Abstract
The development of cancer stems from genetic instability and changes in genomic sequences, and hence the heterogeneity exhibited by tumors is integral to the nature of cancer itself. Tumor heterogeneity can be further altered by factors that are not cancer cell intrinsic, i.e. by the microenvironment, including the patient’s immune responses to tumors and administered therapies (immunotherapies, chemotherapies, and/or radiation therapies). The focus of this review is the impact of tumor heterogeneity on the interactions between immune cells and the tumor, taking into account that heterogeneity can exist at several levels. These levels include heterogeneity within an individual tumor, within an individual patient (particularly between the primary tumor and metastatic lesions), among the subtypes of a specific type of cancer, or within cancers that originate from different tissues. Because of the potential for immunity (either the natural immune system, or via immunotherapeutics) to halt the progression of cancer, major clinical significance exists in understanding the impact of tumor heterogeneity on the associations between immune cells and tumor cells. Increased knowledge of why, whether, and how immune-tumor interactions occur provides the means to guide these interactions and improve outcomes for patients.
Keywords: cancer, heterogeneity, immune, interactions, tumor
1. Introduction
Tumor heterogeneity is derived partially from the instability of the cancer cell genome and from the selection that cancers undergo in their development, and can be modified by selection processes that result from immunological attacks on tumor cells (Fig. 1). Furthermore, tumor heterogeneity goes beyond the genetic changes within the tumor cells to transcriptional signs of altered proliferation or apoptosis in these cells [1]. Such non-genetic differences can potentially be due to cancer cell responses to microenvironmental factors in tumors, such as changes in cell density or angiogenesis. Notably, multiplexed investigation has revealed that tumor evolution in the process of metastasis is also influenced by the metastatic site’s immunological context, leading to immune-evasive tumors irrespective of lymphocyte infiltration [2]. Such immune evasion is enabled by features that are extrinsic to tumor cells, as well as ones that are intrinsic to them [2]. Taking into account the perspectives of tumor heterogeneity as encompassing genetic changes in tumor cells, non-genetic alterations in tumor cell phenotypes, and the variability of immune cell infiltration into tumors, many advancements in comprehension of tumor/immune interactions have been made which will be crucial in furthering the development of cancer prognosis and treatment. In recent years, vital strides have been made in understanding the consequences of tumor heterogeneity on the interactions between immune system components and tumors, as described in this review.
Fig. 1.
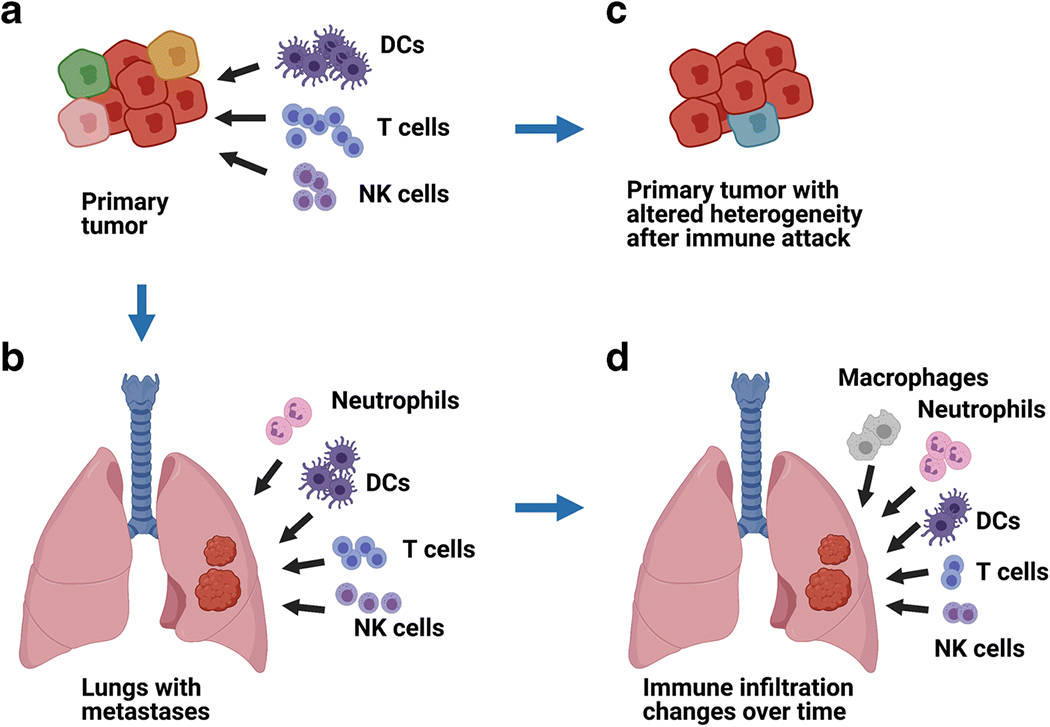
Immune cells (including T cells, DCs, NKs and others) can defend against primary tumors (A) or metastatic lesions (B). Immune cell infiltrates can differ in composition in primary tumors versus metastases, in terms of numbers, types, activation levels, and/or specificities (A, B). In some instances, immune cells can potentially modify tumor heterogeneity by selective targeting (C). Immune cell infiltration can be variable in location within tumors, between tumors, and/or can change in quantity or composition within the same tumors over time (D).
2. Strategies to assess the impact of tumor heterogeneity on immune-tumor interactions
Investigation of the effects of tumor heterogeneity on tumor/immune associations requires defining the parameters for quantification: which immune cells will be counted, and in what sections of which tumors (e.g., primary and/or metastases)? Each tumor can potentially exhibit an internal distribution of immune cells that is heterogeneous in location. The distribution of immune cells can also be heterogeneous in comparisons between patient-matched primary tumor and metastasis, or among various metastases derived from the same patient. Immune infiltration, both within tumors and among tumors, was analyzed by Obeid et al. [3] in a study assessing CD8+ T cell presence in melanoma tissue arrays. These investigators reported great variance in the numbers of CD8+ T cells found in separate core samples derived from the same tumors. Although CD8+ T cell numbers were similar in tumors that had arisen simultaneously, they varied drastically among tumors that had developed at varied points in time. These findings have implications that are methodologically valuable for the study of immune/tumor interactions (in terms of tumor sample size and sources of samples taken). They also underscore the need for acquiring tumor biopsies of sufficient size in clinical trials that are designed to assess the success of novel immunotherapies.
In terms of approach, exploration of the influences of tumor heterogeneity on tumor/immune associations also takes into account spatial and longitudinal analysis of immune cells within the local tumor microenvironment. Immune cells are not necessarily confined to a singular compartment within a tumor, but rather can occupy a range of potential locations in the tumor’s structure [4,5]. Immune cells may be located deep within the tumor core or at the leading edge, or they may be restricted to the peritumoral border. Characteristically, the central portion (as in addition to areas in the periphery) of tumors may have necrotic foci (often caseous), which can still exhibit infiltration with myeloid cells. Regarding peritumoral borders, tumors (especially those which are benign) are typically encapsulated by a fibrous tissue sheath, or at minimum (for malignant tumors) exhibit a rim of compressed, capsular-like connective tissue. Thus, there are perceptible boundaries of tumors, at which immune cells can sometimes be detected (e.g., by immunohistochemistry) as barred from entry. Furthermore, it should be noted that a specific intratumoral location is not necessarily a requirement for tumor cell influence on immune cells, since tumor cells can influence immune cells either by direct contact (including receptor-mediated interaction) or via secreted factors, such as cytokines or metabolic products that can alter the local pH [4]. Longitudinal differences in immune cell presence that are observed over the duration of cancer development and/or therapy can be informative about the kinetics of immune responses and/or the effectiveness of treatments [5,6].
Progress in comprehending tumor/immune cell interaction is due, in large part, to innovative advances in sequencing approaches [7]. In addition, new strategies for data analysis have provided tools for handling complex sequencing data for tumors and types of infiltrating immune cells. Widespread usage by investigators of the Cell Type Identification by Estimating Relative Subsets of Known RNA Transcripts (CIBERSORT) computational approach has contributed to progress in the investigations of tumor/immune interaction. CIBERSORT allows estimation of the amounts of specific RNA transcripts, and by those approximations, it provides results on the relative numbers of various types of cells in a source of mixed cells, such as a tumor [8]. A striking example of the utility of CIBERSORT was the analysis of patterns of expression from ~18,000 separate patient-derived tumors, representing 39 types of cancer [9]. Across a wide spectrum of cancers, connections were drawn between specific intratumoral immune cell populations and the duration of patient survival, noting either a positive correlation (especially with high numbers of gamma-delta T cells) or a negative one (having increased numbers of neutrophils was strongly linked to adverse outcomes). The findings in this report provided a clearer overall picture of tumor/immune cell association, as well as fresh impetus for investigation of the intratumoral roles of the cell types found in the largest quantities in these cancers.
Another recently generated resource for approaching research questions related to tumor heterogeneity and immune/tumor interaction is a database termed The Cancer Immunome Atlas [10]. This database includes immune cell infiltration and tumor antigen data, as well as tumor clonality/heterogeneity results, for more than 8,000 tissue samples representing 20 cancers listed in The Cancer Genome Atlas. By the application of machine learning, Charoentong et al. generated ratings that they referred to as immunophenoscores [10]. The immunophenoscore adds to the utility of the previously established immunoscore for disease prognosis (developed by Mlecnik et al. [11]), in that the immunophenoscore has also been demonstrated to serve as a forecaster of therapeutic responsiveness to immune checkpoint inhibitor treatment.
3. Murine models provide evidence for role of tumor heterogeneity in immune-tumor interactions
Animal models have been indispensable in the analysis of the relationship between heterogeneity in tumors and immune cell associations with tumors, since such models allow detailed examination of changes in tumor composition over time and at various in vivo locations to an extent that is not feasible in human patients. Early reports by Fidler and colleagues provided an initial understanding of selective pressures involved in tumor evolution, and the influence of that selection on metastasis [12,13]. From their investigation of mouse melanoma models, Price et al. described metastasis as dependent on a subset of tumor cells having a set of distinctive features that allow their persistence during spread to distant sites [12]. This view was also supported by work from the same research group that characterized metastasis in mouse melanoma models as “sequential and selective and contains stochastic elements” [13].
Non-genetic heterogeneity of tumors has also been shown by variations in the growth, maintenance, and responsiveness to chemotherapy of human xenografted colorectal tumor samples that lacked mutational changes after multiple serial passages in mice [14]. The use of immunodeficient mice xenografted with limiting dilutions of cancer cell populations containing putative stem cells has also enabled the development of the concept of cancer stemness [15]. Evidence has been presented in a murine breast cancer model that cancer stem cells themselves are heterogeneous populations [16]. Characteristics of cancer stemness are linked to intratumoral heterogeneity (in terms of tumor clone diversity) and also to deficiency in intratumoral immune cells [17,18].
Analysis of mouse models has also revealed that a set of immune response-related genes for which expression can vary in tumor cells is that encoding the immune messengers called chemokines. A chemokine was found to have a pivotal role in an investigation of intratumoral immune cell infiltration conducted with congenic tumor cell clones derived from KPCY mice backcrossed to C57BL/6 [19]. The KPC strain is a mouse model that mimics the progression of human pancreatic cancer, due to pancreas-specific expression of K-ras and p53 mutations; for KPCY mice, the “Y” represents yellow fluorescent protein [20,21]. The implantation of individual clones resulted in tumors that either had T cell-rich microenvironment or T cell-poor microenvironment; thus, the heterogeneity of these clones was maintained following transplantation. The expression of the chemokines CXCL15, CCL20, and CXCL1 had differential expression in T cell-rich versus T cell-poor tumors. Furthermore, CXCL1 was identified as having a high tumor cell-intrinsic expression level. Better accessibility, as found for the CXCL1 gene’s promoter in T cell-poor versus T cell-high tumors, is consistent with an epigenetic mechanism of control in these pancreatic cancer cells. Transcriptional regulation of the gene for CXCL1 by MYC was also evident in this model. The CXCL1 chemokine acts as a ligand for the receptor CXCR2, which is involved in the infiltration of myeloid cells into pancreatic tumors [22,23]. These data, together with the investigators’ discovery that CXCL1 knockout increased T cell infiltration, suggests that CXCL1 attraction of myeloid-lineage cells could be a major factor in shaping the intratumoral T cell population.
Heterogeneous production of cytokines by tumors can also regulate immune/tumor interactions, as demonstrated by murine models. In addition to originating in various types of immune cells, several cytokines (e.g., IL-15) can be produced by tumor cells [24]. The supposition that tumor cell-secreted IL-15 has profound effects has been supported by studies in mouse models showing that IL-15 can contribute to tumor eradication by T cells [24] and by NK cells [25]. Conversely, variation in cytokine production by immune cells can also influence heterogeneity of the tumor cells. As an example, the cytokine IFN-gamma has also been shown to influence tumor heterogeneity in mouse tumor models [26]. An investigation by Takeda et al. of IFN-gamma’s functions in intratumoral diversity utilized murine tumor models of mammary cancer and lymphoma (each expressing an exogenous antigen) and of fibrosarcoma (expressing a known endogenous tumor antigen) [26]. Only if IFN-gamma was present in the tumor microenvironment did mouse CD8+ T cell activity lead to the development of cancer clones that were resistant due to antigen loss. Furthermore, CD8+ T cells secreting IFN-gamma exerted genetic effects on tumor cells, such as changes in gene copy numbers and expression of DNA repair-related genes. These data suggest that T cells may, in some instances, alter diversity in tumor cells and induce immune resistance in the tumors cells by IFN-gamma.
By employing a mouse melanoma cell line, Wolf et al. [27] examined the effects of mutational burden and intratumoral heterogeneity on immune responses. In these experiments, the murine melanoma cell line B2905 was irradiated with UVB to augment the mutational burden, and then the irradiated B2905 cells (or non-irradiated B2905 cells, as a control) were implanted into mice. Compared to the non-irradiated cells, the irradiated cells generated tumors with a faster growth rate. The tumors were then subsequently treated with anti-PD-1, and the mice that received the UVB-irradiated B2905 cells had less of a therapeutic response to the immune checkpoint inhibitor than those mice that received the non-irradiated cells. However, when the mice were each injected with a single cell-derived UVB-irradiated B2905 clone, the tumors had a reduced rate of growth. Through examination of the growth rates of single cell clones in immunocompetent versus immunodeficient mice, it was discerned that the single cell clones grew at a relatively slower pace because they were undergoing immune rejection. The non-heterogeneous nature of the single cell clone-initiated tumors resulted in more T cell infiltration and higher levels of T cell reactivity. These discoveries indicate that tumor heterogeneity detracts from anti-tumor immune responsiveness.
Results published on an investigation of B cell lymphoma support the supposition that immunity depresses tumor heterogeneity [28]. The employed model used was lymphoma derived from Eμ-myc transgenic mice; these mice consistently generate B cell lymphomas early in life. Lymphoma cells were taken from an Eμ-myc mouse and fluorescently tagged to create populations with distinct colors, and mixtures of the tagged cells were made that had matched growth rates. The labeled mixtures were then administered to a series of new mouse hosts. By intravital two-photon imaging, lymphomas were observed to be present as spatially separated areas in the bone marrow. Areas that had increased T cell infiltration had a lesser number of lymphoma cells, perhaps due to cytolysis. Whole-exome sequencing of these subclones revealed that those that had intratumoral evidence of more active immunity also had more limited lymphoma cell heterogeneity. Furthermore, anti-PD-1 therapy also acted to depress the genetic diversity of the lymphoma cells.
In mouse model studies, connections have been drawn between tumor characteristics (e.g., tumor burden and stage), growth factor secretion, and both myeloid cell expansion and T cell depletion. For example, Donkor et al. [29] reported a positive correlation between tumor burden and an increased number of spleen and tumor myeloid-derived suppressor cells (MDSCs), as well as a diminishment in T lymphocyte frequency at the same sites. Cyclophosphamide chemotherapy treatment of mice, with subsequent adoptive transfer of tumor-immune cells, led to abrogation of subcutaneous tumors [30], likely due to the pre-transplant lymphodepletion and increased IL-7 production caused by cyclophosphamide allowing effective expansion of the transplanted T cells [30].
Mathematical modeling based on the autochthonous mouse prostate cancer TRAMP model has been generated by Robertson-Tessi et al. to take into account changes in metabolism that occur with tumorigenesis, the interactions of metabolites with the tumor microenvironment, and the influence of chemotherapy on tumor metabolic stratification and growth patterns [31]. Thus, investigations in the field have moved from using mouse models in the very first steps in understanding the nature of multiple influences (including immunological pressures) on metastasis, to the use of data obtained from a well established mouse tumor model (the TRAMP model) to create simulations to extrapolate multiple events and interactions in the cancer microenvironment. From this point forward in this review, we will turn our attention from mouse models to human patient-based studies.
4. The impact of intratumoral heterogeneity on immune cell interactions with the tumor
4.1. Molecular phenotypes of tumors influence immune infiltration and immunotherapy response
Among individual tumor cells present within each tumor mass, there can be variation in the expression of immune response-related genes, such as ligands for immune checkpoint proteins. Programmed cell death protein-ligand 1 (PD-L1) can be expressed on tumor cells and bind to the immune checkpoint programmed cell death protein-1 (PD-1) on the surface of T cells, thereby inhibiting effector T cell functions. Several immune checkpoint inhibitors are now FDA approved, and the range of cancers for which their use is permitted is steadily increasing. Due to the potential for immunotherapies as viable treatments for cancer, numerous studies have been conducted to monitor both the expression of PD-L1 in tumors and potential pathways of resistance to anti-PD-1 exhibited by tumors.
As an example of the latter type of investigation, Hugo et a. delineated an expression pattern in melanoma patients that was characteristic of resistance to anti-PD-1 treatment [32]. They found that responsiveness to anti-PD-1 checkpoint inhibitor treatment did not show positive correlation with an overall elevation in the number of tumor mutations, but they observed some association with increased mutation of BRCA2. Since BRCA2 has a role in DNA repair, this may indicate linkage to a distinctive set of mutations or simply increased cellular stress conducive to cell death [32]. Using the same bioinformatics strategy for gene expression analysis that was utilized in the aforementioned melanoma study, comprehensive gene expression data from three to four separate regions per tumor obtained from a total of ten non-small cell lung cancer tumors was evaluated to determine the enrichment scores for anti-PD-1 resistance signatures [33]. In the separate samples from discrete regions of the same tumor, there was some consistency in the anti-PD-1 resistance co-enrichment score, but four out of ten patients had intra-tumoral variation in the extent to which an anti-PD-1 resistance signature was exhibited.
To further address the question of what tumor cell phenotypes influence responsiveness to immunotherapy, whole-exome sequencing was performed on anti-PD-1 resistant melanoma metastases [34]. Samples from four patients were evaluated, and two of the patients had mutations resulting in loss of function of the Janus kinase 1 or 2 proteins (JAK1/2) along with loss of the corresponding wild-type JAK1/2. These mutations caused impairment of IFN-gamma signaling, and therefore of IFN-gamma-mediated arrest of proliferation. A third patient among the four had a homozygous loss of function mutation (due to truncation) in the gene that encodes beta 2-microglobulin (β2m). Because the β2m subunit is required for proper folding and expression of the major histocompatibility complex (MHC) class I molecule that presents tumor antigens to T cells to trigger lysis of cancer cells, the localization of MHC class I molecules to the plasma membrane was deficient in the anti-PD-1 therapy-resistant cancer cells derived from this patient. The fourth patient in the group had no evident tumor cell genetic defect that could be deemed responsible, yet had no up-regulated PD-L1 expression. Thus, molecular phenotypes affecting anti-PD-1 resistance were identified, but were heterogeneous among the patients, with 50% having resistant tumors bearing JAK1/2 mutations, 25% having the mutation in the β2m gene, and 25% having a lack of PD-L1 expression but no evident genetic basis.
In addition to the presence of JAK1/2 mutations in melanoma, such a mutation was also identified in one of sixteen patients who had mismatch repair-deficient colon cancer and who had been unresponsive to anti-PD-1 treatment [35]. This observation is consistent with the notion that JAK1/2 mutation can lead to anti-PD-1 resistance in a subset of patients, but that this phenotype is heterogeneous among the population of patients treated with immune checkpoint inhibitors. Corresponding to the aforementioned results from investigation of anti-PD-1-resistant melanoma patient-derived samples [34], a recent case study of a patient with mismatch repair-deficient colorectal cancer that was anti-PD-1-resistant also described a β2m mutation in one allele and loss of the wild-type β2m allele [36]. Such findings suggest that loss of intratumoral β2m could be a recurring challenge for some patients in the clinical implementation of immune checkpoint inhibitor therapy.
Another recent report described the impact of heterogeneous MHC class I expression by tumor cells on the localization of T cells in Stage III metastatic melanoma tissue samples [37]. In this study, the researchers used a multiplexed tissue imaging platform that allows repeated rounds of antibody staining and imaging on each individual sample to assemble detailed information about relative locations. High expression of MHC class I molecules on the tumor cells was discovered to be necessary, yet not sufficient, for elevated levels of T cells to be present. In addition to tumor cell MHC class I molecule expression influencing the overall number of T cells within the tumor, the presence of Treg cells and B cells was also a factor. High MHC class I expression was associated with longer overall survival, but only in cases with concurrent elevated numbers of intratumoral T cells. These data on positive correlations of MHC class I expression with the intratumoral presence of T cells are consistent with other investigations of melanoma tumor samples [34,38,39].
Cancer stemness molecular features are associated with the degree of tumor immune infiltration. Analyzing gene expression data from 8,290 primary tumors from twenty-one different solid tumor types, Miranda et al. [40] described a negative correlation across cancer types between tumor stemness and survival and also between tumor stemness and infiltration of CD8+ T cells, natural killer (NK) cells, and B cells. Notably, other immune cell types had heterogeneous association with stemness; these cell types included neutrophils, CD4+ T cells, and T regulatory cells (Tregs). An immune cell pattern representing the presence of NK cells, CD8+ T cells, and B cells was observed to have a negative correlation with cancer-testis antigens [40], possibly indicating that cells expressing these antigens had been successfully diminished by these immune components. In this study, cancer stemness was also found to correlate negatively with an IFN-α/β signaling signature while corresponding positively with expression of certain genes associated with immunosuppression (CD276/B7-H3, CD155/PVR, and TGFB1). In total, these findings are consistent with stemness paralleling increased intratumoral heterogeneity and deleterious effects on immunity.
4.2. Tumor heterogeneity’s impact on neutrophils and MDSCs
Single cell RNA sequencing of tissue samples from human colorectal liver metastases was performed to assess tumor microenvironment heterogeneity [41]. The frequencies of T helper cells, CD8+ cytotoxic T cells, and memory T cells were reduced in the tumor samples, relative to the normal tissue samples, whereas Treg and CD4+ T cell frequencies were increased, and neutrophil marker expression was also augmented. A molecular change in colorectal cancer cells that is associated with neutrophil infiltration is SMAD4 loss [42–44]. Deficiency in SMAD4 corresponds with an influx of CCR1+ myeloid cells. The predominant CCR1+ cell type infiltrating primary colorectal tumors was found to be MDSCs, and the CCR1+ cells infiltrating metastatic colorectal cancer samples principally had neutrophil markers [43–44]. Factors affecting the presence of MDSCs were evaluated by Diaz-Montero [45]; in samples from solid tumor patients, these cells were found to be elevated in parallel with the tumor burden and stage, and to impair T cell proliferation. Additional studies, focused on breast cancer and pancreatic cancer, showed an increase in MDSCs with worsening cancer stage [46–47].
4.3. Tumor heterogeneity can be altered by immune-tumor interactions
Two scenarios exist for the cause-and-effect relationships of tumor heterogeneity and immune/tumor interactions (Fig. 2). The extent of heterogeneity for a tumor can potentially dictate immune responses, via stimulating reactivity to tumor neo-antigens and thereby causing increased immune infiltration if heterogeneity is elevated. On the other hand, the interactions and influence of intratumoral immune cells can possibly influence the degree to which tumors are heterogeneous by deleting tumor cells expressing certain antigens. Work by McDonald et al. [48] provides backing for the latter, i.e., that immunity guides heterogeneity. On breast cancer patient data from The Cancer Genome Atlas, these researchers used a calculation called mutant allele tumor heterogeneity (MATH) to determine the likely heterogeneity of the tumors, and CIBERSORT was used as the tool to assess the immunological components in the tumors. The observations from this research were that more genetically heterogeneous tumors (those with a higher MATH score) had greatly reduced CD8+ T cells (and markers of cytotoxicity, such as granzyme A) and CD4+ T cells, an increased number of Treg cells, and fewer markers of T cell exhaustion. Therefore, the more heterogeneous tumors had impaired immune/tumor interactions and reduced immune reactivity. In this case, increased mutational diversity was not facilitating the immune response targeting the tumor, and relatively weak anti-tumor responses had failed to limit tumor heterogeneity.
Fig. 2.
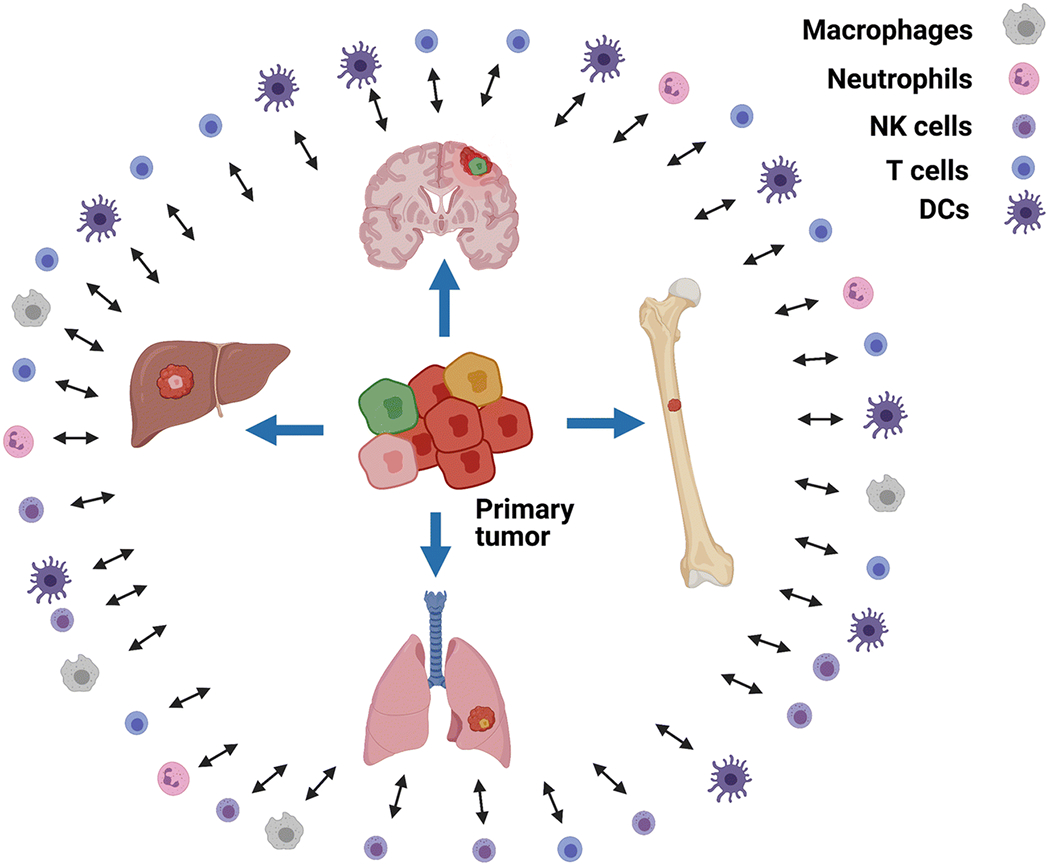
Scenarios for relationships of tumor heterogeneity and immune/tumor interactions. Heterogeneous primary tumors give rise to metastases with expression patterns that are potentially overlapping but distinct from the primary tumor. Tumor heterogeneity can induce immune cell proliferation, activation, and differentiation, along with intratumoral immune infiltration and tumor immunoediting. Cytokines and growth factors in the peripheral blood, due to secretions by immune cells in lymph node and spleen, can result in modifications of tumor growth and heterogeneity.
For high-grade serous ovarian cancer, support has also been found for immune/tumor interactions constraining tumor heterogeneity [49]. In patient tumor samples, three types of immune-infiltrating cell patterns were identified: N-TIL (few tumor-infiltrating lymphocytes), S-TIL (tumor-infiltrating lymphocytes in the stroma), and ES-TIL (having lymphocytes present in both stroma and epithelium, and more lymphocytes closely proximal to tumor cells). Among these types, the ES-TIL immunologic pattern had a negative correlation with tumor heterogeneity. In addition, certain genomic abnormalities were discovered as being linked to immune cell infiltration, indicating that restrictions on tumor development were potentially imposed by immune reactivity.
Lung squamous cell carcinoma was discovered to have extensive non-genetically induced heterogeneity, in addition to genetically mediated heterogeneity [1]. Within local areas, and in some cases even within subclones of lung squamous cell carcinomas, alterations were identified in expression relevant to cancer signaling pathways [1]. By examination of immune cell heterogeneity, the frequency of tumor neo-antigens was found inversely correlated with the extent of immune cell infiltration. This is consistent with destruction of tumor cells expressing neo-antigens that had been recognized by the local lymphocytes and with immune escape by those tumor cells with a lesser degree of non-genetic, as well as genetic, diversity.
In clinical assessments of outcomes, IFN-gamma receptor signaling defects have been demonstrated in anti-PD-1 therapy-resistant tumor cells from some patients [34,35,50]. The effects of IFN-gamma expression on tumor heterogeneity are complicated, as illustrated in a study by Williams et al. [51]. In an in vitro T cell assay, expression of the genes encoding IFN-gamma receptor and JAK1 (which is involved in IFN-gamma receptor signaling) contributed to susceptibility of melanoma cell line lysis. Despite this, when the same IFN-gamma receptor-knockout and JAK1-knockout cells that had been evaluated in vitro were grown in mice, they were more sensitive (rather than less susceptible) to CD8+ T cell attack, and this in vivo phenomenon was linked to lower PD-L1 expression by the tumor cells (as shown by RNA-Seq).
4.4. Neo-antigens are heterogeneous among tumor cells and influence T cell recognition
In an analysis of 45 discrete regions in 11 tumors classified as lung adenocarcinoma, neo-antigen intratumoral heterogeneity had a positive correlation with T cell receptor intratumoral heterogeneity [52]. This relationship may indicate that neo-antigen presence might dictate the composition of the local intratumoral T cell receptor repertoire. Greater intratumoral heterogeneity of T cell receptors corresponded to more likelihood of relapse and a briefer duration of survival post-surgery. Therefore, these data suggest that although more diverse neo-antigens may attract a wider range of T cells, if the T cells do not successfully eradicate the tumor then patient survival will not be prolonged.
Lung adenocarcinoma patient tumors and tumor-derived cell lines were monitored by RNA-Seq to gain information about the intratumoral heterogeneity of immune-response gene expression in these samples [53]. Genes integral to the IFN-gamma pathway were diversely expressed. Notably, decreased expression of these genes positively correlated with resistance to vandetanib, a small molecule drug with inhibitory activity against the constitutively active RET tyrosine kinases generated by gene fusions in some lung adenocarcinomas. The expression of neo-antigens was also heterogeneous, and the presence of fewer neo-antigens also corresponded to vandetanib resistance. MHC class II gene expression was up-regulated in a heterogeneous manner in these samples. According to these investigators’ analysis of data from the The Cancer Genome Atlas, increased expression of MHC class II, more than MHC class I, is a positive prognostic marker, suggesting possible presentation of the heterogeneous neo-antigens on MHC class II molecules expressed on these tumors to T cells.
Probing the intratumoral heterogeneity of antigens in primary liver cancer samples from patients indicated that tumor neo-epitopes were the predominant antigens leading to lymphocyte infiltration, rather than cancer-testis antigens or viral (hepatitis B) antigens [54]. Distribution of somatic mutations (leading to neo-antigen production) across tumor areas was heterogeneous, as was the distribution of CD8+ T cells, CD4+ T cells, macrophages, and memory B cells. Comparisons of the neo-antigen pool with tumor-infiltrating lymphocyte numbers gave indications of pruning of tumor cells to constrain immunogenic diversity in certain areas. Upregulated expression of genes encoding immune checkpoint proteins corresponded to tumor-infiltrating lymphocyte quantity, which suggests a tumor counter-response to the lymphocytes’ attack.
By analyzing the heterogeneity and antigenicity of colorectal cancer antigens, as well as the immune reactivity induced by these antigens, Angelova et al. demonstrated that the infiltrating immune cell populations were linked with specific molecular phenotypes of tumor cells [55]. Many cancer-germline antigens were present in tumors from all 598 patients whose cancers were included in this study, but the neo-antigens that were identified in the tumors were typically unique to specific patients. Tumors with signs of having undergone hypermutation had increased levels of immunoinhibitory proteins (e.g., CTLA-4) and only limited numbers of infiltrating immunosuppressive immune cells (such as MDSCs and Tregs). Microsatellite instable high (MSI-H) tumors, which were all hypermutated, contained elevated numbers of lymphocytes indicative of an adaptive immune response. In contrast, tumors that were not hypermutated tended to have lower expression of MHC class I molecules and immunoinhibitory proteins, in addition to infiltration by immunosuppressive cells.
4.5. Treatment of cancer can correspond with changes in intratumoral immune cell interactions
The treatment of cancer, by any type of modality, can potentially lead to the evolution of tumor heterogeneity by selection mechanisms. Hanin et al. developed a detailed computational model for prediction of the natural history of cancer metastases in individual patients [56]. Various studies have explored whether, and in what manner, this evolution is subsequently followed by corresponding changes in immune/tumor cell interactions within the tumor. Since chemotherapy can be toxic to lymphocytes, in addition to cancer cells, the balance of tumor diminishment versus maintenance of the patient’s immunocompetence has been explored. In silico modeling has been developed by Park et al. for the purpose of attaining equilibrium in tumor attack and immunity protection [57]. The optimal dosing (balancing therapeutic effectiveness and lack of harm to immunity) that was the goal of this modeling was termed by these authors as the “Goldilocks Window,” indicating the point at which the personalized level of therapy would be neither too little nor too much, and thus most favorable for each individual patient.
Jiménez-Sánchez et al. reported their evaluation of a specific patient suffering from high-grade serous ovarian cancer who had received several types of chemotherapeutic drugs [58]. The patient experienced growth of some metastases but regression of others; those that regressed were demonstrated to contain T cell infiltrates. Several years after tumor resection, the patient’s peripheral blood had T cells capable of recognition of presumptive ovarian cancer neo-antigens. Taken together, these data present the coupled effects of chemotherapy and heterogeneous immunological responsiveness on patient outcome. In a later study, Jiménez-Sánchez et al. undertook additional investigation of high-grade serous ovarian cancer patient samples, with immunogenomic assessment prior to, and following, administration of chemotherapeutic agents [59]. They showed both “hot” (i.e., inflammatory) and “cold” (immune cell-depleted) tumor microenvironments were present even within the same regions of tumors in patients that had not yet undergone chemotherapy; the presence of “cold” areas was linked to Wnt signaling. After neoadjuvant chemotherapy, NK cell infiltration and T cell expansion increased. Overall, this study portrays chemotherapy as an activator of immunity in intratumoral locales, with the potential to cause the entry of anti-tumor lymphocytes into ovarian cancer areas deficient in immune cells.
In the treatment of human hematological malignancies, lymphodepletion can be utilized prior to transfer of donor cells, in order to facilitate the proliferation of the adoptively transferred cells. Analysis of the impact on cytokine levels following myeloma patient melphalan depletion of lymphocytes revealed elevation in circulating IL-6, IL-7, and IL-15 [60]. Likewise, fludarabine pre-treatment prolonged the survival of cytotoxic T lymphocytes transplanted into melanoma patients, and the conditioning regimen increased blood levels of IL-7 and IL15 [61].
5. Primary tumors versus metastasis heterogeneity: impact on immune/tumor interactions
Several studies have investigated the effects of heterogeneity between primary tumors and metastases on the expression of immune checkpoint proteins, which are capable of influencing the extent of immune cell interactions in tumors. Matched primary tumor and metastases from lung cancer patients were immunostained for PD-L1 and PD-L2, and heterogeneous PD-L1 ligand expression was found for primary versus metastatic non-small cell lung cancer sites in instances where the primary tumor expressed PD-L1 [62]. However, this was not found to be true when the primary tumor lacked PD-L1 expression; typically there was also an absence of PD-L1 in the paired metastasis. However, for small cell lung cancer, PD-L1 was not expressed either in primary or in metastatic lesions. Thus, there was substantial heterogeneity in PD-L1 distribution noted in non-small cell lung cancer, whereas this was not the case for small cell lung cancer. In contrast, PD-L2 showed comparable heterogeneity for both the small cell lung cancer and the non-small cell lung cancer tissue samples. Immune infiltration was not directly assessed in this study, and no correlation was discovered between PD ligand expression and the stage of disease or the number of metastatic lesions for either the small cell lung cancer cases or the non-small cell lung cancer cases.
Evaluation of patient-matched primary and metastasis samples of squamous cell carcinoma of the lung also revealed frequent heterogeneity in PD ligand expression between the two sets of samples [63]. Although the expression of both PD-L1 and PD-L2 was retained in a majority of the metastases from the same patients, in some cases expression was lost (~18.9% for PD-L1 and 6.5% for PD-L2) or gained (in 10.8% of the samples for PD-L1 and 31.2% for PD-L2). Furthermore, in their investigation of squamous cell carcinoma of the lung, the samples that were PD-L1-high had a strongly positive correlation with greater numbers of infiltrating CD8+ T cells, but a similar positive correlation was not found for PD-L2-high samples [63]. Therefore, the heterogeneity exhibited by primary tumor versus metastasis influenced the resultant degree of CD8+ T cell interactions with the squamous cell carcinoma tumors.
In order to understand the variability of PD-L1 and T cell presence in primary tumors versus metastases, both primary lung cancer samples and patient-matched brain metastases were tested for PD-L1 and for the CD3 T cell marker by the use of immunostaining [64]. Since PD-L1 can be expressed on T cells and tumor cells, both cell types were evaluated for PD-L1 expression. Out of 176 tumor pairs (from 73 patients), PD-L1 expression was discordant on tumor cells in 14% of the pairs and on the tumor-infiltrating lymphocytes in 26% of the pairs. Disparities were most frequent in cases for which the samples were resected with a time difference of at least six months. A substantial fraction of the brain metastases did not express PD-L1 and/or did not have lymphocytes present. These results indicate the existence of heterogeneity in many of the paired samples, implying that identification of immune checkpoint protein expression in the primary lung tumor may not necessarily indicate similar expression of the corresponding protein in the metastases. In addition, because of the necessity for tumor cell expression of MHC class I molecules for successful tumor cell lysis by cytotoxic T lymphocytes, paired primary lung tumors and brain metastases were evaluated for MHC class I expression by immunostaining [65]. In a group of 51 patients, 24 brain metastases and 27 primary tumors expressed detectable MHC class I molecules, with discordance in pairs found with samples for 11 patients. This degree of MHC class I expression difference in primary tumors versus metastases should be considered in cases in which immunotherapies are found to be poorly effective.
To determine whether there is heterogeneity in the expression of PD-L1 in the primary tumors versus synchronous axillary lymph node tumors in patients with triple negative breast cancer, immunostaining for PD-L1 was employed [66]. Evidence was obtained of considerable heterogeneity in PD-L1-staining spatial localization both for immune cells and for tumor cells in the primary tumors and lymph node metastases. Notably, PD-L1 expression was increased on both the tumor cells and the lymphocytes in the lymph node metastases, relative to the primary tumors. Correspondence was observed between PD-L1 expression and the quantity of lymphocytes infiltrating the tumor stroma. The expression of PD-L1 at either the primary tumor site or the lymph node metastasis site was a negative prognostic indicator. Overall, these findings suggest that the presence of PD-L1 expression by a lymph node metastasis may be a valid biomarker for implementation of immune checkpoint inhibitor therapy for patients with triple-negative breast cancer.
Although T cell infiltration of primary ovarian tumors is known to be a favorable sign, less is understood about lymphocyte infiltration of ovarian tumor metastases. Dötzer, et al. undertook an immunohistochemical assessment of 38 metastases to the omentum and 49 patient-matched primary tumors, finding heterogeneity in the comparisons of patient-matched samples for a variety of immune cell types [67]. In the stromal areas, staining of omental metastases had more extensive immune infiltration, relative to the primary ovarian tumors; this was shown by staining for markers of total leukocytes, T cells, cytotoxic T cells, as well as for PD-1. Notably, increased infiltration of leukocytes and T cells into omentum metastasis stroma positively correlated with the presence of metastasis in the lymph node. Furthermore, for ovarian cancers that were responsive to platinum-based therapy, there was a positive correspondence between increased CD8+ T cell presence in metastases to the peritoneum, whereas increased PD-1-expressing cells in the stroma of peritoneal metastases were linked to poorer platinum-based drug response. Thus, the positive correlations between diversity in immune cell presence and chemotherapy sensitivity may provide improved guidance for ovarian cancer treatment.
Closely related to the issue of disparity between primary versus metastasis lesions is the effect of stage on tumor heterogeneity and immune/tumor cell interactions. The influence of tumor stage on infiltration by many types of immune cells was assessed by review of patient immunohistochemistry and outcome data of a period spanning eight years [68]. Overall, longer duration of survival was positively correlated with markers of T cells (cytotoxic, T helper 1, and T-gamma/delta), mast cells, and macrophages, whereas poorer survival correlated with infiltration by eosinophils, T helper 2 cells, T central memory cells, T helper-17 cells, Tregs, and NK cells. With increasing stage, most T cells were present at diminishing levels. B cell numbers rose at later stages of tumor progression, and the presence of B cell and T follicular helper (Tfh) markers correlated with longer survival. In addition, these researchers utilized mouse orthotopic colon cancer models with targeted gene knockouts to confirm the importance of B and T cells (including Tfh cells) to immune protection against colon cancer progression and to prolongation of survival.
6. The impact of heterogeneity in cancer subtypes and types on immune/tumor interactions
Variations in cancer subtypes can distinctly influence immune cell interactions. By a novel virtual biopsy approach, variability in immune infiltration was investigated in breast cancer subtypes [69]. To produce virtual biopsies, images of three whole-tumor hematoxylin and eosin-stained sections were combined with automated image analysis to generate representations of 998 human primary breast tumors. For the human epidermal growth factor receptor 2-positive (Her2+) subtype of breast cancer, the virtual biopsies tended to reveal more variability in lymphocyte infiltrate, as compared to other breast cancer subtypes.
Several transcriptomic classifications of pancreatic cancer subtypes have been generated [70–72]. New pancreatic ductal adenocarcinoma liver metastases subtypes have been recently delineated by proteomics analysis, which were classified as metabolic, progenitor-like, proliferative, and inflammatory [73]. The inflammatory subtype contains increased numbers of proteins relevant to the adaptive immune response, complementary activation, and IL-8, which has been established as an immunosuppressive factor in the tumor microenvironment [74]. Thus, pancreatic cancer subtypes are heterogeneous in regard to immunologically relevant cells, activated immune-related pathways, and cytokine expression.
The molecular subtypes of glioblastoma (known as classical, neural, mesenchymal, and proneural) exhibit heterogeneity in immune cell infiltration [75–77]. Engler et al. reported that elevated expression of immune response-related genes was characteristic of the mesenchymal subtype of adult glioblastoma and of pediatric high-grade gliomas [75]. Furthermore, these investigators discovered an increase in expression of microglia/macrophage-related genes in mesenchymal-subtype glioblastoma tumor samples from both adult and pediatric patients. By immunostaining of glioblastoma tumor samples from adults, the presence of higher numbers of microglia/macrophages in the mesenchymal subtype was confirmed. Elevated expression of microglia/macrophage-related genes in glioblastoma tumors from adults (though not from pediatric patients) was observed to be a negative prognostic factor. In related findings, Doucette et al. noted that the glioblastoma mesenchymal subtype had increased gene expression characteristic of immunosuppressive responses, innate immune cells, and cells involved in the adaptive immune response [76]. Wang et al. also found correlation between a microglia/macrophage signature and a mesenchymal subtype [77]. In addition, they determined that abrogation of NF1 expression (as is often characteristic of mesenchymal glioblastoma) was linked to chemotaxis of microglia/macrophages, although the mechanism underlying the attraction is still unknown.
Heterogeneity in immune checkpoint protein expression was examined across cancer types [78]. Notable correspondences were found for various cancers between duration of survival and immune checkpoint protein expression, and also between patterns of tumor immune infiltrate and expression of immune checkpoint proteins. For breast cancer patients, the expression of CTLA-4, LAG-3, PD-1, and TIM-3 was associated with intratumoral infiltration by CD8+ and CD4+ T lymphocytes, B lymphocytes, dendritic cells, and neutrophils. Additionally, the expression of each of these immune checkpoint markers was associated with longer patient survival. PD-1 expression positively correlated with the presence of all immune cell types examined in lung cancer and breast cancer, and it had weaker positive correspondence with most immune cell types in the ovarian cancer cases. Patient survival was improved in the ovarian cancer cases in which CTLA-4 and PD-1 intratumoral expression was increased. Immune checkpoint protein expression was not universally a positive factor; for example, TIM-3 expression correlated with worse survival for lung cancer patients. Altogether, this examination of immune checkpoint protein heterogeneity yielded guidance for several cancers as to which immune responses and interactions correspond to the expression of particular immune checkpoint protein markers and what prognosis may be expected for patients with these diseases.
In comparisons of immune cell infiltration across heterogeneous cancer types, tumors that had progressed to more advanced stages were found to have fewer cytotoxic immune cells, indicating that the cytotoxic cells were likely restricting the advancement of the cancers [79]. For this study, the set of genes chosen to indicate the presence of cytotoxic cells was focused on those highly expressed in CD8+ T cells, NK cells, and gamma-delta T cells. For several of the cancers examined, patient tumors having greater heterogeneity in tumor genomic status had positive correspondence with diminished cytotoxic cell presence, suggesting that immune-mediated pruning of tumor cell diversity had reduced tumor clonal variation. Intratumoral cytotoxic immunophenotypes were associated with increased expression of MHC class I and II genes, proinflammatory chemokines and cytokines, and cancer germline antigens. Prolonged patient survival positively correlated with increased presence of cytotoxic immune cells, except in the case of low-grade gliomas. For low-grade gliomas, patient survival was shorter; conceivably, larger gliomas may disrupt the blood-brain barrier and allow for further entry of these immune cells. This broad view of a spectrum of cancers reveals major, concurrent changes in cytotoxic cell populations, tumor expression profiles, and patient outcomes.
7. Conclusions and future directions
As described above, new approaches in analyzing the relationship between tumor heterogeneity and immune/tumor interactions have led to great progress in comprehending the complexity of this field. The tumor microenvironment has been described as an ecosystem, which aptly depicts the complicated and dynamic interconnectedness of tumor cells, stroma cells, immune cells, and other cells [80, 81]. Cutting-edge genomic, transcriptomic, proteomic, and bioinformatics strategies to characterize the tumor microenvironment have been rapidly yielding data with consequences for scientific understanding and for patient prognosis and treatment [82]. A developing new frontier, in terms of strategy for new investigations, lies in the application of in silico analysis techniques to tumor heterogeneity studies. As mentioned above, by mathematical modeling, Park et al. are developing the means to personalize the “Goldilocks Window” of chemotherapy for patients, in order to clear the tumor microenvironment preferentially of tumor cells while retaining immune cells [57]. As another example of a novel approach using in silico technology, Puniya et al. have generated computational modeling, beginning from microenvironmental cues, of signal transduction leading to CD4+ T cell subtype differentiation, thus providing a means to assess how T cell subtypes may be altered by their surroundings [83]. In addition, Wells et al. have produced a computer model of a developing tumor that includes the major attributes of tumor/immune cell associations and reflects the influences of spatial and functional effects [84]. By their modeling, increased heterogeneity in cell types and locations resulted in immunosuppressive patterns of expression. This model also proved to be useful by allowing computational testing of cell-mediated treatments to alleviate such immunosuppression. Although in silico models will not fully replace cell models and, in particular, animal models of the tumor microenvironment, they are already driving progress forward in grasping the nature of the challenges and seeking clinically relevant solutions.
Acknowledgements
The figures were created with the BioRender.com program.
Grant Support
This work was supported by funding from the National Institutes of Health (NIH) (R21 CA223429, P30 CA036727, P50 CA127297, T32 CA009476, and NIGMS U54 GM115458), the UNMC Pediatric Cancer Research Center, and by UNMC Graduate Studies Office fellowships. The contents of this manuscript are the authors’ responsibility and do not represent NIH’s official views.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of interests
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- 1.Sharma A, Merritt E, Hu X, Cruz A, Jiang C, Sarkodie H, et al. (2019). Non-genetic intratumor heterogeneity is a major predictor of phenotypic heterogeneity and ongoing evolutionary dynamics in lung tumors. Cell Reports, 29(8), 2164–2174.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelova M, Mlecnik B, Vasaturo A, Bindea G, Fredriksen T, Lafontaine L, et al. (2018). Evolution of metastases in space and time under immune selection. Cell, 175, 751–765. [DOI] [PubMed] [Google Scholar]
- 3.Obeid JM, Hu Y, Erdag G, Leick KM, Slingluff CL Jr. (2017). The heterogeneity of tumor-infiltrating CD8+ T cells in metastatic melanoma distorts their quantification: how to manage heterogeneity? Melanoma Research 27(3), 211–217. [DOI] [PubMed] [Google Scholar]
- 4.Shembrey C, Huntington ND, Hollande F (2019). Impact of tumor and immunological heterogeneity on the anti-cancer immune response. Cancers, 11(9), 1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsujikawa T, Mitsuda J, Ogi H, Miyagawa-Hayashino A, Konishi E, Itoh K, et al. (2020). Prognostic significance of spatial immune profiles in human solid cancers. Cancer Science, 111(10), 3426–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jamal-Hanjani M, Quezada SA, Larkin J, Swanton C (2015). Translational implications of tumor heterogeneity. Clinical Cancer Research, 21(6), 1258–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caswell DR, Swanton C (2017). The role of tumour heterogeneity and clonal cooperativity in metastasis, immune evasion and clinical outcome. BMC Medicine 15(1), 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA (2015). Robust enumeration of cell subsets from tissue expression profiles. Nature Methods, 12(5), 453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, et al. (2015). The prognostic landscape of genes and infiltrating immune cells across human cancers. Nature Medicine, 21(8), 938–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charoentong P, Finotello F, Angelova M, Mayer C, Efremova M, Rieder D, et al. (2017). Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Reports, 18(1), 248–262. [DOI] [PubMed] [Google Scholar]
- 11.Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, et al. (2011). Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. Journal of Clinical Oncology, 29(6), 610–618. [DOI] [PubMed] [Google Scholar]
- 12.Price JE, Aukerman SL, Fidler IJ (1986). Evidence that the process of murine melanoma metastasis is sequential and selective and contains stochastic elements. Cancer Research, 46(10), 5172–5178. [PubMed] [Google Scholar]
- 13.Price JE, Naito S, Fidler IJ (1988). Growth in an organ microenvironment as a selective process in metastasis. Clinical & Experimental Metastasis, 6(1), 91–102. [DOI] [PubMed] [Google Scholar]
- 14.Kreso A, O’Brien CA, van Galen P, Gan OI, Notta F, Brown AM et al. (2013). Variable clonal repopulation dynamics influence chemotherapy response in colorectal cancer. Science, 339(6119), 543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Brien CA, Kreso A, Jamieson CHM (2010). Cancer stem cells and self-renewal. Clinical Cancer Research, 16(12):3113–3120. [DOI] [PubMed] [Google Scholar]
- 16.Mukhopadhyay P, Farrell T, Sharma G, McGuire TR, O’Kane B, Sharp JG (2013). Heterogeneity of functional properties of clone 66 murine breast cancer cells expressing various stem cell phenotypes. PLoS One, 8(11):e78725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreso A, Dick JE (2014). Evolution of the cancer stem cell model. Cell Stem Cell, 14(3), 275–291. [DOI] [PubMed] [Google Scholar]
- 18.Greaves M (2013). Cancer stem cells as ‘units of selection’. Evolutionary Applications, 6(1), 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Byrne KT, Yan F, Yamazoe T, Chen Z, Baslan T, et al. (2018). Tumor cell-intrinsic factors underlie heterogeneity of immune cell infiltration and response to immunotherapy. Immunity, 49(1), 178–193e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrne KT, Vonderheide RH (2016). CD40 stimulation obviates innate sensors and drives T cell immunity in cancer. Cell Reports, 15(12), 2719–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, et al. (2012). EMT and dissemination precede pancreatic tumor formation. Cell, 148(1–2), 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chao T, Furth EE, Vonderheide RH (2016). CXCR2-dependent accumulation of tumor-associated neutrophils regulates T-cell immunity in pancreatic ductal adenocarcinoma. Cancer Immunology Research, 4(11), 968–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steele CW, Karim SA, Leach JDG, Bailey P, Upstill-Goddard R, Rishi L, et al. (2016). CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell, 29, 832–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu RB, Engels B, Schreiber K, Ciszewski C, Schietinger A, Schreiber H, et al. (2013). IL-15 in tumor microenvironment causes rejection of large established tumors by T cells in a noncognate T cell receptor-dependent manner. Proceedings of the National Academy of Sciences of the United States of America, 110(20), 8158–8163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu RB, Engels B, Arina A, Schreiber K, Hyjek E, Schietinger A, et al. (2012). Densely granulated murine NK cells eradicate large solid tumors. Cancer Research, 72(8), 1964–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeda K, Nakayama M, Hayakawa Y, Kojima Y, Ikeda H, Imai N, et al. (2017). IFN-γ is required for cytotoxic T cell-dependent cancer genome immunoediting. Nature Communications, 8, 14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolf Y, Bartok O, Patkar S, Eli GB, Cohen S, Litchfield K, et al. (2019). UVB-induced tumor heterogeneity diminishes immune response in melanoma. Cell, 179(1), 219–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milo I, Bedora-Faure M, Garcia Z, Thibaut R, Périé L, Shakhar G, et al. (2018). The immune system profoundly restricts intratumor genetic heterogeneity. Science Immunology, 3(29), eaat1435. [DOI] [PubMed] [Google Scholar]
- 29.Donkor MK, Lahue E, Hoke TA, Shafer LR, Coskun U, Solheim JC, Gulen D, Bishay J, Talmadge JE (2009). Mammary tumor heterogeneity in the expansion of myeloid-derived suppressor cells. International Immunopharmacology, 9(7–8), 937–948. [DOI] [PubMed] [Google Scholar]
- 30.Bracci L, Moschella F, Sestili, La Sorsa V, Valentini M, Canini I, et al. (2007). Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clinical Cancer Research, 13(2), 644–653. [DOI] [PubMed] [Google Scholar]
- 31.Robertson-Tessi M, Gillies RJ, Gatenby RA, Anderson ARA (2015). Impact of metabolic heterogeneity on tumor growth, invasion, and treatment outcomes. Cancer Research 75(8): 1567–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Liekovan S, et al. (2016). Genomic and transcriptomic feastures of response to anti-PD-1 therapy in metastatic melanoma. Cell, 165(1), 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee W-C, Diao L, Wang J, Zhang J, Roarty EB, Varghese S, et al. (2018). Multiregion gene expression profiling reveals heterogeneity in molecular subtypes and immunotherapy response signatures in lung cancer. Modern Pathology, 31(6), 947–955. [DOI] [PubMed] [Google Scholar]
- 34.Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, et al. (2016). Mutations associated with acquired resistance to PD-1 blockade in melanoma. New England Journal of Medicine, 375(9), 819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shin DS, Zaretsky JM, Escuin-Ordinas H, Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, et al. (2017). Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discovery, 7(2), 188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gurjao C, Liu D, Hofree M, AlDubayan SH, Wakiro I, Su M-J, et al. (2019). Intrinsic resistance to immune checkpoint blockade in a mismatch repair-deficient colorectal cancer. Cancer Immunology Research, 7(8), 1230–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan Y, Leontovich AA, Gerdes MJ, Desai K, Dong J, Sood A, et al. (2019). Understanding heterogeneous tumor microenvironment in metastatic melanoma. PLoS One, 14(6), e0216485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Houdt IS, Sluijter BJ, Moesbergen LM, Vos WM, de Gruijl TD, Molenkamp BG, et al. (2008). Favorable outcome in clinically stage II melanoma patients is associated with the presence of activated tumor infiltrating T-lymphocytes and preserved MHC class I antigen expression. International Journal of Cancer, 123(3), 609–615. [DOI] [PubMed] [Google Scholar]
- 39.Kageshita T, Hirai S, Ono T, Hicklin DJ, Ferrone S (1999). Down-regulation of HLA class I antigen-processing molecules in malignant melanoma: association with disease progression. American Journal of Pathology, 154(3), 745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miranda A, Hamilton PT, Zhang AW, Pattnaik S, Becht E, Mezheyeuski T, et al. (2019). Cancer stemness, intratumoral heterogeneity, and immune response across cancers. Proceedings of the National Academy of Sciences of the United States of America, 116(18), 9020–9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Song J, Zhao Z, Yang M, Chen M, Liu C, et al. (2020). Single-cell transcriptome analysis reveals tumor immune microenvironment heterogenicity and granulocytes enrichment in colorectal cancer liver metastases. Cancer Letters, 470, 84–94. [DOI] [PubMed] [Google Scholar]
- 42.Ogawa R, Yamamoto T, Hirai H, Hanada K, Kiyasu Y, Nichikawa G, et al. (2019). Loss of SMAD4 promotes colorectal cancer progression by recruiting tumor-associated neutrophils via the CXCL1/8-CXCR2 axis. Clinical Cancer Research, 25(9), 2887–2899. [DOI] [PubMed] [Google Scholar]
- 43.Itatani Y, Kawada K, Fujishita T, Kakizaki F, Hirai H, Matsumoto T, et al. (2013). Loss of SMAD4 from colorectal cancer cells promotes CCL15 expression to recruit CCR1+ myeloid cells and facilitate liver metastasis. Gastroenterology, 145(5), 1064–1075. [DOI] [PubMed] [Google Scholar]
- 44.Inamoto S, Itatani Y, Yamamoto T, Minamiguchi S, Hirai H, Iwamoto M, et al. (2016). Loss of SMAD4 promotes colorectal cancer progression by accumulation of myeloid-derived suppressor cells through the CCL15-CCR1 chemokine axis. Clinical Cancer Research, 22(2), 492–501. [DOI] [PubMed] [Google Scholar]
- 45.Diaz-Montero C, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ (2009). Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunology Immunotherapy, 58(1), 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Safarzadeh E, Hashemzadeh S, Duijf PHG, Mansoori B, Khaze V, Mohammadi A, et al. (2019). Circulating myeloid-derived suppressor cells: An independent prognostic factor in patients with breast cancer. Journal of Cell Physiology, 234(4), 3515–3525. [DOI] [PubMed] [Google Scholar]
- 47.Xu X-D, Hu J, Wang M, Peng F, Tian R, Guo X-J, et al. (2016). Circulating myeloid-derived suppressor cells in patients with pancreatic cancer. Hepatobiliary & Pancreatic Diseases International 15(1), 99–105. [DOI] [PubMed] [Google Scholar]
- 48.McDonald K-A, Kawaguchi T, Qi Q, Peng X, Asaoka M, Young J, et al. (2019). Tumor heterogeneity correlates with less immune response and worse survival in breast cancer patients. Annals of Surgical Oncology, 26(7), 2191–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang AW, McPherson A, Milne K, Kroeger DR, Hamilton PT, Miranda A, et al. (2018). Interfaces of malignant and immunologic clonal dynamics in ovarian cancer. Cell, 173(7), 1755–1769.e22. [DOI] [PubMed] [Google Scholar]
- 50.Sucker A, Zhao F, Pieper N, Heeke C, Maltaner R, Stadtler N, et al. (2017). Acquired IFNgamma resistance impairs anti-tumor immunity and gives rise to T-cell-resistant melanoma lesions. Nature Communications, 8, 15440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams JB, Li S, Higgs EF, Cabanov A, Wang X, Huang H, et al. (2020). Tumor heterogeneity and clonal cooperation influence the immune selection of IFN-γ-signaling mutant cancer cells. Nature Communications 11(1), 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reuben A, Gittelman R, Gao J, Zhang J, Yusko EC, Wu C-J, et al. (2017). TCR repertoire intratumor heterogeneity in localized lung adenocarcinomas: an association with predicted neoantigen heterogeneity and postsurgical recurrence. Cancer Discovery, 7(10), 1088–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma K-Y, Schonnesen AA, Brock A, Van Den Berg C, Eckhardt SG Liu Z, et al. (2019). Single-cell RNA sequencing of lung adenocarcinoma reveals heterogeneity of immune-response genes. JCI Insight, 4(4), e121387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Losic B, Craig AJ, Villacorta-Martin C, Martins-Filho SN, Akers N, Chen X, et al. (2020). Intratumoral heterogeneity and clonal evolution in liver cancer. Nature Communications, 11(1), 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Angelova M, Charoentong P, Hackl H, Fischer ML, Snajder R, Krogsdam AM, et al. (2015). Characterization of the immunophenotypes and antigenomes of colorectal cancers reveals distinct tumor escape mechanisms and novel targets for immunotherapy. Genome Biology 16(1), 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hanin L, Rose J, Zaider M (2006). A stochastic model for the sizes of detectable metastases. Journal of Theoretical Biology, 243(3), 407–417. [DOI] [PubMed] [Google Scholar]
- 57.Park DS, Robertson-Tessi M, Luddy KA, Maini PK, Bonsall MB, Gatenby RA, et al. (2019). The Goldilocks Window of personalized chemotherapy: getting the immune response just right. Cancer Research, 79(20), 5302–5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiménez-Sánchez A, Memon D, Pourpe S, Veeraraghavan H, Li Y, Vargas HA, et al. (2017). Heterogeneous tumor-immune microenvironments among differentially growing metastases in an ovarian cancer patient. Cell, 170(5), 927–938.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiménez-Sánchez A, Cybulska P, Mager KL, Koplev S, Cast O, Couturier D-L, et al. (2020). Unraveling tumor-immune heterogeneity in advanced ovarian cancer uncovers immunogenic effect of chemotherapy. Nature Genetics, 52(6), 582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Condomines M, Veyrune J-L, Larroque M, Quittet P, Latry P, Lugagne C, et al. (2010). Increased plasma-immune cytokines throughout the high-dose melphalan-induced lymphodepletion in patients with multiple myeloma: a window for adoptive immunotherapy. Journal of Immunology, 184, 1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wallen H, Thompson JA, Reilly JZ, Rodmyre RM, Cao J, Yee C (2009). Fludarabine modulates immune response and extends in vivo survival of adoptively transferred CD8 T cells in patients with metastatic melanoma. PLoS One, 4(3), e4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pinato DJ, Shiner RJ, White SDT, Black JRM, Trivedi P, Stebbing J et al. (2016). Intra-tumoral heterogeneity in the expression of programmed-death (PD) ligands in isogeneic primary and metastatic lung cancer: implications for immunotherapy. OncoImmunology, 5(9), e1213934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim MY, Koh J, Kim S, Go H, Jeon YK, Chung DH (2015). Clinicopathological analysis of PD-L1 and PD-L2 expression in pulmonary squamous cell carcinoma: comparison with tumor-infiltrating T cells and the status of oncogenic drivers. Lung Cancer, 88(1), 24–33. [DOI] [PubMed] [Google Scholar]
- 64.Mansfield AS, Aubry MC, Moser JC, Harrington SM, Dronca RS, Park SS, et al. (2016). Temporal and spatial discordance of programmed cell death-ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Annals of Oncology, 27(10), 1953–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Failing JJ, Aubry MC, Mansfield AS (2020). Human leukocyte antigen expression in paired primary lung tumors and brain metastases in non-small cell lung cancer. Cancer Immunology, Immunotherapy, 70(1), 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li M, Li A, Zhou S, Xu Y, Xiao Y, Bi R, et al. (2018). Heterogeneity of PD-L1 expression in primary tumors and paired lymph node metastases of triple negative breast cancer. BMC Cancer 18(1), 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dötzer K, Schlüter F, Schoenberg MB, Bazhin AV, von Koch FE, Schnelzer A, et al. (2019). Immune heterogeneity between primary tumors and corresponding metastatic lesions and response to platinum therapy in primary ovarian cancer. Cancers, 11(9), 1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, et al. (2013). Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 39(4), 782–795. [DOI] [PubMed] [Google Scholar]
- 69.Khan AM, Yuan Y (2016). Biopsy variability of lymphocytic infiltration in breast cancer subtypes and the ImmunoSkew score. Scientific Reports, 6, 36231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, et al. (2011). Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nature Medicine, 17(4), 500–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moffitt RA, Marayati R, Flate EL, Volmar KE, Loeza SG, Hoadley KA et al. (2015). Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nature Genetics, 47(10), 1168–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, et al. (2016). Genomic analyses identify molecular subtypes of pancreatic cancer. Nature, 531(7592), 47–52. [DOI] [PubMed] [Google Scholar]
- 73.Law HC-H, Lagundzin D, Clement EJ, Qiao F, Wagner ZS, Krieger KL, et al. (2020). The proteomic landscape of pancreatic ductal adenocarcinoma liver metastases identifies molecular subtypes and associations with clinical response. Clinical Cancer Research, 26(5), 1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bakouny Z, Choueiri TK (2020). IL-8 and cancer prognosis on immunotherapy. Nature Medicine, 26, 650–654. [DOI] [PubMed] [Google Scholar]
- 75.Engler JR, Robinson AE, Smirnov I, Hodgson JG, Berger MS, Gupta N, et al. (2012). Increased microglia/macrophage gene expression in a subset of adult and pediatric astrocytomas. PLoS One, 7(8), e43339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Doucette T, Rao G, Rao A, Shen L, Aldape K, Wei J, et al. (2013). Immune heterogeneity of glioblastoma subtypes: extrapolation from the Cancer Genome Atlas. Cancer Immunology Research, 1(2), 112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Q, Hu B, Hu X, Kim H, Squatrito M, Scarpace L, et al. (2017). Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell, 32(1), 42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tu L, Guan R, Yang H, Zhou Y, Hong W, Ma L, et al. (2020). Assessment of the expression of the immune checkpoint molecules PD-1, CTLA4, TIM-3 and LAG-3 across different cancers in relation to treatment response, tumor-infiltrating immune cells and survival. International Journal of Cancer, 147(2), 423–439. [DOI] [PubMed] [Google Scholar]
- 79.Tamborero D, Rubio-Perez C, Muiños F, Sabarinathan R, Piulats JM, Muntasell A, et al. (2018). A pan-cancer landscape of interactions between solid tumors and infiltrating immune cell populations. Clinical Cancer Research, 24(15), 3717–3728. [DOI] [PubMed] [Google Scholar]
- 80.Gonzalez H, Hagerling C, Werb Z (2018). Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes & Development, 32(19–20), 1267–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wagner J, Rapsomaniki MA, Chevrier S, Anzeneder T, Langwieder C, Dykgers A, et al. (2019). A single-cell atlas of the tumor and immune ecosystem of human breast cancer. Cell, 177(5), 1330–1345.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rosenthal R, McGranahan N, Herrero J, Swanton C (2017). Deciphering genetic intratumor heterogeneity and its impact on cancer evolution. Annual Review of Cancer Biology, 1, 223–240 [Google Scholar]
- 83.Puniya BL, Tood RG, Mohammed A, Brown DM., Barberis M, Helikar T (2018). A mechanistic computational model reveals that plasticity of CD4+ T cell differentiation is a function of cytokine composition and dosage. Frontiers in Physiology, 9, 878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wells DK, Chuang Y, Knapp LM, Brockmann D, Kath WL, Leonard JN (2015). Spatial and functional heterogeneities shape collective behavior of tumor-immune networks. PLoS Computational Biology, 11(4), e1004181. [DOI] [PMC free article] [PubMed] [Google Scholar]


