Abstract
Purpose
To evaluate the effect of a one-time dose of insulin or insulin-like growth factor 1 (IGF-1) on cellular proliferation and migration of subacromial bursa tissue (SBT) over time.
Methods
SBT was harvested from over the rotator cuff tendon in 4 consecutive patients undergoing primary arthroscopic rotator cuff repair. SBT was cultured for 3 weeks in complete media until reaching confluence. The culture dishes were stored in a humidified, low oxygen tension (5% CO2) incubator at 37°C. SBT of each patient underwent treatment with a one-time dose of insulin or IGF-1, whereas nontreated SBT served as a negative control. Cellular proliferation and migration were evaluated after 24, 48, 72, and 96 hours of incubation. SBT-derived cells migrated in the detection field were visualized using fluorescent microscopy.
Results
Cellular proliferation at 24, 48, 72, and 96 hours was 1.40 ± 0.27, 1.00 ± 0.20, 1.47 ± 0.31, and 1.68 ± 0.28 for IGF-1; 1.44 ± 0.24, 1.15 ± 0.27, 1.60 ± 0.36, and 1.61 ± 0.32 for insulin; and 1.51 ± 0.35, 1.29 ± 0.33, 1.53 ± 0.35, and 1.57 ± 0.38 for nontreated SBT. Untreated SBT demonstrated a significantly greater proliferation when compared with IGF-1 and insulin within the first 48 hours, although this effect was found to subside by 96 hours. Cellular migration at 24, 48, 72, and 96 hours was 575.7 ± 45.0, 641.6 ± 77.7, 728.3 ± 122.9, and 752.3 ± 114.5 for IGF-1; 528.4 ± 31.3, 592.5 ± 69.8, 664.2 ± 115.2, and 695.6 ± 148.2 for insulin; and 524.4 ± 41.9, 564.4 ± 49.8, 653.2 ± 81.5, and 685.7 ± 115.5 for nontreated SBT. Insulin showed no difference in migration at each timepoint compared to nontreated SBT (P > .05, respectively).
Conclusions
Insulin and IGF-1 initially inhibit cellular proliferation of human SBT, although this effect was found to subside by 96 hours. Further, neither insulin nor IGF-1 changed the slope of cellular migration over time. However, each treatment group demonstrated a significant increase in cellular proliferation and migration.
Clinical Relevance
In the setting of biologic augmentation of rotator cuff repair, the compatibility and synergistic effect of insulin on human SBT is highly limited.
As the high failure rates following rotator cuff repair remain a major concern in shoulder surgery, biologic adjuvants have been proposed in an attempt to support the endogenous healing potential of the repaired tendon as well as reduce retear rates.1, 2, 3, 4 Recent in vitro characterizations of human subacromial bursa tissue (SBT) have shown that these cells fulfill the characteristics of mesenchymal stem cells (MSCs), including similar surface antigen expression profiles and multilineage differentiation, along with superior proliferation potential compared with other tissues within the shoulder, suggesting its use as an easily accessible, inexpensive, and viable augment for arthroscopic rotator cuff repair.5, 6, 7, 8, 9, 10, 11, 12
In the setting of tendon injury, insulin-like growth factor 1 (IGF-1) has come to the forefront, as IGF-1 is active at different stages of the healing process.13,14 During the early inflammatory phase, IGF-1 is highly expressed, exerting an anabolic effect on tendon fibroblast production of collagen.13,14 More importantly, IGF-1 has been shown to enhance the cellular proliferation and migratory potential of fibroblasts.13,15 As this previous work highlights the importance of IGF-1 for sufficient healing of a torn tendon while boosting the ability of involved cells to proliferate and migrate, its effect on and compatibility with SBT is still unknown.
Although IGF-1 has been widely used in basic science research, it is currently not approved for the use in human subjects, as it is an exogenous, engineered growth factor.13 Consequently, the use of insulin, an endogenous hormone approved by the Food and Drug Administration for human use, has been proposed as an attractive alternative due to the similar molecular makeup, having only a difference of 6 amino acids when looking at their respective receptors.13 Further, the application of insulin also may be transferable from translational basic science research to the clinical use in the real-time setting in the operating room. If greater cellular migratory potential and growth is attained with insulin treatment, an improvement in healing might be achieved.
Thus, the purpose of this study was to evaluate the effect of a one-time dose of insulin or IGF-1 on cellular proliferation and migration of SBT over time. The authors hypothesized that a one-time physiologic dose of insulin or IGF-1 would significantly increase the cellular proliferation and migratory potential of SBT over time compared with the negative control and that there would be no significant difference between insulin and IGF-1.
Methods
Patient Selection
This study was approved by the institutional review board before commencement (institutional review board no. 20X-081-1). SBT was collected from 4 consecutive patients (a 63-year-old male, 22-year-old male, 59-year-old female, and 57-year-old male) undergoing primary arthroscopic rotator cuff repair by a single senior surgeon (A.D.M) in May 2019. Patients were eligible for inclusion if they were older than 18 years of age and were indicated for primary arthroscopic rotator cuff repair after not responding to conservative treatment. Patients with a history of diabetes, smoking, or systemic infectious diseases (hepatitis or human immunodeficiency virus) were excluded from the study. Three groups were compared: a control group, an insulin group, and an IGF-1 group.
Harvest and Processing of SBT
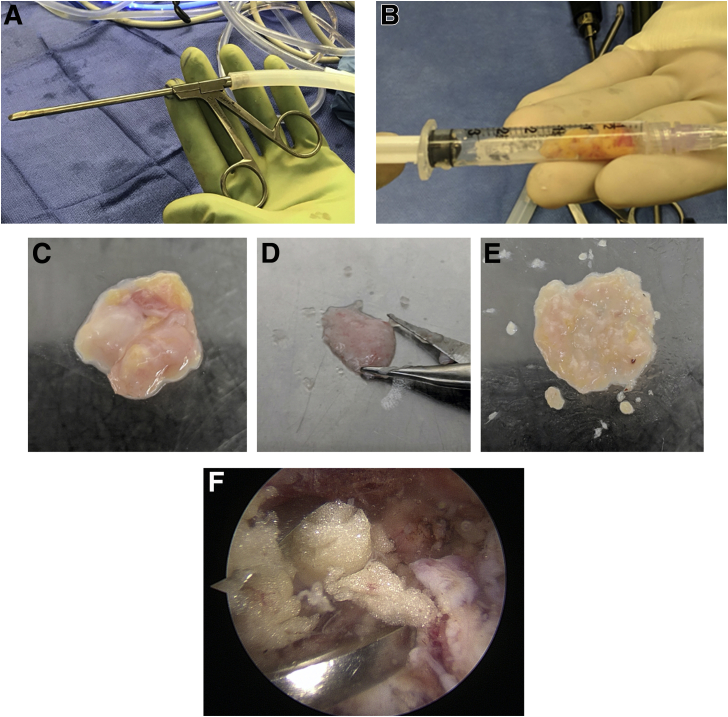
During the patient’s arthroscopic rotator cuff repair, subacromial bursa from directly over the rotator cuff tendon (supraspinatus/infraspinatus) was harvested using an arthroscopic grasper device, (Fig 1A), placed into sterile 3-mL syringes, and measured for reference (Fig 1B).10,11,16, 17, 18 The samples were immediately transported from the operating room to a laminar flow hood for processing. One 200-mg sample of each bursa specimen was carefully weighed for plating. Similar to Baldino et al. and Muench et al., the sample was placed in a culture dish and mechanically digested for 60 seconds using tenotomy scissors (Fig 1 C and D) sterilized in 100% ethanol to maximize cell escape from the surrounding matrix, which has been reported to be feasible in the clinical setting.10,11,16 When the tissue sample resembled a finely minced liquified particulate (Fig 1E), it was resuspended in 10 mL of complete Dulbecco’s Modified Eagle’s Medium (1X; Gibco, Life Technologies Limited, Paisley, United Kingdom), containing 10% fetal bovine serum (Gibco) and 1% penicillin/streptomycin. The culture dishes were stored in a humidified, low oxygen tension (5% CO2) incubator at 37°C. To allow time for the cells to adhere to the culture dish, the media was not replaced during the first week, being followed by replacing it twice a week thereafter until cells reached confluence, which usually occurred after 3 to 4 weeks.9, 10, 11 Figure 1F shows a representative area of where the tendon bursa was routinely collected.
Fig 1.
Subacromial bursa tissue from directly over the rotator cuff tendon (supraspinatus/infraspinatus) was removed using an arthroscopic grasper device (A), placed into sterile 3-mL syringes, and measured for reference (B). A 200-mg sample of each bursa specimen was placed in a culture dish (C) and mechanically digested for 60 seconds using tenotomy scissors (D) until resembling a finely minced liquified particulate (E). A representative area of where the tendon bursa was routinely collected is demonstrated in (F).
Experimental Design
Following harvest and processing of the 4 SBT samples, the culture dishes were stored in a humidified, low oxygen tension (5% CO2) incubator at 37°C until the bursa cells reached confluence. Samples and their credentials were assessed by an experienced research assistant (M.B.M.). To define and ensure correct plating density, confluent bursa cells were then trypsinized with 0.5 mL of 0.01% trypsin–0.04% ethylenediaminetetraacetic acid for 15 minutes at room temperature (25°C) and counted using a Coulter counter (Coulter Electronics, Hialeah, FL). Cellular proliferation and migration assays were performed for each patient (N = 4). Bursa cells were either treated with a one-time dose of insulin or IGF-1, whereas cells without treatment served as a negative control. Proliferation and migration for each treatment group (insulin, IGF-1, negative control) were assessed after 24, 48, 72, and 96 hours of incubation. For each assay, 4 wells per treatment group per time point (total of 4 × 3 × 4 = 48 wells per assay) were used and discarded after the measurements.
Cellular Proliferation Assay
For the proliferation portion of the study, cells were plated at 20,000 cells/cm2 into 48-well Falcon plates for 24 hours to ensure that the cells had attached to the tissue culture plastic. After 24 hours in culture, the cells were treated with a one-time dose of either 10-10 mM insulin from bovine pancreas (Sigma-Aldrich, St. Louis, MO) or 10 nM IGF-1 (R&D Systems, Minneapolis, MN) and were incubated for 24, 48, 72, and 98 hours, respectively.13 Cells without treatment served as a negative control. The ability of the cells to proliferate was determined by an XTT (2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide) assay (Roche Diagnostics, Mannheim, Germany) as described by Morikawa et al.9 Eight hours before each time point, cells were incubated with a combination of the XTT labeling mixture and growth medium. At the end of the incubation period, absorbance was measured using an automated plate reader (BIO Tek, Bad Friedrichshall, Germany) at 450 nm with a reference wavelength of 650 nm.9 This assay is based on the cleavage of the tetrazolium salt XTT to a soluble formazan salt by mitochondrial dehydrogenases only being active in metabolically intact cells. As the increase in formazan formed is measured by the absorbance, this directly correlates to the number of viable cells within the sample.
Two-Dimensional Migration Assay
To determine the bursa cells ability to migrate, a 2-dimensional migration assay was used. After trypsinization and counting, cells were resuspended at 1 × 106 cells/mL in growth medium and 5 μL of Vybrant DiO cell-labeling solution (Thermo Fisher Scientific) per milliliter of cell suspension. The suspension was incubated for 20 minutes at 37°C and then centrifuged for 5 minutes at 1500 RPM and 37°C. The supernatant was removed, and the cells were gently resuspended in the same volume of media. The washing procedure was repeated twice before the cells were finally resuspended at 0.1 × 106 cells/ml. Then, 100-μL samples of suspended cells were pipetted into the wells (10,000 cells/well) of a collagen I–coated cell migration assay plate (Enzo Life Sciences, Inc., Farmingdale, NY). The seeded plate was incubated at 37°C and 5% CO2 for 24 hours to permit cell attachment to the well floor. Cells were then treated for 24 hours with either 10-10 mM insulin or 10 nM IGF-1 in growth medium.13 Again, cells without treatment served as a negative control. At the end of the time, media was aspirated and replaced with growth medium from each well and the well stoppers were removed from the plate, allowing cells to migrate into the detection field. Cells were assayed for migration at 24, 48, 72, and 96 hours.
At the end of each time point, media were carefully aspirated with a multichannel micropipette, and wells were washed with 100 μL of sterile phosphate-buffered saline to remove any unattached cells. Then, 100 μL of α- Minimum Essential Medium was added back to each well. Cell migration for each well was evaluated by measuring the corrected absorbance at a wavelength of 484 nm using an automated plate reader (BIO Tek, Bad Friedrichshall, Germany) with a reference wavelength of 650 nm at 24, 48, 72, and 96 hours of incubation.11 An increase in measured absorbance directly correlates to the number of fluorescent viable cells having migrated into the detection field. The fluorescent cells in the detection field were visualized using a Leica DMI 6000B fluorescent microscope (Leica Microsystems, Buffalo Grove, IL).
Statistical Analysis
Descriptive statistics including mean and standard deviation as well as median and interquartile range were calculated to characterize the groups. Differences in migration and proliferation potential between treatment groups (IGF-1, insulin, no treatment) were assessed using mixed effects linear regression. Models included main effects for group and time as well as group by time interactions to discern whether the amount of change between time-points differed between groups. For migration, time zero values were included in the model as a covariate to account for differences at baseline. Residuals for each model were examined to ensure that large deviations from normality were not present. Pairwise comparisons of marginal mean values were carried out with adjustment for multiple comparisons using the Holm-Bonferroni sequential correction method. An alpha level of <0.05 was set for all comparisons. 95% confidence intervals (CI) were calculated. All statistical analyses were performed using Stata 15.1 (StataCorp 2017 Stata Statistical Software, Release 15; StataCorp LP, College Station, TX).
Results
Cellular Proliferation
Untreated SBT demonstrated a significantly greater cellular proliferation when compared with SBT treated with either IGF-1 or insulin at 24 (P = .003 and P = .01) and 48 (P < .001 and P = .001) hours of incubation (Tables 1 and 2). However, treatment with IGF-1 (difference: 0.268; 95% confidence interval [CI] 0.185-0.351; P < .001) or insulin (difference: 0.160; 95% CI 0.075-0.244; P < .001) resulted in a significant increase in cellular proliferation of SBT over time when comparing the measurements after 24 hours and 96 hours of incubation (Fig 2). For SBT without treatment, there was only a nonsignificant increase in proliferation within this time interval (difference: 0.047; 95% CI –0.039 to 0.133; P = .858). In addition, only treatment with IGF-1 (difference: 0.169; 95% CI 0.084-0.253; P < .001) was able to significantly increase cellular proliferation between 72 hours and 96 hours of incubation when compared with the insulin (difference: –0.013; 95% CI –0.097 to 0.070; P = .754) and no-treatment group (difference: 0.042; 95% CI –0.042 to 0.127; P = .858). Further, there was a significant decrease in proliferation between 24 and 48 hours for each of the 3 groups (P < .001, respectively).
Table 1.
Cellular Proliferation Potential Over Time
| Cellular Proliferation Potential Over Time (Absorbance), h |
||||
|---|---|---|---|---|
| 24 | 48 | 72 | 96 | |
| No treatment, | ||||
| Mean ± SD | 1.51 ± 0.35 | 1.29 ± 0.33 | 1.53 ± 0.35 | 1.57 ± 0.38 |
| Median | 1.28 | 1.19 | 1.33 | 1.55 |
| IQR | 0.59 | 0.65 | 0.69 | 0.60 |
| IGF-1 | ||||
| Mean ± SD | 1.40 ± 0.27 | 1.00 ± 0.20 | 1.47 ± 0.31 | 1.68 ± 0.28 |
| Median | 1.38 | 1.05 | 1.35 | 1.67 |
| IQR | 0.49 | 0.26 | 0.59 | 0.58 |
| Insulin | ||||
| Mean ± SD | 1.44 ± 0.24 | 1.15 ± 0.27 | 1.60 ± 0.36 | 1.61 ± 0.32 |
| Median | 1.61 | 1.07 | 1.59 | 1.55 |
| IQR | 0.46 | 0.54 | 0.72 | 0.60 |
IGF-1, insulin-like growth factor 1; IQR, interquartile range; SD, standard deviation.
Table 2.
Statistical Comparison of Cellular Proliferation Between Groups at Each Time Point
| Time Point, h | Comparison | P Value |
|---|---|---|
| 24 | No treatment vs IGF-1 | .003∗ |
| No treatment vs insulin | .010∗ | |
| IGF-1 vs insulin | .553 | |
| 48 | No treatment vs IGF-1 | <.001∗ |
| No treatment vs insulin | .001∗ | |
| IGF-1 vs insulin | <.001∗ | |
| 72 | No treatment vs IGF-1 | .426 |
| No treatment vs insulin | .426 | |
| IGF-1 vs insulin | .060 | |
| 96 | No treatment vs IGF-1 | .184 |
| No treatment vs insulin | .808 | |
| IGF-1 vs insulin | .162 |
IGF-1, insulin-like growth factor 1.
Indicates statistical significance.
Fig 2.
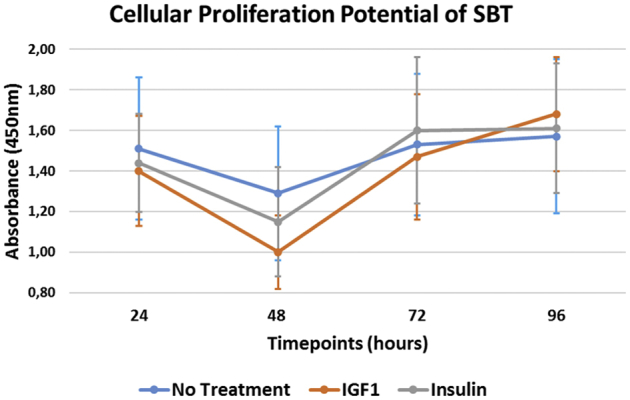
The cellular proliferation potential of SBT over time. At the end of each incubation period, absorbance was measured using an automated plate reader at a wavelength of 450 nm. This assay is based on the cleavage of the tetrazolium salt XTT to a soluble formazan salt by mitochondrial dehydrogenases, only being active in metabolically intact cells. As the increase in formazan formed is measured by the absorbance, this directly correlates to the number of viable cells within the sample. (IGF-1, insulin-like growth factor 1; SBT, subacromial bursa tissue.)
When we looked at the changes between time points, proliferation significantly decreased between 24 and 48 hours in each of the groups (P < .001, respectively). There was a significantly greater increase in proliferation within the time interval from 48 to 72 hours of incubation for SBT treated with IGF-1 (95% CI –0.372 to –0.134; P < .001) or insulin (95% CI –0.305 to –0.068; P = .004) when compared with the no-treatment group. No difference was found between either treatment group (95% CI –0.185 to 0.051; P = .267). However, there was no significant difference in increase of proliferation between 72 hours and 96 hours of incubation, when comparing SBT treated with IGF-1 (95% CI: –0.246 to –0.007; P = .076) or insulin (95% CI –0.063 to 0.174; P = .358) to the control group.
Cellular Migration
SBT treated with IGF-1 (difference: 176.3; 95% CI 142.3-210.4; P < .001) or insulin (difference: 167.3; 95% CI 132.6-201.9; P < .001) as well as those without treatment (difference: 163.0; 95% CI 128.1-197.9; P < .001) demonstrated a significant increase in cellular migration over time when we compared the measurements after 24 hours and 96 hours of incubation (Fig 3). However, there was no significant increase in cellular migration in any of the groups, when we compared the measurements after 72 hours of incubation (P > .05, respectively). In addition, there was no significant increase in migration observed in the control group (difference: 39.8; 95% CI 4.6-75.0; P > .05) between 24 hours and 48 hours of incubation, whereas the treatment with IGF-1 (difference: 64.1; 95% CI 30.4-97.9; P < .001) or insulin (difference: 64.2; 95% CI 30.7-97.6; P < .001) resulted in a significant increase in cellular migration during the early phase. Descriptives are demonstrated in Table 3 along with statistical comparisons in Table 4.
Fig 3.
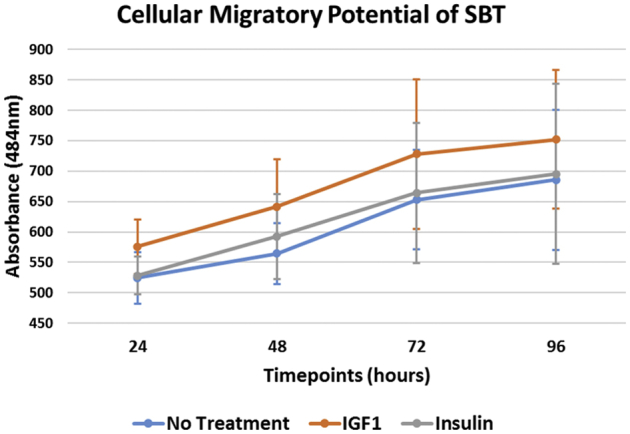
The cellular migratory potential of SBT over time. At each time point, cell migration for each well was evaluated by measuring the absorbance at a wavelength of 484 nm using an automated plate reader. An increase in measured absorbance directly correlates to the number of fluorescent viable cells having migrated into the detection field. (IGF-1, insulin-like growth factor 1; SBT, subacromial bursa tissue.)
Table 3.
Cellular. Migratory Potential Over Time
| Cellular Migratory Potential Over Time (Absorbance), h |
||||
|---|---|---|---|---|
| 24 | 48 | 72 | 96 | |
| No treatment | ||||
| Mean ± SD | 524.4 ± 41.9 | 564.4 ± 49.8 | 653.2 ± 81.5 | 685.7 ± 115.5 |
| Median | 524.0 | 576.5 | 668.0 | 660.0 |
| IQR | 57.0 | 71.5 | 113.0 | 195.0 |
| IGF-1 | ||||
| Mean ± SD | 575.7 ± 45.0 | 641.6 ± 77.7 | 728.3 ± 122.9 | 752.3 ± 114.5 |
| Median | 569.5 | 637.0 | 685.5 | 715.5 |
| IQR | 54.5 | 49.0 | 122.0 | 141.0 |
| Insulin | ||||
| Mean ± SD | 528.4 ± 31.3 | 592.5 ± 69.8 | 664.2 ± 115.2 | 695.6 ± 148.2 |
| Median | 524.0 | 563.5 | 624.0 | 643.0 |
| IQR | 44.0 | 74.0 | 194.0 | 249.0 |
IGF-1, insulin-like growth factor 1; IQR, interquartile range; SD, standard deviation.
Table 4.
Statistical Comparison of Cellular Migration Between Groups at Each Time Point
| Time Point, h | Comparison | P Value |
|---|---|---|
| 24 | No treatment vs IGF-1 | .126 |
| No treatment vs insulin | .683 | |
| IGF-1 vs insulin | .198 | |
| 48 | No treatment vs IGF-1 | .015∗ |
| No treatment vs insulin | .244 | |
| IGF-1 vs Insulin | .244 | |
| 72 | No treatment vs IGF-1 | .009∗ |
| No treatment vs insulin | .494 | |
| IGF-1 vs insulin | .040∗ | |
| 96 | No treatment vs IGF-1 | .033∗ |
| No treatment vs insulin | .643 | |
| IGF-1 vs insulin | .076 |
IGF-1, insulin-like growth factor 1; IQR, interquartile range; SD, standard deviation.
Indicates statistical significance.
There was a significantly greater migration for SBT treated with IGF-1 at 48 hours (P = .015), 72 hours (P = .009), and 96 hours (P = .033) of incubation when compared with SBT without treatment. In addition, the application of IGF-1 was found to result in a significantly greater migration index compared with insulin at 72 hours of incubation (P = .040); however, there was no significant difference at 24 hours (P = .198), 48 hours (P = .244), and 96 hours (P = .076). Insulin treatment further showed no significant difference in cellular migration at each timepoint when compared to SBT without treatment (P > .05, respectively).
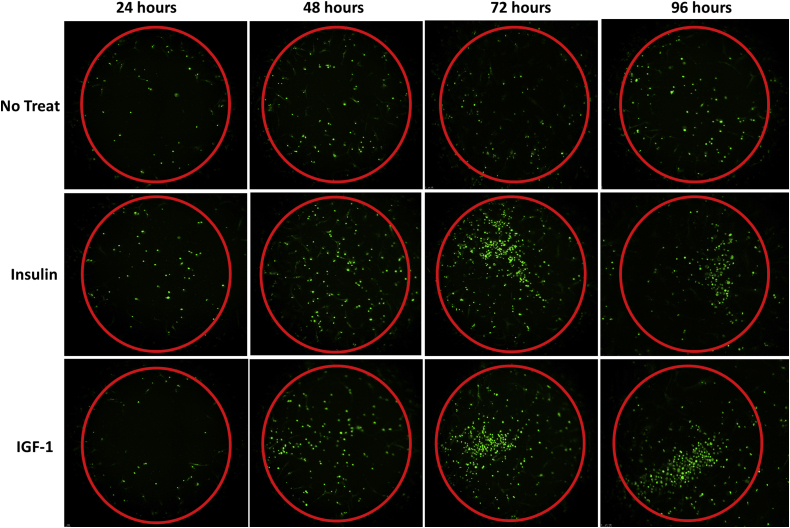
When we looked at the increase in cellular migration between time points, there was no significant difference during any of the time intervals (24 hours to 48 hours, 48 hours to 72 hours, and 72 hours to 96 hours) when comparing the three study groups (P > .05, respectively). Fluorescence microscopy pictures demonstrate the migration of fluorescent SBT cells into the detection field over time (Fig 4).
Fig 4.
Fluorescence microscopy pictures demonstrating the migration of fluorescent SBT cells into the detection field of each well (red circle) over time. (IGF-1, insulin-like growth factor 1; SBT, subacromial bursa tissue.)
Discussion
The most important finding of this study was that that IGF-1 and insulin were actually deleterious to cellular proliferation of human SBT within the first 48 hours of incubation when compared with nontreated SBT, although this effect was found to subside by 96 hours. Further, insulin showed no significant difference in cellular migration at each timepoint when compared to nontreated SBT, whereas treatment with IGF-1 resulted in a significantly greater cellular migration at 48, 72, and 96 hours. However, all groups demonstrated a significant increase in cellular proliferation and migration over time.
Previous studies have already demonstrated that the cellular proliferation potential of SBT appears to be superior not only compared with other soft tissues within the shoulder,8 but also when compared to concentrated bone marrow aspirate obtained from the proximal humerus.10 Morikawa et al.9 demonstrated significantly increased differentiation ability and gene expression of subacromial cells over time compared with standard augmentation of bone marrow aspirate. Further, cellular proliferation of SBT has been shown to be dependent on the anatomic location, with bursa harvested from over the tendon demonstrating a significantly greater proliferation potential than bursa obtained from over the muscle.11 This may be particularly important in the treatment of massive rotator cuff tears with concomitant high-grade tissue degeneration, which have shown healing rates as low as 47% to 50% of cases.19,20
In the setting of tendon injury, IGF-1 has garnered recent attention, with in vitro studies showing its stimulating effect on the proliferation and migration of fibroblasts, suggesting its importance for biologic healing.13,15 However, IGF-1 is currently not approved for use in human subjects, as it is an exogenous, engineered growth factor.13 Consequently, the use of insulin, an endogenous hormone approved for human use by the Food and Drug Administration, has been proposed as an attractive alternative due to its similar biochemical composition, which may also be practical for the use in the real-time setting of the operating room.13 Thus, the already convincing cellular characteristics of SBT as a contributor to tendon healing may be further enhanced by applying a one-time dose of IGF-1 or insulin.
Cells contributing to tendon healing have been found to originate from loose connective tissue surrounding the tendon fascicles, especially the paratenon.21, 22, 23 As the rotator cuff tendon does not seem to be enclosed by a typical paratenon, the surrounding bursal tissue may emulate this function and may be one of the main contributors to endogenous tendon healing.11 This train of thought may be supported by previously published literature reporting that spontaneous healing of the rotator cuff has most noticeably been observed along the subacromial bursal wall, with the cells infiltrating the defects being continuous with the epitenon of the bursa and with minimal contribution from the healing enthesis.22,24,25 Further, Uhthoff et al. stated that the extension of subacromial bursa should rather be considered a reparative response more so than a degenerative change.15 The authors go on to recommend against radical debridement of the bursa during surgery, as it may remove a primary source of neovascularizing signals and fibroblastic cells being necessary for biological repair of the torn tendon.25 These in vitro studies indicate that maximization of cellular proliferation and migratory potential of SBT is critical to provide a sufficient amount of potent cells at the tear site. As healing at the enthesis involves the recruitment or migration, proliferation, and differentiation of cells at the repair site, these are important features to study.26,27
As multiple in vitro studies have indicated that maximization of cellular proliferation and migratory potential of SBT may be critical to provide a sufficient number of potent cells at the tear site,5,7,8,22,24 the application of the endogenous hormones may be an easy, inexpensive, and practicable option to further enhance the biologic activity of SBT. However, the present study demonstrated that insulin and IGF-1 initially inhibit cellular proliferation, although this effect was found to subside by 96 hours. Further, neither insulin nor IGF-1 changed the slope of the cellular migration line over 96 hours when compared with nontreated SBT. Clinically, it is uncertain what impact a small incremental increase in cellular migration over 96 hours might have on rotator cuff tendon healing, especially since tendon healing takes weeks, but an initial slow-down in cellular proliferation after insulin exposure may argue against its use.
In contrast, Mazzocca et al.13 showed that a one-time dose of insulin resulted in a significant increase in gene expression of tendon-specific markers, content of tendon-specific proteins, and receptors on the cell surface in MSCs. Thus, the application of physiologic insulin, when used as a biologic augment, has been suggested to hold various benefits for improved rotator cuff healing. This includes increasing the potential of differentiating MSCs into tendon, as well as stimulating fibroblasts and SBT to proliferate at and migrate to the tear site.13, 14, 15 However, based on the results of the present study, the compatibility and synergistic effect of insulin on SBT in the setting of biologic augmentation of rotator cuff repair appears to be highly limited.
Limitations
There were several limitations to the study. With this being an in vitro study, the findings may not reflect the cellular proliferation and migratory potential of SBT in an in vivo shoulder environment and conclusions regarding the impact of SBT-derived cells on rotator cuff tendon healing cannot be drawn. In addition, only SBT samples from 4 patients were included in the study. Further, patient characteristics, including age, sex, level of physical activity as well as rotator cuff tear characteristics, may have influenced the results. Lastly, the SBT had already been cultured for 3 weeks before being used for the experiment. Thus, this study may not account for differences in cellular characteristics immediately after harvesting the bursa sample.
Conclusions
Insulin and IGF-1 initially inhibit cellular proliferation of human SBT, although this effect was found to subside by 96 hours. Further, neither insulin nor IGF-1 changed the slope of cellular migration over time. However, each treatment group demonstrated a significant increase in cellular proliferation and migration.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: A.D.M. receives consulting fees as well as research support from Arthrex. The company had no involvement in the study design, data collection, or final manuscript. M.P.C. receives personal fees from the Arthroscopy Association of North America (AANA) for the Arthroscopy Journal, outside the submitted work. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Investigation performed at the Department of Orthopaedic Surgery, University of Connecticut, Farmington, Connecticut, U.S.A.
Supplementary Data
References
- 1.Imam M.A., Holton J., Horriat S., et al. A systematic review of the concept and clinical applications of bone marrow aspirate concentrate in tendon pathology. SICOT J. 2017;3:58. doi: 10.1051/sicotj/2017039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zumstein M.A., Ladermann A., Raniga S., Schar M.O. The biology of rotator cuff healing. Orthop Traumatol Surg Res. 2017;103:S1–S10. doi: 10.1016/j.otsr.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Carr J.B., 2nd, Rodeo S.A. The role of biologic agents in the management of common shoulder pathologies: Current state and future directions. J Shoulder Elbow Surg. 2019;28:2041–2052. doi: 10.1016/j.jse.2019.07.025. [DOI] [PubMed] [Google Scholar]
- 4.Carballo C., Lebaschi A., Rodeo S.A. Cell-based approaches for augmentation of tendon repair. Tech Shoulder Elbow Surg. 2017;18:e6–e14. doi: 10.1097/BTE.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song N., Armstrong A.D., Li F., Ouyang H., Niyibizi C. Multipotent mesenchymal stem cells from human subacromial bursa: Potential for cell based tendon tissue engineering. Tissue Eng Part A. 2014;20:239–249. doi: 10.1089/ten.tea.2013.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinert A.F., Kunz M., Prager P., et al. Characterization of bursa subacromialis-derived mesenchymal stem cells. Stem Cell Res Ther. 2015;6:114. doi: 10.1186/s13287-015-0104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dyrna F., Zakko P., Pauzenberger L., McCarthy M.B., Mazzocca A.D., Dyment N.A. Human subacromial bursal cells display superior engraftment versus bone marrow stromal cells in murine tendon repair. Am J Sports Med. 2018;46:3511–3520. doi: 10.1177/0363546518802842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Utsunomiya H., Uchida S., Sekiya I., Sakai A., Moridera K., Nakamura T. Isolation and characterization of human mesenchymal stem cells derived from shoulder tissues involved in rotator cuff tears. Am J Sports Med. 2013;41:657–668. doi: 10.1177/0363546512473269. [DOI] [PubMed] [Google Scholar]
- 9.Morikawa D., Johnson J.D., Kia C., et al. Examining the potency of subacromial bursal cells as a potential augmentation for rotator cuff healing: an in vitro study. Arthroscopy. 2019;35:2978–2988. doi: 10.1016/j.arthro.2019.05.024. [DOI] [PubMed] [Google Scholar]
- 10.Morikawa D., Muench L.N., Baldino J.B., et al. Comparison of preparation techniques for isolating subacromial bursa-derived cells as a potential augment for rotator cuff repair. Arthroscopy. 2020;36:80–85. doi: 10.1016/j.arthro.2019.07.024. [DOI] [PubMed] [Google Scholar]
- 11.Baldino J.B., Muench L.N., Kia C., et al. Intraoperative and in vitro classification of subacromial bursal tissue. Arthroscopy. 2020;36:2057–2068. doi: 10.1016/j.arthro.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 12.Muench L.N., Berthold D.P., Kia C., et al. Concentrated bone marrow aspirate and subacromial bursa-derived cells demonstrate similar cellular adhesion and proliferation potential on demineralized bone matrix scaffolds for biologic augmentation of rotator cuff repair. Muscle Ligaments Tendons J. 2020;10 [Google Scholar]
- 13.Mazzocca A.D., McCarthy M.B., Chowaniec D., et al. Bone marrow-derived mesenchymal stem cells obtained during arthroscopic rotator cuff repair surgery show potential for tendon cell differentiation after treatment with insulin. Arthroscopy. 2011;27:1459–1471. doi: 10.1016/j.arthro.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 14.Molloy T., Wang Y., Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003;33:381–394. doi: 10.2165/00007256-200333050-00004. [DOI] [PubMed] [Google Scholar]
- 15.Abrahamsson S.O. Similar effects of recombinant human insulin-like growth factor-I and II on cellular activities in flexor tendons of young rabbits: Experimental studies in vitro. J Orthop Res. 1997;15:256–262. doi: 10.1002/jor.1100150215. [DOI] [PubMed] [Google Scholar]
- 16.Muench L.N., Baldino J.B., Berthold D.P., et al. Subacromial bursa-derived cells demonstrate high proliferation potential regardless of patient demographics and rotator cuff tear characteristics. Arthroscopy. 2020;36:2794–2802. doi: 10.1016/j.arthro.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Muench L.N., Kia C., Berthold D.P., et al. Preliminary clinical outcomes following biologic augmentation of arthroscopic rotator cuff repair using subacromial bursa, concentrated bone marrow aspirate, and platelet-rich plasma. Arthrosc Sports Med Rehabil. 2020;2:E803–E813. doi: 10.1016/j.asmr.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landry A., Levy B.J., McCarthy M.B., et al. Analysis of time to form colony units for connective tissue progenitor cells (stem cells) harvested from concentrated bone marrow aspirate and subacromial bursa tissue in patients undergoing rotator cuff repair. Arthrosc Sports Med Rehabil. 2020;2:e629–e636. doi: 10.1016/j.asmr.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nho S.J., Delos D., Yadav H., et al. Biomechanical and biologic augmentation for the treatment of massive rotator cuff tears. Am J Sports Med. 2010;38:619–629. doi: 10.1177/0363546509343199. [DOI] [PubMed] [Google Scholar]
- 20.Ecklund K.J., Lee T.Q., Tibone J., Gupta R. Rotator cuff tear arthropathy. J Am Acad Orthop Surg. 2007;15:340–349. doi: 10.5435/00124635-200706000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Dyment N.A., Hagiwara Y., Matthews B.G., Li Y., Kalajzic I., Rowe D.W. Lineage tracing of resident tendon progenitor cells during growth and natural healing. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshida R., Alaee F., Dyrna F., et al. Murine supraspinatus tendon injury model to identify the cellular origins of rotator cuff healing. Connect Tissue Res. 2016;57:507–515. doi: 10.1080/03008207.2016.1189910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dyment N.A., Galloway J.L. Regenerative biology of tendon: mechanisms for renewal and repair. Curr Mol Biol Rep. 2015;1:124–131. doi: 10.1007/s40610-015-0021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirose K., Kondo S., Choi H.R., Mishima S., Iwata H., Ishiguro N. Spontaneous healing process of a supraspinatus tendon tear in rabbits. Arch Orthop Trauma Surg. 2004;124:374–377. doi: 10.1007/s00402-004-0663-8. [DOI] [PubMed] [Google Scholar]
- 25.Uhthoff H., Sarkar K. Surgical repair of rotator cuff ruptures—the importance of the subacromial bursa. J Bone Joint Surg Br. 1991;73:399–401. doi: 10.1302/0301-620X.73B3.1670436. [DOI] [PubMed] [Google Scholar]
- 26.Gulotta L.V., Rodeo S.A. Growth factors for rotator cuff repair. Clin Sports Med. 2009;28:13–23. doi: 10.1016/j.csm.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Angeline M.E., Rodeo S.A. Biologics in the management of rotator cuff surgery. Clin Sports Med. 2012;31:645–663. doi: 10.1016/j.csm.2012.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.