Worldwide, since the beginning of the COVID-19 pandemic, patients with acute respiratory distress syndrome are managed in intensive care units. During the hospitalization, the physicians' efforts are focus on vital functions, and sometimes making forget to compensate for other deficiencies, such as dry eye. Indeed, during patients’ sedation, the eyes are particularly exposed to the risk of corneal damage. Sixty percent of sedated patients have a palpable malocclusion, with the lower part of the cornea remaining uncovered, which can cause exposure keratitis with a variable prevalence up to 60% [1]. A secondary infection with microbial keratitis may lead to a permanent visual impairment [2]. Patients in intensive care units may be placed in a temporary prone position. In this position, eyelids closure cannot be controlled, and patients may have their eyes open, which can significantly worsen surface eye damage. To our knowledge, the effect of prone positioning on the ocular surface is poorly documented [3].
The main objective of the present study was to describe the pathologies of the ocular surface in COVID-19 patients hospitalized in an intensive care unit according to their positioning.
All consecutive patients diagnosed positive for SARS-CoV2 by nasal swab PCR or typical images on computerized tomography, which were hospitalized in an intensive care unit at the Rothschild Foundation (Paris, France) from March to May 2020 were eligible for the study. A French ethics committee (Comité de Protection des Personnes Sud-Ouest et Outre-Mer) approved this study. The study was registered at clinicaltrial.gov under the number NCT 04386810. All patients or their representatives gave a written informed consent before study participation. At the admission in the intensive care unit, severity was assessed by scores SAPS II [4], SOFA [5], and the severity of the acute respiratory distress syndrome was assessed by the Berlin score [6]. During the hospitalization in the intensive care unit, eyes were kept closed and ophthalmological lubricant (Carbomer eye gel) instilled twice a day by nurse team. In prone position, lid taping was associated with ocular lubrication with special care for tape positioning. All the patients included underwent at least one examination of the ocular surface of both eyes and had a weekly clinical examination (i.e. looking for a palpebral malocclusion according to Suresh Score, a chemosis according to Ezra grades, measures of break-up time test after fluorescein dye instillation, Schirmer II test, and Oxford grading [7] after fluorescein dye instillation) by an ophthalmologist. When patients had repeated ophthalmologic examinations, the worst value of each item from one of the 2 eyes was retained.
In our study, 40 eyes of 20 patients were included. The median age was 66.3 years (IQR: 62.6–73.1). On the day of admission in the intensive care unit, the acute respiratory distress syndrome according to the Berlin definition [6] was moderate for 10 patients (50%), severe for 9 patients (45%), and mild for 1 patient (5%). The median scores of SAPS II [3] and SOFA [5] were respectively 55.5 (IQR: 48.5–68.0) and 6.5 (IQR: 3.0–7.5). The median duration of intensive care was 20 days (IQR: 14–31.75). All included patients survived. During the hospitalization, 15 patients (75%) were positioned at least once in the prone position, with a median duration of prone positioning of 32 hours (IQR: 18–45).
Only 2 patients (10%) had unilateral corneal injury. Both patients had corneal epithelial defect (Fig. 1 ) fully resolved within 24 hours after application of vitamin A ointment and eyelid occlusion. The first patient had a 5 mm × 1 mm inferior corneal ulceration with less than 10% corneal stromal thinning and the risk factor was eyelid malocclusion. The second patient had a 2mm nasal corneal epithelial defect located near a pinguecula, and the risk factor was chronic ocular surface inflammation associated with eyelid malocclusion. Sixteen patients (80%) had a superficial punctate keratitis without ulcer.
Fig. 1.
65 years old patient with Covid-19 admitted in intensive care unit since 1 day placed twice during 6 h in prone position. Photograph with fluorescein stain shows a corneal ulcer (a) fully resolved within 24 hours (b). 53 years old patient with Covid-19 in intensive care unit since 5 days placed only in supine position. Photograph with fluorescein stain shows a corneal epithelial defect (c).
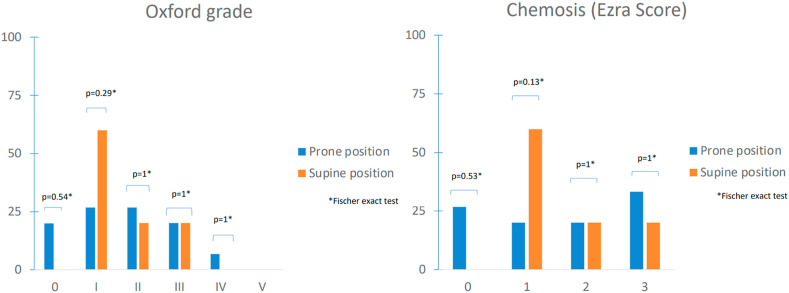
Eyelid malocclusion (Suresh score), Oxford grade [7], chemosis (Graph 1 ), break-up time, and Schirmer II test, were not worsened by positioning. One prone patient had bilateral subconjunctival hemorrhages.
Graph 1.
Comparison of percentages of corneal staining (Oxford grade) and chemosis grade (Ezra score) between Covid19 patients placed in supine and prone position. Raw data were compared using Fischer exact test. Significance: P < 0.05.
This study is the first to describe the characteristics of the ocular surface of patients infected by SARS-CoV-2 hospitalized in an intensive care unit in the prone position. In the overall sample of 40 eyes from 20 patients, only 2 patients (10%) had unilateral uninfected corneal ulceration resolving after eyelid occlusion and healing ointment.
The main strength of our study is its prospective design. The inclusion of patients was dependent on the epidemic, our hospital welcoming COVID-19 patients only at the first peak of the epidemic in France.
The Schirmer II test was not lower than normal while the break-up time test was shortened than normal. This could be due to an evaporative drought, the sedated patients no longer blinking. In addition, Bell's phenomenon and rapid eye movement (REM) are altered by sedation.
REM sleep is the fifth and last stage of sleep that occurs in the sleep cycle. This could lead to corneal complications, because cornea is immobile under the eyelids.
Physiologically, lipids secreted by the meibomian glands are incorporated into the tears during blinking. Indeed, lipids are an important layer of tears and have a major role in the protection of the ocular surface. It would be interesting to see if patients sedated a for long time present meibomian glands dysfonction linked to their absence of stimulation.
More generally, the eyelids, eyelashes and the tear film constitute a protective barrier of the ocular surface, while tears have both a lubricating and an antimicrobial role.
The main limitation of the study is the small sample size in the non-prone group. While the study is not powered to determine a significant difference between both groups, a larger sample may show more severe chemosis with prone position. The direct compression on the eye may elevate intraocular pressure and results in frequent periorbital edema and chemosis. On the other hand, patients in prolonged supine position in intensive care unit have diffuse swelling including face swelling due to vasoplegia that may also lead to chemosis.
In summary, our study shows that patients with COVID-19 hospitalized in intensive care unit with prone position did not have important lesions of the ocular surface. Due to sedation, no blinking and poor eyelid occlusions are risk factors for eye damage. In order to prevent the complications of exposure keratopathy, we recommend keeping the eyelids closed with any lubrificant eye drop, associated why lid taping for prone position, and regularly examined the eyes of all sedated patients, weekly for prone patients in ICU, with the aim of quickly detecting and treating complications.
No meeting presentation.
Declaration of competing interest
No conflicting relationship exists for any author.
References
- 1.Grixti A., Sadri M., Edgar J., Datta A.V. Common ocular surface disorders in patients in intensive care units. Ocul Surf. 2012 Jan;10(1):26–42. doi: 10.1016/j.jtos.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Kirwan J.F., Potamitis T., el-Kasaby H., Hope-Ross M.W., Sutton G.A. Microbial keratitis in intensive care. BMJ. 1997;314(7078):433–434. doi: 10.1136/bmj.314.7078.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanghi P., Malik M., Hossain I.T. Manzouri B ocular complications in the prone position in the critical care setting: the COVID-19 pandemic. J Intensive Care Med. 2021 Mar;36(3):361–372. doi: 10.1177/0885066620959031. [DOI] [PubMed] [Google Scholar]
- 4.Le Gall J.R., Lemeshow S., Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. J Am Med Assoc. 1993;270(24):2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 5.Moreno R., Vincent J.L., Matos R., et al. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study. Working Group on Sepsis related Problems of the ESICM. Intensive Care Med. 1999;25(7):686–696. doi: 10.1007/s001340050931. [DOI] [PubMed] [Google Scholar]
- 6.ARDS Definition Task Force, Ranieri V.M., Rubenfeld G.D., et al. Acute respiratory distress syndrome: the Berlin Definition. J Am Med Assoc. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 7.Bron A.J., Evans V.E., Smith J.A. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22(7):640–650. doi: 10.1097/00003226-200310000-00008. [DOI] [PubMed] [Google Scholar]