Abstract
Background
This study aimed to explore the preoperative risk factors related to blood transfusion after hip fracture operations and to establish a nomogram prediction model. The application of this model will likely reduce unnecessary transfusions and avoid wasting blood products.
Methods
This was a retrospective analysis of all patients undergoing hip fracture surgery from January 2013 to January 2020. Univariate and multivariate logistic regression analyses were used to evaluate the association between preoperative risk factors and blood transfusion after hip fracture operations. Finally, the risk factors obtained from the multivariate regression analysis were used to establish the nomogram model. The validation of the nomogram was assessed by the concordance index (C-index), the receiver operating characteristic (ROC) curve, decision curve analysis (DCA), and calibration curves.
Results
A total of 820 patients were included in the present study for evaluation. Multivariate logistic regression analysis demonstrated that low preoperative hemoglobin (Hb), general anesthesia (GA), non-use of tranexamic acid (TXA), and older age were independent risk factors for blood transfusion after hip fracture operation. The C-index of this model was 0.86 (95% CI, 0.83–0.89). Internal validation proved the nomogram model’s adequacy and accuracy, and the results showed that the predicted value agreed well with the actual values.
Conclusions
A nomogram model was developed based on independent risk factors for blood transfusion after hip fracture surgery. Preoperative intervention can effectively reduce the incidence of blood transfusion after hip fracture operations.
Keywords: Blood transfusion, Hip fracture, Risk factor, Nomogram
Background
The incidence of hip fracture is increasing yearly, and research has also been conducted worldwide [1]. However, bone mineral density is decreased in elderly individuals, and low-energy trauma can lead to hip fracture. Cooper et al. [2] illustrated that by 2050, there would be 6.26 million incident hip fractures worldwide. The disability and death rates of hip fractures are high. Patients with a hip fracture will suffer a loss of independent living ability, which has caused a significant burden to family members and society [3].
Elderly patients who undergo surgical treatment have higher odds of receiving blood transfusion, and the probability of blood transfusion after hip fracture surgery can be as high as 56% [4]. There are many complications after hip fracture, such as low hemoglobin and albumin levels [5]. Blood transfusion increases the risk of joint infection around the incision and surgical area while potentially increasing the risk of infectious diseases, hemolysis, and immune reactions [6]. Therefore, early intervention before surgery based on patients’ preoperative status and laboratory indicators can greatly reduce the risk of postoperative blood transfusion, and avoid the waste of blood products [7]. Hemostatic polymeric materials or hemostatic agents have been used in clinical practice for a long time, but there are drawbacks to the current clinical treatment with these materials [8]. Recently, Wang et al. [9] established a model by investigating the influencing factors of postoperative blood transfusion in femoral neck fracture and the obtained independent risk factors associated with postoperative blood transfusion. However, this study involved a single fracture type. The number of cases was relatively small, so the possibility of offset existed because the prediction model’s construction lacked considerable sample support.
Unlike previously reported studies, the present investigation included a large sample size, focusing on collecting preoperative risk factors for hip fracture patients. Independent risk factors related to postoperative blood transfusion were obtained using regression analysis, and the nomogram model was established. Through the scientific and systematic evaluation of the probability of postoperative blood transfusion, the nomogram model’s application before surgery can more accurately guide surgeons to intervene and treat patients in advance, reduce the occurrence of postoperative blood transfusion.
Methods
Patients
In total, 862 patients who underwent surgery for hip fracture between January 2013 and January 2020 were enrolled, and the ethics committee approved the trial at our hospital. The inclusion criteria were as follows: (1) low-energy injury (e.g., osteoporotic fracture); (2) unilateral fracture without other severe injuries; and (3) no history of antiplatelet drugs, non-steroidal anti-inflammatory, or vasoactive drug application within 1 week preoperatively. The exclusion criteria were as follows: (1) multiple, high-energy injuries; (2) apparent bone destruction, pathological fracture, or severe hematologic disorders; (3) open fracture with increased risk of infection; (4) American Society of Anesthesiologists scores greater than grade III. Of these, 42 patients were excluded: 26 developed severe delirium symptoms or severe procedure-related complications postoperatively (such as dislocation and deep infection), and 16 were transferred to the intensive care unit for continued treatment.
Ultimately, 820 patients were included in the study, and they were randomly divided into a training set (70%) and a testing set (30%). Patients in the training set were used to develop the nomogram model, whereas patients in the testing set were used to validate the resulting nomogram. The use of TXA involved a single dose of intravenous TXA (15 mg/kg) 30 min prior to surgery in the study [10]. According to the World Health Organization criteria, the degree of anemia was classified as mild (male, 11-12.9 g/dl; female, 11-11.9 g/dl), moderate (8-10.9 g/dl), or severe (< 8 g/dl) [11]. The transfusion of blood products should occur through different transfusion strategy according to the patient’s actual situation rather than merely using a certain transfusion strategy [12]. The standard of blood transfusion used in our hospital was that severe or moderate patients with heart rate > 100 bpm, systolic blood pressure < 90 mmHg, depressed mood, or extreme weakness would be considered for blood transfusion.
Data collection
In this study, the preoperative factors affecting postoperative blood transfusion were collected through the electronic medical record system. All preoperative factors were taken from previous studies and included age, sex, BMI, preoperative hemoglobin (Hb), TXA (used or not used), type of anesthesia (GA or combined spinal-epidural anesthesia [CSEA]), preoperative waiting time, type of operation (hip arthroplasty, internal fixation), and type of fracture (femoral neck fracture or inter-trochanteric fracture). In addition, we reviewed the previous history of the patients, including diabetes, hypertension, smoking (> 10 years), and drinking of alcohol (> 10 years).
Statistical analysis
Data were analyzed using the SPSS 26 software for Windows (IBM Corp., Armonk, NY, USA). First, the risk factors that may affect postoperative blood transfusion were classified. Student’s t test or the Mann-Whitney U test was used to perform transfusion and non-transfusion group comparisons for quantitative variables. Categorical variables were compared using the chi-square or Fisher’s exact test. Second, all the training set factors were included in the univariate and multivariate logistic regression analyses to exclude unrelated risk factors. The independent risk factors obtained based on the multivariate regression method were used to construct a nomogram model with the “rms” package of R software (version 3.6.1).
Finally, the C-index, the area under the ROC curve (AUC), calibration curve, and DCA were used to evaluate the predictive ability and performance of the risk model. The C-index enables evaluation of the predictive accuracy and discriminative ability of nomograms [13]. The C-index values ranged from 0.5-1.0, with low accuracy (< 0.5), moderate accuracy (0.5- 0.7), high accuracy (0.7 -0.9), and extreme accuracy (> 0.9). A calibration curve was used to compare the actual risk and predicted risk. The clinical usefulness of the nomogram was estimated by DCA based on the net benefit and threshold probabilities. Statistical tests used p <0.05 as a significance level.
Results
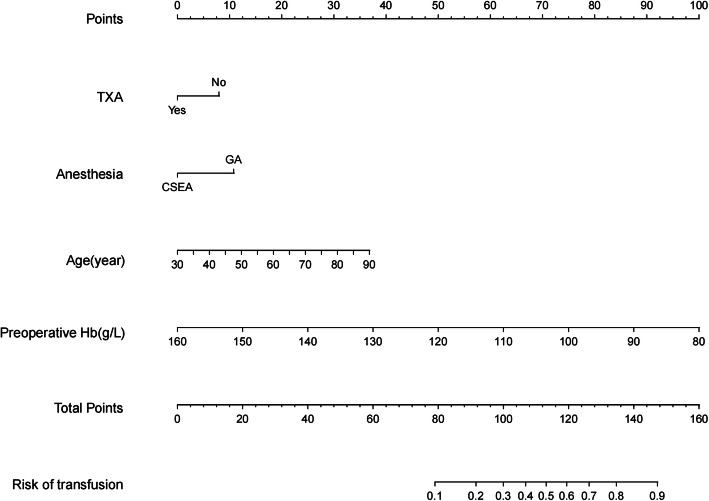
A total of 820 patients were included in this study: 576 patients were included in the training set, and 244 patients were included in the testing set. In the training set, the transfusion group was compared with the non-transfusion group, and the results showed that age, sex, BMI, preoperative Hb, TXA, diabetes, and smoking were correlated with postoperative blood transfusion. However, there was no correlation between the type of anesthesia and postoperative blood transfusion (Table 1). Multivariate analysis showed that the type of anesthesia was an independent risk factor for blood transfusion after hip fracture surgery, so this factor was included in the final modeling. Risk factors (low preoperative Hb, GA, non-use of TXA, older age) for postoperative blood transfusion were obtained by multivariate regression in the training set (Table 2). A new nomogram was constructed to evaluate the postoperative blood transfusion probability after hip fracture (Fig. 1).
Table 1.
Preoperative demographic characteristics in the training set
| Characteristics | Transfusion (n = 111) | Non-transfusion (n = 465) | t/z/χ2 | P |
|---|---|---|---|---|
| Age (year) | 77 (69-82) | 66 (56-77) | 7.87 | < 0.01* |
| Sex | 7.49 | < 0.01* | ||
| Female | 83 (22.7%) | 283 (77.3%) | ||
| Male | 28 (13.3%) | 182 (86.7%) | ||
| BMI (kg/m2) | 22 (20.3-24) | 23.2 (20.8-25.4) | 3.16 | < 0.01* |
| Hb (g/L) | 105.96 ± 14.08 | 126.62 ± 15.18 | 11.07 | < 0.01* |
| TXA | 13.29 | < 0.01* | ||
| No | 79 (24.6%) | 242 (75.4%) | ||
| Yes | 32 (12.5%) | 223 (87.5%) | ||
| Type of anesthesia | 1.33 | 0.25 | ||
| GA | 69 (20.9%) | 261 (79.1%) | ||
| CSEA | 42 (17.1%) | 204 (82.9%) | ||
| Waiting time (d) | 5 (4-7) | 5 (4-7) | 1.83 | 0.70 |
| Hypertension | 1.69 | 0.19 | ||
| No | 70 (17.8%) | 323 (82.2%) | ||
| Yes | 41 (22.4%) | 142 (77.6%) | ||
| Diabetes | 5.08 | 0.02* | ||
| No | 85 (17.6%) | 397 (82.4%) | ||
| Yes | 26 (27.7%) | 68 (72.3%) | ||
| Smoking | 5.08 | 0.02* | ||
| No | 99 (21.0%) | 372 (79%) | ||
| Yes | 12 (11.4%) | 93 (88.6%) | ||
| Alcohol | 2.93 | 0.09 | ||
| No | 96 (20.6%) | 369 (79.4%) | ||
| Yes | 15 (13.5%) | 96 (86.5%) | ||
| Surgical approach | 0.09 | 0.75 | ||
| HA | 87 (19.6%) | 358 (80.4%) | ||
| IF | 24 (18.3%) | 107 (81.7%) | ||
| Type of fracture | 3.63 | 0.06 | ||
| FNF | 87 (21.3%) | 322 (78.7%) | ||
| ITF | 24 (14.4%) | 143 (85.6%) |
Abbreviations: BMI body mass index, Hb hemoglobin, TXA tranexamic acid, GA general anesthesia, CSEA combined spinal and epidural anesthesia, HA hip arthroplasty, IF, internal fixation, FNF femoral neck fracture, ITF inter-trochanteric fracture
*: Statistically significant difference
Table 2.
Univariate and multivariate logistic analysis of risk factors for blood transfusion after hip fracture surgery
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Age (year) | 1.07 (1.044-1.09) | < 0.01* | 1.03 (1.01-1.06) | 0.02* |
| Sex | ||||
| Female | Ref. | Ref. | ||
| Male | 0.53 (0.33-0.84) | 0.01* | 1.08 (0.56-2.10) | 0.82 |
| BMI (kg/m2) | 0.90 (0.84-0.96) | < 0.01* | 0.92 (0.85-1.01) | 0.06 |
| Hb (g/L) | 0.92 (0.90-0.93) | 0.01* | 0.92 (0.90-0.94) | < 0.01* |
| TXA | ||||
| No | 2.28 (1.45-3.57) | < 0.01* | 1.92 (1.11-3.34) | 0.02* |
| Yes | Ref. | Ref. | ||
| Type of anesthesia | ||||
| GA | 1.28 (0.84-1.96) | 0.25 | 2.08 (1.21-3.57) | < 0.01* |
| CSEA | Ref. | Ref. | ||
| Waiting time (d) | 1.11 (1.01-1.21) | 0.04* | 0.93 (0.82-1.04) | 0.19 |
| Hypertension | ||||
| No | Ref. | Ref. | ||
| Yes | 1.33 (0.86-2.05) | 0.19 | 1.14 (0.66-1.96) | 0.65 |
| Diabetes | ||||
| No | Ref. | Ref. | ||
| Yes | 1.79 (1.07-2.97) | 0.03* | 1.55 (0.83-2.92) | 0.17 |
| Smoking | ||||
| No | Ref. | Ref. | ||
| Yes | 0.49 (0.26-0.92) | 0.03* | 0.55 (0.22-1.41) | 0.22 |
| Alcohol | ||||
| No | Ref. | Ref. | ||
| Yes | 0.60 (0.33-1.08) | 0.09 | 1.10 (0.45-2.73) | 0.83 |
| Surgical approach | ||||
| HA | Ref. | Ref. | ||
| IF | 0.92 (0.56-1.52) | 0.75 | 1.62 (0.45-5.78) | 0.46 |
| Type of fracture | ||||
| FNF | Ref. | Ref. | ||
| ITF | 0.62 (0.38-1.02) | 0.06 | 0.51 (0.14-1.85) | 0.30 |
Abbreviations: BMI body mass index, Hb hemoglobin, TXA tranexamic acid, GA general anesthesia, CSEA combined spinal and epidural anesthesia, HA hip arthroplasty, IF internal fixation, FNF femoral neck fracture, ITF inter-trochanteric fracture
*: Statistically significant difference
Fig. 1.
Nomogram for predicting postoperative blood transfusion in patients with hip fracture
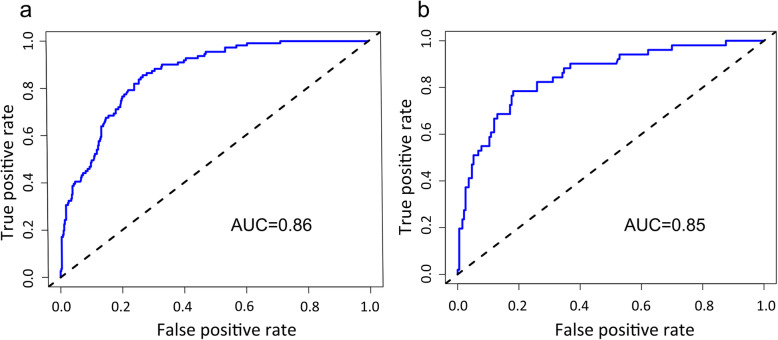
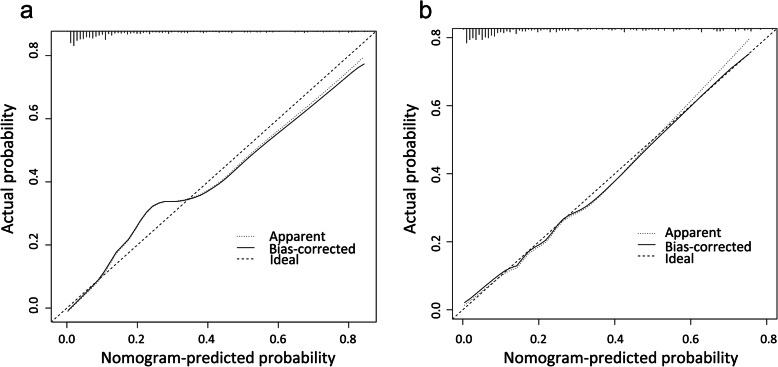
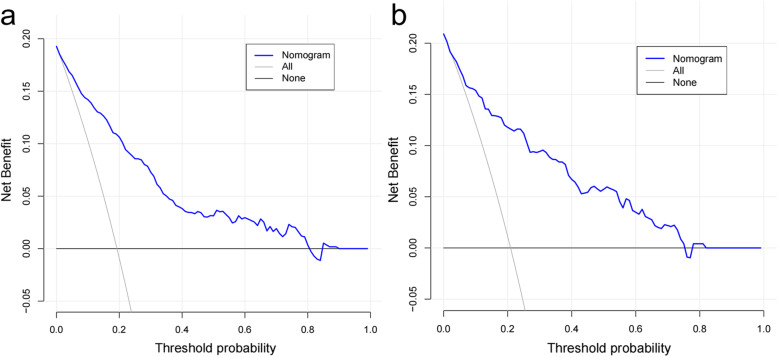
The accuracy of the nomogram model was estimated by internal validation in the training set and testing sets. This model’s C-index was 0.86 (95% CI, 0.83~0.89), which indicated that the model was predictive with high accuracy. Furthermore, the ROC curve was constructed, and the AUC was calculated for both the training and testing sets. The AUC was 0.86 in the training set (Fig. 2a) and 0.85 in the testing set (Fig. 2b), illustrating that the model had high discrimination. The calibration curves demonstrated good consistency between the model’s actually observed probability and the predicted probability (Fig. 3). DCA indicated that this nomogram model could be an excellent prediction tool for blood transfusion after hip fracture operation (Fig. 4).
Fig. 2.
Comparison of the area under the receiver operating characteristic curve between nomogram-independent predictors in the training set (a) and the testing set (b)
Fig. 3.
Comparison of calibration curves between the training set (a) and the testing set (b)
Fig. 4.
Comparison of decision curve analyses between the training set (a) and the testing set (b)
The use of the nomogram is straightforward. The total score of prognostic indicators can be obtained by adding the individual scores, and then the total score can be used to calculate the probability of blood transfusion. For example, a 70-year-old patient whose preoperative hemoglobin was 100 g/L decided to use general anesthesia during the operation and did not use TXA during the perioperative period. The patient’s age score was 25, the preoperative Hb score was 75, the general anesthesia score was 11, and the not used TXA score was 8. The total score was 25 + 75 + 11 + 8 = 119, which was equivalent to 60% of the risk of blood transfusion.
Postoperative patients received routine treatment. Antibiotics were used to prevent infection within 3 days after the operation; symptomatic support was given to patients with mild fever after blood transfusion; all mild skin infections were cured after debridement and dressing changes. Patients received low-molecular-weight heparin to prevent postoperative deep vein thrombosis 12 h after the operation and were changed to rivaroxaban after discharge to 1 month after the operation.
Discussion
Among the complications of hip fracture, 40-80% of patients show symptoms of anemia, which is considered one of the threatening complications in hip fracture [14], and correcting anemia is beneficial to improve the patient prognosis. However, related studies have pointed out that perioperative blood transfusion cannot reduce the postoperative mortality of hip fracture but will further increase the postoperative infection risk [15]. Therefore, reducing the risk of blood transfusion is a problem for all surgeons. Therefore, constructing a nomogram in this study can make it more intuitive to evaluate a patient’s probability of postoperative blood transfusion.
Using the nomogram model, preoperative risk factors can be assessed at an early stage, and surgeons can apply interventions to reduce the waste of blood products. This study concluded that low preoperative Hb was one of the independent risk factors for postoperative blood transfusion, which is consistent with previous studies [9, 16]. The preoperative Hb of the non-transfused group was 126.62 ± 15.18, which was significantly higher than that of the transfused group (105.96 ± 14.08) (P < 0.05). Hip surgery mainly involved GA or CSEA, and the univariate results showed that the type of anesthesia was not statistically significant. In contrast, the multivariate analysis showed that the type of anesthesia was statistically significant, and GA was 2.08 (95% CI, 1.21-3.57). First, CSEA may decrease intraoperative bleeding by keeping patients hypotensive intraoperatively through reducing the pressure on the arteriovenous system [17]. Second, the inhaled anesthetic mixture during GA hinders the formation of erythrocytes during the recovery of erythrocyte endogenesis, which eventually leads to the aggravation of anemia in patients [18]. Previous studies have also reported similar results [9]. Therefore, CSEA can reduce intraoperative bleeding and improve surgical safety.
TXA is widely used in clinical work, especially in orthopedic fields involving the joints and the spine, thereby reducing the perioperative bleeding risk [19]. Evidence shows that the safety and effectiveness of tranexamic acid play an important role in the fast-track procedures [20]. TXA stabilizes fibrin clots by binding plasmin to fibrin to inhibit plasminogen activation, resulting in more stable hemostasis [21]. Yang et al. [22] confirmed that after the application of TXA, the blood transfusion rate of hip replacement decreased from 22.4 to 5.7%. Research has shown that patients who receive TXA have significantly less total blood loss than patients who do not [23].
Among the patients who were used to develop the model, those older than 65 years who received blood transfusion accounted for 27.4% of the elderly patients, and those younger than 65 years who received a transfusion accounted for 6.3% of their age group. There was a significant difference between the two groups. Some authors similarly consider older age of hip fracture patients as a risk factor for postoperative blood transfusion [16, 24, 25]. Gruson et al. [26] suggested that each 5-year increase in age is associated with a 32% relative increase in the risk of transfusion, with an almost threefold increase in the risk of transfusion among patients older than 65. Elderly patients are prone to develop decreased hematopoietic activity, decreased platelet function, and a lower transfusion threshold by physicians [27]. Therefore, advanced age is an independent risk factor for postoperative blood transfusion. There is no consensus on whether sex is an independent risk factor for postoperative transfusion. Some authors have suggested that female patients have a higher risk of postoperative transfusion than male patients [26]. But in the present study, the results were shown not to be independent risk factors by binary regression analysis, in keeping with previous findings [12].
The present study has some shortcomings: (1) It was adopted to be a retrospective study. The data were from a single center, there was no multicenter analysis, and the discriminatory ability of this model in prediction needs to be confirmed by external tests. (2) The study period was relatively long, and there may be differences regarding surgical techniques and surgical medication; therefore, a cross-sectional study should be conducted.
Conclusions
This study found that risk factors affecting postoperative blood transfusion were highly correlated with preoperative Hb, type of anesthesia, TXA, and age. This study developed a nomogram model; the advantages of which can transform complex regression equations into a visual and straightforward graph, make the results of the prediction model readable, and have a higher use value. Our established nomogram enables a relatively accurate assessment of the risk of postoperative blood transfusion in hip fracture, guides the surgeon to preoperative interventional treatment, and reduces the rate of postoperative blood transfusion.
Acknowledgements
Not applicable
Abbreviations
- C-index
Concordance index
- ROC curve
Receiver operating characteristic curve
- DCA
Decision curve analysis
- Hb
Hemoglobin
- GA
General anesthesia
- CSEA
Combined spinal-epidural anesthesia
- TXA
Tranexamic acid
- BMI
Body mass index
- HA
Hip arthroplasty
- IF
Internal fixation
- FNF
Femoral neck fracture
- ITF
Inter-trochanteric fracture
Authors’ contributions
Authors FC B and YS A designed the study. Authors XK C and FC B collected the clinical data and conducted the statistical analysis. Author FC B wrote the manuscript. Author FC B and XK C revised the manuscript. All authors critically read the manuscript to improve intellectual content. The authors read and approved the final manuscript.
Funding
This study did not receive any external funding.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of Chengde Medical University Affiliated Hospital research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
All participants gave their informed consent in writing prior to inclusion in the study.
Consent for publication
All participants agreed with the data and publication of the manuscript.
Written informed consent was obtained from all participants.
Competing interests
The authors declare that they have no competing interests.
Footnotes
FuCheng Bian and XiaoKang Cheng are co-first authors.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Peng G, Guan Z, Hou Y, Gao J, Rao W, Yuan X, Guo J, Huang X, Zhong Z, Lin J. Depicting developing trend and core knowledge of hip fracture research: a bibliometric and visualised analysis. J Orthop Surg Res. 2021;16(1):174. doi: 10.1186/s13018-021-02292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper C, Campion G, Melton LJ., 3rd Hip fractures in the elderly: a world-wide projection. Osteoporos Int. 1992;2(6):285–289. doi: 10.1007/BF01623184. [DOI] [PubMed] [Google Scholar]
- 3.Lin JC, Liu ZG, Liu RR, Xie LW, Xie HL, Cai HG. The increase of osteopontin and beta-carboxy-terminal cross-linking telopeptide of type I collagen enhances the risk of hip fracture in the elderly. J Clin Lab Anal. 2020;34(5):e23204. doi: 10.1002/jcla.23204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verlicchi F, Desalvo F, Zanotti G, Morotti L, Tomasini I. Red cell transfusion in orthopaedic surgery: a benchmark study performed combining data from different data sources. Blood Transfus. 2011;9(4):383–387. doi: 10.2450/2011.0095-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higashikawa T, Shigemoto K, Goshima K, Usuda D, Okuro M, Moriyama M, Inujima H, Hangyou M, Usuda K, Morimoto S, Matsumoto T, Takashima S, Kanda T, Sawaguchi T. Risk factors for the development of aspiration pneumonia in elderly patients with femoral neck and trochanteric fractures: a retrospective study of a patient cohort. Medicine (Baltimore) 2020;99(7):e19108. doi: 10.1097/MD.0000000000019108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newman ET, Watters TS, Lewis JS, Jennings JM, Wellman SS, Attarian DE, Grant SA, Green CL, Vail TP, Bolognesi MP. Impact of perioperative allogeneic and autologous blood transfusion on acute wound infection following total knee and total hip arthroplasty. J Bone Joint Surg Am. 2014;96(4):279–284. doi: 10.2106/JBJS.L.01041. [DOI] [PubMed] [Google Scholar]
- 7.Roberts M, Ahya R, Greaves M, Maffulli N. A one-centre prospective audit of peri- and postoperative blood loss and transfusion practice in patients undergoing hip or knee replacement surgery. Ann R Coll Surg Engl. 2000;82(1):44–48. [PMC free article] [PubMed] [Google Scholar]
- 8.Franceschini G, Visconti G, Di Leone A, Masetti R. “Warning” to postoperative complications when using hemostatic agents! Acta Neurochir (Wien) 2019;161(5):871–872. doi: 10.1007/s00701-019-03863-y. [DOI] [PubMed] [Google Scholar]
- 9.Wang JQ, Chen LY, Jiang BJ, Zhao YM. Development of a nomogram for predicting blood transfusion risk after hemiarthroplasty for femoral neck fractures in elderly patients. Med Sci Monit. 2020;26:e920255. doi: 10.12659/MSM.920255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikolaou VS, Masouros P, Floros T, Chronopoulos E, Skertsou M, Babis GC. Single dose of tranexamic acid effectively reduces blood loss and transfusion rates in elderly patients undergoing surgery for hip fracture: a randomized controlled trial. Bone Joint J. 2021;103-B(3):442–448. doi: 10.1302/0301-620X.103B3.BJJ-2020-1288.R1. [DOI] [PubMed] [Google Scholar]
- 11.Chang WK, Tai YH, Lin SP, Wu HL, Chan MY, Chang KY. Perioperative blood transfusions are not associated with overall survival in elderly patients receiving surgery for fractured hips. J Chin Med Assoc. 2019;82(10):787–790. doi: 10.1097/JCMA.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 12.Zhu C, Yin J, Wang B, Xue Q, Gao S, Xing L, Wang H, Liu W, Liu X. Restrictive versus liberal strategy for red blood-cell transfusion in hip fracture patients: a systematic review and meta-analysis. Medicine (Baltimore) 2019;98(32):e16795. doi: 10.1097/MD.0000000000016795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23(13):2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 14.Spahn DR. Anemia and patient blood management in hip and knee surgery: a systematic review of the literature. Anesthesiology. 2010;113(2):482–495. doi: 10.1097/ALN.0b013e3181e08e97. [DOI] [PubMed] [Google Scholar]
- 15.Shokoohi A, Stanworth S, Mistry D, Lamb S, Staves J, Murphy MF. The risks of red cell transfusion for hip fracture surgery in the elderly. Vox Sang. 2012;103(3):223–230. doi: 10.1111/j.1423-0410.2012.01606.x. [DOI] [PubMed] [Google Scholar]
- 16.Dai CQ, Wang LH, Zhu YQ, Xu GH, Shan JB, Huang WC, Wei LH, Zhou FL, Li Y. Risk factors of perioperative blood transfusion in elderly patients with femoral intertrochanteric fracture. Medicine (Baltimore) 2020;99(15):e19726. doi: 10.1097/MD.0000000000019726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Modig J. Regional anaesthesia and blood loss. Acta Anaesthesiol Scand Suppl. 1988;89:44–48. doi: 10.1111/j.1399-6576.1988.tb02842.x. [DOI] [PubMed] [Google Scholar]
- 18.Borghi B, Casati A, Iuorio S, Celleno D, Michael M, Serafini P, Pusceddu A, Fanelli G, Study Group on Orthopedic Anesthesia of the Italian Society of Anesthesia A, Intensive C Frequency of hypotension and bradycardia during general anesthesia, epidural anesthesia, or integrated epidural-general anesthesia for total hip replacement. J Clin Anesth. 2002;14(2):102–106. doi: 10.1016/S0952-8180(01)00362-2. [DOI] [PubMed] [Google Scholar]
- 19.Lin ZX, Woolf SK. Safety, efficacy, and cost-effectiveness of tranexamic acid in orthopedic surgery. Orthopedics. 2016;39(2):119–130. doi: 10.3928/01477447-20160301-05. [DOI] [PubMed] [Google Scholar]
- 20.Pennestri F, Maffulli N, Sirtori P, Perazzo P, Negrini F, Banfi G, Peretti GM. Blood management in fast-track orthopedic surgery: an evidence-based narrative review. J Orthop Surg Res. 2019;14(1):263. doi: 10.1186/s13018-019-1296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye W, Liu Y, Liu WF, Li XL, Shao J. The optimal regimen of oral tranexamic acid administration for primary total knee/hip replacement: a meta-analysis and narrative review of a randomized controlled trial. J Orthop Surg Res. 2020;15(1):457. doi: 10.1186/s13018-020-01983-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, Lv YM, Ding PJ, Li J, Ying-Ze Z. The reduction in blood loss with intra-articular injection of tranexamic acid in unilateral total knee arthroplasty without operative drains: a randomized controlled trial. Eur J Orthop Surg Traumatol. 2015;25(1):135–139. doi: 10.1007/s00590-014-1461-9. [DOI] [PubMed] [Google Scholar]
- 23.Ma H, Wang H, Long X, Xu Z, Chen X, Li M, He T, Wang W, Liu L, Liu X. Early intravenous tranexamic acid intervention reduces post-traumatic hidden blood loss in elderly patients with intertrochanteric fracture: a randomized controlled trial. J Orthop Surg Res. 2021;16(1):106. doi: 10.1186/s13018-020-02166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brunskill SJ, Millette SL, Shokoohi A, Pulford EC, Doree C, Murphy MF, Stanworth S. Red blood cell transfusion for people undergoing hip fracture surgery. Cochrane Database Syst Rev. 2015;4:CD009699. doi: 10.1002/14651858.CD009699.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karademir G, Bilgin Y, Ersen A, Polat G, Buget MI, Demirel M, Balci HI. Hip fractures in patients older than 75 years old: retrospective analysis for prognostic factors. Int J Surg. 2015;24(Pt A):101–104. doi: 10.1016/j.ijsu.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Gruson KI, Accousti KJ, Parsons BO, Pillai G, Flatow EL. Transfusion after shoulder arthroplasty: an analysis of rates and risk factors. J Shoulder Elbow Surg. 2009;18(2):225–230. doi: 10.1016/j.jse.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Jones CI. Platelet function and ageing. Mamm Genome. 2016;27(7-8):358–366. doi: 10.1007/s00335-016-9629-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.