Abstract
Background:
Neuropsychiatric symptoms can impact decision-making in patients with Alzheimer disease (AD).
Methods:
Using a simple decision-making task, a variant of the ultimatum game (UG) modified to control feelings of unfairness, this study investigated rejection responses among responders to unfair offers. The UG was administered to 11 patients with AD, 10 comparably demented patients with behavioral variant frontotemporal dementia (bvFTD), and 9 healthy controls (HC). The results were further compared with differences on the caregiver Neuropsychiatric Inventory (NPI).
Results:
Overall, patients with AD significantly rejected more total offers than did the patients with bvFTD and the HC (P < .01). On the NPI, the only domain that was significantly worse among the patients with AD compared to the other groups was dysphoria/depression.
Conclusions:
These results suggest that early AD can be distinguished based on increased rejections of offers in decision-making, possibly consequent to a heightened sense of unfairness from dysphoria/depression.
Keywords: Alzheimer disease, behavioral variant frontotemporal dementia, decision-making, dementia, emotion, ultimatum game
Introduction
Decision-making capacity depends on cognition, particularly frontal-executive functions, but also varies with neuropsychiatric symptoms (NPSs) such as depression and anxiety.1,2 Previous research on decision-making in Alzheimer disease (AD) has focused on the impact of cognitive deficits, but not on the impact of their NPSs.3 Recognizing NPS effects on decision-making is especially important because they may be reversible with treatment. The presence of depression, in particular, can bias decision-making toward negative feelings and a heightened sense of unfairness.4-8 In addition, these NPS effects upon decision-making may help distinguish patients with early AD compared to other dementias, such as behavioral variant frontotemporal dementia (bvFTD).
Investigators can test the simplest forms of decision-making using neuroeconomic decision-making simulation games, such as the dictator game and the ultimatum game (UG). Despite their simplicity, very few investigators have used these games to evaluate decision-making in AD.9 In these games, a player (“proposer”) receives a set amount of money and must decide how much to share with another player. In the dictator game, the “receiver” can only accept the money, but in the UG, the “responder” must decide to reject or accept the sums of money from the proposer. Using the UG, most, but not all studies, report that acceptance rates of depressed patients are lower than those of healthy controls (HC) and that depressed patients judged the stimuli more negatively.10-13 In these depressed patients, the responders’ behavior in the UG is motivated by a negative emotional bias toward increased feelings of unfairness, thus altering rational decision-making.
We sought to clarify how NPS-like depression affects decision-making on the UG among patients with AD. This study used a variant of the UG modified to control feelings of unfairness, yet allow NPS to affect the patients in their role of responders to unfair offers. Given the greater prevalence of depression and anxiety in patients with AD compared to those with bvFTD,14-16 we predicted that these NPSs would result in a negative bias to unfair proposals in the UG and greater rejection rates in AD compared to patients with bvFTD or HC. The results of this study supported this prediction.
Methods
Participants
This study recruited 11 patients with early AD enrolled in a university clinic after being diagnosed per Institutes of Aging-Alzheimer Association criteria for clinically probable AD.17 A comparison group of 10 patients with early bvFTD were enrolled after meeting International Consensus Criteria for Clinically Probable bvFTD.18 On entry, all patients had an extensive neurological assessment and neuroimaging, along with the Mini-Mental State Examination (MMSE) and a Montreal Cognitive Assessment (MoCA). There were an additional 9 HC matched by age (within 3 years), sex, and education (within 3 years), with the 2 patient groups. Exclusionary criteria included individuals with major medical or established premorbid (nondementia) psychiatric illnesses. University of California at Los Angeles institutional review board approved the protocol and confirmed its ethical compliance to the Declaration of Helsinki (ClinicalTrails.gov NCT01147679). All patients provided informed consent and their private health information was stored with a Health Insurance Portability and Accountability Act compliant storage system.
Ultimatum Game Procedures
Participants were presented a modified, computerized form of the UG, previously set to have an acceptance rate of 100% by level 2 and 75% at level 1 among 20 normal participants. This computerized version was sufficiently simple enough so that cognitively impaired patients could understand and respond. The computerized version muted the effect of an actual human participant as the proposer, thus minimizing report feelings of unfairness among the normal participants and maximizing acceptance rates. The participants viewed a screen displaying several prompts. A prerecorded narrator also read these prompts during the experiment so that both a vocal and a visual prompt informed the patient of the first offer (“Offer-Initial”). This first offer involved splitting US$20.00 such that the participant responder received 10% of the money while the computerized proposer retained 90%. The participant could either verbally accept this offer to receive a hypothetical US$2.00 or reject the offer leaving neither party with income in the game. Those who refused this offer received a second offer so that only those who rejected the initial offer knew and experienced the second offer. The second offer, a 20% to 80% split, involved the responder receiving US$4.00 while the computerized proposer retained US$16.00. The final combined results of the first and second offer were analyzed as “Offer-Total.” After each presentation, the participants were asked to explain, in their own words, the nature of the decision they made, hence assuring comprehension of the task. All participants were able to describe the task and the offers.
Neuropsychiatric Inventory
The dementia participants’ caregivers (AD and bvFTD) completed the Neuropsychiatric Inventory (NPI),19,20 a well-validated instrument designed for screening the wide variety of NPSs in dementia. This instrument evaluated the following 12 domains: delusions, hallucinations, agitation/aggression, dysphoria/depression, anxiety, euphoria, apathy, disinhibition, irritability/lability, aberrant motor activity, nighttime behavioral disturbances, and appetite and eating abnormalities. The caregivers were asked a screening question in each domain, and, if present, the caregivers were then asked 7 to 9 domain questions used to rate each domain by frequency on a 4-point scale and by severity on a 3-point scale. The final domain score was obtained by multiplying the frequency and severity scores, and the total NPI score was the sum of all of the individual domain scores (0-144).
Statistical Analyses
Each statistical analysis utilized SPSS version 23.0 software. First, the investigators checked assumptions to run parametric statistics. Subsequently, the investigators generated basic (eg, age, sex, education, age of onset, MMSE, MoCA) statistics for each group. Next, these characteristics were compared between the groups using χ2 and analysis of variance or t tests for categorical and continuous variables, respectively. A final step involved logistic regression of acceptance rates with group and NPI domains as predictor variables.
Results
The groups did not differ by age, gender, or education (see Table 1). In addition, the 2 dementia groups did not differ in their overall cognitive status on the MMSE and MoCA (t = 0.69 and 0.49, respectively).
Table 1.
Group Characteristics: Alzheimer Disease (AD), Behavioral Variant Frontotemporal Dementia (bvFTD), and Healthy Controls (HC).a,b
| AD, n = 11 | bvFTD, n = 10 |
HC, n = 9 | |
|---|---|---|---|
| Age of testing | 61.36 (5.70) | 62.40 (11.51) | 59.77 (10.08) |
| Sex, male (%) | 36 | 32 | 33.3 |
| Education (years) | 16.55 (1.86) | 16.0 (1.89) | 16.64 (1.85) |
| Age of onset | 58.09 (6.11) | 57.0 (10.18) | NA |
| Mini-Mental State Examination | 22.67 (7.57) | 24.83 (6.65) | NA |
| Montreal Cognitive Assessment | 17.33 (6.35) | 19.0 (9.20) | NA |
Abbreviations: AD, Alzheimer disease; NA, not applicable.
Means and standard deviations except percentage for sex.
No significant group differences in these variables.
Ultimatum Game
Patients with AD had significantly lower acceptance rates on the UG. There were overall group differences in both Offer-Initial (χ2 = 7.13, P < .05) and Offer-Total (χ2 = 10.03, P < .01). On Offer-Initial, acceptance rates were 2 (18%) of 11 for the AD group, 5 (50%) of 10 for the bvFTD group, and 7 (78%) of 9 for the HC group. On Offer-Total, acceptance rates were 3 (27.3%) of 11 for the AD group, 9 (90%) of 10 for the bvFTD group, and 7 (78%) of 9 for the HC group. Further, between-group analysis showed significant differences between AD and HC on Offer-Initial (χ2 = 7.10, P < .01) and on Offer-Total (χ2 = 5.05, P < .05) and between AD and bvFTD groups on Offer-Total (χ2 = 8.42, P < .01; see Figure 1).
Figure 1.
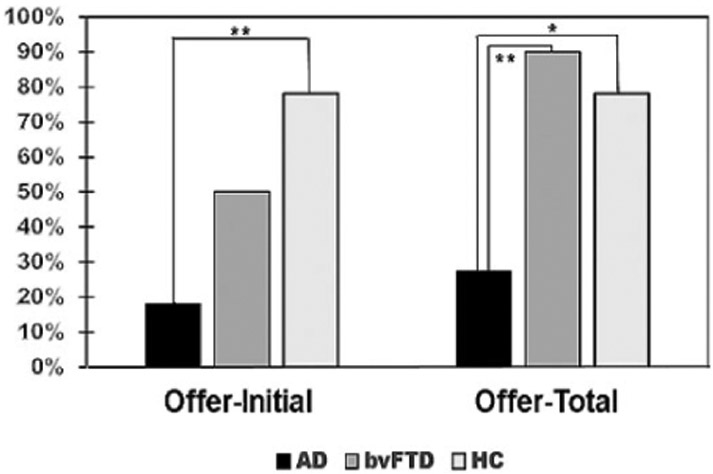
Acceptance rates between patients with Alzheimer disease (AD), behavioral variant frontotemporal dementia (bvFTD), and healthy controls (HC). *Significant differences at the <.05 level; **significant differences at the <.01 level.
Neuropsychiatric Inventory
As anticipated, the patients with bvFTD had worse total NPI scores reflecting worse performance in the behavioral domains that define bvFTD: apathy/indifference, disinhibition, aberrant motor behavior, and appetite/eating as well agitation/aggression (see Table 2).18 In contrast, the only domain where patients with AD scored worse than patients with bvFTD was dysphoria/depression. On the NPI, 0 (absent NPS) is a normal score19; therefore, the patients with AD had additional positive symptoms in anxiety, apathy/indifference, irritability, aberrant motor, sleep/nighttime, and appetite/eating.
Table 2.
Neuropsychiatric Inventory Domain and Total Scores: Alzheimer Disease (AD), Behavioral Variant Frontotemporal Dementia (bvFTD), and Healthy Controls (HC).a,b
| NPI Item | AD | bvFTD | t Test | P Value |
|---|---|---|---|---|
| Delusions | 0 | 1.88 (4.224) | 1.169 | ns |
| Hallucinations | 0 | 1.13 (2.80) | 1.058 | ns |
| Agitation/aggression | 0 | 3.25 (3.576) | 2.393 | <.05 |
| Dysphoria/depression | 2.57 (2.57) | 0.38 (1.061) | −2.22 | <.05 |
| Anxiety | 2.14 (3.76) | 2.13 (3.944) | −0.009 | ns |
| Elation/euphoria | 0 | 1.75 (3.284) | 1.403 | ns |
| Apathy/indifference | 1.43 (3.36) | 6.66 (4.307) | 2.575 | <.05 |
| Disinhibition | 0 | 6.50 (4.84) | 3.536 | <.01 |
| Irritability | 0.43 (1.134) | 1.88 (2.295) | 1.509 | ns |
| Aberrant motor | 1.29 (2.984) | 6.50 (4.751) | 2.498 | <.05 |
| Sleep/nighttime | 2.15 (3.40) | 3.38 (4.926) | 0.491 | ns |
| Appetite/eating | 1.14 (3.02) | 8.75 (4.268) | 3.924 | <.01 |
| Total | 11.14 (11.99) | 44.19 (27.43) | 3.64 | .0017 |
Abbreviation: ns, nonsignificant.
Means and standard deviations for frequency × severity scores.
Normal responses are “0” or absent the neuropsychiatry symptom(s).
A logistic regression of acceptance rates did not reveal significant differences for predicting acceptance rates across groups based on NPI domains. However, among the 8 patients with AD who persisted in rejected the offer (Offer-Total), all scored 2 or more on the NPI dysphoria/depression domain (2 × 2 Fisher test, P < .01).
Discussion
This study explored NPS effects on decision-making in AD by responses to a version of the UG, modified to control the sense of unfairness. The patients with AD more frequently rejected unfair offers in comparison to both patients with bvFTD and HC, whereas the patients with bvFTD did not differ from the normal patients. The patients with AD also had significant dysphoria/depression on the NPI. Together, these findings suggest that a heightened sense of unfairness, such as from depression, can significantly influence decision-making beyond cognitive impairments in AD and that treating these emotional aspects may improve decision-making among patients with dementia.
Neuropsychiatric symptoms alter the feelings of fairness feelings from offers and, by extension, alter the likelihood of acceptance or rejection of the offers. Depression appears to be the most important NPS to alter the feelings of fairness. On the UG, in most, but not all reports, the acceptance rates of depressed patients, who judge the stimuli more negatively, are lower than those for normal controls.10-12 Nondepressed people reject many offers that they see as unfair regardless of poor outcomes for themselves, and depressed people reject even more of these offers because they see them as even more unfair.21 Thus, depression is associated with a negative emotional bias that can alter decision-making in patients with dementia as well as in nondemented individuals.
Although this study did not establish depression as the cause of the decreased acceptance rates among the patients with AD, the frequent presence of depression in AD, and in our AD group, supports this association.14-16,22 After apathy, depression is the most common NPS in AD, followed by aggression, anxiety, and sleep disorders.16 Depression occurs in 42% to 50% of patients with AD and can occur as one of the earliest symptoms of dementia or even precede the clinical diagnosis for years.14-16 In a meta-analysis of 63 studies, the prevalence of depression with dementia was 42% (confidence interval, 38-45), over 3 times more than expected for depression in general.14 There is further evidence that depression may correlate with τ pathology and amyloid-beta accumulation and that antidepressants may alleviate dysphoria/depression in AD.15
The difference in UG acceptance rates in the patients with AD versus patients with bvFTD was particularly notable, given the primary deficits in apathy, disinhibition, and executive cognition that are characteristic of bvFTD. Moreover, patients with bvFTD have early degradation of the anterior cingulate cortex, anterior insula, and ventromedial prefrontal cortex (vmPFC),16,23-28 areas involved in decision-making. However, the patients with bvFTD in this study did not have higher acceptance rates than HC. Other investigators have shown that patients with bvFTD did not differ in overall acceptance rates from HC but had decreased acceptance rates in fairer or prosocial scenarios and increased acceptance rates in punishment conditions.29 Two lesion studies involving the vmPFC showed higher rejection rates as compared to controls,28,30,31 but a third lesion study showed reduced sensitivity to fairness and increased acceptance of unfair offers.32 This discrepancy among lesion studies and those with bvFTD may reflect differential difficulty with decreased emotional reactivity,33,34 or, in some patients, elevated baseline emotional reactivity to unfair treatment.30,31
There are limitations to this study. First, the study’s sample size limits the generalizability of the results. This study needs to be confirmed among a larger cohort of patients with dementia. Second, the participants responded to a computer-generated presentation, which decreased the sense of the “humanness” of the proposer; however, this helped maximize acceptance rates in a methodology that was simple enough for patients with cognitively impaired dementia. Third, the link between decreased acceptance rates and dysphoria/depression among the patients with AD needs further documentation.
Conclusions
This study examined responses to the UG in patients with AD and those with bvFTD, as well as HC. In the role of “responders,” the patients with AD rejected unfair offers at a higher rate than the other groups and had more dysphoria/depression, suggesting an increased experience of the unfairness of offers due to negative emotional reactivity from depression. This study emphasizes the importance of evaluating patients with AD for depression that might benefit from treatment, with psychiatric assessment and further measures of depression in dementia. Further studies may clarify whether treating these patients for depression diminishes their feelings of unfairness and improves the decision-making of patients with early AD.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute of Aging grant numbers R01AG050967 and R01AG034499 (Mario F. Mendez, Principal Investigator). The funding source did not influence the performance of this research.
Footnotes
The data are included within this article or available on request from authors.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Paulus MP. Decision-making dysfunctions in psychiatry—altered homeostatic processing? Science. 2007;318(5850):602–606. [DOI] [PubMed] [Google Scholar]
- 2.Rubinsztein JS, Michael A, Underwood BR, Tempest M, Sahakian BJ. Impaired cognition and decision-making in bipolar depression but no ‘affective bias’ evident. Psychol Med. 2006;36(5):629–639. [DOI] [PubMed] [Google Scholar]
- 3.de Siqueira AS, Yokomizo JE, Jacob-Filho W, Yassuda MS, Aprahamian I. Review of decision-making in game tasks in elderly participants with Alzheimer disease and mild cognitive impairment. Dement Geriatr Cogn Disord. 2017;43(1-2):81–88. [DOI] [PubMed] [Google Scholar]
- 4.Harle KM, Allen JJ, Sanfey AG. The impact of depression on social economic decision making. J Abnorm Psychol. 2010;119(2):440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Must A, Szabo Z, Bodi N, Szasz A, Janka Z, Keri S. Sensitivity to reward and punishment and the prefrontal cortex in major depression. J Affect Disord. 2006;90(2-3):209–215. [DOI] [PubMed] [Google Scholar]
- 6.Smoski MJ, Lynch TR, Rosenthal MZ, Cheavens JS, Chapman AL, Krishnan RR. Decision-making and risk aversion among depressive adults. J Behav Ther Exp Psychiatry. 2008;39(4):567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cella M, Dymond S, Cooper A. Impaired flexible decision-making in major depressive disorder. J Affect Disord. 2010;124(1-2):207–210. [DOI] [PubMed] [Google Scholar]
- 8.Adida M, Jollant F, Clark L, et al. Trait-related decision-making impairment in the three phases of bipolar disorder. Biol Psychiatry. 2011;70(4):357–365. [DOI] [PubMed] [Google Scholar]
- 9.Bosch-Domenech A, Nagel R, Sanchez-Andres JV. Prosocial capabilities in Alzheimer’s patients. J Gerontol B Psychol Sci Soc Sci. 2010;65B(1):119–128. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Zhou Y, Li S, Wang P, Wu GW, Liu ZN. Impaired social decision making in patients with major depressive disorder. BMC Psychiatry. 2014;14:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheele D, Mihov Y, Schwederski O, Maier W, Hurlemann R. A negative emotional and economic judgment bias in major depression. Eur Arch Psychiatry Clin Neurosci. 2013;263(8):675–683. [DOI] [PubMed] [Google Scholar]
- 12.Destoop M, Schrijvers D, De Grave C, Sabbe B, De Bruijn ER. Better to give than to take? Interactive social decision-making in severe major depressive disorder. J Affect Disord. 2012;137(1-3):98–105. [DOI] [PubMed] [Google Scholar]
- 13.Hinterbuchinger B, Kaltenboeck A, Baumgartner JS, Mossaheb N, Friedrich F. Do patients with different psychiatric disorders show altered social decision-making? A systematic review of ultimatum game experiments in clinical populations. Cogn Neuropsychiatry. 2018;23(3):117–141. [DOI] [PubMed] [Google Scholar]
- 14.Chi S, Wang C, Jiang T, Zhu XC, Yu JT, Tan L. The prevalence of depression in Alzheimer’s disease: a systematic review and meta-analysis. Curr Alzheimer Res. 2015;12(2):189–198. [DOI] [PubMed] [Google Scholar]
- 15.Chi S, Yu JT, Tan MS, Tan L. Depression in Alzheimer’s disease: epidemiology, mechanisms, and management. J Alzheimers Dis. 2014;42(3):739–755. [DOI] [PubMed] [Google Scholar]
- 16.Zhao QF, Tan L, Wang HF, et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: systematic review and meta-analysis. J Affect Disord. 2016;190:264–271. [DOI] [PubMed] [Google Scholar]
- 17.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34(7):939–939. [DOI] [PubMed] [Google Scholar]
- 18.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(pt 9):2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. [DOI] [PubMed] [Google Scholar]
- 20.Kaufer DI, Cummings JL, Christine D, et al. Assessing the impact of neuropsychiatric symptoms in Alzheimer’s disease: the Neuropsychiatric Inventory Caregiver Distress Scale. J Am Geriatr Soc. 1998;46(2):210–215. [DOI] [PubMed] [Google Scholar]
- 21.Chase HW, Camille N, Michael A, Bullmore ET, Robbins TW, Sahakian BJ. Regret and the negative evaluation of decision outcomes in major depression. Cogn Affect Behav Neurosci. 2010; 10(3):406–413. [DOI] [PubMed] [Google Scholar]
- 22.Novais F, Starkstein S. Phenomenology of depression in Alzheimer’s disease. J Alzheimers Dis. 2015;47(4):845–855. [DOI] [PubMed] [Google Scholar]
- 23.Kipps C, Nestor P, Acosta-Cabronero J, Arnold R, Hodges J. Understanding social dysfunction in the behavioural variant of frontotemporal dementia: the role of emotion and sarcasm processing. Brain. 2009;132(pt 3):592–603. [DOI] [PubMed] [Google Scholar]
- 24.Ibañez A, Manes F. Contextual social cognition and the behavioral variant of frontotemporal dementia. Neurology. 2012;78(17):1354–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies RR, Kipps CM, Mitchell J, Kril JJ, Halliday GM, Hodgels JR. Progression in frontotemporal dementia: identifying a benign behavioral variant by magnetic resonance imaging. Arch Neurol. 2006;63(11):1627–1631. [DOI] [PubMed] [Google Scholar]
- 26.Rilling JK, Sanfey AG. The neuroscience of social decision-making. Annu Rev Psychol. 2011;62:23–48. [DOI] [PubMed] [Google Scholar]
- 27.Gabay AS, Radua J, Kempton MJ, Mehta MA. The ultimatum game and the brain: a meta-analysis of neuroimaging studies. Neurosci Biobehav Rev. 2014;47:549–558. [DOI] [PubMed] [Google Scholar]
- 28.Shamay-Tsoory SG, Suleiman R, Aharon-Peretz J, Gohary R, Hirschberger G. Sensitivity to fairness and intentions of others in the ultimatum game in patients with ventromedial prefontal lesions. J Int Neuropsychol Soc. 2012;18(6):952–961. [DOI] [PubMed] [Google Scholar]
- 29.O’Callaghan C, Bertoux M, Irish M, et al. Fair play: social norm compliance failures in behavioural variant frontotemporal dementia. Brain. 2016;139(pt 1):204–216. [DOI] [PubMed] [Google Scholar]
- 30.Koenigs M, Kruepke M, Newman JP. Economic decision-making in psychopathy: a comparison with ventromedial prefrontal lesion patients. Neuropsychologia. 2010;48(7):2198–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koenigs M, Tranel D. Irrational economic decision-making after ventromedial prefrontal damage: evidence from the ultimatum game. J Neurosci. 2007;27(4):951–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu X, Wang X, Hula A, et al. Necessary, yet dissociable contributions of the insular and ventromedial prefrontal cortices to norm adaptation: computational and lesion evidence in humans. J Neurosci. 2015;35(2):467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mendez MF, Shapira JS. Loss of emotional insight in behavioral variant frontotemporal dementia or “frontal anosodiaphoria”. Conscious Cogn. 2011;20(4):1690–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carr AR, Samimi MS, Paholpak P, Jimenez EE, Mendez MF. Emotional quotient in frontotemporal dementia vs. Alzheimer’s disease: the role of socioemotional agnosia. Cogn Neuropsychiatry. 2017;22(1):28–38. [DOI] [PubMed] [Google Scholar]


