’s exact test or Wilcoxon rank-sum test, where applicable. OS and time to imDC (TTimDC) symptom survival were estimated using Kaplan-Meier method, and death was considered as censor for TTimDC. Survival was calculated from ICI start date to event or last follow-up date. Log-rank test was used to compare OS and TTimDC between IBD and control groups. This study includes patients with multiple cancers and different ICI therapy regimens at different cancer stages, and as such, survival analyses and TTimDC should be carefully interpreted. Besides using individually matched cohorts, we explored whether cancer stage and history of chemotherapy or radiation therapy were statistically different between cohorts; we also performed a multivariate Cox proportional-hazards model to evaluate the impact of preexisting IBD on OS and TTimDC. Since cancer stage was not a pre-specified matching criterion, we ran a sensitivity analysis for the primary outcomes of OS and TTimDC using data from patients with only stage 4 cancers.
Multivariate Cox proportional-hazards models were fit for OS and TTimDC. Backwards elimination procedure was used to identify final Cox models, starting from all factors in the univariate summary table. Only significant factors were kept in the final models, but age, gender, and group were forced into the model to show their effects on OS. For OS final model, cancer type and cancer medication were included as strata (matched by these two factors and age). TTimDC model was not stratified due to limited event numbers. It is worth noting that these multivariate Cox proportional-hazards models analyze the entire cohort of the study and thus significant results do not relate particularly to the IBD cohort. All tests were two-sided and p values of 0.05 or less were considered statistically significant. Statistical analyses were performed using SAS Studio V.3.7 (SAS Institute, Cary, NC) and R 3.6 (R Foundation, Vienna, Austria).
Results
Patient characteristic
We identified 30 patients who had a diagnosis of IBD prior to the initiation of immunotherapy, and compared their outcomes, using a 5:1 matched cohort design, with 150 control patients based on cancer type, ICI type, as well as age (table 1). No statistical differences were observed between groups in terms of gender, race, smoking status, history of rheumatologic disorders, cancer stage, prior cancer-related treatment frequencies, prior steroid usage, or total number of ICI doses. We explored the six cases of stage 2 disease that were only in the control cohort (despite no statistical difference in overall stage among both groups, who were stage 4 in almost 90% of cases), and found that five of the six patients underwent ICI therapy due to otherwise non-resectable tumors that have progressed on prior first-line therapies for the respective cancers. The other patient required immunotherapy as part of adjuvant treatment for resected stage 2 disease. Median age at initiation of ICI treatment was 67 across both groups. Cancer diagnoses were equally distributed across multiple organ systems. ICI therapy was mostly PD-1/PD-L1 inhibitors (154/180, 85.6%) while the rest included the combination of anti-CTLA-4/PD-1 or PD-L1. The IBD cohort demonstrated significantly higher rates of small bowel (30% vs 4.7%) and large bowel surgeries (46.7% vs 10.7%).
Table 1.
Summary of patient characteristics by patient group
| Group | All | P value | ||
| Ctrl | IBD | |||
| N (%) | N (%) | N (%) | ||
| Gender | ||||
| Female | 60 (40) | 18 (60) | 78 (43.33) | 0.07 |
| Male | 90 (60) | 12 (40) | 102 (56.67) | |
| Race | ||||
| Black | 19 (12.67) | 1 (3.33) | 20 (11.11) | 0.31 |
| Caucasian | 128 (85.33) | 29 (96.67) | 157 (87.22) | |
| Multiracial | 3 (2) | 0 (0) | 3 (1.67) | |
| Smoking status | ||||
| Former smoker | 83 (55.33) | 16 (53.33) | 99 (55) | >0.99 |
| Never smoked tobacco | 43 (28.67) | 9 (30) | 52 (28.89) | |
| Smokes tobacco daily | 24 (16) | 5 (16.67) | 29 (16.11) | |
| History of other autoimmune disorders | ||||
| No | 124 (82.67) | 23 (76.67) | 147 (81.67) | 0.44 |
| Yes | 26 (17.33) | 7 (23.33) | 33 (18.33) | |
| Small bowel surgery | ||||
| No | 143 (95.33) | 21 (70) | 164 (91.11) | 0.0002 |
| Yes | 7 (4.67) | 9 (30) | 16 (8.89) | |
| Large bowel surgery | ||||
| No | 134 (89.33) | 16 (53.33) | 150 (83.33) | <0.0001 |
| Yes | 16 (10.67) | 14 (46.67) | 30 (16.67) | |
| Cancer stage | ||||
| 2 | 6 (4) | 0 (0) | 6 (3.33) | 0.48 |
| 3 | 12 (8) | 4 (13.33) | 16 (8.89) | |
| 4 | 132 (88) | 26 (86.67) | 158 (87.78) | |
| Cancer type | ||||
| Cancer of bladder | 4 (2.67) | 2 (6.67) | 6 (3.33) | 0.95 |
| Cancer of breast | 6 (4) | 1 (3.33) | 7 (3.89) | |
| Cancer of bronchus; lung | 80 (53.33) | 15 (50) | 95 (52.78) | |
| Cancer of colon | 11 (7.33) | 2 (6.67) | 13 (7.22) | |
| Cancer of head and neck | 5 (3.33) | 1 (3.33) | 6 (3.33) | |
| Cancer of kidney and renal pelvis | 7 (4.67) | 2 (6.67) | 9 (5) | |
| Cancer of other urinary organs | 3 (2) | 1 (3.33) | 4 (2.22) | |
| Cancer of rectum and anus | 3 (2) | 1 (3.33) | 4 (2.22) | |
| Melanomas of skin | 24 (16) | 4 (13.33) | 28 (15.56) | |
| Melanoma of orbit | 7 (4.67) | 1 (3.33) | 8 (4.44) | |
| Cancer treatment | ||||
| Atezolizumab | 22 (14.67) | 5 (16.67) | 27 (15) | 0.996 |
| Atezolizumab, followed by ipilimumab, nivolumab combination | 1 (0.67) | 0 (0) | 1 (0.56) | |
| Durvalumab | 5 (3.33) | 1 (3.33) | 6 (3.33) | |
| Ipilimumab, nivolumab combination | 17 (11.33) | 3 (10) | 20 (11.11) | |
| Ipilimumab, nivolumab combination, followed by pembrolizumab | 4 (2.67) | 1 (3.33) | 5 (2.78) | |
| Nivolumab | 36 (24) | 8 (26.67) | 44 (24.44) | |
| Pembrolizumab | 65 (43.33) | 12 (40) | 77 (42.78) | |
| No of doses, N (median, range) | 6 (1–83) | 6 (1–42) | 6 (1–83) | 0.88 |
| History of chemotherapy | ||||
| No | 52 (34.67) | 10 (33.33) | 62 (34.44) | >0.99 |
| Yes | 98 (65.33) | 20 (66.67) | 118 (65.56) | |
| History of radiation therapy | ||||
| No | 130 (86.67) | 27 (90) | 157 (87.22) | 0.77 |
| Yes | 20 (13.33) | 3 (10) | 23 (12.78) | |
| Prior steroid use | ||||
| No | 19 (12.67) | 5 (16.67) | 24 (13.33) | 0.56 |
| Yes | 131 (87.33) | 25 (83.33) | 156 (86.67) | |
| Age at treatment, years (median, range) | 67 (23–84) | 67 (39–81) | 67 (23–84) | 0.54 |
Patients with IBD and controls were propensity score matched by cancer type, cancer mediation, and age. P values by Fisher’s exact test. Summary of age and number of doses by patient group. P value by Wilcoxon rank-sum test.
IBD, inflammatory bowel disease.
Pre-treatment IBD characteristics
Data regarding individual patients with IBD is found in table 2 and online supplemental table 1. Of 30 patients with IBD, 19 had Crohn’s disease (CD), 10 had ulcerative colitis (UC), and 1 was indeterminate colitis. A minority (7/30, 23%) had a history of other autoimmune disorders, and only 3/30 (10%) were considered to have active IBD disease at start of ICI. Those with active disease were deemed only mildly active by their gastroenterologist. Fifteen patients (50%) in our IBD cohort were on IBD therapy (biologic, azathioprine, mercaptopurine, budesonide, or aminosalicylic acid (5-ASA) derivatives) at cancer diagnosis. Nine of those patients needed to stop one or more therapies due to cancer diagnosis (four were on biologics and five on corticosteroid-sparing immunosuppressive agents). At the time of ICI treatment initiation, two of those nine patients were actively receiving 5-ASA derivatives, one was on budesonide and one on infliximab. The remaining six patients continued their IBD therapy without interruptions (5-ASA in three, budesonide, mercaptopurine, and infliximab in each of the others). Other supportive medications included antidiarrheals (5), probiotics (3), and rifaximin (1). Thirteen were not on any bowel-related therapy. Half (15/30, 50%) had undergone some form of bowel surgery.
Table 2.
IBD cohort incidence of imDC, colitis, and diarrhea
| N (%) | Incidence of imDC | % | |
| Total IBD cohort | 30 (100) | 11 | 37 |
| Gender | |||
| Female | 18 (60) | 8 | 44 |
| Male | 12 (40) | 3 | 25 |
| IBD type | |||
| CD | 19 (63) | 6 | 32 |
| UC | 10 (33) | 5 | 50 |
| IBD-U | 1 (3) | 0 | 0 |
| History of other autoimmune diseases | |||
| Yes | 7 (23) | 5 | 71 |
| No | 23 (77) | 6 | 26 |
| History of extra-intestinal manifestations | |||
| Yes | 6 (20) | 3 | 50 |
| No | 24 (80) | 8 | 33 |
| Smoking status | |||
| Smoker | 5 (17) | 2 | 40 |
| Ex-smoker | 16 (53) | 5 | 31 |
| Never smoker | 9 (30) | 4 | 44 |
| Small bowel surgery | |||
| Yes | 9 (30) | 4 | 44 |
| No | 21 (70) | 7 | 33 |
| Large bowel surgery | |||
| Yes | 14 (47) | 4 | 29 |
| No | 16 (53) | 7 | 44 |
| Any bowel surgery | |||
| Yes | 15 (50) | 4 | 27 |
| No | 15 (50) | 7 | 47 |
| IBD medications held due to cancer diagnosis | |||
| Yes | 9 (30) | 5 | 56 |
| No | 21 (70) | 6 | 29 |
| Active disease at ICI start | |||
| Yes | 3 (10) | 1 | 33 |
| No | 27 (90) | 10 | 37 |
| IBD treatment at ICI start | |||
| Yes | 10 (33) | 3 | 30 |
| No | 20 (67) | 8 | 40 |
CD, Crohn’s disease; IBD-U, inflammatory bowel disease—undeterminate; ICI, immune checkpoint inhibitor; imDC, immune-mediated diarrhea and colitis; UC, ulcerative colitis.
jitc-2021-002567supp001.pdf (456.6KB, pdf)
Immune-related toxicity in the IBD cohort
Following ICI therapy, all irAEs were monitored (table 3). Except for immune-mediated colitis and immune-mediated diarrhea, which were analyzed as time-to-event endpoints and thus were not included in this table, both groups had overall comparable rates of irAEs overall. When patients who discontinued ICI therapy were compared, the reason to stop ICI in the IBD group was more likely to be due to irAEs as opposed to death, infection, or treatment holiday, as compared with the control group (p=0.006). Only 28/180 (15.6%) of the entire cohort were still on therapy by the end of the follow-up period.
Table 3.
Summary of patient immunotherapy-related adverse events by patient group
| Group | All | P value* | ||
| Ctrl | IBD | |||
| N (%) | N (%) | N (%) | ||
| irAEs† | ||||
| No | 73 (48.67) | 11 (36.67) | 84 (46.67) | 0.32 |
| Yes | 77 (51.33) | 19 (63.33) | 96 (53.33) | |
| ICI stop reason | ||||
| Colitis | 6 (4) | 7 (23.33) | 13 (7.22) | 0.04 |
| Disease progression | 51 (34) | 8 (26.67) | 59 (32.78) | |
| Other irAE | 23 (15.33) | 6 (20) | 29 (16.11) | |
| Death | 32 (21.33) | 4 (13.33) | 36 (20) | |
| Complete remission | 1 (0.67) | 0 (0) | 1 (0.56) | |
| Still receiving | 23 (15.33) | 5 (16.67) | 28 (15.56) | |
| Infection | 5 (3.33) | 0 (0) | 5 (2.78) | |
| Treatment break | 9 (6) | 0 (0) | 9 (5) | |
| ICI stop reason (condensed) | ||||
| N/A (still receiving) | 23 (15.33) | 5 (16.67) | 28 (15.56) | |
| All other reasons | 98 (65.33) | 12 (40) | 110 (61.11) | 0.006 |
| irAE (including imDC) | 29 (19.33) | 13 (43.33) | 42 (23.33) | |
IBD and control cohorts were propensity score matched by cancer type, cancer mediation, and age.
*P values by Fisher’s exact test.
†ICI colitis and ICI diarrhea were analyzed as time-to-event endpoints and were not included in this table.
IBD, inflammatory bowel disease; ICI, immune checkpoint inhibitor; imDC, immune-mediated diarrhea and colitis; irAE, immune-related adverse events.
Among the 11/30 patients with IBD who developed imDC, the most common GI symptoms were abdominal pain (8/11, 73%), increased frequency of stool (7/11, 64%), and nausea/vomiting (6/11, 55%). Melena or hematochezia was reported in 3/11 patients, and fever in 2 patients. Although colonoscopies were not required for diagnosis, they were performed in three of the patients with ICI colitis and showed non-ulcer inflammation, chronic active colitis, and focal active colitis with chronic active colitis, respectively. None of the imDC events led to life-threatening conditions that needed surgical intervention, and no death was related to imDC. Treatment included corticosteroids (8/11, 73%), 5-ASA in one patient, and vedolizumab in another. The remaining three patients who did not require corticosteroids had improvement after stopping the ICI therapy only and so did not end up requiring a colonoscopy. Almost half (5/11, 55%) needed hospitalization. Three patients with IBD who subsequently developed imDC restarted the same ICI after resolution of the irAE, all of which were anti-PD-1/L1. The first patient with grade 3 colitis underwent colonoscopy revealing moderate active colitis in the cecum and chronic colitis with erosions in the descending colon and rectosigmoid junction, and was treated with oral corticosteroid taper with no recurrence; the second with grade 2 diarrhea was diagnosed based on symptoms with subsequent CT showing no colonic thickening, and was treated with ICI interruption without recurrence after ICI resumption. Only one recurrence of imDC occurred in the third patient who was diagnosed with grade 2 colitis based on symptoms and CT evidence of left-sided colitis, without colonoscopy, initially managed with rectal corticosteroids, and whose recurrence was managed with PO corticosteroids.
It is worth noting that no imDC developed in the cohort of six patients who did continue their immunosuppressive regimens; however, all had disease progression or death. In contrast, five of the nine patients with IBD therapy interruptions at the time of cancer diagnosis developed imDC, and four of these nine patients also had either no progression of disease or partial remission at time of imDC or at the end of study follow up. In particular, three of the five patients who developed imDC had partial remission or no progression.
Time to imDC
One patient in each group developed imDC on the first day of ICI therapy: a 67-year-old white male with inactive left colon UC who was on 5-ASA on ipilimumab/nivolumab initiation for stage 4 melanoma (methotrexate had been interrupted after melanoma diagnosis), and a 57-year-old black female without IBD and with stage 4 lung adenocarcinoma who received pembrolizumab. Since both of these patients’ survival times were 0, they were excluded from TTimDC analysis; 149 control patients and 29 patients with IBD were included in the time to IMDC analysis. Median follow-up time was 16.4 months (range: 1.2–50.0 months), but median TTimDC was not reached. Patients with IBD had significantly worse TTimDC, in regards to both time-to-event and in total number, than did control patients, with p<0.0001 by log rank test (figure 1). Limiting this analysis to stage 4 only cancers resulted in no change for IBD versus control TTimDC (online supplemental figure 1). Cumulative incidence rates of ICI colitis, ICI diarrhea, and imDC at 6 and 12 months are reported in table 4. Multivariate Cox proportional-hazards model was fit for TTimDC analysis (table 5) and patients with IBD still had significantly worse imDC outcomes (1-year imDC-free rate 67% vs 93%; HR 7.59, 95% CI 3.00 to 19.15, p<0.0001). Patients who were steroid naive (p=0.047) had shorter TTimDC than those who had prior steroid exposure. Non-smokers and current smokers had shorter TTimDC than ex-smokers (p=0.01).
Figure 1.
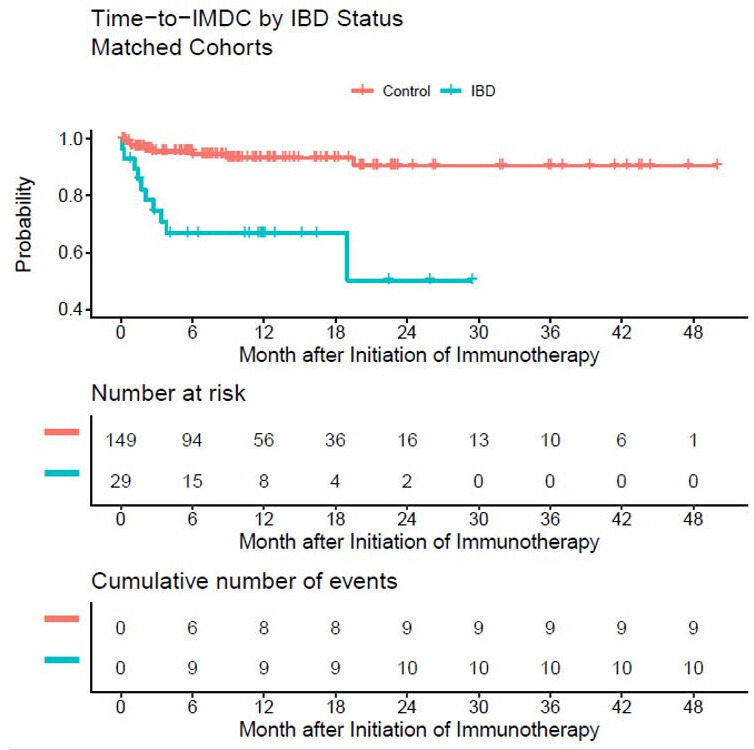
Table 4.
Incidence rates of immune-mediated diarrhea and colitis in IBD and control cohorts
| Groups | Total N | Number of events at 6 months (rate %) | Number of events at 1 year (rate %) | ||||
| ICI diarrhea | ICI colitis | imDC | ICI diarrhea | ICI colitis | imDC | ||
| All patients | 180 | 6 (3.3%) | 12 (6.7%) | 18 (10%) | 7 (3.9%) | 14 (7.8%) | 21 (11.7%) |
| Control | 150 | 3 (2%) | 4 (2.7%) | 7 (4.7%) | 4 (2.7%) | 6 (4%) | 10 (6.7%) |
| IBD | 30 | 3 (10%) | 8 (26.7%) | 11 (36.7%) | 3 (10%) | 8 (26.7%) | 11 (36.7%) |
Event, ICI colitis or ICI diarrhea; IBD, inflammatory bowel disease; ICI, immune checkpoint inhibitor; imDC, immune-mediated diarrhea and colitis; N, total sample size.
Table 5.
Multivariate Cox proportional-hazards models for TTimDC and OS for IBD and control cohorts
| Variable | HR (95% CI) | P value |
| Endpoint: TTimDC (N=178, E=19) | ||
| IBD vs control | 7.583 (3.003 to 19.148) | <0.0001 |
| Never smoker vs former | 4.075 (1.35 to 12.303) | 0.01 |
| Current smoker vs former | 6.432 (1.734 to 23.859) | 0.005 |
| Prior steroids (yes vs no) | 2.807 (1.013 to 7.776) | 0.047 |
| Endpoint: OS (N=180, E=113) | ||
| IBD vs control | 0.894 (0.541 to 1.477) | 0.66 |
| 1-year increase in age | 1.001 (0.986 to 1.016) | 0.91 |
| Female vs male | 1.439 (0.986 to 2.102) | 0.06 |
N means total sample size; E means total number of events. TTimDC event=immune-mediated diarrhea or colitis, death is considered censor. OS event=death by any cause.
History of radiation exposure was balanced in both groups (13.33% vs 10%, p=0.77). We explored this factor to understand if higher imDC occurred among patients with abdominopelvic radiation as opposed to radiation elsewhere. In 23 patients with prior radiotherapy, 15 (65%) had lung cancer, and 22 (96%) had metastatic disease. The most common areas of radiation were head and neck in 12 (52%), chest and thoracic spine in 12 (52%), lumbar spine and pelvis in 9 (39%), and one (4%) in the distal tibia. imDC occurred in two of three patients with baseline inactive IBD and radiation in the sacroiliac area. History of radiation exposure itself, however, was not a significant marker of imDC in multivariate Cox proportional-hazards models (online supplemental table 2).
Overall survival
Using multivariate Cox proportional-hazards model (table 5), there was no significant difference in OS between IBD and control cohorts (p=0.66). In addition, a log-rank test of OS between IBD and control groups corroborated the lack of difference in OS where median OS of both groups was 12.3 months (95% CI 10.0 to 16.2 months, p=0.90). Kaplan-Meier curves are presented in online supplemental figure 2. Limiting this analysis to stage 4 only cancers resulted in no change for IBD versus control OS (online supplemental figure 3). More than half of all patients (113/180, 62.8%) had died by the end of follow-up period (median: 16.4 months; range: 1.2–50.0 months). Age and gender did not significantly affect OS (p=0.91 and p=0.06, respectively).
Discussion
Our study highlights that the increased risk of colitis and diarrhea in patients with IBD (including higher event incidence and shorter time to event) does not result in a higher rate of adverse outcomes of surgery or death. Nonetheless, imDC can occur as soon as the first day of ICI therapy, with or without IBD, as our study shows. Our dataset does not show a signal for higher mortality in patients with IBD compared with a propensity-matched non-IBD cohort (median OS of both groups=12.3 months, p=0.90). It is worth noting that the total number of administered ICI doses were similar among both groups (p=0.88), although causes of interruption were dissimilar (irAEs in the IBD group vs other causes in the control group, p=0.006, table 1). Given multi-drug and multi-cancer survival data, we used a 5:1 statistical matching algorithm to account for the potential confounding of cancers types and drugs. In addition, we orthogonally verified this survival analysis by using a multivariate Cox proportional-hazards model (p=0.66). In fact, due to the propensity matching algorithm, six cases of limited stage 2 disease were included in the control cohort. All cancers within the IBD cohort were stage IV. Despite potentially improved survival by stage in the control group, no statistical difference in overall stage was observed between non-IBD and IBD groups, further underlining the safety of immunotherapy in the IBD group in terms of overall survival. In short, our data affirm the current AGA current practice update for ICI colitis, favoring treating patients with IBD and cancer with immunotherapy if that is otherwise the appropriate cancer therapy.18
When considering IBD-related characteristics, our IBD cohort is similar to general IBD cohorts in terms of IBD-related surgical rates, although it is composed mostly of patients with inactive CD and UC.19 Because IBD is a heterogeneous disease, it is challenging to compare across studies and assume these cohorts are equivalent. It is, however, worth noting that 27/30 (90%) of our patients had silent IBD at start of the disease, and 10/30 (33%) were on IBD-specific therapy. These proportions parallel other studies whereby patients who were symptomatic from their autoimmune disorders at baseline were more likely to experience a flare on ICI therapy.4 20
By using a time-dependent outcome (TTimDC) and propensity matching, we were able to make stronger correlations as compared with using imDC as a binary outcome. We saw rates of imDC that are similar to prior studies on patients with preexisting autoimmune disorders or IBD who received ICI therapy. In a systematic review of 123 patients with preexisting autoimmune disorders who received ICI therapy for various cancers, 14% developed imDC. Specifically, within this cohort, 8 out of 13 patients with IBD (62%) suffered either a flare or a developed de novo imDC.11 Another review of 112 patients with autoimmune disorders included 14 patients with IBD, half of whom developed colitis.12 In two retrospective studies that included around 50 patients each with underlying autoimmune disorders on anti-PD1/PD-L1 agents, disease flares were recorded in 13% to 38% of patients.4 20 More recently, two studies on patients with IBD observed a 19% (4/21) incidence of immune-mediated colitis in one study21 and a 21% (21/102) incidence of grade 3 or 4 diarrhea in another.14 In addition, in previously published studies, discontinuation rates from all irAEs ranged from 0% to 29% in patients with autoimmune disorders, with anti-CTLA4 resulting in higher rates of irAEs than anti-PD1 monotherapy.11 16 20 22 23 Among patients with IBD, discontinuation rates were 33% to 86%, and our study rates, 83.33%, fell on the higher end of that range.11 16 The rate of imDC recurrence after ICI resumption in the IBD cohort was also similar to the general population literature, in fact, where imDC recurred in a third of patients.24
This study offered three main insights into how patients with IBD may behave once they receive ICI therapy, yet these findings remain speculative due to the limited number of patients with IBD (n=30) and those who had events of interest (imDC, n=11). First, as might be expected, baseline immunosuppression for autoimmune disorders at the time of ICI initiation has shown some protection from a flare or irAE. While the number of patients in our cohort were smaller, no imDC developed in four patients who had three clinical findings in common: (1) no interruption of IBD therapy at cancer diagnosis, (2) IBD maintenance therapy continued at time of ICI therapy start, and (3) inactive disease at start of ICI therapy. This observation is only speculative and warrants more highly powered studies to confirm, but it could suggest that better control of IBD may reduce the risk of developing imDC. Similarly, a systematic review of 123 patients with preexisting autoimmune disorders, in which 44.7% were on anti-CTLA4 therapy and of which 13 had IBD, did not show an increased risk for immune-related toxicity.11 Interestingly, this study also showed no differences in irAEs between patients with active versus inactive autoimmune disease.11 Another more recent study with 102 patients with IBD who received ICI therapy did not show an association between active GI disease within 3 months of immunotherapy and increased risk of GI adverse events (OR 1.53, 95% CI 0.52 to 4.46, p=0.437).14 Second, we found that two of three patients with baseline inactive IBD and radiation history developed imDC but had a radiation exposure specifically to the sacroiliac area. Although history of radiation exposure itself did not show a signal for imDC in the entire cohort by multivariate Cox proportional-hazards model, it would be relevant to perform larger prospective studies to understand the interaction between abdominopelvic radiation and rates of imDC in the general population, as other studies have shown an implication for radiation therapy on both rates of irAEs and OS.25 Finally, we found that patients who maintained IBD immunosuppressive therapies at cancer diagnosis and subsequently ICI administration may less likely suffer from imDC, but this may be at the cost of more disease progression. Although this needs to be confirmed prospectively, a conversation with patients with IBD about the interaction between IBD therapy and ICI therapy, as well as the potential trade-off between imDC and OS, should be discussed before ICI therapy. It is important to mention that inferences from these findings are premature given very small numbers. A prospective study using anti-PD1/PD-L1 therapy in a cohort with preexisting autoimmune diseases including IBD is currently accruing to help answer these clinical questions (NCT03816345).
The debate over the safety of corticosteroids either prior to or while undergoing ICI therapy remains controversial, especially in patients with preexisting autoimmune conditions.26–28 In multivariate Cox proportional analysis for TTimDC for the entire cohort, patients who were naive to steroids prior to ICI therapy were likely to have worse TTimDC (p=0.047), regardless of IBD status. This finding may strengthen the idea that ICI therapy without prior immune suppression induces a stronger immune activation, which then may lead to notable irAEs. Other studies suggest steroids are harmful to cancer prognosis. A study by Faje et al sheds light on the worse OS outcomes with higher-dose corticosteroids in patients with immune-mediated hypophysitis.29 Similarly, a systematic review of 16 studies showed that steroid exposure did increase the risk of death and cancer progression when administered for palliative symptom control such as dyspnea or brain metastasis, but not when used for management of irAEs.30 Our cohort was predominantly advanced cancer (158/180, 88% stage IV), and we did not see evidence of worsening mortality with steroid use using multivariate Cox proportional-hazards model. Our findings within this advanced disease set could support the hypothesis that steroid-associated poor prognosis is not driven by corticosteroid use in the irAE setting but perhaps specifically for palliative indications.28 These findings bear a plausible biological explanation: when used prior to ICI therapy administration, corticosteroids are immunosuppressive for the initial immune activation phase of ICI therapy, thereby dampening the survival benefit in patients with cancer. When used to treat irAEs, on the other hand, ICI immune activation had already occurred. The need for corticosteroids is thus to reduce the overactivation of the immune system, rather than hindering the immune activation in the first place. What is still not known, however, is whether immunotherapy would be enough to benefit patients over a short period of time (ie, the average time to first irAE and initation of corticosteroids) and whether longer duration of ICI treatment after corticosteroid therapy would be unhelpful. Equally, prolonged high-dose corticosteroids may also drive a suppressed OS in patients with irAEs. This is an active area of research currently. Further studies should assess the mechanisms connecting irAEs to antitumor immunity (such as shared antigens, shared adjuvants, or differential regulation of immunity by PD-1/CTLA-4 in different people), which may allow us to better titrate corticosteroids and minimize OS trade-off with irAE treatment.
This study still, however, has limitations that stem from its retrospective design. Extracted data on certain variables, such as gender, race, and smoking history, were based on electronic coding as opposed to manual chart review and thus may be susceptible to extraction errors. Similarly, 10 of 30 cases were historically diagnosed with IBD (as detailed in Methods section); however, we had no access to the original documentation of pathologic diagnosis. Due to the small size of this cohort, we were unable to power observations for comparison of patient subsets such as those with active IBD or baseline use of biologics at the beginning of ICI therapy. Finally, our cohort was mostly exposed to PD-1/PD-L1 inhibitors (154/180, 85.6%) and thus may not be as descriptive of the risk of imDC in relation to anti-CTLA4 therapy.
Conclusion
Growing knowledge around the safety and cancer outcomes of ICIs in patients with cancer and preexisting autoimmune diseases is in response to a major challenge in the management of a growing population of patients with cancer undergoing treatment with immunotherapy. Our study shows that patients with IBD have significantly worse time to imDC, both in regards to incidence and time-to-event, compared with patients without a history of IBD. Importantly, no significant difference in OS between IBD and control patients was observed, despite the higher imDC event rate. We have not seen conclusive data that corticosteroid use for irAE are deleterious; however, not treating significant irAEs such as colitis appropriately would certainly prove harmful. Such results do not suggest liberal use of ICI therapy in patients with IBD, but do underline the need to carefully screen and select such patients before possible ICI therapy initiation. Ultimately, we will need prospective studies to develop better means to stratify the imDC risk in patients with IBD and determine which subgroups may receive the therapy safely. We also need to develop means to mediate this risk so that patients with IBD may receive these important therapies which improve quality and duration of life for patients with cancer.
Footnotes
Twitter: @JosephHabibi_MD
Contributors: Conception and design: JS, JP, PF. Administrative support: WW. Provision of study materials or patients: WW, JS, PF. Collection and assembly of data: JS, RS, MSF. Data analysis and interpretation: WW, MSF, JS, RS, PF. Manuscript writing: all authors. Final approval of manuscript: all authors. Accountable for all aspects of the work: all authors.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information. Additional data are available on reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study was approved under IRB review as a minimal risk study.
References
- 1. Khan F, Funchain P, Bennett A, et al. How should we diagnose and manage checkpoint inhibitor-associated colitis? Cleve Clin J Med 2018;85:679–83. 10.3949/ccjm.85a.18020 [DOI] [PubMed] [Google Scholar]
- 2. Collins M, Michot JM, Danlos FX, et al. Inflammatory gastrointestinal diseases associated with PD-1 blockade antibodies. Ann Oncol 2017;28:2860–5. 10.1093/annonc/mdx403 [DOI] [PubMed] [Google Scholar]
- 3. Shivaji UN, Jeffery L, Gui X, et al. Immune checkpoint inhibitor-associated gastrointestinal and hepatic adverse events and their management. Therap Adv Gastroenterol 2019;12:175628481988419. 10.1177/1756284819884196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leonardi GC, Gainor JF, Altan M, et al. Safety of programmed death-1 pathway inhibitors among patients with non-small-cell lung cancer and preexisting autoimmune disorders. J Clin Oncol 2018;36:1905–12. 10.1200/JCO.2017.77.0305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chaput N, Lepage P, Coutzac C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol 2017;28:1368–79. 10.1093/annonc/mdx108 [DOI] [PubMed] [Google Scholar]
- 7. Prieux-Klotz C, Dior M, Damotte D, et al. Immune checkpoint inhibitor-induced colitis: diagnosis and management. Target Oncol 2017;12:301–8. 10.1007/s11523-017-0495-4 [DOI] [PubMed] [Google Scholar]
- 8. Trac B, Chen HH, Muhlbauer A. IpiColitis: ipilimumab-induced colitis with a wide spectrum of histological features. Am J Dig Dis 2018. [Google Scholar]
- 9. Yanai S, Nakamura S, Kawasaki K, et al. Immune checkpoint inhibitor-induced diarrhea: clinicopathological study of 11 patients. Dig Endosc 2020;32:616-620. 10.1111/den.13555 [DOI] [PubMed] [Google Scholar]
- 10. Cassol CA, Owen D, Kendra K, et al. Programmed cell death-1 (PD-1) and programmed death-ligand 1 (PD-L1) expression in PD-1 inhibitor-associated colitis and its mimics. Histopathology 2020;77:240–9. 10.1111/his.14115 [DOI] [PubMed] [Google Scholar]
- 11. Abdel-Wahab N, Shah M, Lopez-Olivo MA, et al. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: a systematic review. Ann Intern Med 2018;168:121–30. 10.7326/M17-2073 [DOI] [PubMed] [Google Scholar]
- 12. Tison A, Quéré G, Misery L, et al. Safety and efficacy of immune checkpoint inhibitors in patients with cancer and preexisting autoimmune disease: a nationwide, multicenter cohort study. Arthritis Rheumatol 2019;71:2100–11. 10.1002/art.41068 [DOI] [PubMed] [Google Scholar]
- 13. Kyi C, Carvajal RD, Wolchok JD, et al. Ipilimumab in patients with melanoma and autoimmune disease. J Immunother Cancer 2014;2. 10.1186/s40425-014-0035-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abu-Sbeih H, Faleck DM, Ricciuti B, et al. Immune checkpoint inhibitor therapy in patients with preexisting inflammatory bowel disease. J Clin Oncol 2020;38:S450–1. 10.14309/01.ajg.0000592636.29532.cc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bergqvist V, Hertervig E, Gedeon P, et al. Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis. Cancer Immunol Immunother 2017;66:581–92. 10.1007/s00262-017-1962-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kähler KC, Eigentler TK, Gesierich A, et al. Ipilimumab in metastatic melanoma patients with pre-existing autoimmune disorders. Cancer Immunol Immunother 2018;67:825–34. 10.1007/s00262-018-2134-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cancer Therapy Evaluation Program (CTEP) . Common terminology criteria for adverse events (CTCAE).v.5.0 [5x7], 2017. [Google Scholar]
- 18. Dougan M, Wang Y, Rubio-Tapia A, et al. AGA clinical practice update on diagnosis and management of immune checkpoint inhibitor colitis and hepatitis: expert review. Gastroenterology 2021;160:1384–93. 10.1053/j.gastro.2020.08.063 [DOI] [PubMed] [Google Scholar]
- 19. Burisch J, Jess T, Martinato M, et al. The burden of inflammatory bowel disease in Europe. J Crohns Colitis 2013;7:322–37. 10.1016/j.crohns.2013.01.010 [DOI] [PubMed] [Google Scholar]
- 20. Menzies AM, Johnson DB, Ramanujam S, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 2017;28:368–76. 10.1093/annonc/mdw443 [DOI] [PubMed] [Google Scholar]
- 21. Grover S, Ruan AB, Srivoleti P, et al. Safety of immune checkpoint inhibitors in patients with pre-existing inflammatory bowel disease and microscopic colitis. JCO Oncol Pract 2020;16:e933–42. 10.1200/JOP.19.00672 [DOI] [PubMed] [Google Scholar]
- 22. Gutzmer R, Koop A, Meier F, et al. Programmed cell death protein-1 (PD-1) inhibitor therapy in patients with advanced melanoma and preexisting autoimmunity or ipilimumab-triggered autoimmunity. Eur J Cancer 2017;75:24–32. 10.1016/j.ejca.2016.12.038 [DOI] [PubMed] [Google Scholar]
- 23. Danlos F-X, Voisin A-L, Dyevre V, et al. Safety and efficacy of anti-programmed death 1 antibodies in patients with cancer and pre-existing autoimmune or inflammatory disease. Eur J Cancer 2018;91:21–9. 10.1016/j.ejca.2017.12.008 [DOI] [PubMed] [Google Scholar]
- 24. Abu-Sbeih H, Ali FS, Naqash AR, et al. Resumption of immune checkpoint inhibitor therapy after immune-mediated colitis. J Clin Oncol 2019;37:2738–45. 10.1200/JCO.19.00320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andreatos N, Roopkumar J, Khorana A, et al. 197 Survival outcomes and toxicity among patients treated with concomitant radiotherapy and immunotherapy for advanced melanoma: two faces of the abscopal effect? J Immunother Cancer 2020;8:A212–A116. 10.1136/jitc-2020-SITC2020.0197 [DOI] [Google Scholar]
- 26. Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non–small-cell lung cancer. J Clin Oncol 2018;36:2872–8. 10.1200/JCO.2018.79.0006 [DOI] [PubMed] [Google Scholar]
- 27. Horvat TZ, Adel NG, Dang T-O, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol 2015;33:3193–8. 10.1200/JCO.2015.60.8448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ricciuti B, Dahlberg SE, Adeni A, et al. Immune checkpoint inhibitor outcomes for patients with non-small-cell lung cancer receiving baseline corticosteroids for palliative versus nonpalliative indications. J Clin Oncol 2019;37:1927–34. 10.1200/JCO.19.00189 [DOI] [PubMed] [Google Scholar]
- 29. Faje AT, Lawrence D, Flaherty K, et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 2018;124:3706–14. 10.1002/cncr.31629 [DOI] [PubMed] [Google Scholar]
- 30. Petrelli F, Signorelli D, Ghidini M, et al. Association of steroids use with survival in patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Cancers 2020;12. 10.3390/cancers12030546. [Epub ahead of print: 27 Feb 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2021-002567supp001.pdf (456.6KB, pdf)
Data Availability Statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information. Additional data are available on reasonable request.


