Abstract:
Blood-primed cardiopulmonary bypass circuits are frequently necessary to achieve safe support during pediatric open-heart surgery. Literature is lacking regarding suitable prime constituents or methods for achieving a physiologically appropriate blood-primed circuit. We examined the chemistry and hematology of neonatal blood-primed circuits from the conclusion of the priming procedure until the initiation of bypass. Base deficit/excess, pH, pO2, pCO2, HCO3, glucose, sodium, potassium, calcium, hematocrit, lactate, and osmolality were analyzed. Any deviation over time from the original prime value was compared for significance. Statistically significant changes were found between T0 and all time points for all parameters, except for pH and pO2 out to 1 hour. Among all parameters, various rates of change were observed. Although most changes in the parameters were found to be statistically significant, those changes may not be clinically significant based on clinician interpretation. Attention to the prime quality beyond the immediate post-priming period may be beneficial. Should the time period between validation of the prime quality and initiation of bypass be extended, it may be advisable to reevaluate the prime quality.
Keywords: cardiopulmonary bypass, blood prime, neonatal, prime quality
Blood products alter the patient’s acid–base status, electrolytes, fluid balance, oxygenation, and hemodynamics (1). Maintaining a normal physiologic profile throughout the course of cardiopulmonary bypass (CPB) is crucial to the patient’s outcome. Setting a standard for a safe and appropriate CPB blood prime is an important component of that process. Institutional protocols set the conduct of CPB, which includes adjusting prime constituents to within appropriate ranges (2). Little data currently exist regarding the quality of the CPB circuit blood prime (3). The neonatal circuit prime used in this study is approximately 244 mL. Using a conventional calculation of 85 mL/kg, some of the smallest congenital heart surgery patients have a circulating blood volume of less than 325 mL (2). This high ratio of circuit prime volume to patient blood volume makes it important to have a physiologically balanced CPB blood primed circuit. Historically, our blood priming process concluded with a sample drawn to evaluate the prime hematocrit, electrolyte, osmolality, and acid–base status. Based on the result, appropriate changes may be made, and the prime was reevaluated at the discretion of the perfusionist. To have a completed CPB circuit prime before surgical incision, priming occurs at the beginning of the case. Therefore, the prime may recirculate for an extended period of time before the initiation of CPB. This study examines the quality of the CPB blood primed circuit from the conclusion of the CPB priming procedure until the initiation of CPB.
METHODS
The arterial-venous (A-V) loop of a CPB circuit used for patients requiring 1,400 mL/min of flow or less consisted of a Terumo FX05 oxygenator (Terumo Cardiovascular, Ann Arbor, MI) with a hard-shell venous reservoir, a 1/4″ or 3/16″ pump boot, a 3/16″ × 1/4″ A-V loop (Terumo Cardiovascular), and a DHF02 hemofilter (LivaNova, London, UK) attached to the sampling manifold.
The CPB circuit was primed, and blood prime quality was verified according to departmental guidelines. The total prime volume for the pediatric CPB circuit was 268 mL for the circuit with the 1/4″ pump boot and 244 mL for the circuit including the 3/16″ pump boot. The CPB circuit was primed with Plasmalyte-A (Baxter, Deerfield, IL) containing 2 mEq NaHCO3 and 20 mg CaCl2 per 100 mL of Plasmalyte-A. A pre-bypass filter was removed after the crystalloid prime process was complete, and 50 mL of 25% albumin was added. The circuit volume was hemoconcentrated to a dynamic reservoir level of 30 mL. Two thousand (2,000) units of heparin was added to the prime. Fresh frozen plasma in the amount of 120 mL was added to the reservoir. All banked packed red blood cells (PRBCs) were washed with Plasmalyte-A in a Fresenius CATS autotransfusion device (Fresenius, Lake Zurich, IL) before being added to the CPB circuit as per departmental guidelines. Washed PRBCs in the amount of 120 mL were added to the prime to ensure a target on pump hematocrit of 25% or greater. The prime volume was again hemoconcentrated to a dynamic reservoir level of 30 mL. One hundred fifty mL of .45% saline was added to the prime and removed via hemoconcentration again to a reservoir level of 30 mL. Six mEq of NaHCO3 and 60 mg CaCl2 were added to the prime. The prime volume was recirculated at 500 mL/min without restriction, and sweep gas was applied until pCO2 of 55 mmHg was achieved as reported by the gas calibrated CDI 500 blood gas analyzer (Terumo) at which point the sweep gas was discontinued. The first blood gas was drawn as per clinical routine at 5 minutes after prime completion and designated as time 0 (T0). The prime continued to recirculate at 500 mL/min, and subsequent blood samples occurred within a four-minute window until the initiation of CPB at 15, 30, 45, 60, 90, and 120 minutes from T0. All prime quality data including pH, pO2, pCO2, HCO3, base deficit/excess, glucose, sodium, potassium, calcium, hematocrit, hemoglobin, lactate, and osmolality (calculated) were collected in a REDCap database. No changes were made to the CPB prime based on the samples drawn for this study. The institutional review board approved this study.
STATISTICAL ANALYSIS
Values were summarized using means and standard deviations for continuous variables and frequencies, respectively, and percent for categorical variables. Changes in blood values over time were visualized with scatterplots showing individual changes over time overlaid with the overall trend. Paired sample t-tests were used to test for differences between baseline and the last follow-up values of acid–base status, electrolyte levels, fluid status, oxygenation, and hemodynamic status. Linear mixed models were used to analyze the change of blood values over time, allowing subjects to have specific initial value (random intercept) with a first-order continuous autoregressive error structure. Changes from baseline were compared using Dunnett’s test. p-values <.05 were considered significant.
RESULTS
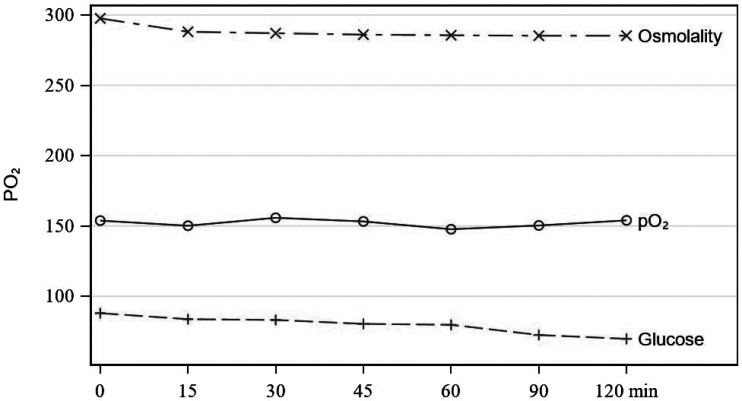
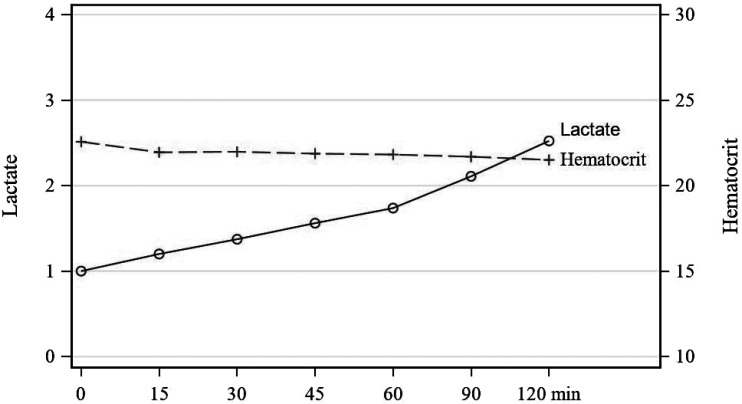
Ninety-nine CPB blood primes were analyzed. Statistically significant changes were found between T0 and all time points for all parameters, except for pH and pO2 through the first hour (Table 1). Change in values for pH were only statistically significant after T1, whereas changes for pO2 were significant at all time points (Figure 1). Among parameters, various rates of change were observed. There is a continual and steady rise in pH throughout the recirculation time studied (Figure 2). Within the first thirty minutes of recirculation, pCO2, HCO3, base excess, calcium, and sodium dropped significantly. This was followed by a steady but less dramatic decrease throughout the remainder of the recirculation period (Figures 3 and 4). These changes were analyzed and determined to be statistically and clinically significant within the 30-minute window identified. There was a consistent decrease in glucose that was statistically significant (Figure 5). The change in hematocrit was significant until T4, at which point the change was no longer significant. Potassium decreased throughout the time studied at a gradual rate (Figure 3). Lactates, even though increasing significantly over time, remained less than 2.5 mmol/L (Figure 6). Last, osmolality results dropped significantly within the first 30 minutes of recirculation and continued to drop statistically while staying within normal range throughout the recirculation of the prime (Figure 5).
Table 1.
Change in value from previous time point.
| Variable | T1 (n = 99) | T2 (n = 84) | T3 (n = 66) | T4 (n = 49) | T5 (n = 25) | T6 (n = 9) |
|---|---|---|---|---|---|---|
| pH | .00899 ± .0498 | .0194 ± .0235 | .0159 ± .0174 | .0173 ± .0143 | .03 ± .0171 | .0189 ± .0105 |
| pCO2 | −9.16 ± 5.93 | −2.8 ± 1.76 | −2.13 ± 1.02 | −1.8 ± .853 | −2.71 ± 1.01 | −2 ± .962 |
| pO2 | −3.77 ± 27.3 | 5.89 ± 27.2 | 4.36 ± 28.9 | −.388 ± 3.49 | 2.44 ± 4.67 | 3 ± 9.95 |
| HCO3 | −5 ± 2.35 | −.942 ± 1.03 | −.665 ± .356 | −.592 ± .358 | −.876 ± .376 | −.667 ± .235 |
| Base | −4.33 ± 1.9 | −.535 ± .995 | −.338 ± .301 | −.233 ± .236 | −.308 ± .158 | −.311 ± .162 |
| Ca | −.127 ± .066 | −.0144 ± .0238 | −.00864 ± .0091 | −.00571 ± .00816 | −.0076 ± .00436 | −.00444 ± .00527 |
| Na | −4.72 ± 2.07 | −.393 ± 1.25 | −.258 ± .73 | −.204 ± .79 | −.28 ± .458 | −.111 ± .333 |
| K | −.0566 ± .0745 | −.0226 ± .0567 | −.0227 ± .0576 | −.0184 ± .0441 | −.008 ± .0572 | −.0444 ± .0527 |
| Glu | −4.12 ± 2.34 | −2.37 ± 1.56 | −2.61 ± 1.57 | −2.55 ± 1.43 | −3.76 ± 1.23 | −3.78 ± .972 |
| Hct | −.622 ± .415 | −.0798 ± .278 | −.097 ± .316 | −.0714 ± 0.5 | −.076 ± .231 | −.122 ± .273 |
| Hgb | −.198 ± .142 | −.0333 ± .101 | −.0258 ± .106 | −.0327 ± .17 | −.028 ± .0891 | −.0333 ± .112 |
| Lac | .201 ± .0814 | .2 ± .0677 | .183 ± .0776 | .176 ± .048 | .34 ± .0764 | .3 ± .05 |
| Osmo | −9.42 ± 4.28 | −1.03 ± 2.32 | −.802 ± 1.39 | −.431 ± 1.33 | −.668 ± .828 | −.433 ± .817 |
| p-Values Using Paired t-Test (no p-value Adjustment for Multiple Tests) | ||||||
| T1 | T2 | T3 | T4 | T5 | T6 | |
| pH | .6727 | .0000 | .0000 | .0000 | .0000 | .0039 |
| pCO2 | .0000 | .0000 | .0000 | .0000 | .0000 | .0002 |
| pO2 | .4585 | .1247 | .5943 | .8757 | .0003 | .7031 |
| HCO3 | .0000 | .0000 | .0000 | .0000 | .0000 | .0000 |
| Base | .0000 | .0000 | .0000 | .0000 | .0000 | .0004 |
| Ca | .0000 | .0000 | .0000 | .0000 | .0000 | .1250 |
| Na | .0000 | .0006 | .0044 | .1250 | .0156 | 1.0000 |
| K | .0000 | .0000 | .0002 | .0117 | .5703 | .1250 |
| Glu | .0000 | .0000 | .0000 | .0000 | .0000 | .0000 |
| Hct | .0000 | .0421 | .0396 | .8529 | .1168 | .2159 |
| Hgb | .0000 | .0167 | .0214 | .2551 | .2246 | .3972 |
| Lac | .0000 | .0000 | .0000 | .0000 | .0000 | .0039 |
| Osmo | .0000 | .0000 | .0000 | .0035 | .0000 | .0781 |
Note: Values are mean ± SD.
Figure 1.
pO2.
Figure 2.
pH.
Figure 3.
Calcium, potassium, and sodium.
Figure 4.
Base, HCO3, and pCO2.
Figure 5.
pO2, glucose, and osmolality.
Figure 6.
Lactate and hematocrit.
DISCUSSION
Many institutions measure the prime gas in a similar fashion to our historical practice as we described (4). However, as expected, we found the prime blood values change over time. We concluded that it may be beneficial to prepare the prime with the estimated time between priming and the initiation of CPB in mind. Most changes occurred within 30 minutes (T2) of T0. Beyond T2, the rate of change became less yet was still statistically significant for most values. Although the change beyond T2 was statistically significant, the clinical significance decreases with time. Although every patient comes to the operating room with a different acid–base and hematological condition, there is a way to standardize the process of achieving a reliable and safe prime.
The parameters hypothesized to change significantly over time were lactate, pCO2, acid–base balance, and sodium. These parameters were mostly affected by time and simultaneously easier to manipulate. The lactate increased with time; however, it was not felt to be clinically significant as the changes were minimal and were less than 2.5 mmol/L for all prime samples. Values for pCO2 exhibited the most change between time point T0 and T2. After 30 minutes, the changes are still statistically significant, but the rate of change is less than that in the first 30 minutes. Similarly, the most statistically significant and clinically significant changes for sodium, bicarbonate, and base deficit parameters occurred within the first 30 minutes of prime recirculation. After approximately 30 minutes, the rate of change is significantly reduced for most values. The statistical data show a high sodium prime will fall to within a physiologically acceptable range within 30 minutes of prime recirculation.
After considering the statistical data and their clinical relevance, a change to our priming practice was implemented. As a result of pCO2 and sodium falling significantly within the first 30 minutes, it was decided that eight mEq of NaHCO3 should be supplemented in place of the six mEq of NaHCO3 originally described in the protocol. Blood gases are now drawn at approximately 5 minutes as described by the priming protocol, and in addition at 30 minutes from the time that the first blood prime gas was drawn. The 5-minute blood analysis has been maintained as a safety measure to ensure the appropriate medications were administered to the prime and an expected blood gas profile resulted. The results from these changes yield a 5-minute prime value marginally outside of normal physiologic ranges. However, the prime values at 30 minutes result parameters that fall into normal physiologic ranges. For most surgeries, the pump is primed and prepared for support well before the 30-minute window, thus exposing the patient to a safe and optimized prime.
LIMITATIONS
A limitation identified in this study was the difference in pump boot sizes. Twenty-eight of the pump circuits included a 3/16″ pump boot, whereas seventy-two incorporated a 1/4″ pump boot. Because of the small number of circuits in each subset, an analysis of the two groups may not have been beneficial. However, both circuit designs were treated the same, and pump recirculation was standardized at 500 mL/min across both circuits. As such, both circuit designs were included in the analysis.
Although the conduct of circuit prime and the recipe used to blood prime are the same across the department, there were seven different perfusion team members who primed circuits during this study. Minor variation in the practice of those perfusionists and the conduct of prime may not be ruled out as a confounding factor.
CONCLUSION
Although most of the changes in parameters were found to be statistically significant, changes after 30 minutes may not be clinically significant based on clinician interpretation. Attention to the prime quality beyond the immediate post-priming period may be beneficial. Should the time period between validation of the prime quality and initiation of bypass be extended, it may be advisable to reevaluate the quality of the prime and treat any outlying parameters.
REFERENCES
- 1.Valleley MS, Buckley KW, Hayes KM, et al. Are there benefits to a fresh whole blood vs. packed red blood cell cardiopulmonary bypass prime on outcomes in neonatal and pediatric cardiac surgery? J Extra Corpor Technol. 2007;39:168–76. [PMC free article] [PubMed] [Google Scholar]
- 2.Matte GS. Perfusion for Congenital Heart Surgery Notes on Cardiopulmonary Bypass for a Complex Patient Population. Hoboken, NJ: John Wiley & Sons; 2015. [Google Scholar]
- 3.Lilley A The selection of priming fluids for cardiopulmonary bypass in the UK and Ireland. Perfusion. 2002;17:315–9. [DOI] [PubMed] [Google Scholar]
- 4.Reagor JA, Clingan S, Kulat BT, et al. The norwood stage 1 procedure - conduct of perfusion: 2017 survey results from NPC-QIC member institutions. Perfusion. 2018;33:667–78. [DOI] [PubMed] [Google Scholar]