Abstract
Diffuse midline glioma (DMG) is an incurable malignancy with the highest mortality rate among pediatric brain tumors. While radiotherapy and chemotherapy are the most common treatments, these modalities have limited promise. Due to their diffuse nature in critical areas of the brain, the prognosis of DMG remains dismal. DMGs are characterized by unique phenotypic heterogeneity and histological features. Mutations of H3K27M, TP53, and ACVR1 drive DMG tumorigenesis. Histological artifacts include pseudopalisading necrosis and vascular endothelial proliferation. Mouse models that recapitulate human DMG have been used to study key driver mutations and the tumor microenvironment. DMG consists of a largely immunologically cold tumor microenvironment that lacks immune cell infiltration, immunosuppressive factors, and immune surveillance. While tumor-associated macrophages are the most abundant immune cell population, there is reduced T lymphocyte infiltration. Immunotherapies can stimulate the immune system to find, attack, and eliminate cancer cells. However, it is critical to understand the immune microenvironment of DMG before designing immunotherapies since differences in the microenvironment influence treatment efficacy. To this end, our review aims to overview the immune microenvironment of DMG, discuss emerging insights about the immune landscape that drives disease pathophysiology, and present recent findings and new opportunities for therapeutic discovery.
Keywords: Diffuse midline glioma, Microenvironment, Macrophage, Microglia, Immunotherapy, H3K27M
1. Introduction
Diffuse midline glioma (DMG) is a devastating pediatric brainstem tumor accounting for 10–15% of brain tumors and 80% of brain stem tumors in children and adolescents [1,2]. A DMG diagnosis is made in approximately 300 children per year in the United States with the median age at diagnosis between 6 and 7 [3]. Children with DMG survive 9 to 11 months after diagnosis and have a 99% 5-year mortality [4], [5], [6]. To date, there is no effective treatment for DMG. Chemotherapy and targeted molecular agents have proved to be minimally effective treatments, and surgical resection of the tumor is difficult due its location in the pons, thalamus, and spinal cord [7]. DMGs are infiltrative in nature, predominantly involving the pons but can be thalamic in location invading into surrounding brain and spinal cord. Fractionated external beam radiotherapy (RT), the standard of care for DMG, has only been successful in providing limited disease control or improving symptoms and confers a survival benefit of approximately 3 months. In the absence of standard RT, the median survival is 6 months [8].
Like many other central nervous system (CNS) tumors, DMGs have several intrinsic mechanisms to inhibit host antitumor responses. DMGs have a unique immune landscape characterized by nonpolarized resident immune cells and immune-induced secretions (Fig. 1). This landscape impacts DMG pathophysiology, prognosis, treatment options, and outcomes. In this review, we will explore the heterogeneity of the DMG immune microenvironment and contributions of specific immune subpopulations to DMG pathology.
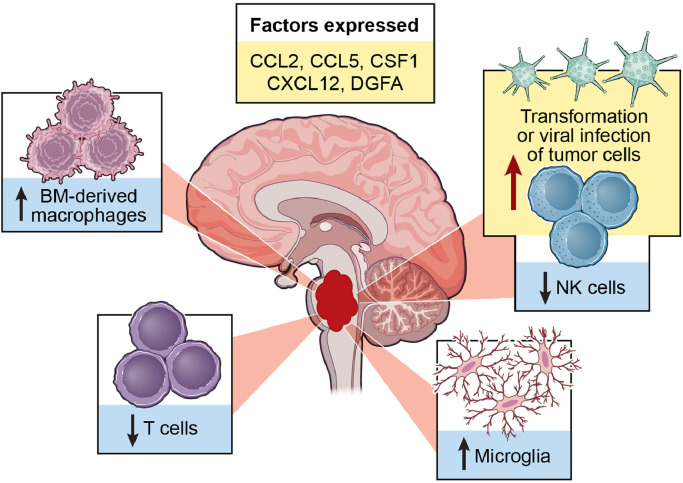
Fig. 1.
Summary of DMG-immune system interactions. DMG is an immunologically cold tumor with very limited T cell and NK cell infiltration. Glioma associated macrophages consisting of bone marrow-derived macrophages and microglia are the primary immune cells that reside in the tumor microenvironment. Select chemokines and cytokines are also expressed in DMG.
1.1. Cellular origin
Because DMG has spatial-temporal homogeneity and tends to arise during middle childhood, aberrant neurodevelopmental processes trigger tumor cell development and proliferation [9]. Both cellular origin and microenvironmental signaling is necessary for tumor growth [10]. DMG originates from oligodendrocyte progenitor or neural stem cells [3]. Oligodendrocytes play a role in myelin development during childhood and are responsible for supporting and insulating axons in the CNS [4]. The DMG cell of origin was identified via histological staining which revealed that oligodendrocyte precursor cells were enriched at the location of DMG origination and around the median age of DMG diagnosis [3]. To further validate oligodendrocytes as the cell of origin for DMG, transcriptional and chromatin landscape studies conveyed that oligodendrocyte genes are transcriptionally and epigenetically upregulated in DMG [11], [12], [13]. Furthermore, single cell RNA sequencing has shown that proliferative stem-like cells of primary DMG tumors are phenotypically similar to oligodendrocyte precursor cells [14]. Most notably, DMG proliferative stem-like cells express nestin and vimentin, markers found in neural stem or precursor cells, and olig2, a transcription factor commonly associated with oligodendrocyte precursors [3].
1.2. Molecular alterations
The understanding of DMG's biological underpinnings has been used to identify genetic and epigenetic signatures that are present in patient subpopulations [7]. Mutations in genes encoding for histones prevent histone methylation and have been identified in a plethora of cancers. Approximately 80% of DMG tumors exhibit a characteristic substitution of lysine-to-methionine at position 27 of histones 3.1 and 3.3 [15], [16], [17]. Deregulation of such histone proteins impedes polycomb repressive complex-2 (PRC2) methyltransferase complex functioning, causes systemic hypomethylation of the lysine at position 27 of the H3 protein (H3K27), and hinders gene expression [15,[18], [19], [20]]. The H3.1K27M and H3.3K27M DMG subgroups are characteristically associated with unique genetic alterations. In addition to direct mutations in H3.1-K27M and H3.3-K27M, DMGs harbor indirect mutations in H3.3G34RV that alter post-translationally modified residues [6]. H3.3K27M tumors are associated with mutations in tumor protein p53 (TP53), while H3.1K27M tumors often harbor mutations in activin A receptor type 1 (ACVR1) or have phosphoinositide 3-kinase (PI3K) pathway dysregulation [21].
Secondary associated mutations that contribute to cancer formation are seen in addition to histone mutations and lead to unique oncogenic outcomes [6]. Forty-two percent of DMG tumors harbor mutations in TP53, the gene encoding the tumor suppressor protein p53 [22]. P53 modulates cell survival and apoptosis in the developing nervous system. The preferential expression of p53 in neural progenitor cells (NPCs) is critical for regulating cell cycle progression and apoptosis [22]. Together, platelet-derived growth factor beta (PDGFB) signaling and TP53 loss frequently promotes tumor formation. Mechanisms underlying p53 mutations in DMG cells include disruption of p53 protein stability and gene expression and an increased rate of neural stem cell proliferation [23].
ACVR1 is a developmental regulator that is mutated in approximately 24% of patients with DMG and has been reported at a younger age of diagnosis [22]. ACVR1 encodes for the ALK2 (activin receptor-like kinase-2) receptor in the bone morphogenetic protein (BMP) signaling pathway. ACVR1 is also responsible for patterning during late gastrulation in embryogenesis and regulating craniofacial and cardiac development [24]. Six mutations have been described in two domains (glycine–serine rich domain and kinase domain) of ACVR1. The G328V mutation is the most common ACVR1 mutation in DMG, however, all mutations commonly segregate with H3.1K27M mutations [22].
Recurrent truncating mutations in the gene encoding protein phosphatase Mg2+/Mn2+dependent 1D (PPM1D) has been identified in 9–23% of DMG cases [25]. PPM1D mutations are often present concurrently with Histone H3 mutations (H3K27M) and are mutually exclusive with tumor suppressor protein 53 (TP53)-inactivating mutations [26]. PPM1D is critical for neurodevelopment and plays a role in dephosphorylating checkpoint kinases ATM (ataxia–telangiectasia mutated), ATR (ataxia telangiectasia and Rad3-related protein), and Chk1/2 to achieve homeostatic regulation of DNA damage response [27]. However, the phosphatase PPM1D is considered an oncogenic phosphatase because of its role in inactivating p53. In addition to DMG, PPM1D amplification or overexpression has been found in many carcinomas, including medulloblastoma, neuroblastoma, ovarian cancer, and breast cancer [27].
DMGs also harbor amplifications in genes involved in cell cycle regulation, specifically cyclin dependent kinase inhibitors CDK4 and CDK6 and cyclin D family members CCND1, CCND2, and CCMD3 [28]. Cyclin dependent kinases and cyclins form complexes that are critical for neurogenesis and phosphorylates the retinoblastoma (Rb) protein [29]. Specifically, overexpression of Cyclin D and CDK4 in NPCs inhibits neurogenesis and shortens the G1 phase of the cell cycle suggesting Cyclin D and CDK4 are involved in the G1-mediated switch from proliferation to neurogenesis [30]. Moreover, inhibition of CDK4 and CDK6 can trigger cell cycle arrest at the G1 checkpoint and has been explored as a potential therapeutic option for DMG [31].
Funato et al. and Larson et al. have revealed that increased PDGFRA expression is associated with DMG tumor formation. Studies found that the combination of PDGFRA activation and p53 loss was sufficient to induce neoplastic transformation in human embryonic stem cells and form genetically engineered mouse model (GEMM) brainstem gliomas [21,32].
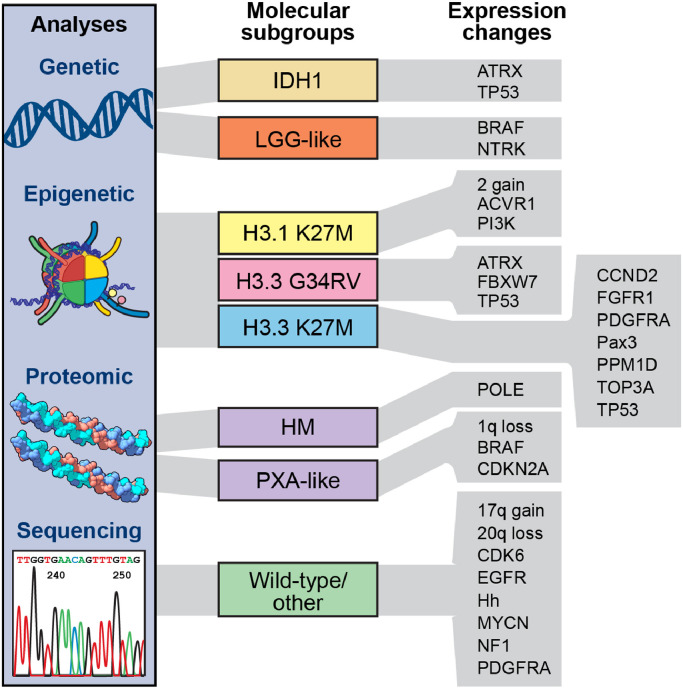
As noted above, DMG harbors many genetic alterations that comprise unique molecular subgroups (eg, isocitrate dehydrogenase 1 (IDH1), H3.1/3.3, pleomorphic xanthoastrocytoma (PXA-like)) and drive tumorigenesis (Fig. 2). While heterogeneity of DMG subgroups has previously been relegated to histone mutations, a seminal large-scale integrated analysis identified comprehensive risk subgroups of DMG [6].
Fig. 2.
Schematic representation of different biological subgroups exhibiting unique mutational profiles and transcriptional states in DMG. Molecular subgroups include IDH1, LGG-like, H3.1K27M, H3.3G34RV, H3.3K27M, HM (hypermutator phenotype), PXA-like, and H3/IDH1 wild type. These subgroups are associated with variable expression changes. Abbreviations: 2 gain: chromosome 2 gains; ATRX: α thalassemia/mental retardation syndrome X-linked; ACVR1: Activin A receptor, type I; BRAF: v-raf murine sarcoma viral oncogene homolog B1; CCND2: Cyclin D2; CDK6: Cyclin Dependent Kinase 6; CDKN2A: Cyclin Dependent Kinase Inhibitor 2A; EGFR: epidermal growth factor receptor; FBXW7: F-box/WD repeat-containing protein 7; FGFR1: Fibroblast growth factor receptor 1; Hh: Hedgehog; HM: Hypermutator phenotype; IDH1: Isocitrate dehydrogenase 1; NF1: Neurofibromatosis Type 1 ; NTRK: Neurotrophic Tyrosine Kinase; PAX3: Paired box gene 3; PDGFRA: Platelet Derived Growth Factor Receptor Alpha; PI3K: Phosphoinositide 3-kinase; POLE: DNA Polymerase Epsilon Catalytic Subunit; PPM1D: Protein phosphatase 1D; PXA: Pleomorphic xanthoastrocytoma ; TOP3A: DNA topoisomerase 3-alpha; TP53: Tumor protein P53.
1.3. Immune landscape of DMG
1.3.1. Natural killer cells
Natural killer (NK) cells, phenotypically marked by CD3−, CD56+, and CD16+, are effective cytotoxic lymphocytes that contain perforin-rich and granzyme-rich granules and can kill cancer cells and virally infected cells. NK cells classically have limited existence and function in the brain tumor microenvironment because of the immunosuppressive factors released by tumor cells [33]. Similar levels of NK cells are seen across all DMG subtypes. While a recent study reported that NK cells are low and defective in patients with DMG, induction of NK cells in the brain has the potential to kill brain tumor cells [34]. A recent study has shown that transformed or virally infected brain tumor cells can recruit NK cells leading to subsequent tumor cell killing [35]. A DMG-specific study noted that expression of one or more activating ligands for NKG2D (natural-killer group 2, member D) causes a chain reaction of cytokine production, targeted cytotoxic granule release, and NK cell activation. Combining DMG and NK cells resulted in effective killing of DMG cells in vitro [36]. NK cells also have the potential to serve as a prognostic marker for DMG. A clinical study revealed that tumors wild-type for H3.3K27M with NK cell infiltration are positively prognostic for DMG [37].
1.3.2. Microglia and macrophages
Microglia are myeloid cells accounting for 10–20% of the non-neuronal cell population that support and protect neuronal function [38]. Microglia and CNS-border associated macrophages, such as perivascular, choroid plexus-associated, and meningeal macrophages, have similar ontogeny and are commonly referred to as resident macrophages (or tumor-associated macrophages (TAMs)) residing in the CNS [39]. Of the CNS-border associated macrophages, choroid plexus-associated macrophages are extrastriatal bone marrow-derived macrophages (BMDMs). These immune cells are responsible for maintaining brain homeostasis and immunological responses [38].
TAMs display pro-tumorigenic effects in DMG [40]. Infiltration of TAMs is partially mediated through PDGFB signaling [40]. In a DMG GEMM, it has been demonstrated that bone marrow-derived macrophages are the predominant TAM sub-population in the tumor microenvironment [40]. TAMs are a large part of the DMG immune environment as indicated by the high expression of CD45, CD68, and CD163, markers commonly expressed in microglia and peripheral BMDMs. Furthermore, knockout of CC chemokine ligand 3 (CCL3), an important driver of TAM recruitment and accumulation, in DMG GEMMs resulted in fewer BMDMs and consequently conferred survival benefit [40]. TAMs in DMG differ from TAMs in adult CNS tumors since they display a lower expression of IL6, IL1A, IL1B, CCL3, CCL4 [41]. Moreover, immunohistochemistry has revealed that DMG has decreased myeloid infiltration compared to adult CNS tumors [36].
Once BMDMs in DMG are activated, they undergo morphological changes and alterations in gene expression profile [42]. DMG tumor BMDMs and microglia in the tumor parenchyma have unique molecular characteristics distinct from normal brain microglia and macrophages. Moreover, DMG tumor microglia have unique morphology since these microglia have shorter processes and enlarged cell bodies in the tumor micronevironment [42].
The functionality of DMG TAMs differ from BMDMs seen in glioblastoma (GBM). Gene ontology analysis of the top 50 genes enriched in GBM and DMG myeloid cells revealed that genes enriched in DMG are involved in cell adhesion, angiogenesis, and extracellular matrix organization [42]. Genes enriched in GBM are involved in processes such as monocyte chemotaxis, neutrophil chemotaxis, and chemokine-mediated signaling pathway [42]. These findings suggest that DMG consists of distinctive inflammatory mechanisms. Additional gene ontology analysis identified genes differentially expressed in DMG-associated BMDMs compared to cortical microglia [42]. This analysis revealed that DMG-associated BMDMs have undergone an activation process consistent with their morphological changes.
1.3.3. Lymphocytes
The DMG immune environment is largely non-inflammatory and does not consist of an adaptive immune component. The lack of tumor infiltrating lymphocytes (TILs) in the tumor microenvironment has been indicated by decreased expression of CD3+ lymphocytes [42]. The few T cells that are present within the tumor microenvironment are found in perivascular spaces and around areas of necrosis [43].
Furthermore, the brain's inability to initiate an antitumor immune response to DMG has been attributed to the absence of myeloid antigen presenting cells that are essential for recruiting effector lymphocytes [36]. In addition to the lack of lymphocyte recruitment, studies have shown that DMG may have an undefined mechanism of evading T lymphocyte recognition. The evasion process was demonstrated when allogeneic T cells were unable to mediate DMG cell killing when both were combined in culture [36].
1.3.4. Pre-clinical in vivo models of DMG
The generation of diverse DMG modeling systems that recapitulate tumor growth and invasion is imperative to pre-clinically evaluating potential treatments and advancing research on DMG biology. While in vitro experiments provide critical findings about the cellular and molecular characterization of DMG, they have limitations such as the inability to model invasion, angiogenesis, metastasis, and the response of the tumor microenvironment to treatments [44]. As a result, in vivo models, such as patient derived xenografts (PDXs) and GEMMs, have been designed to better understand tumor biology and develop optimal treatment modalities for DMG that can be easily translated to patients.
1.3.5. Xenograft models
PDX models are commonly used as DMG tumor models in which DMG cells from patient samples are implanted into immunocompromised mice [45]. Patient samples can be freshly harvested via biopsy or obtained from postmortem tissue. While fresh tissue is preferred for orthotopic implantation, the diffuse nature and location of DMG can preclude a safe biopsy [46,47]. However, within the last ten years, efforts have been made to improve the feasibility and safety of DMG stereotactic biopsies [48].
The first attempts to generate a PDX DMG model involved implanting human adult cerebral cortex GBM cells into the brainstem of mice and rats [49,50]. These cells were derived from serially transplanted xenografts or expanded patient cell lines. These models were designed to evaluate therapeutic response rates of DMG given the brainstem's specialized microenvironment and blood brain barrier (BBB) [49]. Although these tumors mimicked DMG anatomy and histology, they were not capable of fully recapitulating molecular and cellular characteristics [49].
Before DMG PDX models were established, DMG xenograft cell lines were derived from autopsy tissue [3]. These cells grew into neurospheres and were expanded and stabilized before transplantation [3]. In vitro culturing methods involved propagating cells in culture medium conducive to neural stem cell tissue growth containing factors that promote the expression of Nestin, glial fibrillary acidic protein (GFAP), Vimentin, Sox2, Olig2, and CD1333. Consequently, the cultured neurospheres resembled primitive neural precursor cell types. Once sufficiently expanded, neurospheres were dissociated and stereotactically injected into the fourth ventricle of immunodeficient neonatal mice [3]. These mice developed hindbrain tumors diffusely infiltrating the brainstem, cerebellum, and cerebrum, with histopathology replicating DMG [3].
Isolation of fresh tissue can be difficult, however, there are a number of groups that have cultured and established xenograft cell lines from tumor biopsies [51], [52], [53]. These cultures were mainly used to test the efficacy of targeted agents and combination treatments of RT and small molecule inhibitors. Cultures have also been used to study DMG biology [53]. Chan et al. used biopsy-implanted PDX models to study the role of H3K27M mutations in regulating methylation and gene expression pattern changes in DMG.
While most PDX modeling methods involve culturing patient-derived cells before implantation, there have been direct xenograft transplantations where cells are directly transplanted into immunocompromised mice [49]. This method produced tumors that resembled DMG but comprised of cells with mouse rather than human origin. The mechanism for this cellular transformation is unknown. A more recent study concurrently generated two DMG models: one where DMG cells were directly transplanted into the brainstem of mice upon biopsy and another where cells were expanded before implantation. While neither of these models formed tumor masses, models mimicked the epigenetic variability observed in the patients with DMG [54]. Overall, direct transplantation of patient-derived cells is less favorable for developing PDX models because there is a greater possibility of losing cells during the transplantation process [55].
The site of injection and the preparation of cells before xenograft implantation has varied. One group has implanted DMG cells into the striatum rather than brainstem [56] and another infected cells with human telomerase reverse transcriptase (hTERT) and a luciferase reporter before implantation [57]. The hTERT-Luc mouse model generated by Hashizume et al. expressed key genes for human DMG including GFAP, Nestin, Olig2, and PDGFA. Upon gene expression and copy number analysis, this model was compared to previous analyses of human DMG samples and used to evaluate a preclinical efficacy of RT and inhibitor MK-1775 in vivo [52,57].
Larger studies have used both autopsy and biopsy specimens to generate DMG PDX models. Grasso et al. used biopsy and patient derived DMG cells to generate in vitro and in vivo model systems and run a large-scale drug screen including 83 drugs. It was found that panobinostat, a histone deacytelase (HDAC) inhibitor, has a synergistic effect with GSK-J4, a histone de-methylase inhibitor, and is cytotoxic to DMG cells in vitro and can be used to effectively treat orthotopic DMG tumors in vivo [58].
1.3.6. Genetically engineered mouse models
In addition to PDXs, GEMMs have become important in vivo tools for DMG research (Table 1). It was first suspected that GEMM could be used to model DMG because previous findings have indicated that the RCAS (replication-competent avian sarcoma-leucosis virus long-terminal repeat with splice acceptor system) could be used to generate gliomas outside of the subventricular zone. GEMMs involve the introduction of genetic alterations to a specific cell-of-origin. Because immunocompetent mice are used, primordial growth and development of tumors in a functional tumor microenvironment can be observed. Concerted efforts towards generating GEMMs have contributed to further investigate DMG cells-of-origin and molecular underpinnings associated with tumor initiation, growth, histology, and treatment response.
Table 1.
GEMMs of DMG.
| Model | Technical approach | Incidence | Genotype | Cell of Origin | Reference |
|---|---|---|---|---|---|
| GEMM | |||||
| RCAS/tv-a | |||||
| 80% by 3 months | PDGF-B, p16 loss | nestin-expressing NPCs (hindbrain) | [59] | ||
| 77% in 1 month | PDGF-B, p53 loss | nestin-expressing NPCs (hindbrain) | [61] | ||
| 72% by 3 months | H3.3K27M, p53 loss | nestin-expressing NPCs (hindbrain) | [19] | ||
| 95% by 3 months | H3.3K27M, p53 loss, PDGF-B | nestin-expressing NPCs (hindbrain) | [111] | ||
| 43% by 3 months | H3.3K27M, p53 loss, PDGF-B | Pax3-expressing NPCs (hindbrain) | [64] | ||
| 100% by 1–1.5 months | H3.3K27M, p53 loss, PDGF-B | nestin-expressing NPCs (hindbrain) | [112] | ||
| In utero electroporation | |||||
| 100% by 4 months | H3.3K27M, p53 loss, PDGFRA, ATRX loss | periventricular NPCs (forebrain/hindbrain) | [113] | ||
| 100% by 6–8 months | H3.3K27M, p53 loss | periventricular NPCs (forebrain/hindbrain) | [113] | ||
| 100% by 4 months | H3.3K27M, p53 loss, ATRX loss | periventricular NPCs (forebrain/hindbrain) | [113] | ||
| >90% by 1.5–2 months | H3.3K27M, DNp53, PDGFRAD842V | periventricular NPCs (forebrain) | [114] | ||
| 100% by 1 month | H3.3K27M, p53 loss, PDGF-B | periventricular NPCs (hindbrain) | [115] | ||
| 100% by 1 month | H3.3WT, p53 loss, PDGF-B | periventricular NPCs (hindbrain) | [115] | ||
| 100% by 1 month | H3.3WT, p53 loss, PDGFRAD842V | hindbrain periventricular NPCs (hindbrain) | [115] | ||
| 100% by 1 month | H3.3K27M, p53 loss, PDGFRAWT | hindbrain periventricular NPCs (hindbrain) | [115] | ||
| Transgenic | |||||
| 100% by 4 months | H3.3K27M, p53 loss | nestin-expressing NPCs | [113] | ||
| 100% by 4 months | H3.3K27M, p53 loss | GFAP-expressing NPCs | [113] | ||
| 86% by 3 months | H3.3K27M, p53 loss | nestin-expressing NPCs | [21] | ||
| 96% by 3 months | p53 loss, PDGFRAV544ins | nestin-expressing NPCs | [21] | ||
| 80% by 3 months | H3.1K27M, ACVR1G328V, PIK3CAH1047R | Olig2-expressing OPCs | [116] | ||
| 80% by 3 months | H3.1K27M, ACVR1G328V | Olig2-expressing OPCs | [116] |
GEMMs are generally designed using the RCAS/tumor virus A (TVA) modeling system and genetic aberrations associated with the human disease of interest. The RCAS–TVA system uses the retroviral avian leucosis and sarcoma virus family as a vector to deliver the gene of interest. Subsequently, the virus selectively infects cells expressing the corresponding surface receptor TVA.
Because Nestin-expressing cells are suspected to be the cells-of-origin for DMG, one of the first GEMM for DMG involved using germline Ink4a-ARF loss and PDGFB overexpression targeted to Nestin-expressing cells in the pons of neonatal mice [3,59]. While these GEMMs tumors grew in the brainstem and were similar in histology to human DMG, they were not formed exclusively in the pons, so this model was considered to be a brain stem glioma (BSG) GEMM rather than a DMG model. Although tumors were generated in the brainstem, this model is one of the few GEMMs that closely mimics human DMG.
GEMMs have been used as a preclinical tool to test potential therapeutics and study tumor biology. The BSG GEMM has been further characterized by comparing it to other glioma GEMMs. Specifically, Hambardzumyan et al. compared the BSG GEMM to gliomas of the cerebral cortex to define subsets of DMG and understand mechanisms driving tumor growth [60]. This study found that found that the BSG GEMM had a high expression of transcription factor Pax3 that is essential for gliomagenesis [60].
The BSG GEMM has also been used to test tumor response to treatments. Becher et al. used this model to test the therapeutic efficacy of RT and AKT signaling inhibitor perifosine, while Barton et al. studied the therapeutic effect of cyclin-dependent kinase (CDK) 4/6 inhibitor PD0332991 alone and in combination with RT [59,61]. A survival benefit was found when combining perofisine and RT Becher et al.’s study; however, the study conducted by Barton et al. was the first to report that a treatment other than RT alone resulted in survival benefit. Most recently this model has been used to detect the effects of systemic administration and convection enhanced delivery (CED) of HDAC inhibitor panobinostat in treating DMG [62,63].
In addition to the BSG GEMM, a GEMM that resembled DMG was generated by initiation in the pons and PDGF signaling overexpression, p53 loss, and H3.3K27M mutation [19]. This model has many advantages because of its location in the pons and harbors a global knockout of H3K27me3 that mimics patient DMGs characterized by H3K27me3 mutations [19].
In 2016, DMG GEMM was established by injecting Pax3-Tv-a;Trp53fl/fl mice with RCAS-PDGFB and RCASCre, with or without RCAS-H3.3K27M [64]. The RCAS plasmid-produced avian retroviruses expressing Cre and PDGFB infect mouse cells expressing Pax3 and RCAS virus receptor Tv-a. The generation of this model was used to further investigate the cell-of-origin and possible cancer stem cells in DMG.
Another unique DMG GEMM involves the use of human embryonic stem cells to create neural precursor-like cells (NPCs) that are altered with activated PDGFRA, H3.3K27M, and p53 knockdowns[32]. The addition of these genes to NPCs induced tumorigenesis. This study provided valuable information on NPC response to oncogenes in vitro and in vivo [32].
Recently, Larson et al. developed a neuro-specific, promoter-driven conditional H3f3aK27M knock-in DMG GEMM and demonstrated that H3.3K27M cooperates with PDGFRA mutations and loss of p53 to induce brainstem gliomas molecularly resembling human DMG [21]. The K27M mutation, and the subsequent H3K27me3 loss, led to discrete transcriptional changes with selective regulation of bivalent promoters in tumors. Upregulated genes were enriched for association with neural development, while genes that encoded homeodomain transcription factors were downregulated, thus suggesting that H3.3K27M acclimatizes a more undifferentiated phenotype21L.
1.4. Existing and upcoming therapies for DMG
1.4.1. Standard-of-care treatments
Conventional fractionated external beam RT is the principal treatment modality for DMG (Fig. 3). RT minimally extends patient survival and temporarily relieves symptoms [65]. The standard RT dose administered for DMG is 54 Gy that is delivered as 30 fractions of 1.8 Gy [66]. The rationale for RT being used as a treatment is based on studies reporting that DMG spreads congruously and tumor recurrence is most often local and within the fields of RT [67], [68], [69].
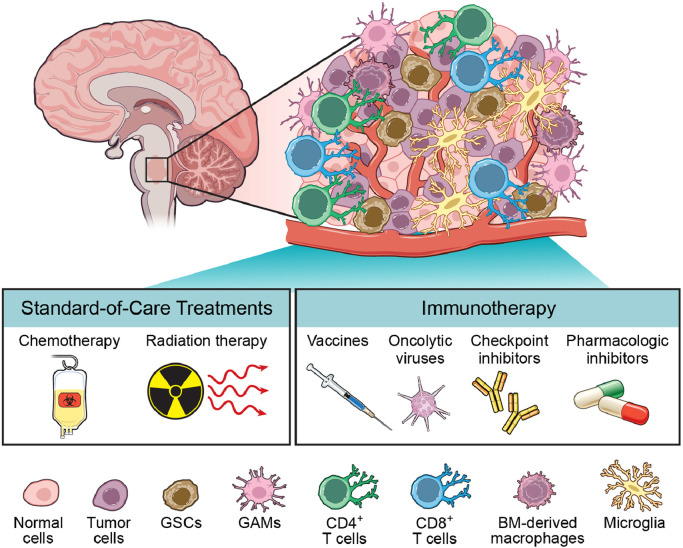
Fig. 3.
DMG is a heterogeneous tumor with diverse treatment modalities. The mainstay of treatment for DMG is fractionated RT with intermittent chemotherapy. Emerging immunotherapies, such as vaccines, oncolytic viruses, checkpoint inhibitors, and pharmacologic inhibitors, have been met with preclinical success and are undergoing clinical trials.
RT damages proliferating cancer cells and induces hypoxia [70]. Within the tumor microenvironment, hypoxia induces a cascade of events that lead to an increase in chemokine CXCL12 and subsequent recruitment of bone marrow-derived monocytes and hematopoietic progenitor cells that co-express CXCR4 and CXCR7 [70]. These stromal cells undergo differentiation to become tumor-promoting macrophages that mediate angiogenesis and tumor recurrence [71], [72], [73]. Accordingly, preclinical studies have found that blocking CXCL12 [74] and CXCR7 [75] impedes tumor recurrence upon irradiation. In addition to inhibiting cell proliferation and inducing tumor hypoxia, RT also upregulates PD-L1 at the surface of tumor-infiltrating myeloid cells [76]. Programmed death-ligand 1 (PD-L1) is a coinhibitory ligand expressed in many types of tumor cells. Inhibition of the PD-L1 and programmed cell death protein 1 (PD-1) checkpoint can serve as a potential immunotherapy [77]. The addition of immunotherapies and novel therapeutic inhibitors can have a concomitant effect when combined with RT to treat DMG.
Chemotherapy is also a primary mode of treatment for DMG despite having minimal impact on prognoses. Chemotherapy has the potential to mitigate RT-induced damage and improve neuro-cognitive outcomes when combined with RT [78]. However, chemotherapy damages the bone marrow and eventually impacts the number and activation state of resident immune cells [79]. The most common chemotherapy used to treat brain cancer is temozolomide (TMZ). TMZ directly targets cancer cells and has immunomodulatory effects [80]. TMZ induces lymphopenia, which interestingly can be harnessed to improve immunotherapy. This was confirmed by findings indicating that lymphoablative doses of TMZ increase tumor antigen-specific immune responses in GBM patients [81,82] and GBM-bearing mice [83]. The mechanism underpinning this synergistic effect is that upon administration of TMZ, there are compensatory homeostatic cytokines reducing the T-cell activation threshold and induction of proliferation which in turn heightens immune responses [83]. While TMZ has shown improved survival in GBM characterized by a O6-methylguanine-DNA methyl-transferase (MGMT) promoter methylation, this drug has not been successful in patients with DMG. In addition to TMZ, many clinical trials for DMG treatment have involved myelosuppressive chemotherapy, including a high-dose chemotherapy trial with stem-cell rescue [84,85]. These trials have not shown improvement in overall survival.
Although chemotherapy has proven to be ineffective in the treatment of DMG, there are diverse perspectives about whether or not to use chemotherapy [86,87]. A recent survey found that 44% of physicians suggested that patients undergo adjuvant chemotherapy after RT [88]. Overall, the efficacy of chemotherapy is limited because DMG tumors characteristically have an intact BBB which makes CNS penetration difficult [89]. The administration of therapeutics that increase BBB permeability along with chemotherapy could enhance the efficacy of chemotherapy for DMG.
1.4.2. Immune factors involved in DMG tumor growth and development
Malignant brain cancers commonly undergo infiltration of immune cells. In the tumor, immune cells become polarized, acquire new properties that support tumor growth, and facilitate the secretion of a variety of growth factors and pro-angiogenic cytokines [90]. While interactions between brain tumor and immune cells tend to be diverse, secretory immune cells are not a part of the DMG milieu [91]. Hierarchical clustering analysis has demonstrated that DMG cultures resemble human neural precursor cells and secrete substantially fewer cytokines and chemokines than GBM cells [42]. On a transcriptional level, DMG cell cultures do not express cytokine genes and only express a limited number of chemokines and growth factors that may contribute to the immune infiltration in the tumor [42]. Interleukin-2 (IL2) is an example of a key cytokine that has decreased expression in DMG. Reduced IL2-mediated signaling is indicative of low levels of T lymphocytes that may have antitumorigenic properties. The immunosuppressive growth factor transforming growth factor beta (TGF-β) and the neutrophil chemotactic factor IL8 are two of the few factors readily expressed in DMG [40]. TGF-β contributes to the tumor's non-inflammatory nature, while IL2 strongly induces CXC chemokine receptor 2 (CXCR2).
Recently, it has been reported that DMGs have higher expressions of leukocyte-attracting chemokines CXCL1, CXCL2, CXCL5, and CXCL6 compared to other pediatric high-grade gliomas (pHGGs) [40]. These chemokines exert their biological effects by interacting with the CXCR2 receptor which is also overexpressed in DMGs [40]. Interestingly, although DMGs have been universally considered “immune cold,” DMG tumors express a subset of chemokines and growth factors. RNA sequencing and gene expression analysis has shown that patient-derived DMG cell cultures express high levels of CCL2, CCL5, CSF1, CXCL12, and PDGFA [42].
CCL2 and CCL5 are both chemoattractant proteins critical for monocyte and lymphocyte chemotaxis. CCL2 plays a key role in regulating the migration of TAMs, myeloid derived suppressor cells (MDSCs), and regulatory T cells (Tregs) to tumor sites [92]. Despite the ability for CCL2-expressing DMG cells to regulate migration, MDSCs and Tregs have not been reported to infiltrate DMG tumors which may be attributed to underlying tumor cell-intrinsic factors. Similarly, CCL5 has been associated with CD8+ T cell infiltration in various carcinomas; however, DMG tumors contain very few infiltrating T-cells [42].
CSF1 is a cytokine associated with M2 TAMs. M2 TAMs are characterized by a pro-tumorigenic phenotype that is immune regulatory and anti-inflammatory [42]. Despite DMG tumor cells producing CSF1, TAMs in DMG tumors cannot be distinguished by the M1 (classically activated) or M2 (alternatively activated) macrophage phenotype [93].
PDGFRA is a genetic alteration that is common in DMG. The contributions of PDGFRA to DMG tumor development has been described in preclinical models. PDGFRA activation induces multiple cellular activities including cell proliferation, migration, transformation, and survival [94]. PDGFRA activation and p53 loss have been found to induce neoplastic transformation in human embryonic stem cells and induce GEMMs of brainstem gliomas [21,43].
CXCL12 is a chemoattractant expressed in various tumors and its receptor, CXCR4, is overexpressed in at least 20 different cancers types, including breast cancer, ovarian cancer, and melanoma [95]. The CXCL12/CXCR4 interaction contributes to tumor cell growth, survival and angiogenesis in cancers and is critical for homing and metastatic mediation of secondary growth in organs [96]. However, the role of CXCL12/CXCR4 axis in tumor growth and organ development has often been debated [97,98].
1.4.3. Recent immunotherapeutic discoveries
Immunotherapy has become an emerging therapeutic option for DMG and several of these approaches have been implemented in clinical trials (Table 2). However, this specific treatment poses a number of challenges since the DMG tumor microenvironment is immunosuppressive and has impaired immune surveillance. When combined with additional therapeutic interventions, immunotherapies can enhance the endogenous immune response and activate the intrinsic antitumor response.
Table 2.
Recent and current immunotherapy clinical trials for DMG.
| Intervention | Administration | Clinical Trial | Tumor Eligibility | Phase | Recruitment Status |
|---|---|---|---|---|---|
| C7R-GD2 CAR T cells | IV | NCT04099797 | Newly diagnosed; recurrent/refractory | I | Recruiting |
| GD2 CAR T cells | IV | NCT04196413 | Newly diagnosed | I | Recruiting |
| B7-H3 CAR T cells | Intratumoral; intraventricular | NCT04185038 | Newly diagnosed | I | Recruiting |
| Autologous dendritic cell vaccines (ADCV) | Intradermal | NCT02840123 | Newly diagnosed | I | Unknown |
| DNX-2401 | Intratumoral | NCT03178032 | Newly diagnosed | I | Active, not recruiting |
| DC vaccine/TMZ | IV | NCT03396575 | Newly diagnosed | I | Recruiting |
| cemiplimab (REGN2810) + RT | IV | NCT03690869 | Newly diagnosed; recurrent/refractory | I | Recruiting |
| H3K27M vaccine + nivolumab | IV | NCT02960230 | Newly diagnosed | I | Recruiting |
| Pembrolizumab | IV | NCT02359565 | Recurrent/refractory | I | Recruiting |
| APX005M [CD40 agonistic Ab] | IV | NCT03389802 | Newly diagnosed; recurrent/refractory | I | Recruiting |
| indoximod + RT/TMZ | PO | NCT04049669 | Newly diagnosed | II | Recruiting |
| IL12 adenovirus | Intratumoral | NCT03330197 | Newly diagnosed | I/II | Recruiting |
Abbreviations: IV (intravenous); PO (oral).
Peptide vaccines involving the injection of tumor-specific antigens stimulate immune response and have the potential to provide clinical benefit for tumors and produce an antitumor effect [99]. Ochs et al. designed a peptide vaccine (27-mer peptide) targeting the H3.3K27M mutation in a major histocompatibility complex-humanized DMG mouse model [100]. Administration of this vaccine resulted in a cytotoxic T cell and T helper (Th) cell-mediated immune response that was induced by interferon-gamma (IFNγ).
Dendritic cell (DC) vaccines have also been studied as a potential immunotherapy for DMG. Because DCs are robust antigen-presenting cells (APCs) situated at the interface between the innate and adaptive immune system, they are capable of inducing antigen-specific T cell responses and functioning as cellular adjuvants [101]. DC vaccines are made by leukapheresis of monocytic cells, exposing these cells to tumor-cell antigens, and administering the cells as a vaccine. DC vaccines have been reported as a potential therapy for DMG and have been shown to recruit tumor-specific T cells in several studies [101,102].
The effectiveness of peptide and DC vaccines may be countered by the lack of immune cell infiltration that is characteristic of DMG. As a result, the potential for DMG to stimulate T lymphocyte expansion and recruitment must be explored since very few T lymphocytes exist around the tumor site.
Recent studies have shown that oncolytic virus modification can be used to destroy tumorigenic cells. Martínez-Vélez et al. has reported that the administration of Delta-24-RGD (DNX-2401 in the clinic), a replicative oncolytic adenovirus, in the pons has a good safety profile and results in a significant increase in the survival of DMG mouse models [103]. Specifically, DNX-2401 administration induces T lymphocyte infiltration in the delineated tumor mass, leading to immune recognition of the tumor site. A preclinical study has explored the antitumor efficacy of DNX-2401 viral infection and replication in vitro. Intratumoral administration of DNX-2401 was nontoxic in both immunodeficient and immunocompetent mouse models of DMG and led to a significant increase in animal survival [102]. Additionally, DMG tumor cells have shown sensitivity to Newcastle disease virus (NDV) and infecting DMG cell lines with NDV decreases cell viability. Clinical studies where NDV was administered to patients with DMG showed an increase of IFNγ secreting T cells in the tumor microenvironment indicating that NDV could be used as a potential immunotherapy [104].
Delivery of CD40L-expressing adenovirus (Ad-CD40L) has been found to induce immune-mediated antitumor response [105]. Conventionally, CD40/CD40L interactions stimulate an immune response through Th cells, provide proliferation and differentiation signals to B cells, and facilitate APC maturation leading to the induction of cytotoxic T lymphocytes. Studies reported that Ad-CD40L induces both adaptive and humoral antitumor immune responses [106]. As a part of the adaptive immune response, Ad-CD40L induced infiltration of CD45+ cells composed of CD4+ and CD8+ T cells, CD19+ B cells, and NK cells. Increased IgG levels was indicative of an active humoral immune response upon AD-CD40L delivery. Ad-CD40L administration also upregulated genes involved in signaling pathways of neuroinflammation, T and B cell signaling, Th activation, and DC saturation [105]. The study also explored the effects of replication competent adenovirus since the use of standard adenoviral vectors can be associated with proinflammatory off-target effects. The replication competent adenovirus proved to be a more effective cancer treatment and mitigated proinflammatory cytokine and chemokine production. Upon delivery, cure rates in patient-derived DMG xenograft mouse models were up to 50% and weight loss in these mice was minimal [105].
Identification of DMG neoantigens has spurred the development of adoptive T cell therapies and immune checkpoint inhibitors. A recent study found that DMGs express B7-H3, a checkpoint molecule, which can be targeted using chimeric antigen receptor (CAR) T cell therapy. CAR T cells directed at B7-H3 produce IFNγ, IL2, and tumor necrosis factor-alpha (TNF-α) to induce tumor cell killing [106]. CAR T cell therapy has also been developed for DMG tumors with mutated H3K27M and high expression of GD2 (disialoganglioside-glycolipid antigen) devoid of neurotoxicity and deleterious side effects [107]. Similar to the B7-H3 CAR T cell therapy, CAR T cells directed at GD2 produce IFNγ, IL2, and TNF-α to induce tumor cell killing and cause inflammation in neuronal tissue [107]. This therapy has been approached with hesitance because of its adverse effects such as ventriculomegaly in vivo which is consistent with hydrocephalus in patients.
Pharmacologic inhibition of lysine specific demethylase (LSD1) is a potential immunotherapy for DMG because it is selectively cytotoxic and promotes an immune gene signature associated with NK cell killing [37]. LSD1 is a potential target in DMG because LSD1 regulates the histone mark H3K4me1 which is known to be enriched in intergenic regions of DMG and LSD1 may control access to enhancers of genes important in DMG pathology [37].
While checkpoint inhibitors against PD-L1 and PD1 have shown great promise as a cancer immunotherapy, minimal efficacy has been demonstrated when using PD-L1 for DMG therapy since DMG is immunologically cold with low endogenous expression of PD-L1 [36]. In order for PD-L1 to be an effective treatment for DMG, it must be combined with adjuvant therapies to enhance systemic immune response. Previous retrospective studies involving children diagnosed with DMG who received a combination of RT and nivolumab (PD-1 inhibitor) showed that these patients experienced slightly improved prognoses with no off-targeting or mitigating factors compared to those patients only received RT [108].
Immune modulating monoclonal antibodies (mAbs) have become novel oncologic therapies. The efficacy of immune modulating antibody MDV9300 (pidilizumab) in pediatric hematological malignancies has prompted its effects to be evaluated on DMG [108]. Pidilizumab augments endogenous antitumor response by acting as an immune modulator for humanized IgG1 mAb and having secondary inhibitory effects on PD-1. A clinical study involving the administration of pidilizumab to nine DMG patients observed an improvement in survival by 6.3 months compared to patients who underwent RT alone [109]. It is important to note that additional clinical trials involving the safety, tolerability, and benefit of pidilizumab must be conducted in order to confirm the aforementioned findings.
1.4.4. Outstanding questions
While this review comprehensively highlights the immune microenvironment of DMG, advanced knowledge about immune cell subpopulations that modulate DMG progression is imperative. This knowledge can be acquired from studies exploring the interplay between immune cell subpopulations and the tumor microenvironment.
Given the intratumoral heterogeneity of DMG, there is a paucity of information on the immune microenvironment associated with different DMG subgroups. This lack of information raises the question: how does tumor location and molecular identity of DMG impact the immune landscape? The recent characterization of DMG subgroups is the tip of the iceberg and more work must be done to explore how DMG's epigenetic and genetic expression attunes the immune microenvironment [6]. These findings are critical since the development of novel immunotherapies hinges on understanding the ways in which tumor heterogeneity influences the immune landscape.
2. Conclusion
Despite our knowledge on cell intrinsic mechanisms driving tumor growth, there is a paucity of information on the immune landscape of pHGGs, let alone DMG [110]. To date, there is insufficient research on the tumor immune microenvironment of DMG, yet this scope of research is imperative to developing effective therapeutic strategies. Current research has revealed that aggressive brain tumor subtypes, such as DMG, have high myeloid signatures, low expression of immune modulatory factors, and minimal infiltration of lymphocytes and NK cells responsible for tumor elimination. The lack of inflammatory cells in the DMG tumor microenvironment has made immune surveillance nonexistent. Currently, immunotherapy approaches for DMG have limited success because DMG has a low mutation burden and immunosuppressive microenvironment. The absence of antigen presenting cells, downregulation of the major histocompatibility complex, and presence of BBB have dampened antitumor immune responses, rendering most DMG tumors immunologically ‘cold’ and unresponsive to the use of existing immunotherapies alone [35]. However, when combined with adjuvant therapies, immunotherapies have the potential to elicit an antitumor response. Immunotherapy in combination with a neoadjuvant therapy can potentially eliminate cancer cells while sparing critical structures within the brain. Continued research endeavors involving the immune microenvironment and the emergence of innovative immunotherapies provide a critical background on future studies related to DMG.
Search strategy and selection criteria
Data for this review were identified by searches of PubMed and Scopus, and references from relevant articles using the search terms “diffuse midline glioma”, “diffuse intrinsic pontine glioma”, “H3K27M”, “immune microenvironment”, “immunotherapies”, “DIPG/DMG mouse models” and related terms. Searches were also formed based on investigator names. Abstracts and reports from meetings were excluded. Only articles published in English were included. Articles published in English between 1993 and 2021 were included. Articles were chosen according to their relevance to the theme as perceived by the authors.
Contributors
GP and CH conceptualized the review and were the primary authors revising the final manuscript. GP wrote the manuscript. DH and AB reviewed and edited the manuscript and provided insight on the figure design. All authors read and approved the final version of the manuscript.
Declaration of Competing Interest
All authors declare that they have no competing interests.
Acknowledgments
We would like to thank and acknowledge Mr. Dave Schumick for the figure illustrations.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2021.103453.
Appendix. Supplementary materials
References
- 1.Chintagumpala M., Eckel S.P., Krailo M. A pilot study using carboplatin, vincristine, and temozolomide in children with progressive/symptomatic low-grade glioma: a children's oncology group study†. Neuro Oncol. 2015;17(8):1132–1138. doi: 10.1093/neuonc/nov057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrom Q.T., Gittleman H., Fulop J. CBTRUS statistical Report: primary brain and central nervous system tumors diagnosed in the United States. Neuro Oncol. 2015;17 doi: 10.1093/neuonc/nov189. 2008-2012. iv1-iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monje M., Mitra S.S., Freret M.E. Hedgehog-responsive candidate cell of origin for diffuse intrinsic pontine glioma. Proc Natl Acad Sci U S A. 2011;108(11):4453–4458. doi: 10.1073/pnas.1101657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooney T., Lane A., Bartels U. Contemporary survival endpoints: an international diffuse intrinsic pontine glioma registry study. Neuro Oncol. 2017;19(9):1279–1280. doi: 10.1093/neuonc/nox107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffman L.M., Van Zanten S.E.M.V., Colditz N. Clinical, radiologic, pathologic, and molecular characteristics of long-term survivors of diffuse intrinsic pontine glioma (DIPG): a collaborative report from the International and European Society for pediatric oncology DIPG registries. J Clin Oncol. 2018;36(19):1963–1972. doi: 10.1200/JCO.2017.75.9308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackay A., Burford A., Carvalho D. Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell. 2017;32(4):520–537. doi: 10.1016/j.ccell.2017.08.017. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valvi S, Gottardo NG. Diffuse intrinsic pontine glioma. In: Brain tumors - An update. InTech; 2018. 10.5772/intechopen.78578. [DOI]
- 8.Johung T.B., Monje M. Current neuropharmacology send orders for reprints to reprints@benthamscience.ae diffuse intrinsic pontine glioma: new pathophysiological insights and emerging therapeutic targets. Curr Neuropharmacol. 2017;15:88–97. doi: 10.2174/1570159×14666160509123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikbakht H., Panditharatna E., Mikael L.G. Spatial and temporal homogeneity of driver mutations in diffuse intrinsic pontine glioma. Nat Commun. 2016;7 doi: 10.1038/ncomms11185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loveson K.F., Fillmore H.L. Intersection of brain development and pediatric diffuse midline gliomas: potential role of microenvironment in tumor growth. Brain Sci. 2018;8(11) doi: 10.3390/brainsci8110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagaraja S, Vitanza NA, Woo P, et al. Transcriptional dependencies in Diffuse Intrinsic Pontine Glioma Graphical abstract HHS Public Access. doi:10.1016/j.ccell.2017.03.011 [DOI] [PMC free article] [PubMed]
- 12.Tate M.C., Lindquist R.A., Nguyen T. Postnatal growth of the human pons: a morphometric and immunohistochemical analysis. J Comp Neurol. 2015;523(3):449–462. doi: 10.1002/cne.23690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballester L.Y., Wang Z., Shandilya S. Morphologic characteristics and immunohistochemical profile of diffuse intrinsic pontine gliomas. Am J Surg Pathol. 2013;37(9):1357–1364. doi: 10.1097/PAS.0b013e318294e817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filbin M.G., Tirosh I., Hovestadt V. Developmental and oncogenic programs in H3K27M gliomas dissected by single-cell RNA-seq. Science. 2018;360(6386):331–335. doi: 10.1126/science.aao4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu G., Broniscer A., McEachron T.A. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44(3):251–253. doi: 10.1038/ng.1102. Published 2012 Jan 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartzentruber J., Korshunov A., Liu X.Y. Driver mutations in histone H3.3 and chromatin remodeling genes in pediatric glioblastoma. Nature. 2012;482(7384):226–231. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 17.Khuong-Quang D.A., Buczkowicz P., Rakopoulos P. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 2012;124(3):439–447. doi: 10.1007/s00401-012-0998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bender S., Tang Y., Lindroth A.M. Reduced H3K27me3 and DNA hypomethylation are major drivers of gene expression in K27M mutant pediatric high-grade gliomas. Cancer Cell. 2013;24(5):660–672. doi: 10.1016/j.ccr.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Lewis P.W., Müller M.M., Koletsky M.S. Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science. 2013;340(6134):857–861. doi: 10.1126/science.1232245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venneti S., Garimella M.T., Sullivan L.M. Evaluation of Histone 3 Lysine 27 trimethylation (H3K27me3) and enhancer of Zest 2 (EZH2) in pediatric glial and glioneuronal tumors shows decreased H3K27me3 in H3F3A K27M mutant glioblastomas. Brain Pathol. 2013;23(5):558–564. doi: 10.1111/bpa.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larson J.D., Kasper L.H., Paugh B.S. Histone H3.3 K27M accelerates spontaneous brainstem glioma and drives restricted changes in bivalent gene expression in brief cancer cell article histone H3.3 K27M accelerates spontaneous brainstem glioma and drives restricted changes in bivalent gene expression. Cancer Cell. 2019;35:140–155. doi: 10.1016/j.ccell.2018.11.015. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor K.R., Vinci M., Bullock A.N., Jones C. ACVR1 mutations in DIPG: lessons learned from FOP. Cancer Res. 2014;74(17):4565–4570. doi: 10.1158/0008-5472.CAN-14-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jansen S., Geuer S., Pfundt R. De novo truncating mutations in the last and penultimate exons of PPM1D cause an intellectual disability syndrome. Am J Hum Genet. 2017;100(4):650–658. doi: 10.1016/j.ajhg.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komatsu Y., Scott G., Nagy A., Kaartinen V., Mishina Y. BMP type I receptor ALK2 is essential for proper patterning at late gastrulation during mouse embryogenesis. Dev Dyn. 2007;236(2):512–517. doi: 10.1002/dvdy.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu G., Diaz A.K., Paugh B.S. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet. 2014;46(5):444–450. doi: 10.1038/ng.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L., Chen L.H., Wan H. Exome sequencing identifies somatic gain-of-function PPM1D mutations in brainstem gliomas. Nat Genet. 2014;46(7):726–730. doi: 10.1038/ng.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pecháčková S., Burdová K., Macurek L. WIP1 phosphatase as pharmacological target in cancer therapy. J Mol Med. 2017;95(6):589–599. doi: 10.1007/s00109-017-1536-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zarghooni M., Bartels U., Lee E. Whole-genome profiling of pediatric diffuse intrinsic pontine gliomas highlights platelet-derived growth factor receptor α and poly (ADP-ribose) polymerase as potential therapeutic targets. J Clin Oncol. 2010;28(8):1337–1344. doi: 10.1200/JCO.2009.25.5463. [DOI] [PubMed] [Google Scholar]
- 29.Giacinti C., Giordano A. RB and cell cycle progression. Oncogene. 2006;25(38):5220–5227. doi: 10.1038/sj.onc.1209615. [DOI] [PubMed] [Google Scholar]
- 30.Lange C., Huttner W.B., Calegari F. Cdk4/CyclinD1 overexpression in neural stem cells shortens G1, delays neurogenesis, and promotes the generation and expansion of basal progenitors. Cell Stem Cell. 2009;5(3):320–331. doi: 10.1016/j.stem.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 31.Sun Y., Sun Y., Yan K. Potent anti-tumor efficacy of palbociclib in treatment-naïve H3.3K27M-mutant diffuse intrinsic pontine glioma. EBioMedicine. 2019;43:171–179. doi: 10.1016/j.ebiom.2019.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Funato K, Major T, Lewis PW, Allis CD, Tabar V. Use of human embryonic stem cells to model pediatric gliomas with H3.3K27M histone mutation. Science (80-). 2014;346(6216):1529–33. doi:10.1126/science.1253799 [DOI] [PMC free article] [PubMed]
- 33.Fares J., Fares M.Y., Fares Y. Natural killer cells in the brain tumor microenvironment: defining a new era in neuro-oncology. Surg Neurol Int. 2019;10(43):1–4. doi: 10.25259/SNI-97-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L., Yu H., Xue Y., Liu Y. Decreased natural killer cells in diffuse intrinsic pontine glioma patients. Child's Nerv Syst. 2020:1345–1346. doi: 10.1007/s00381-020-04665-9. [DOI] [PubMed] [Google Scholar]
- 35.Kim Y., Yoo J.Y., Lee T.J. Complex role of NK cells in regulation of oncolytic virus–bortezomib therapy. Proc Natl Acad Sci U S A. 2018;115(19):4927–4932. doi: 10.1073/pnas.1715295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lieberman N.A.P., Degolier K., Kovar H.M. Characterization of the immune microenvironment of diffuse intrinsic pontine glioma: implications for development of immunotherapy. Neuro Oncol. 2019;21(1):83–94. doi: 10.1093/neuonc/noy145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bailey C.P., Figueroa M., Gangadharan A. Pharmacologic inhibition of lysine-specific demethylase 1 as a therapeutic and immune-sensitization strategy in pediatric high-grade glioma. Neuro Oncol. 2020:1–13. doi: 10.1093/neuonc/noaa058. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guillemin G.J., Brew B.J. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J Leukoc Biol. 2004;75(3):388–397. doi: 10.1189/jlb.0303114. [DOI] [PubMed] [Google Scholar]
- 39.Lopez-Atalaya J.P., Askew K.E., Sierra A., Gomez-Nicola D. Development and maintenance of the brain's immune toolkit: microglia and non-parenchymal brain macrophages. Dev Neurobiol. 2018;78(6):561–579. doi: 10.1002/dneu.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ross J., Chen Z., Herting C. Brain; 2020. Platelet-Derived growth factor beta is a potent inflammatory driver in pediatric High- Grade Glioma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gieryng A., Pszczolkowska D., Walentynowicz K.A., Rajan W.D., Kaminska B. Immune microenvironment of gliomas. Lab Investig. 2017;97(5):498–518. doi: 10.1038/labinvest.2017.19. [DOI] [PubMed] [Google Scholar]
- 42.Lin G.L., Nagaraja S., Filbin M.G., Suvà M.L., Vogel H., Monje M. Non-inflammatory tumor microenvironment of diffuse intrinsic pontine glioma. Acta Neuropathol Commun. 2018;6(1):51. doi: 10.1186/s40478-018-0553-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu C., Ward P.S., Kapoor G.S. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483(7390):474–478. doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hambardzumyan D., Parada L.F., Holland E.C., Charest A. Genetic modeling of gliomas in mice: new tools to tackle old problems. Glia. 2011;59(8):1155–1168. doi: 10.1002/glia.21142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hermans E., Hulleman E. Patient-derived orthotopic xenograft models of pediatric brain tumors: in a mature phase or still in its infancy? Front Oncol. 2020;9:1418. doi: 10.3389/fonc.2019.01418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Z.J., Rao L., Bhambhani K. BRIEF REPORT Diffuse intrinsic pontine glioma biopsy: a single institution experience. Pediatr Blood Cancer. 2015;62:163–165. doi: 10.1002/pbc.25224. [DOI] [PubMed] [Google Scholar]
- 47.Roujeau T., Machado G., Garnett M.R. Stereotactic biopsy of diffuse pontine lesions in children. J Neurosurg. 2007;107(1 SUPPL):1–4. doi: 10.3171/PED-07/07/001. [DOI] [PubMed] [Google Scholar]
- 48.Williams J.R., Young C.C., Vitanza N.A. Progress in diffuse intrinsic pontine glioma: advocating for stereotactic biopsy in the standard of care. Neurosurg Focus. 2020;48(1):1–8. doi: 10.3171/2019.9.FOCUS19745. [DOI] [PubMed] [Google Scholar]
- 49.Caretti V., Zondervan I., Meijer D.H. Monitoring of tumor growth and post-irradiation recurrence in a diffuse intrinsic pontine glioma mouse model. Brain Pathol. 2011;21(4):441–451. doi: 10.1111/j.1750-3639.2010.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aoki Y., Hashizume R., Ozawa T. An experimental xenograft mouse model of diffuse pontine glioma designed for therapeutic testing. J Neurooncol. 2012;108(1):29–35. doi: 10.1007/s11060-011-0796-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Truffaux N., Philippe C., Paulsson J. Preclinical evaluation of dasatinib alone and in combination with cabozantinib for the treatment of diffuse intrinsic pontine glioma. Neuro Oncol. 2015;17(7):953–964. doi: 10.1093/neuonc/nou330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mueller S., Hashizume R., Yang X. Targeting wee1 for the treatment of pediatric high-grade gliomas. Neuro Oncol. 2014;16(3):352–360. doi: 10.1093/neuonc/not220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chan K.M., Fang D., Gan H. The histone H3.3K27M mutation in pediatric glioma reprograms H3K27 methylation and gene expression. Genes Dev. 2013;27(9):985–990. doi: 10.1101/gad.217778.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Plessier A., Le Dret L., Varlet P. New in vivo avatars of diffuse intrinsic pontine gliomas (DIPG) from stereotactic biopsies performed at diagnosis. Oncotarget. 2017;8(32):52543–52559. doi: 10.18632/oncotarget.15002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Welby J.P., Kaptzan T., Wohl A. Current murine models and new developments in H3K27M diffuse midline gliomas. Front Oncol. 2019;9(FEB):92. doi: 10.3389/fonc.2019.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thirant C., Bessette B., Varlet P. Clinical relevance of tumor cells with stem-like properties in pediatric brain tumors. PLoS ONE. 2011;6(1):e16375. doi: 10.1371/journal.pone.0016375. Gullberg D, editor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hashizume R., Smirnov I., Liu S. Characterization of a diffuse intrinsic pontine glioma cell line: implications for future investigations and treatment. J Neurooncol. 2012;110(3):305–313. doi: 10.1007/s11060-012-0973-6. [DOI] [PubMed] [Google Scholar]
- 58.Grasso C.S., Debily M.A., Quist M.J. Functionally-defined therapeutic targets in diffuse intrinsic pontine glioma: a report of the children's oncology group DIPG Preclinical consortium HHS public access. Nat Med. 2015;21(6):555–559. doi: 10.1038/nm.3855. Functionally-defined. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Becher O.J., Hambardzumyan D., Walker T.R. Preclinical evaluation of radiation and perifosine in a genetically and histologically accurate model of brainstem glioma. Cancer Res. 2010;70(6):2548–2557. doi: 10.1158/0008-5472.CAN-09-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hambardzumyan D., Amankulor N.M., Helmy K.Y., Becher O.J., Holland E.C. Modeling adult gliomas using RCAS/t-va technology. Transl Oncol. 2009;2(2):89–95. doi: 10.1593/tlo.09100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barton K.L., Misuraca K., Cordero F. PD-0332991, a CDK4/6 inhibitor, significantly prolongs survival in a genetically engineered mouse model of brainstem glioma. PLoS ONE. 2013;8(10):e77639. doi: 10.1371/journal.pone.0077639. Alonso MM, editor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hennika T., Hu G., Olaciregui N.G. Pre-Clinical Study of Panobinostat in Xenograft and Genetically Engineered Murine Diffuse Intrinsic Pontine Glioma Models. Castro MG. PLoS ONE. 2017;12(1) doi: 10.1371/journal.pone.0169485. editor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tosi U, Kommidi H, Adeuyan O, et al. PET, image-guided HDAC inhibition of pediatric diffuse midline glioma improves survival in murine models.; 2020. http://advances.sciencemag.org/. Accessed September 26, 2020. [DOI] [PMC free article] [PubMed]
- 64.Misuraca K.L., Hu G., Barton K.L., Chung A., Becher O.J. A Novel Mouse Model of Diffuse Intrinsic Pontine Glioma Initiated in Pax3-Expressing Cells. Neoplasia. 2016;18(1):60–70. doi: 10.1016/j.neo.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gallitto M., Lazarev S., Wasserman I. Role of radiation therapy in the management of diffuse intrinsic pontine glioma: a systematic review. Adv Radiat Oncol. 2019;4(3):520–531. doi: 10.1016/j.adro.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bradley K.A., Zhou T., McNall-Knapp R.Y. Motexafin-gadolinium and involved field radiation therapy for intrinsic pontine glioma of childhood: a children's oncology group phase 2 study. Int J Radiat Oncol Biol Phys. 2013;85(1):e55. doi: 10.1016/j.ijrobp.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mantravadi R.V.P., Phatak R., Bellur S., Liebner E.J., Haas R. Brain stem gliomas: an autopsy study of 25 cases. Cancer. 1982;49(6):1294–1296. doi: 10.1002/1097-0142(19820315)49:6<1294::AID−CNCR2820490636>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 68.Halperin E.C. Pediatric brain stem tumors: patterns of treatment failure and their implications for radiotherapy. Int J Radiat Oncol Biol Phys. 1985;11(7):1293–1298. doi: 10.1016/0360-3016(85)90244-5. [DOI] [PubMed] [Google Scholar]
- 69.Freeman C.R., Krischer J.P., Sanford R.A. Final results of a study of escalating doses of hyperfractionated radiotherapy in brain stem tumors in children: a pediatric oncology group study. Int J Radiat Oncol Biol Phys. 1993;27(2):197–206. doi: 10.1016/0360-3016(93)90228-N. [DOI] [PubMed] [Google Scholar]
- 70.Rita A, Antunes P, Scheyltjens I, et al. Understanding the glioblastoma immune microenvironment as basis for the development of new immunotherapeutic strategies. doi:10.7554/eLife.52176 [DOI] [PMC free article] [PubMed]
- 71.Tabatabai G., Frank B., Möhle R., Weller M., Wick W. Irradiation and hypoxia promote homing of haematopoietic progenitor cells towards gliomas by TGF-β-dependent HIF-1α-mediated induction of CXCL12. Brain. 2006;129(9):2426–2435. doi: 10.1093/brain/awl173. [DOI] [PubMed] [Google Scholar]
- 72.Kioi M., Harsh G.R., Martin Brown J. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest. 2010;120(3) doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tseng D., Vasquez-Medrano D., Brown J. Targeting SDF-1/CXCR4 to inhibit tumour vasculature for treatment of glioblastomas. Br J Cancer. 2011;104:1805–1809. doi: 10.1038/bjc.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu X., Xiao Q., Bai X. Activation of STAT3 is involved in malignancy mediated by CXCL12-CXCR4 signaling in human breast cancer. Oncol Rep. 2014;32(6):2760–2768. doi: 10.3892/or.2014.3536. [DOI] [PubMed] [Google Scholar]
- 75.Walters M.J., Ebsworth K., Berahovich R.D. Inhibition of CXCR7 extends survival following irradiation of brain tumours in mice and rats. Br J Cancer. 2014;110(5):1179–1188. doi: 10.1038/bjc.2013.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang J.Y., Yan Y.Y., Li J.J., Adhikari R., Fu L.W. PD-1/PD-L1 based combinational cancer therapy: icing on the cake. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.00722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zou W., Wolchok J.D., Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328) doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jalali R. 2015. pp. 63–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mathios D., Kim J.E., Mangraviti A. Anti-PD-1 antitumor immunity is enhanced by local and abrogated by systemic chemotherapy in GBM. Sci Transl Med. 2016;8(370) doi: 10.1126/scitranslmed.aag2942. 370ra180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karachi A., Dastmalchi F., Mitchell D.A., Rahman M. Temozolomide for immunomodulation in the treatment of glioblastoma. Neuro Oncol. 2018;20(12):1566–1572. doi: 10.1093/neuonc/noy072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sampson J.H., Aldape K.D., Archer G.E. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol. 2011;13(3):324–333. doi: 10.1093/neuonc/noq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Batich K.A., Reap E.A., Archer G.E. Long-term survival in glioblastoma with cytomegalovirus pp65-targeted vaccination. Clin Cancer Res. 2017;23(8):1898–1909. doi: 10.1158/1078-0432.CCR-16-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sanchez-Perez L.A., Choi B.D., Archer G.E. Myeloablative temozolomide enhances CD8+ T-cell responses to vaccine and is required for efficacy against brain tumors in mice. PLoS ONE. 2013;8(3) doi: 10.1371/journal.pone.0059082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Osorio F.G., Rosendahl Huber A., Oka R. Somatic mutations reveal lineage relationships and age-related mutagenesis in human hematopoiesis. Cell Rep. 2018;25(9):2308–2316. doi: 10.1016/j.celrep.2018.11.014. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jansen M.H.A., Van Vuurden D.G., Vandertop W.P., Kaspers G.J.L. Antitumour treatment diffuse intrinsic pontine gliomas: a systematic update on clinical trials and biology. CANCER Treat Rev. 2011 doi: 10.1016/j.ctrv.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 86.Cohen K.J., Heideman R.L., Zhou T. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the children's oncology group. Neuro Oncol. 2011;13(4):410–416. doi: 10.1093/neuonc/noq205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chassot A., Canale S., Varlet P. Radiotherapy with concurrent and adjuvant temozolomide in children with newly diagnosed diffuse intrinsic pontine glioma. J Neurooncol. 2012;106(2):399–407. doi: 10.1007/s11060-011-0681-7. [DOI] [PubMed] [Google Scholar]
- 88.El-Khouly F.E., Veldhuijzen van Zanten S.E.M., Santa-Maria Lopez V. Diagnostics and treatment of diffuse intrinsic pontine glioma: where do we stand? J Neurooncol. 2019;145(1):177–184. doi: 10.1007/s11060-019-03287-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.El-Khouly F.E., van Vuurden D.G., Stroink T. Effective drug delivery in diffuse intrinsic pontine glioma: a theoretical model to identify potential candidates. Front Oncol. 2017;7(OCT):254. doi: 10.3389/fonc.2017.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smith H.A., Kang Y. The metastasis-promoting roles of tumor-associated immune cells. J Mol Med. 2013;91(4):411–429. doi: 10.1007/s00109-013-1021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Glass R., Synowitz M. CNS macrophages and peripheral myeloid cells in brain tumors. Acta Neuropathol. 2014;128(3):347–362. doi: 10.1007/s00401-014-1274-2. [DOI] [PubMed] [Google Scholar]
- 92.DeCordova S., Shastri A., Tsolaki A.G. Molecular heterogeneity and immunosuppressive microenvironment in glioblastoma. Front Immunol. 2020;11:1402. doi: 10.3389/fimmu.2020.01402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martinez F.O., Gordon S., Locati M., Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177(10):7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 94.Paugh B.S., Zhu X., Qu C. Novel oncogenic PDGFRA mutations in pediatric high-grade gliomas. Cancer Res. 2013;73(20):6219–6229. doi: 10.1158/0008-5472.CAN-13-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4(7):540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 96.Zhao D., ping Li X, Gao M., Zhao C., liu Wang J, hui Wei L. Stromal cell-derived factor 1α stimulates human endometrial carcinoma cell growth through the activation of both extracellular signal-regulated kinase 1/2 and Akt. Gynecol Oncol. 2006;103(3):932–937. doi: 10.1016/j.ygyno.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 97.Zeelenberg I.S., Ruuls-Van Stalle L., Roos E. The chemokine receptor CXCR4 is required for outgrowth of colon carcinoma micrometastases. Cancer Res. 2003;63(13):3833–3839. [PubMed] [Google Scholar]
- 98.Airoldi I., Raffaghello L., Piovan E. CXCL12 does not attract CXCR4+ human metastatic neuroblastoma cells: clinical implications. Clin Cancer Res. 2006;12(1):77–82. doi: 10.1158/1078-0432.CCR-05-1376. [DOI] [PubMed] [Google Scholar]
- 99.Tsukahara T., Hirohashi Y., Kanaseki T. Peptide vaccination therapy: towards the next generation. Pathol Int. 2016;66(10):547–553. doi: 10.1111/pin.12438. [DOI] [PubMed] [Google Scholar]
- 100.Ochs K., Ott M., Bunse T. K27M-mutant histone-3 as a novel target for glioma immunotherapy. Oncoimmunology. 2017;6(7):1–7. doi: 10.1080/2162402X.2017.1328340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Benitez-Ribas D., Cabezón R., Flórez-Grau G. Immune response generated with the administration of autologous dendritic cells pulsed with an allogenic tumoral cell-lines lysate in patients with newly diagnosed diffuse intrinsic pontine glioma. Front Oncol. 2018;8(APR):1–9. doi: 10.3389/fonc.2018.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vandenberk L., Belmans J., Van Woensel M., Riva M., Van Gool S.W. Exploiting the immunogenic potential of cancer cells for improved dendritic cell vaccines. Front Immunol. 2016;6(JAN):1–15. doi: 10.3389/fimmu.2015.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martínez-Vélez N., Garcia-Moure M., Marigil M. The oncolytic virus delta-24-RGD elicits an antitumor effect in pediatric glioma and DIPG mouse models. Nat Commun. 2019;10(1) doi: 10.1038/s41467-019-10043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Van Gool S.W., Makalowski J., Bonner E.R. Addition of multimodal immunotherapy to combination treatment strategies for children with DIPG: a single institution experience. Medicines. 2020;7(5):29. doi: 10.3390/medicines7050029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wongthida P., Schuelke M.R., Driscoll C.B. Neuro Oncol; 2020. Ad-CD40L mobilizes CD4 t cells for the treatment of brainstem tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Majzner R.G., Theruvath J.L., Nellan A. CAR T cells targeting B7-H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin Cancer Res. 2019;25(8):2560–2574. doi: 10.1158/1078-0432.CCR-18-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mount C.W., Majzner R.G., Sundaresh S. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M+ diffuse midline gliomas letter. Nat Med. 2018;24(5):572–579. doi: 10.1038/s41591-018-0006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kline C., Liu S.J., Duriseti S. Reirradiation and PD-1 inhibition with nivolumab for the treatment of recurrent diffuse intrinsic pontine glioma: a single-institution experience. J Neurooncol. 2018;140(3):629–638. doi: 10.1007/s11060-018-2991-5. [DOI] [PubMed] [Google Scholar]
- 109.Fried I., Lossos A., Ben Ami T. Preliminary results of immune modulating antibody MDV9300 (pidilizumab) treatment in children with diffuse intrinsic pontine glioma. J Neurooncol. 2018;136(1):189–195. doi: 10.1007/s11060-017-2643-1. [DOI] [PubMed] [Google Scholar]
- 110.Ross J.L., Vega J.V., Plant A., MacDonald T.J., Becher O.J., Hambardzumyan D. Tumor immune landscape of pediatric high-grade gliomas.[published online ahead of print, 2021 Apr 15] Brain. 2021:awab155. doi: 10.1093/brain/awab155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lindquist K.A., Satpute A.B., Wager T.D., Weber J., Barrett L.F. The brain basis of positive and negative affect: evidence from a meta-analysis of the human neuroimaging literature. Cereb Cortex. 2016;26(5):1910–1922. doi: 10.1093/cercor/bhv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cordero F.J., Huang Z., Grenier C. Histone H3.3K27M represses p16 to accelerate gliomagenesis in a murine model of DIPG. Mol Cancer Res. 2017;15(9):1243–1254. doi: 10.1158/1541-7786.MCR-16-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pathania M., De Jay N., Maestro N. H3.3K27M cooperates with Trp53 loss and PDGFRA gain in mouse embryonic neural progenitor cells to induce invasive high-grade gliomas. Cancer Cell. 2017;32(5):684–700. doi: 10.1016/j.ccell.2017.09.014. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miklja Z., Yadav V.N., Cartaxo R.T. Everolimus improves the efficacy of dasatinib in PDGFRα-driven glioma. J Clin Invest. 2020;130(10):5313–5325. doi: 10.1172/JCI133310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Patel S.K., Hartley R.M., Wei X. Generation of diffuse intrinsic pontine glioma mouse models by brainstem targeted in utero electroporation. Neuro Oncol. 2019;22(3):381–392. doi: 10.1093/neuonc/noz197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fortin J., Tian R., Zarrabi I. Mutant ACVR1 Arrests glial cell differentiation to drive tumorigenesis in pediatric gliomas. Cancer Cell. 2020;37(3):308–323. doi: 10.1016/j.ccell.2020.02.002. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.