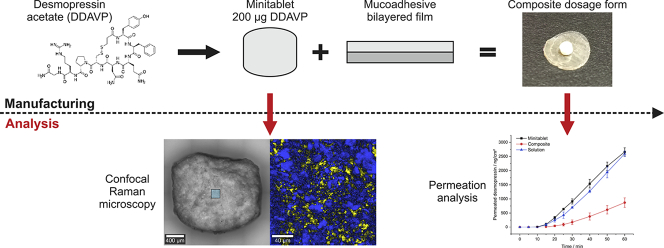
Abstract
Desmopressin acetate (DDAVP) is an oligopeptide indicated for the treatment of primary nocturnal enuresis, for example. The poor oral bioavailability of DDAVP accelerated a shift to alternative routes of administration like nasal and oromucosal, whereby nasal administration results in high fluctuations increasing the risk of undesirable side effects. Aim of the study was to use a new composite dosage form (solid matrix attached to a bilayer mucoadhesive film) to make DDAVP available via oromucosal route, reducing the risk of undesirable side effects through precise dosing. DDAVP was incorporated into a solid matrix in the form of a minitablet, and both direct tableting (AV > 30) and granulation followed by tableting (AV = 17.86) were compared. Minitablets with content uniformity could only be obtained by granulation and loss supplementation (AV = 11.27) with immediate drug release (>80% after 7–8 min) and rapid disintegration (<49 s). Permeation studies were performed with a clinically relevant dose (200 μg) in a time interval of up to one hour, resulting in apparent permeation coefficients of 4.90 × 10−6 cm/s (minitablet) and 2.04 × 10−6 cm/s (composite). Comparable fluctuations showed no inferiority of composite and minitablet regarding dosing accuracy. Thus, a step towards controlled and dose-accurate transmucosal delivery of systemically active DDAVP could be achieved.
Keywords: Desmopressin acetate, Buccal delivery, Minitablet, Mucoadhesive film, Paediatrics, Permeation, Confocal Raman microscopy
Graphical abstract
1. Introduction
Desmopressin acetate (DDAVP, 1-desamino-8-D-arginin-vasopressin) is a synthetic oligopeptide derivative of the endogenous hormone vasopressin, which is used for the treatment of enuresis nocturna and diabetes insipidus centralis in both children and adults (De Waele et al., 2014; Lottmann and Alova, 2007). In addition, it is approved for the treatment of hemophilia A and von Willebrand type I disease (Mannucci et al., 1977). However, orally delivered DDAVP is degraded by the acidic gastric fluid, resulting in the poor oral bioavailability of 0.08% to 0.16% (Hashim and Abrams, 2008). Therefore, parenteral drug administration remains the gold standard for the administration of peptide-based treatments, such as DDAVP (Richard, 2017). However, parenteral administration is inconvenient, which may result in low compliance. Various research is being conducted to explore alternative application routes, such as nasal or transoromucosal administration, which have become increasingly important in recent years (Patel et al., 2011). In addition to parenteral formulations, desmopressin is available in the other dosage forms like tablet, nasal spray, nasal drop and, since 2007, sublingual tablet (Vande Walle et al., 2007). Bypassing the intestinal first-pass effect may result in the increased bioavailability (BA) of desmopressin delivered via the nasal (5%–10%) (Fjellestad-Paulsen et al., 1993) and sublingual routes (0.28%) (Hashim and Abrams, 2008) compared with oral administration. A major drawback of nasal drug administration is high variation in BA, which can lead to dangerous and uncontrollable side effects (hyponatremia), particularly among the pediatric population. Up to 80% of desmopressin-induced hyponatremia cases have been related to nasal application (van Kerrebroeck and Nørgaard, 2009), which has resulted in the withdrawal of drug approvals for previously authorized nasal sprays for the indication of enuresis nocturna in children and adults (FDA, 2007). Sublingual administration led to dangerous hyponatremia in only 1% of investigated cases (van Kerrebroeck and Nørgaard, 2009). In addition, the desmopressin-containing nasal sprays currently available on the market have been completely withdrawn at this time because they showed superpotency. The exact cause of the superpotency could not yet be determined, resulting in unavailability until at least mid to late 2021 (FDA, 2020). Therefore, the alternative route of administration via the oral cavity appears to be effective and less risky than nasal applications. Compared to the local application of drugs in the oral cavity, as already frequently described in the literature, e.g. local anesthetics such as lidocaine, the application of systemically active drugs poses many more challenges. Hydrophilicity/lipophilicity ratio, molecular weight, drug loading (and resulting donor concentration), saliva volume, residence time, application site and others, represent key factors in transmucosal drug absorption (Narang and Sharma, 2011). Clinical studies have reported a significantly increased preference for sublingual desmopressin preparations compared with those using oral tablets, especially among children aged 5–11 years (Lottmann et al., 2007). Other studies have demonstrated the improved effectiveness of sublingual administrations due to reduced food interference compared with oral tablets (De Guchtenaere et al., 2011). However, the absorption of drugs via the oral mucosa requires profound knowledge regarding the amount of drug that permeates through the oral mucosa over a defined application period. Based on the promising potential and good acceptance of orally administered drug delivery systems, some recent research has examined alternative application methods for desmopressin to enhance BA. By using a mucoadhesive submicron emulsion, a 12-fold increase in the BA of DDAVP was observed compared with a pure saline solution (Ilan et al., 1996). The incorporation of DDAVP into mucoadhesive, multi-layer film preparations has also been investigated (Lindert, 2016). However, polymer swelling may have detrimental effects on the velocity of systemic drug absorption by forming a diffusion barrier. In addition, the dosage is typically determined by the size of the film, which can result in dosage limitations, especially for pediatric use. Although clinically relevant doses were not examined in the study performed by Lindert et al., this formulation was considered a promising approach for increasing transoromucosal BA. Therefore, this approach and others should be further investigated to ensure that clinically relevant plasma concentrations can be provided using therapeutic doses. Properties such as disintegration and DDAVP release should be accomplished as quickly as possible to achieve the fastest possible permeation while ensuring constant adhesion to the oral mucosa. One possible solution could be the combination dosage form of a minitablet (MT) and a mucoadhesive bilayer film (MBF), such as that developed by our research group for the local drug delivery of lidocaine hydrochloride, which provided controllable and low levels of drug permeation (Kottke et al., 2020). Minitablets are defined as tablets smaller than 3 mm, which is advantageous for applicability, especially among young children (Freerks et al., 2020), and suitable for substances with stability problems. Although DDAVP-loaded MTs for peroral use are described in the literature (van der Merwe et al., 2004), there are no approaches for buccal drug delivery and certainly not with rapid release properties. To exploit the advantageous properties of each dosage form within the composite dosage form, the individual dosage forms should be combined to eliminate their individual disadvantages. However, the various scientific work has indicated that the production of a low-dose MT is challenging. Hermes et al. investigated this issue in detail and showed that the requirements of the European Pharmacopoeia (Ph. Eur.) regarding content uniformity are difficult to meet when using direct tableting applications (Hermes, 2012). Therefore, the present study aimed to develop a low-dose, solid, DDAVP-loaded matrix by manufacturing an MT with a DDAVP loading in the range of current commercially available tablets (200 μg). These MTs are designed for rapid disintegration and immediate drug release with the goal of systemically effective drug amount permeating across the oral mucosa within defined time interval of less than one hour. The purpose of this advanced study was to investigate whether the composite dosage form is suitable not only for local but also for systemic buccal administration of low-dose, potentially unstable, peptide drugs. Drug permeation tests were performed to determine the permeation profiles of the DDAVP-loaded composite dosage forms compared with a regular, single MT.
2. Materials and methods
2.1. Materials
In this study, desmopressin acetate (PolyPeptide laboratories, Limhamn, Sweden) was used as the active pharmaceutical ingredient.
For the preparation of the MTs, α-lactose monohydrate (Flowlac 100, Meggle, Wasserburg, Germany), magnesium stearate (Parteck LUB MST, Merck, Frankfurt a.M., Germany) and fumed silicon dioxide (Aerosil 200, Evonik, Darmstadt, Germany) were used. The binder used for granulation was povidone 30 (Kollidon® 30, BASF, Ludwigshafen, Germany).
Chitosan (85% deacylated, Sigma Aldrich, Darmstadt, Germany), hypromellose (HPMC, Pharmacoat 603, Shin Etsu, Tokyo, Japan), and ethyl cellulose (EC, Aqualon N 22, Ashland, Kentucky, USA) were chosen as film-forming agents. Glycerol, at 85% (Caelo, Hilden, Germany), and triethylcitrate (Caelo, Hilden, Germany) were selected as plasticizers. Carmellose-natrium (CMC—Na), at 20% (Walocel C 30 PA 09, Dow Wolff Cellulosics, Bomlitz, Germany) in aqueous solution was used as the glue between the MT and the MBF.
Water [high-performance liquid chromatography (HPLC)-grade] was obtained from Fisher Scientific (Hampton, USA). Acetonitrile (HPLC grade), ortho-phosphoric acid (85%, p.a.), and formic acid (98%, p.a.) were purchased from AppliChem (Darmstadt, Germany). Ethanol (99%), hydrochloric acid (0.1 N), and dipotassium hydrogen phosphate were provided by VWR Chemicals (Langenfeld, Germany). The buffer composition used in these studies contained 10 mM dipotassium hydrogen phosphate in distilled water, and the pH value (pH 6.8 or 7.4) was adjusted with orthophosphoric acid.
Biomimetic membrane systems by PermeaPad® (innoME, Espelkamp, Germany) were used as barriers for permeation measurements.
2.2. Methods
2.2.1. Manufacturing of minitablets
2.2.1.1. Granulation
An aqueous 2.5% solution of povidone (Kollidon 30) was used as the polymeric binding solution. The quantitative composition of the granules is presented in Table 1 (see section 2.2.1.3). DDAVP was dissolved in the polymeric solution (120.3 mg/ml), and the resulting solution was divided into two aliquots. The splitting of the polymer solution was required to avoid a resulting pasty mass during production. Therefore, the amount was halved and the granulation was carried out twice in series. Due to the small batch size, the granulation process was performed manually using a mortar and pestle. Initially, the first aliquots of the polymeric solution containing dissolved DDAVP was applied to the filler and granulated using a mortar and pestle. Afterward, the granules were pressed through a size 500 sieve and dried for 30 min at 45 °C in a drying oven (Kendro Laboratory Products, Hanau, Germany). The procedure was repeated with the second aliquots of the polymeric solution to incorporate the entire amount of DDAVP into the granules.
Table 1.
Composition of different DDAVP minitablet formulations (F = formulation, DT = direct tableting, G + T = granulation + tableting, G + T + L = granulation + tableting + loss supplement).
| Substance | Amount (%)a |
||
|---|---|---|---|
| F1 (DT) | F2 (G + T) | F3 (G + T + L) | |
| Desmopressin acetate | 3.0 | 3.0 | 3.7 |
| Magnesium stearate | 3.0 | 3.0 | 3.0 |
| Kollidon 30b | – | 0.025 | 0.025 |
| Flowlac 100c | ad 100.0 | ad 100.0 | ad 100.0 |
(w/w).
Povidone with K = 30.
α-lactose monohydrate/amorphous lactose.
2.2.1.2. Blending
The powder mixture that was prepared for direct compression (F1, see Table 1) was blended for 20 min in a Turbula mixer type T2C (Willy Bachofen, Switzerland) at 49 rpm. Subsequently, 3.0% magnesium stearate was added and mixed for another 2 min. Pre-granulated formulations (F2 and F3, see Table 1) were mixed for 2 min in total after adding 3.0% magnesium stearate.
2.2.1.3. Tableting
Three different MT formulations were compacted using a rotary tablet press (Pressima MX-EU-B/D, IMA Kilian, Cologne, Germany) equipped with a 19-tip, 2-mm tableting tooling (Ritter, Stapelfeld, Germany). The MT weight was set to 6.5 mg, which corresponded to a dose strength of 200 μg DDAVP. The die filling was performed by manual weighing. Table 1 represents the composition of the DDAVP MTs.
2.2.2. Characterization of minitablets
2.2.2.1. Content Uniformity
To test the content uniformity, the acceptance value (AV) according to the Ph. Eur. 2.9.40 was calculated. An HPLC method, which was validated in a previous study (Kottke et al., 2021), was used to determine desmopressin concentration. An HPLC (Dionex, Sunnyvale, California) using an EC 125/4 Nucleosil 120–3 C18 column (Macherey Nagel, Düren, Germany) was coupled to a self-constructed, highly sensitive, coaxial liquid-core waveguide fluorescence detector. The excitation wavelength for desmopressin was 278 nm using an ultraviolet (UV) light-emitting diode (LED), and fluorescence chromatograms were recorded at an acquisition rate of 2 Hz. The chromatographic parameters were set as follows: mobile phase A = water containing 0.08% trifluoroacetic acid (v/v); mobile phase B = acetonitrile; column temperature = 35 °C; and flow rate = 1.0 ml/min. For the gradient elution, mobile phase B was increased from 5% to 50% within 5 min and then changed to 95% within a 0.5 min period. At 10.5 min the concentration of mobile phase B was decreased to 5%, followed by an equilibration time of 4 min.
2.2.2.2. Dissolution
The dissolution tests were performed in a modified USP 1 basket, with a rotation speed of 60 rpm, 100 ml phosphate buffer pH 6.8, and a temperature of 37 ± 0.5 °C. The sampling was performed manually. For each dissolution test, six minitablets were used for quantification. The drug release was quantified using a validated HPLC method, as described in section 2.2.2.1.
2.2.2.3. Disintegration
To investigate the MT disintegration time, a modified test system was used, based on that described by Kleinebudde (1997), using DT2 (Sotax, Basel, Switzerland). Within this method, the MT was inserted into a cylindric tube, sealed on both sides with a mesh (710 μm). Six cylinders, each containing an MT, were then added to a conventional disintegration apparatus and fixed with a metal block. As the disintegration medium, phosphate buffer pH 6.8 at a temperature of 37.0 ± 0.5 °C was selected.
2.2.2.4. Tensile strength
To evaluate the mechanical strength of the MTs, a Texture Analyser TA.Xtplus (Stable Micro Systems, Surrey, England) with a flat punch of 5 mm and a pre-speed of 0.1 mm/s was used. Subsequently, the tensile strength was calculated as described by Fell and Newton (1970).
2.2.2.5. Scanning electron microscopy (SEM)
To assess the morphology, DDAVP, drug-free granules, and DDAVP-containing granules were investigated using a scanning electron microscope Phenom G2 pro (Phenom-World, Eindhoven, Netherlands). An acceleration voltage of 5 kV was adjusted.
2.2.2.6. Confocal Raman microscopy (CRM)
The confocal Raman microscope alpha 300 R (WITec, Ulm, Germany) was used to perform the Raman spectroscopic investigations. A single-mode laser with a wavelength of 532 nm was applied for excitation. Using a Zeiss EC Epiplan-Neofluar HD 20×/0.5 microscope objective, the laser power on the samples was set to 20 mW. The Raman microscope was equipped with a WITec UHTS 300 spectrometer and an Andor iDus Deep Depletion charge-coupled device (CCD) camera. The CCD chip was cooled to −60 °C. By using a reflection grating with 600 lines/mm, an average spectral resolution of 3.8 cm−1/pixel was achieved. Reference Raman spectra of pure substances were acquired with an exposure time of 10 s. Raman images with an area of 200 × 200 μm and a spatial resolution of 1 μm were obtained from the cross-sections of the differently produced MTs. The exposure time was set to 0.05 s per spectrum. In addition, light microscopic bright-field images of the MT cross-sections were obtained using a Zeiss EC Epiplan HD 10×/0.25 microscope objective. The Raman spectra processing and the compilation of Raman images were conducted using the software WITec FIVE (Version 5.2.4.81, WITec, Ulm, Germany). The cosmic rays were first removed, followed by baseline correction and the normalization of Raman intensities. The software OriginPro 2020, version 9.7.0.188 (Origin Lab, Northampton, USA), was finally used to display the Raman spectra.
2.2.3. Manufacturing of bilayer film and composite dosage form
2.2.3.1. Bilayer film preparations
The MBF consisted of a mucoadhesive layer [2.5% chitosan (85% deacetylated), 6.0% hypromellose, 2.0% glycerol (85%), and 0.1 N HCl] (Lindert, 2016) and a shield layer [12% ethyl cellulose, 2.5% triethylcitrate, 5.0% distilled water, and ethanol (99%) ad 100% (V/V)]. The manufacturing process for these films has been previously described (Kottke et al., 2020). In brief, the shield layer was cast discontinuously using a film-casting bench (Erichsen, Hemer, Germany), with a wet film thickness of 500 μm, and dried at 30 °C for two hours. Subsequently, the mucoadhesive layer was cast on top of the shield layer using the same settings.
2.2.3.2. Composite dosage form
To manufacture the composite dosage form, the produced MT (section 2.2.1.3) was glued onto the bilayer film (section 2.2.3.1.) using an adhesive agent consisting of an aqueous 20% CMC-Na solution. A drop of the adhesive was applied to the center of the film, and the MT was placed on top of the adhesive and pressed on with a mass of 2 kg for 30 s.
2.2.4. Permeation studies
2.2.4.1. Experimental implementation of permeation
The permeation measurement was performed using Kerski diffusion cells (Kerski et al., 2015), with a defined permeation area of 0.64 cm2. As a barrier, the biomimetic membrane system PermeaPad® (innoME, Espelkamp, Germany) was selected. Phosphate buffer (pH 7.4) was used as an acceptor medium (8 mL), which was continuously stirred at 350 rpm and tempered to 37 °C. The MTs were placed in the donor chamber, and 100 μL phosphate buffer was added. When examining the composites, the membrane was first moistened with 20 μL buffer solution. Subsequently, the composite was applied, and 80 μL pH 7.4 phosphate buffer was added. Samples were collected at pre-defined time intervals of 5, 10, 15, 20, 25, 30, 40, 50, and 60 min. At each sampling, 1 mL was removed and replaced with 1 mL fresh medium.
2.2.4.2. Quantification method for permeation studies
A self-constructed, highly sensitive, coaxial liquid-core waveguide fluorescence detector (Kottke et al., 2021) was used to quantify the amount of desmopressin that permeated within one hour. Because the detector system allows reliable quantification of desmopressin concentrations down to 10 ng/mL, this system has been successfully applied as an alternative to the more commonly used liquid chromatography-tandem mass spectrometry (LC-MS/MS) detection method for permeation studies (Majid et al., 2021). The fluorescence detector was coupled to an HPLC system (Dionex, Sunnyvale, California) equipped with a quaternary pump (P 580 A) and an autosampler (ASI-100). To perform permeation analysis, the previously described HPLC method was used (section 2.2.2.1).
3. Results and discussion
3.1. Minitablets as solid matrices
To investigate the feasibility of preparing low-dose, composite dosage forms of potentially unstable drugs for systemic buccal application, MTs were chosen as the solid matrix. For this reason, no complete industrial drug development was intended. Currently, no literature has described the preparation of DDAVP minitablets with a dosage of 200 μg. The production of MTs with uniform contents is challenging in the field of tableting; therefore, both direct tableting and granulation followed by tableting should be investigated.
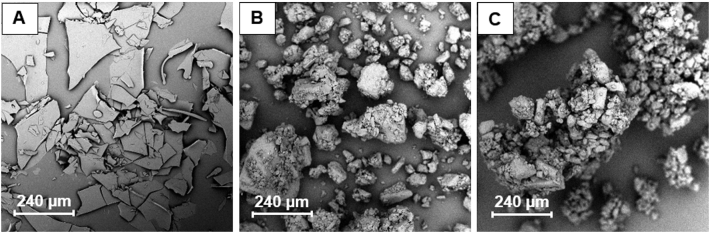
The preliminary assessment of the morphologies of DDAVP, drug-free granules, and drug-loaded granules was performed by acquiring SEM images, which are shown in Fig. 1. The plate-shaped structure of DDAVP (Fig. 1A) could result in segregation, which could cause difficulties with regard to direct tableting. The granules basically show a uniform appearance, which may result in fewer content uniformity problems during granulation followed by tableting.
Fig. 1.
SEM images of (A) DDAVP, (B) drug-free granules, and (C) granules containing DDAVP.
DDAVP MTs were manufactured by direct tableting and by granulation followed by tableting, and both types of tables were examined to determine the uniformity of the contents. The acceptance values (AVs) of the various MT formulations were investigated to assess whether the manufacturing processes used here resulted in the formation of MTs that fulfill the requirements for content uniformity according to Ph. Eur. 2.9.40.
The direct compression of DDAVP MTs resulted in an AV(DC) > 30, which did not fulfill the level 1 requirements of Ph. Eur. 2.9.40 (AV < 15), suggesting that this manufacturing process was not suitable for the production of MTs with uniform contents and or an appropriate manufacturing method for the solid matrix of the composite dosage form. This lack of uniformity can likely be explained by the SEM images and the confocal Raman microscopic images (see section 3.2), which are shown in Fig. 1, Fig. 3, respectively. The plate-shaped structure of DDAVP may cause segregation, preventing a uniform content in response to direct compression. High deviations from the desired claim of 100% (83.96% ± 1.38%) result in increased AV values, preventing the fulfillment of Ph. Eur. requirements (AV(G+T) = 17.86). To improve the content uniformity, a granulation step was added, which resulted in a reduction of AV, but the AV remained above the limit of Ph. Eur. (Level 1). A more precise examination of the data revealed that the deviations from the declared target content were high, suggesting that drug loss likely occurred during the manual granulation process. Based on the evaluated content of the granulated MTs (G + T), a high precision but low accuracy was identified accounting for 20% drug loss. This was addressed by supplementing the corresponding amount of drug applying manual granulation with mortar and pestle. As a result, granulation was repeated in a further test batch, but this time 20% of the desmopressin acetate quantity used was added beforehand to counteract the subsequent losses and drug adhesion to the used tools. This calculated supplement is referred to here as the so-called loss supplement. The previously calculated percentage deviation from the target content was then added as a loss supplement, and the manual granulation process was repeated. By performing an initial granulation step, followed by the addition of a 20% loss supplement, MTs could be produced that fulfilled the requirements of Ph. Eur., with an AV < 15 (AV(G+T+L) = 11.27). The granulation step enabled the production of a uniform structure for the filler and the drug. This process presented a method for the production of a solid, DDAVP-loaded matrix in the form of an MT for further processing into a composite dosage form.
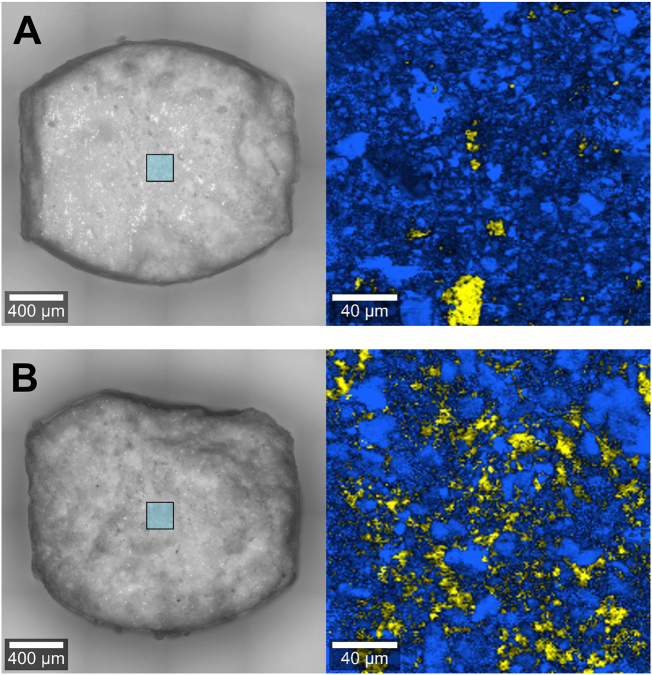
Fig. 3.
Cross-section of directly compacted desmopressin minitablet (A) and the granulated desmopressin minitablet (B), yellow = desmopressin acetate, blue = α-lactose monohydrate. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. DDAVP distribution within the granules
To investigate the distribution of the active ingredient within the MT, cross-sections of the prepared MTs were examined using CRM. First, whether individual substances could be distinguished by their Raman spectra was investigated.
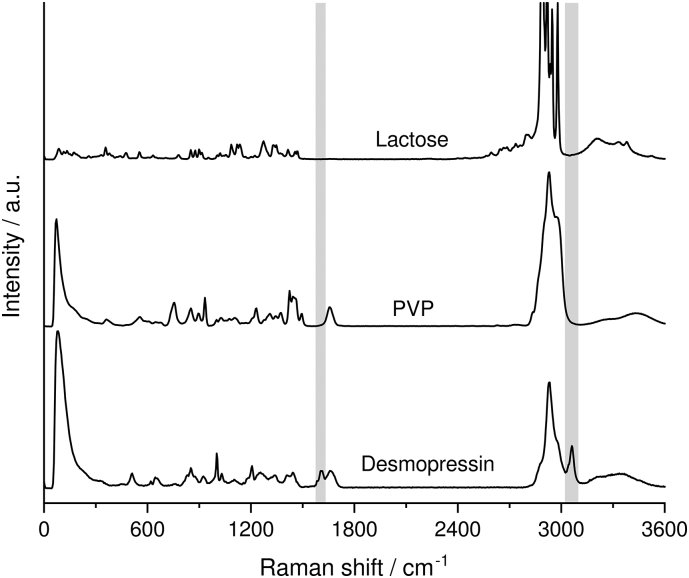
Fig. 2 compares the Raman spectra of the pure substances. The grey areas mark the Raman transitions of desmopressin acetate that do not overlap with the Raman transitions of the excipients. The characteristic marker bands of desmopressin acetate are located at 1610 cm−1 and 3065 cm−1.
Fig. 2.
Raman spectra of desmopressin and the excipients of the minitablet. The grey bars mark the specific Raman transitions of desmopressin. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
To investigate the distribution of desmopressin acetate within the MTs, cross-sections of the differently manufactured MTs were examined by CRM. Fig. 3A shows the false-color Raman image of the directly compressed MT. Individual DDAVP particles were found in the scanned area, reflecting the lack of content uniformity (section 3.1). In comparison, Fig. 3B clearly reveals the uniform distribution of desmopressin acetate, identified between the lactose particles of the granulated MTs. This is probably due to the application of the active pharmaceutical ingredient with the binder solution, which provides improved distribution of the drug among the granules and binder bridges.
Because the drug contained in the MT is meant to be absorbed later within the composite dosage form, via the oral mucosa, the MT should have sufficient strength but disintegrate quickly. The tensile strength of the plain DDAVP MTs was 1.24 ± 0.37 N/mm (n = 10), and the mean disintegration time was 49 s (n = 6), which is far below the Ph. Eur. definition of an orodispersible tablet (3 min). According to the US FDA, however, these MTs cannot be categorized as orodispersible tablets because they exceed the FDA limit of 30 s. However, for the purposes of the present study, the development of an orodispersible MT is not necessary. In addition to disintegration, drug release is of crucial importance. For the further processing of MTs into the composite dosage form, immediate drug release is desirable because the release of the drug from the dosage form can significantly influence subsequent permeation.
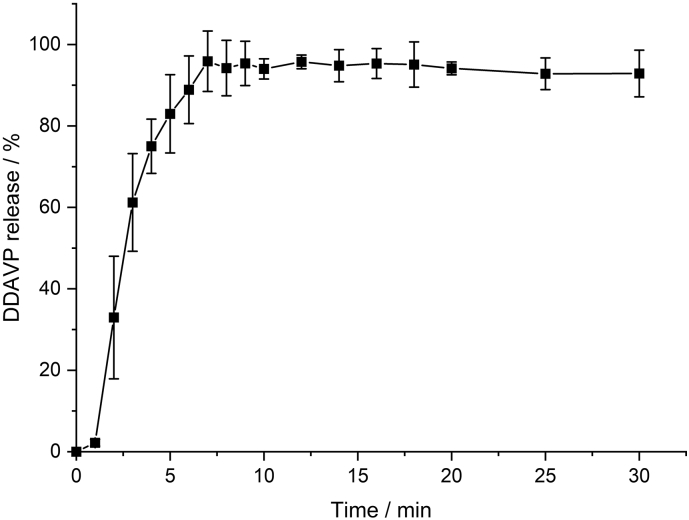
The release profile of the DDAVP MT is shown in Fig. 4. After 7–8 min, on average, a complete drug release could be achieved. The Ph. Eur. defines tablets with 80% release within 15 min as immediate-release tablets. This result can also be transferred to the composite dosage form, as no significant differences were observed between the unprocessed MT and the composite dosage form in the previous work by Kottke et al. Thus, the desired conditions for the MTs designed in the present study were clearly fulfilled. According to literature data, thermodynamic solubility of desmopressin acetate is 50 mg/mL at 25 °C (SCBT, 2021). Considering experimental conditions, the solubility would be 5 g/100 mL. 1/10 of the saturation concentration corresponds to 500 mg/100 mL. The drug loading per minitablet is 200 μg, whereas 6 minitablets were used in the release experiment, which corresponds to a maximum drug concentration of 1.2 mg/100 mL. Therefore, it can be seen that the maximum concentration is less than 1/10 of the saturation concentration, thus fulfilling the sink conditions.
Fig. 4.
Dissolution profile of DDAVP minitablets in phosphate buffer, pH 6.8, mean ± SD, n = 6.
Based on the intended drug release profile, the requirements regarding content uniformity of the solid matrix component, immediate drug release, and rapid disintegration time were fulfilled. Consequently, the manufactured MTs containing DDAVP can be considered a suitable matrix for further processing into composite dosage forms, as described in section 2.2.3.2.
3.3. Permeation
The permeation profile of desmopressin obtained from the plain MT was compared with that from the composite dosage form (area = 0.38 cm2; height = 2.12 ± 0.01 mm; width = 0.7 cm), which is illustrated in Fig. 5. The plain MT was intended to represent a benchmark for an orodispersible formulation, such as the oral lyophilizates that have previously been established for the pharmaceutical market.
Fig. 5.
Image of the DDAVP containing composite dosage form with displayed diameter.
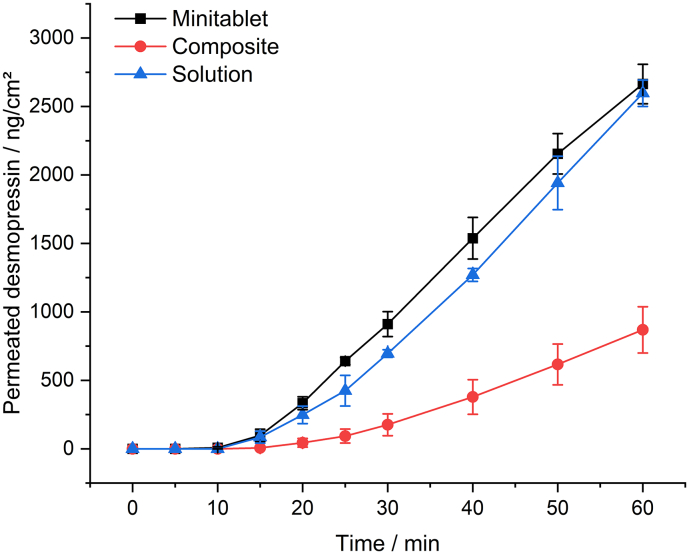
Fig. 6 shows the DDAVP permeation profiles of the composite dosage form and the plain MTs. The permeation tests were performed using biomimetic membranes to allow for the investigation and evaluation of the concepts addressed in this study under facilitated conditions, without the high matrix exposure associated with the use of biological membranes.
Fig. 6.
DDVAP Permeation from minitablets, composite dosage forms and solutions in phosphate buffer pH 7.4 (donor and acceptor media), using PermeaPad® as a biomimetic barrier, mean ± SD, n = 3.
Based on the permeation studies, a flux of 58.79 ng/cm2/min (R2 = 0.9998) was obtained for the MT, and a flux of 24.5 ng/cm2/min (R2 = 0.9997) was obtained for the composite dosage form. These results indicated apparent permeation coefficients of 4.90 × 10−6 cm/s for the MT and 2.04 × 10−6 cm/s for the composite. Examination of a solution of the same concentration resulted in a flux of 62.32 ng/cm2/min (R2 = 0.9998) and an apparent permeation coefficient of 5.19 × 10−6 cm/s. After a lag time of approximately 10 min, the plain MT presented a linear permeation profile. In comparison, in the permeation profile for the composite, the first concentrations can be determined after a lag time of approximately 20 min, which is a prolonged lag time compared with that for the plain MT. These experiments revealed that the plain MT displayed a three-fold higher permeation than that for the composite, which may represent the effects of shielding the MT from the surrounding donor medium and limited wetting, which can negatively impact drug release. It was expected that the plain MT would have a higher flux, since the entire surface is wetted with liquid, and the concentration gradient, due to the rapid disintegration, rapid release and lack of shielding by the carrier film, can occur within a short time interval. Nevertheless, the results demonstrate that despite the rapid drug release (> 80% in 7–8 min) as well as fast disintegration (<49 s), the amount of permeated DDAVP is low. Desmopressin acetate is a basic oligopeptide, which belongs to class 3 BCS system (Ono et al., 2016), hence it is characterized by its high solubility, but low drug permeability. The latter is attributed to different key factors. One decisive point is the relatively high molecular weight of 1069 g/mol. The Lipinski rule of five defines substances with a molecular weight of less than 500 g/mol as having high permeability. Therefore, DDAVP does not meet the Lipinski specification, but shows a cumulative drug concentration of up to almost 2.6 μg/cm2 in the investigated time interval. Furthermore, the permeation of hydrophilic basic molecules such as DDAVP preferentially follows the paracellular pathway across hydrophilic interstitial spaces and tight junctions, which further reduces permeation at the given molecular size. Both factors, high molecular weight and the paracellular route provide an explanation for the low permeation, despite rapid release and fast disintegration. Therefore, in the formulation studied, permeation across the membrane represents the rate-determining step rather than disintegration or drug release. The use of the orodispersible minitablet within the composite dosage form allows a rapid release with a short application and residence time. However, the rate-determining step is the passage through the membrane, which is why shielding by the surrounding film is crucial for drug exposure. At present, we consider a maximum duration of one hour to be realistic and have therefore addressed this in the drug permeation studies. Nevertheless, an excessively long application time (especially in the oral cavity) can negatively influence the compliance of the patients. To answer this question, clinical studies in adults as well as in infants and children are essential.
To represent realistic conditions, the available permeation area must be reduced to the size of the composite dosage form, measuring 0.38 cm2 (Table 2). After adjusting the permeation area, a higher desmopressin permeation value was calculated, resulting in a flux of 38.6 ng/cm2 and an apparent permeation coefficient of 3.22 × 10−6 cm/s. The shielding layer prevented drug dilution by saliva or the ablation of the drug during application in the oral cavity; therefore, shielding represents a major advantage for the composite dosage form. Despite the lower permeation of the composite form, the permeation profile exhibits only minor deviations in the same size range as those observed for the plain MT. The permeation of the composite formulation remained sufficient to potentially enable the administration of a clinically applicable drug level, preventing dilution by saliva, swallowing, or drug degradation within the gastrointestinal tract. It is the successful implementation of permeation that differs from the work of Lindert et al., although identical experimental setups were used (Kerski diffusion cell). There are important differences, especially with regard to the applied dose, which has a direct effect on the permeation when the concentration gradient described in Fick's 1st law of diffusion is taken into account. In order to achieve a sufficient concentration for the analytical method, the loading of the three-layer films had to be increased to 2.5 mg/0.64 cm2. Within our permeation study, this dose increase was not required due to the sensitive detector system (0.2 mg/0.64 cm2), which is why the real therapeutic dosages could be measured. With an applied DDAVP amount of 2.5 μg, a permeated drug amount of almost 10 μg was obtained after one hour, which represented 0.4% of the applied dose (Lindert, 2016). According to Table 2, almost 0.4% of the applied drug amount permeated from the composite; however, in this study, the applied drug amount was only 200 μg. The processing of DDAVP into a composite dosage form was, therefore, able to equal permeation rates as those achieved by three-layered films even at a 12.5-fold dose reduction. Since the resulting concentration gradient has a crucial role in permeation and thus has a direct influence on the rate and extent of permeation, these two formulations can only be compared with each other to a limited extent. Therefore, the use of the composite dosage form may facilitate improved drug safety without the loss of efficacy, especially when used for infants and children.
Table 2.
Permeation of desmopressin was calculated for an area of 0.64 cm2 (MT and composite) and for an area of 0.38 cm2 (composite), mean ± SD, n = 3.
| Time (min) | Drug amount (ng/cm2) |
Percentage of applied dose (%) |
||||
|---|---|---|---|---|---|---|
| MT (0.64 cm2) | Composite (0.64 cm2) | Composite (0.38 cm2) | MT (0.64 cm2) | Composite (0.64 cm2) | Composite (0.38 cm2) | |
| 10 | 7.1 ± 7.5 | – | – | 0.004 ± 0.004 | – | – |
| 30 | 910.7 ± 90.7 | 175.5 ± 80.1 | 292.5 ± 133.4 | 0.46 ± 0.05 | 0.09 ± 0.04 | 0.15 ± 0.07 |
| 60 | 2664.3 ± 144.2 | 868.6 ± 168.4 | 1447.7 ± 280.7 | 1.38 ± 0.07 | 0.43 ± 0.08 | 0.72 ± 0.14 |
4. Conclusion
The development of MTs as a solid matrix containing a precise dosage of 200 μg DDAVP was able to achieve a fast disintegration time and immediate drug release. Due to the plate-shaped morphology of DDAVP crystals, a sufficiently uniform content was not achievable using direct compression. To produce MTs that fulfill the requirements of the Ph. Eur. in terms of content uniformity, a two-step granulation process and the addition of a loss supplement was necessary. Using a combination of the MT and an MBF preparation, a composite dosage form was established, enabling the administration of DDAVP to the oral mucosa over a defined period of time. Moreover, reliable determination of desmopressin delivery was achieved at a clinically relevant application dose and time. The permeation of the composite dosage form was threefold reduced compared with the plain MT. Despite the lower permeation of the composite form, no significant differences in the fluctuation range were observed, suggesting a comparable dosing accuracy. Further investigations will focus on the permeation profiles when using even higher matrix exposure and biological membranes, especially compared with other dosage forms containing DDAVP, such as nasal sprays, oral tablets, or MBFs.
Declaration of Competing Interest
None.
Acknowledgments
The authors are grateful to Meggle (Germany), Ashland (USA), and Shin Etsu (Japan) for the donation of raw materials. We further thank Andrea Michel (Institute of Pharmaceutics and Biopharmaceutics) for the preparation of materials for chromatographic performance and for her support in the performance of drug permeation studies.
References
- De Guchtenaere A., Van Herzeele C., Raes A., Dehoorne J., Hoebeke P., Van Laecke E., Vande Walle J. Oral lyophylizate formulation of desmopressin: superior pharmacodynamics compared to tablet due to low food interaction. J. Urol. 2011;185:2308–2313. doi: 10.1016/j.juro.2011.02.039. [DOI] [PubMed] [Google Scholar]
- De Waele K., Cools M., De Guchtenaere A., Van de Walle J., Raes A., Van Aken S., De Coen K., Vanhaesebrouck P., De Schepper J. Desmopressin lyophilisate for the treatment of central diabetes insipidus: first experience in very young infants. Int. J. Endocrinol. Metab. 2014;12 doi: 10.5812/ijem.16120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA . 2007. Desmopressin FDA Alerts. (Information for Healthcare Professionals) [Google Scholar]
- FDA 2020. https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/ferring-us-issues-voluntary-nationwide-recall-ddavpr-nasal-spray-10-mcg01ml-desmopressin-acetate; Last access: 09.04.2021.
- Fell J.T., Newton J.M. Determination of tablet strength by the diametral-compression test. J. Pharm. Sci. 1970;59:688–691. doi: 10.1002/jps.2600590523. [DOI] [PubMed] [Google Scholar]
- Fjellestad-Paulsen A., Höglund P., Lundin S., Paulsen O. Pharmacokinetics of 1-deamino-8-D-arginine vasopressin after various routes of administration in healthy volunteers. Clin. Endocrinol. 1993;38:177–182. doi: 10.1111/j.1365-2265.1993.tb00990.x. [DOI] [PubMed] [Google Scholar]
- Freerks L., Sommerfeldt J., Löper P.C., Klein S. Safe, swallowable and palatable paediatric mini-tablet formulations for a WHO model list of essential medicines for children compound – A promising starting point for future PUMA applications. Eur. J. Pharm. Biopharm. 2020;156:11–19. doi: 10.1016/j.ejpb.2020.08.014. [DOI] [PubMed] [Google Scholar]
- Hashim H., Abrams P. Desmopressin for the treatment of adult nocturia. Therapy. 2008;5:667–683. [Google Scholar]
- Hermes M. Heinrich Heine University; Düsseldorf, Germany: 2012. Kindgerechte, niedrigdosierte Zubereitungen mit Enalaprilmaleat. Ph. D. thesis. [Google Scholar]
- Ilan E., Amselem S., Weisspapir M., Schwarz J., Yogev A., Zawoznik E., Friedman D. Improved oral delivery of desmopressin via a novel vehicle: mucoadhesive submicron emulsion. Pharm. Res. 1996;13:1083–1087. doi: 10.1023/a:1016023111248. [DOI] [PubMed] [Google Scholar]
- Kerski S., Rathsack W., Stodt G. 2015. Permeationszelle mit Isofillkammer. Patent DE202015004165. [Google Scholar]
- Kleinebudde . University Kiel; Germany: 1997. Pharmazeutische Pellets durch Extrudieren/Sphäronisieren: Herstellung, Eigenschaften, Modifizierung. Habilitation thesis. [Google Scholar]
- Kottke D., Lura A., Lunter D.J., Breitkreutz J. Manufacturing and characterisation of a novel composite dosage form for buccal drug administration. Int. J. Pharm. 2020;589:119839. doi: 10.1016/j.ijpharm.2020.119839. [DOI] [PubMed] [Google Scholar]
- Kottke D., Burckhardt B.B., Breitkreutz J., Fischer B. Application and validation of a coaxial liquid core waveguide fluorescence detector for the permeation analysis of desmopressin acetate. Talanta. 2021:122145. doi: 10.1016/j.talanta.2021.122145. [DOI] [PubMed] [Google Scholar]
- Lindert S. Heinrich Heine University; Düsseldorf, Germany: 2016. Entwicklung und Charakterisierung filmförmiger Zubereitungen zur oromukosalen Anwendung von Peptiden. Ph. D. thesis. [Google Scholar]
- Lottmann H.B., Alova I. Primary monosymptomatic nocturnal enuresis in children and adolescents. Int. J. Clin. Pract. 2007;61:8–16. doi: 10.1111/j.1742-1241.2007.01464.x. [DOI] [PubMed] [Google Scholar]
- Lottmann H., Froeling F., Alloussi S., El-Radhi A.S., Rittig S., Riis A., Persson B.-E. A randomised comparison of oral desmopressin lyophilisate (MELT) and tablet formulations in children and adolescents with primary nocturnal enuresis. Int. J. Clin. Pract. 2007;61:1454–1460. doi: 10.1111/j.1742-1241.2007.01493.x. [DOI] [PubMed] [Google Scholar]
- Majid H., Bartel A., Burckhardt B.B. Development, validation and standardization of oromucosal ex-vivo permeation studies for implementation in quality-controlled environments. J. Pharm. Biomed. Anal. 2021;194:113769. doi: 10.1016/j.jpba.2020.113769. [DOI] [PubMed] [Google Scholar]
- Mannucci P.M., Pareti F.I., Ruggeri Z.M., Capitanio A. 1-deamino-8-D-arginine vasopressin: a new pharmacological approach to the management of haemophilia and von willebrand’s disease. Lancet. 1977;309:869–872. doi: 10.1016/s0140-6736(77)91197-7. [DOI] [PubMed] [Google Scholar]
- Narang N., Sharma J. Sublingual mucosa as a route for systemic drug delivery. Int J Pharm Pharm Sci. 2011;3:18–22. [Google Scholar]
- Ono A., Tomono T., Ogihara T., Terada K., Sugano K. Investigation of biopharmaceutical and physicochemical drug properties suitable for orally disintegrating tablets. ADMET and DMPK. 2016;4:335–360. [Google Scholar]
- Patel V.F., Liu F., Brown M.B. Advances in oral transmucosal drug delivery. J. Control. Release. 2011;153(2):106–116. doi: 10.1016/j.jconrel.2011.01.027. [DOI] [PubMed] [Google Scholar]
- Richard J. Challenges in oral peptide delivery: lessons learnt from the clinic and future prospects. Ther. Deliv. 2017;8:663–684. doi: 10.4155/tde-2017-0024. [DOI] [PubMed] [Google Scholar]
- SCBT 2021. https://www.scbt.com/de/p/desmopressin-acetate-62288-83-9 Last access: 10.04.2021.
- van der Merwe S.M., Verhoef J.C., Kotzé A.F., Junginger H.E. N-Trimethyl chitosan chloride as absorption enhancer in oral peptide drug delivery. Development and characterization of minitablet and granule formulations. Eur. J. Pharm. Biopharm. 2004;57:85–91. doi: 10.1016/s0939-6411(03)00152-8. [DOI] [PubMed] [Google Scholar]
- van Kerrebroeck P., Nørgaard J.P. Desmopressin for the treatment of primary nocturnal enuresis. Pediatr. Health. 2009;3:311–327. [Google Scholar]
- Vande Walle J., Stockner M., Raes A., Nørgaard J.P. Desmopressin 30 years in clinical use: a safety review. Curr. Drug Saf. 2007;2:232–238. doi: 10.2174/157488607781668891. [DOI] [PubMed] [Google Scholar]