Abstract
Introduction
Alport syndrome is a rare genetic disorder that affects as many as 60,000 persons in the USA and a total of 103,000 persons (<5 per 10,000) in the European Union [1, 2]. It is the second most common inherited cause of kidney failure and is characterized by progressive loss of kidney function that often leads to end-stage kidney disease. Currently, there are no approved disease-specific agents for therapeutic use. We designed a phase 3 study (CARDINAL; NCT03019185) to evaluate the safety, tolerability, and efficacy of bardoxolone methyl in patients with Alport syndrome.
Methods
The CARDINAL phase 3 study is an international, multicenter, double-blind, placebo-controlled, randomized registrational trial. Eligible patients were of ages 12–70 years with confirmed genetic or histologic diagnosis of Alport syndrome, eGFR 30–90 mL/min/1.73 m<sup>2</sup>, and urinary albumin to creatinine ratio (UACR) ≤3,500 mg/g. Patients with B-type natriuretic peptide values >200 pg/mL at baseline or with significant cardiovascular histories were excluded. Patients were randomized 1:1 to bardoxolone methyl or placebo, with stratification by baseline UACR.
Results
A total of 371 patients were screened, and 157 patients were randomly assigned to receive bardoxolone methyl (n = 77) or placebo (n = 80). The average age at screening was 39.2 years, and 23 (15%) were <18 years of age. Of the randomized population, 146 (93%) had confirmed genetic diagnosis of Alport syndrome, and 62% of patients had X-linked mode of inheritance. Mean baseline eGFR was 62.7 mL/min/1.73 m<sup>2</sup>, and the geometric mean UACR was 141.0 mg/g. The average annual rate of eGFR decline prior to enrollment in the study was −4.9 mL/min/1.73 m<sup>2</sup> despite 78% of the patient population receiving ACE inhibitor (ACEi) or ARB therapy.
Discussion/Conclusion
CARDINAL is one of the largest interventional, randomized controlled trials in Alport syndrome conducted to date. Despite the use of ACEi or ARB, patients were experiencing significant loss of kidney function prior to study entry.
Keywords: Alport syndrome, Chronic kidney disease, Inflammation
Introduction
Alport syndrome is a rare and serious genetic disease that affects as many as 60,000 persons in the USA and an estimated 103,000 persons (<5 per 10,000) in the European Union [1, 2]. It is the second most common inherited cause of kidney failure after polycystic kidney disease [3]. Alport syndrome is caused by mutations in the COL4A3, COL4A4, and COL4A5 genes, encoding the α3, α4, and α5 chains of type IV collagen. Type IV collagen is a major constituent of basement membranes. More than 500 different mutations have been described, mostly linked to X-linked chromosomal (XLAS, 67%) and autosomal dominant inheritance [4]. The defective type IV collagen in Alport syndrome leads to typical splitting in the glomerular basement membrane, podocyte effacement, glomerulosclerosis with matrix deposition, and kidney fibrosis and end-stage kidney disease (ESKD) early in life. Although the genetic defect affects type IV collagen in the glomerular basement membrane, secondary events lead to tubulointerstitial fibrosis with resulting loss of kidney function [5].
Current treatment recommendations [6, 7] include drugs that block the renin-angiotensin aldosterone system in proteinuric patients with Alport syndrome. While these agents consistently reduce blood pressure and proteinuria across a broad range of glomerular diseases, the effects of renin-angiotensin aldosterone system inhibitors in Alport syndrome have not been previously tested in any randomized controlled trial.
Bardoxolone methyl is a semisynthetic triterpenoid that activates nuclear factor erythroid 2-related factor 2 (Nrf2), a transcription factor that modulates the expression of hundreds of genes involved in inflammation, oxidative stress, and cellular energy metabolism [8, 9]. By activating the Keap1-Nrf2 pathway, bardoxolone methyl also suppresses nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), the primary transcription factor producing proteins that mediate inflammation and the production of reactive oxygen species [10, 11, 12, 13, 14, 15]. Bardoxolone methyl has been studied in multiple clinical trials that together have enrolled >3,000 patients with one of six different forms of CKD, including Alport syndrome, autosomal dominant polycystic kidney disease (ADPKD), IgA nephropathy, focal segmental glomerulosclerosis (FSGS), and diabetic kidney disease. In these clinical studies, treatment with bardoxolone methyl has consistently improved kidney function as assessed by either inulin clearance, creatinine clearance, or estimated GFR (eGFR) [16, 17, 18]. Increases in eGFR with bardoxolone methyl treatment relative to placebo have been observed in multiple long-term clinical studies, which persist approximately 4 weeks after cessation of the drug [16, 17, 19]. These results are thought to reflect the drug's antifibrotic effects and are consistent with beneficial effects on structural remodeling observed in animal models of CKD [20, 21, 22, 23, 24]. In patients with Alport syndrome, the sustained improvement in kidney function could provide a multi-year delay in disease progression to ESKD.
Materials and Methods
Overview
CARDINAL (NCT03019185) is an international, multicenter, phase 2/3 trial designed to assess the safety, tolerability, and efficacy of bardoxolone methyl in qualified patients with Alport syndrome. The CARDINAL phase 2 study was open label and enrolled 30 patients, and it is not the subject of this publication. The CARDINAL phase 3 study is an international, double-blind, randomized, placebo-controlled trial. The general design and participants' baseline characteristics from the CARDINAL phase 3 study are discussed herein.
Patient Population
We enrolled patients aged 12–70 years with Alport syndrome diagnosed histologically (by electron microscopy) or genetically by a documented mutation in disease-associated genes, including COL4A3, COL4A4, or COL4A5. Genetic testing was conducted as part of the trial by an independent vendor (Machaon Diagnostics, Oakland, CA, USA) unless patients provided documentation of prior genetic or histological diagnosis for eligibility. Patients had eGFR between 30 and 90 mL/min/1.73 m2 (inclusive) and urinary albumin to creatinine ratio (UACR) ≤3,500 mg/g. Patients with macroalbuminuria (UACR of 301–3,500 mg/g) were to comprise no >40% of enrolled trial participants. Patients were to have received maximally tolerated labeled doses of an angiotensin-converting enzyme inhibitor (ACEi) and/or angiotensin receptor blocker (ARB), unless medically contraindicated. Patients with clinically significant cardiovascular disease or serum B-type natriuretic peptide concentrations >200 pg/mL during screening were not eligible for the study. Patients with uncontrolled diabetes and/or hypertension were also excluded.
Study Outcomes
The primary efficacy endpoint for CARDINAL is change from baseline in eGFR in bardoxolone methyl-treated patients relative to placebo after 48 and 100 weeks of treatment. Key secondary endpoints were the off-treatment changes from baseline in eGFR at weeks 52 and 104, following a 4-week withdrawal period.
Several exploratory endpoints at the 48- and 100-week time points will also be considered: (1) the proportion of patients who experienced either a 30% increase or decrease from baseline in eGFR; (2) the distribution of changes in eGFR from baseline; (3) Patient Global Impression of Change (PGIC) and Clinical Global Impression-Improvement (CGI-I) scores after 48 and 100 weeks of treatment; and (4) the proportion of patients with a kidney failure event defined as the composite endpoint consisting of confirmed ≥30% decline from baseline in eGFR, confirmed eGFR <15 mL/min/1.73 m2, or ESKD (initiation of maintenance dialysis or kidney transplant).
Study Design
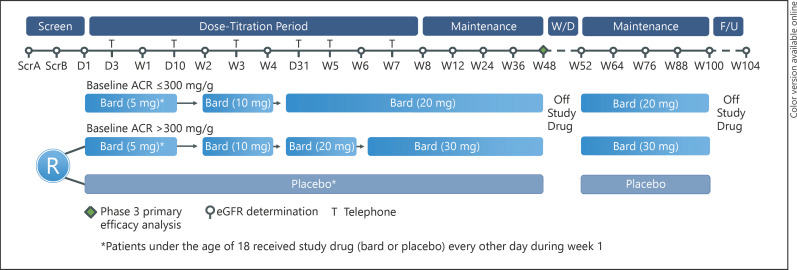
Patients with Alport syndrome who met the inclusion and exclusion criteria were randomized 1:1 to receive either bardoxolone methyl or placebo. Randomization was stratified by baseline UACR (≤300 mg/g, 300 to ≤1,000 mg/g, and 1,000 to ≤3,500 mg/g). Patients randomized to placebo remained on placebo throughout the study, undergoing sham dose titration. The target maximum bardoxolone methyl dose was determined by baseline proteinuria status. Adult patients started once-daily dosing by receiving 5 mg for the first week, and dose escalated to 10 mg at week 2, to 20 mg at week 4, and lastly to 30 mg at week 6 (only if baseline UACR >300 mg/g). Patients <18 years of age started bardoxolone methyl dosing at 5 mg every other day for the first week, followed by 5 mg once daily at week 2, and then increased dosage every 2 weeks following the same aforementioned dose-titration scheme based on baseline UACR at weeks 2, 4, and 6 to achieve the same target doses as adult patients.
All patients in the study follow the same visit and assessment schedule (Fig. 1). Following randomization on day 1, patients are assessed in-person at weeks 1, 2, 4, 6, 8, 12, 24, 36, 48, 52, 64, 76, 88, 100, and 104 and by telephone contact on days 3, 10, 21, 31, 38, and 45. Study drug is discontinued for 4 weeks between weeks 48 and 52. Patients restart treatment at week 52 at the same dose received at week 48, and patients continue study drug treatment through week 100. At week 100 visit study, the drug was discontinued again for 4 weeks, and a follow-up visit is scheduled at week 104.
Fig. 1.
CARDINAL trial design. UACR, urinary albumin to creatinine ratio; Bard, bardoxolone methyl; eGFR, estimated glomerular filtration rate; F/U, follow-up; ScrA, screening visit A; ScrB, screening visit B; W/D, withdrawal.
We calculated eGFR using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation or Bedside Schwartz equation for patients under the age of 18 at the time of consent. Pediatric patients who turned 18 during the conduct of the trial continued to have eGFR calculated with the Bedside Schwartz equation. Clinical lab evaluations, including eGFR, were assessed at each in-person visit. We collected historical eGFR data from all patients for a period of up to 5 years prior to study entry to estimate the annualized change in eGFR. We converted all historical serum creatinine values to mg/dL in order to calculate eGFR according to the appropriate equation using the patient age at laboratory collection for adult patients, or patient height at screening for pediatric patients. Historical serum creatinine and corresponding eGFR values were considered part of a patient's medical history and distinct from central lab assessments collected as part of the trial. We collected first morning void urine samples for UACR at screening and every 4 weeks until week 12 and every 12 weeks thereafter until the end of treatment; UACR was also assessed at weeks 52 and 104 following a 4-week study drug withdrawal period. We conducted pure tone audiology tests at day 1 and weeks 48 and 100 to assess changes in hearing, also an exploratory outcome.
Statistical Analysis
We summarize demographics and baseline clinical characteristics using descriptive statistics; continuous data are summarized with number of mean or geometric mean (where noted) and standard deviation (SD). Categorical data are summarized using proportions.
We defined baseline eGFR for the study as the average of the 2 screening measurements and the day 1 eGFR measurement. For evaluation of the eGFR trajectory before trial participation, we obtained all eGFR values before trial participation going as far back as the eGFR value closest to 5 years but not >5.5 years and calculated the annualized eGFR slope (in mL/min/1.73 m2/year). We employed a longitudinal mixed model with the eGFR at each available time point as the dependent variable and time in years as the covariate. UACR results were log transformed for analysis and summarized using geometric means. In accordance with published guidance, we did not perform inference tests comparing baseline characteristics across groups.
Results
Enrollment
Between August 2017 and November 2018, 371 patients were screened, and of those 157 enrolled at 47 study sites in the USA, Europe, Japan, and Australia. Patients were randomly allocated to bardoxolone methyl (77 patients) or placebo (80 patients). Main reasons for exclusion were screening eGFR values that were <30 or >90 mL/min/1.73 m2, UACR values >3,500 mg/g, and absence of genetic or histological confirmation of Alport syndrome diagnosis.
Demographic and Baseline Characteristics in the Overall, Adult, and Pediatric Populations
Baseline characteristics of the enrolled patient population are shown in Table 1. The mean age was 39.2 years (range, 13–70 years), and 134 of 157 patients (85.0%) were 18 years or older. The majority of patients were female (91/157, 58%) and Caucasian (118/157, 75%).
Table 1.
Baseline characteristics
| Parameter | All | Adult | Pediatric |
|---|---|---|---|
| Patients, n | 157 | 134 | 23 |
| Age, years, mean ± SD | 39.2±15.3 | 43.3±12.5 | 15.3±1.4 |
| Age <18, n (%) | 23 (15) | 0 | 23 (100) |
| Sex (female), n (%) | 91 (58) | 87 (65) | 4 (17) |
| Race, n (%) | |||
| White (Caucasian) | 118 (75) | 109 (81) | 9 (39) |
| Asian | 26 (17) | 19 (14) | 7 (30) |
| Black or African American | 5 (3) | 2 (2) | 3 (13) |
| Others | 8 (5) | 4 (3) | 4 (17) |
| Systolic blood pressure, mm Hg, mean ± SD | 119.7±12.5 | 120.6±12.5 | 114.1±11.4 |
| Diastolic blood pressure, mm Hg, mean ± SD | 73.2±10.0 | 74.6±9.7 | 65.2±8.0 |
| Baseline eGFR, mL/min/1.73 m2, mean ± SD | 62.7±17.9 | 61.4±18.1 | 69.9±15.4 |
| ≤60 mL/min/1.73 m2, n (%) | 66 (42) | 62 (46) | 4 (17) |
| > 60 mL/min/1.73 m2, n (%) | 91 (58) | 72 (54) | 19 (83) |
| Historic annual change in eGFR, mL/min/1.73 m2, mean ± SE | −4.85±0.38 | −4.28±0.39 | −10.74±1.19 |
| ACR, mg/g, geometric mean ± SE | 141.0±23.9 | 129.5±23.9 | 230.9±95.8 |
| <30 mg/g, n (%) | 39 (25) | 36 (27) | 3 (13) |
| ≤300 mg/g | 85 (54) | 75 (56) | 10 (44) |
| >300 mg/g | 72 (46) | 59 (44) | 13 (57) |
| >1,000 mg/g | 36 (23) | 28 (21) | 8 (35) |
| Baseline CKD stage, n (%) | |||
| 1 | 3 (2) | 3 (2) | 0 |
| 2 | 89 (57) | 70 (52) | 19 (83) |
| 3a | 33 (21) | 32 (24) | 1 (4) |
| 3b | 29 (19) | 26 (19) | 3 (13) |
| 4 | 3 (2) | 3 (2) | 0 |
| Age at Alport syndrome diagnosis, years, mean ± SD | 29.7±17.9 | 33.0±17.2 | 10.5±5.1 |
| Confirmed histologic diagnosis of Alport syndrome, n (%) | 32 (20) | 26 (19) | 6 (26) |
| Confirmed genetic diagnosis of Alport syndrome, n (%) | 146 (93) | 126 (94) | 20 (87) |
| Mode of inheritance, n (%) | |||
| X-linked | 98 (62) | 84 (63) | 14 (61) |
| Autosomal (recessive or dominant) | 48 (31) | 42 (31) | 6 (26) |
| Genotype, n (%) | |||
| COL4A3 mutation | 14 (9) | 12 (9) | 2 (9) |
| COL4A4 mutation | 30 (19) | 27 (20) | 3 (13) |
| COL4A3 and COL4A4 mutation | 4 (3) | 3 (2) | 1 (4) |
| COL4A5 mutation | 98 (62) | 84 (63) | 14 (61) |
| COL4A3 and COL4A5 mutation | 1 (1) | 1 (1) | 0 |
| COL4A4 and COL4A5 mutation | 1 (1) | 1 (1) | 0 |
| ACEi/ARB treatment, n (%) | 122 (78) | 105 (78) | 17 (74) |
| Hearing loss, n (%) | 70 (45) | 59 (44) | 11 (48) |
| Hematuria, n (%) | 135 (86) | 116 (87) | 19 (83) |
| Weight, kg, mean ± SD | 75.0±18.0 | 77.4±17.7 | 61.1±13.7 |
| Body mass index, kg/m2, mean ± SD | 26.6±5.9 | 27.5±5.8 | 21.1±3.0 |
eGFR, estimated glomerular filtration rate; UACR, urinary albumin to creatinine ratio; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
The mean age at Alport syndrome diagnosis was 29.7 years for the overall population. Of the enrolled patients, 32 (20%) had a histological diagnosis and 146 (93%) had a genetic diagnosis of Alport syndrome; 98 (62%) had an X-linked genetic subtype and 48 (31%) patients had a non-X-linked (autosomal recessive or dominant) genetic subtype. Most of the patients were receiving an ACEi or ARB (122 [78%]). Seventy (45%) patients reported impaired auditory acuity at the time of screening, and 135 (86%) patients reported hematuria at baseline, which was not systematically assessed during the study. Mean weight and BMI were 75.0 kg and 26.6 kg/m2 at baseline, respectively. Blood pressure was well controlled, with mean systolic and diastolic blood pressures of 120 and 73 mm Hg.
The mean ± SD baseline eGFR for the overall population was 62.7 ± 17.9 mL/min/1.73 m2 with a total of 66 (42%) patients having an eGFR value ≤60 mL/min/1.73 m2. The average rate of annual eGFR decline prior to enrollment in the study was −4.9 mL/min/1.73 m2. The geometric mean UACR at baseline for the overall patient population was 141.0 mg/g with 72 (46%) patients having an UACR >300 mg/g.
A total of 23 (15%) pediatric patients (<18 years of age) were randomized in the trial. The average age at screening for patients in the pediatric population was 15.3 ± 1.4 years. A total of 14 of 23 (61%) patients in the pediatric population had an X-linked mode of inheritance, and 4 (17%) patients were female. The pediatric population had a mean baseline eGFR value of 69.9 mL/min/1.73 m2. Thus, despite their young age, the enrolled pediatric patients had lost a meaningful degree of kidney function prior to study entry. Moreover, the historical average annual eGFR decrease in these patients was −10.7 mL/min/1.73 m2, a more rapid rate of disease progression than that observed in the adult patients (−4.3 mL/min/1.73 m2 per year). The pediatric population had a higher baseline UACR than the adult population, with a mean baseline UACR of 230.9 mg/g, and 13 (57%) patients had UACR >300 mg/g. Eleven (48%) pediatric patients reported impaired auditory acuity at the time of the screening. Mean weight and BMI were slightly lower than the adult population (61.1 kg and 21.1 kg/m2 at baseline, respectively).
Baseline Characteristics according to eGFR and Albuminuria Categories
Of the randomized patients, a total of 66 (42%) patients had baseline eGFR ≤60 mL/min/1.73 m2 (Table 2). Patients with a lower eGFR had a higher average UACR (268.8 mg/g), were older, and were more likely to be treated with ACEi or ARBs (60 [91%] patients) and to have X-linked Alport syndrome (44 [67%] compared to 54 [59%] in the group with higher eGFR). Patients in this subgroup also had numerically higher mean systolic blood pressure compared to the patients with higher eGFR (124 vs. 117 mm Hg, respectively). Patients in the lower eGFR group were more likely to have had hearing loss at baseline (34 [52%] patients) than patients in the higher eGFR group (36 [40%] patients).
Table 2.
Baseline characteristics according to eGFR and albuminuria categories
| Parameter | Baseline eGFR |
Baseline ACR |
||
|---|---|---|---|---|
| ≤60 mL/min/1.73 m2 | >60 mL/min/1.73 m2 | ≤300 mg/g | >300 mg/g | |
| Patients, n | 66 | 91 | 85 | 72 |
| Age, years, mean ± SD | 44.5±14.9 | 35.4±14.4 | 43.3±14.4 | 34.4±15.0 |
| Age <18, n (%) | 4 (6) | 19 (21) | 10 (12) | 13 (18) |
| Sex (female), n (%) | 37 (56) | 54 (59) | 64 (75) | 27 (38) |
| Race, n (%) | ||||
| White (Caucasian) | 55 (83) | 63 (69) | 72 (85) | 46 (64) |
| Black or African American | 1 (2) | 4 (4) | 4 (5) | 1 (1) |
| Asian | 7 (11) | 19 (21) | 8 (9) | 18 (25) |
| Others | 3 (5) | 5 (6) | 1 (1) | 7 (10) |
| Systolic blood pressure, mm Hg, mean ± SD | 123.5±12.9 | 116.9±11.5 | 116.4±11.5 | 123.5±12.7 |
| Diastolic blood pressure, mm Hg, mean ± SD | 76.5±9.8 | 70.8±9.5 | 71.3±9.0 | 75.4±10.7 |
| Baseline eGFR, mL/min/1.73 m2, mean ± SD | 44.5±9.6 | 75.9±8.3 | 68.3±16.6 | 56.1±17.3 |
| ≤60 mL/min/1.73 m2, n (%) | 66 (100) | 0 | 28 (33) | 38 (53) |
| >60 mL/min/1.73 m2, n (%) | 0 | 91 (100) | 57 (67) | 34 (47) |
| ACR, mg/g, geometric mean ± SE | 268.8±66.7 | 88.3±19.3 | 28.3±4.7 | 938.0±69.7 |
| <30 mg/g, n (%) | 9 (14) | 30 (33) | 39 (46) | 0 |
| 30 to ≤300 mg/g, n (%) | 28 (42) | 57 (63) | 85 (100) | 0 |
| >300 to ≤1,000 mg/g, n (%) | 38 (58) | 34 (38) | 0 | 72 (100) |
| >1,000 mg/g, n (%) | 25 (38) | 11 (12) | 0 | 36 (50) |
| Age at Alport syndrome diagnosis, years, mean ± SD | 32.2±19.7 | 27.8±16.3 | 34.4±17.4 | 24.1±16.9 |
| Confirmed histologic diagnosis of Alport syndrome, n (%) | 17 (26) | 15 (17) | 12 (14) | 20 (28) |
| Confirmed genetic diagnosis of Alport syndrome, n (%) | 61 (92) | 85 (93) | 82 (96) | 64 (89) |
| Mode of inheritance, n (%) | ||||
| X-linked | 44 (67) | 54 (59) | 49 (58) | 49 (68) |
| Autosomal (recessive or dominant) | 17 (26) | 31 (34) | 33 (39) | 15 (21) |
| Genotype, n (%) | ||||
| COL4A3 mutation | 4 (6) | 10 (11) | 8 (9) | 6 (8) |
| COL4A4 mutation | 12 (18) | 18 (20) | 22 (26) | 8 (11) |
| COL4A3 and COL4A4 mutation | 1 (2) | 3 (3) | 3 (4) | 1 (1) |
| COL4A5 mutation | 44 (67) | 54 (59) | 49 (58) | 49 (68) |
| COL4A3 and COL4A5 mutation | 1 (2) | 0 | 0 | 1 (1) |
| COL4A4 and COL4A5 mutation | 0 | 1 (1) | 1 (1) | 0 |
| ACEi/ARB treatment, n (%) | 60 (91) | 62 (68) | 54 (64) | 68 (94) |
| Hearing loss, n (%) | 34 (52) | 36 (40) | 30 (35) | 40 (56) |
| Hematuria, n (%) | 54 (82) | 81 (89) | 73 (86) | 62 (86) |
eGFR, estimated glomerular filtration rate; UACR, urinary albumin to creatinine ratio; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Seventy-two (46%) patients had a baseline UACR >300 mg/g (macroalbuminuria). Compared with patients with UACR ≤300 mg/g, patients with macroalbuminuria were younger and were diagnosed with Alport syndrome at a younger age, had a lower mean baseline eGFR (56.1 mL/min/1.73 m2), and were more likely to be on ACEi or ARB (68 [94%] patients). Patients with macroalbuminuria were also more likely to be male (45 [63%] patients compared to 66 [42%] patients in the overall patient population and 21 [25%] patients in the subgroup with lower UACR) and more likely to have X-linked Alport syndrome (49 [68%] patients compared to 49 [58%] in the subgroup with lower UACR). These patients had a numerically higher mean systolic blood pressure compared to the patients with lower UACR (124 vs. 116 mm Hg, respectively) and were also more likely to have had hearing loss (40 [56%] patients vs. 30 [35%] patients, respectively).
Discussion
The CARDINAL trial is a multinational randomized double-blind, placebo-controlled trial evaluating the safety and efficacy of bardoxolone methyl in patients with Alport syndrome. A key secondary endpoint of the trial is the off-treatment change from baseline at week 52 (after 48 weeks of treatment) and again at week 104 after the second year of treatment. The off-treatment endpoint follows recent recommendations by the NKF-FDA-EMA Scientific Working group for evaluating off-treatment changes in eGFR cases where acute eGFR effects are observed [25] and will evaluate bardoxolone methyl's effect on the loss of kidney function in patients with Alport syndrome.
The CARDINAL phase 3 trial has enrolled a total of 157 patients in 16 months, including 134 adult and 23 pediatric patients. The trial studied multiple stages of the disease and has included patients with a wide range of baseline eGFR (30–90 mL/min/1.73 m2). The mean baseline age in the trial was 39.2 years, which supports the notion that Alport syndrome generally afflicts a younger patient population than many other forms of CKD.
Patients with Alport syndrome are usually diagnosed due to onset of hematuria in childhood or early adulthood, followed later by proteinuria and progressive loss of kidney function. Although the severity of disease manifestations differs by the type of causative mutation, all forms of Alport syndrome are characterized by progressive nephropathy and are therefore associated with an increased risk for progression to ESKD. In male patients with X-linked Alport syndrome, the median age at onset of ESKD is 25 years; the incidence of kidney failure increases to 90% by age 40 and nearly 100% by age 60 for these patients [26]. Alport syndrome accounts for an estimated 0.2% of adults and 3% of children with ESKD in the USA [3]. As a result, children and young adults diagnosed with Alport syndrome may experience a dramatic increase in the risk of cardiovascular and other adverse health events and altered growth.
As is the case with other chronic progressive kidney diseases, inflammation has been shown to be a pathogenic feature of Alport syndrome that correlates with declining kidney function [27]. Data from multiple animal models of CKD demonstrate that bardoxolone methyl and closely related structural analogs suppress inflammation and fibrosis, reduce glomerulosclerosis, prevent tubulointerstitial damage, and improve kidney function [20, 21, 22, 23, 24]. Additional studies have demonstrated that acute treatment with bardoxolone methyl reverses endothelial dysfunction and mesangial cell contraction, increases glomerular surface area (Kf), and restores single nephron glomerular filtration rate without changes in intraglomerular pressure [28, 29]. Because of these properties, bardoxolone methyl may be effective at ameliorating the rate of decline in eGFR in patients with Alport syndrome.
A prior trial (BEACON, NCT01351675) showed that patients with type 2 diabetes mellitus and stage 4 CKD randomized to bardoxolone methyl experienced a significant increase in events of heart failure or fluid overload; the excess risk was evident during the first 4 weeks of the trial. In post hoc analyses, patients with a history of heart failure or an elevated BNP (>200 ng/mL) were found to be at increased risk [17, 30]. As a result, subsequent clinical trials, including CARDINAL, excluded patients with those clinical characteristics as well as severe (stage 4) CKD and UACR >3,500 mg/g. Additionally, unlike BEACON, CARDINAL utilized a dose-titration regimen to allow for individual dose optimization based on tolerability and to potentially mitigate some of the tolerability issues thought to be related to fixed dosing in BEACON. The maximum allowable dose was determined based on a patient's albuminuria status at baseline. Results from a prior dose-ranging trial in patients with diabetic kidney disease suggested that higher bardoxolone methyl doses may be required to have an optimal effect on eGFR in patients with macroalbuminuria. Specifically, in patients with macroalbuminuria at baseline, a 30-mg dose was required to produce an eGFR response that was similar to that in patients with microalbuminuria treated at 20 mg. Consequently, CARDINAL included dose titration up to a maximum dose of 20 mg (same dose as that utilized in BEACON) for patients with UACR ≤300 mg/g and a maximum dose of 30 mg for patients with UACR >300 mg/g. The trial also limited enrollment of patients with baseline UACR >300 mg/g to approximately 40% of the randomized population, which may have excluded some patients at highest risk for progression from entering the trial. Nevertheless, despite the majority of patients having baseline UACR ≤300 mg/g, approximately 40% of randomized patients had baseline eGFR ≤60 mL/min/1.73 m2, demonstrating progressive loss of kidney function even in patients with lower levels of albuminuria and despite the use of ACEi or ARB therapy in more than three-quarters of trial participants. Moreover, the average rate of annual eGFR decline prior to enrollment in the study was −4.9 mL/min/1.73 m2, consistent with that observed in a prior Alport syndrome natural history study (−4.0 mL/min/1.73 m2; [31]), and more pronounced than that reported in other forms of CKD, including diabetic kidney disease, hypertensive CKD, and ADPKD [32, 33, 34]. Additional evaluation of the trial population by baseline eGFR or UACR categories showed that patients with Alport syndrome with more advanced kidney disease (either lower eGFR or higher UACR) tended to have X-linked Alport syndrome, were receiving ACEi or ARB, and exhibited other manifestations of the disease, including hearing loss.
While the majority of adult patients randomized into the trial were female, all but four of the pediatric patients were male. The predominance of male sex in the pediatric population is likely due to the fact that a majority of the patients enrolled in the study had an X-linked mode of inheritance, and X-linked male patients typically develop symptoms of Alport syndrome earlier than female patient [35]. Data from CARDINAL phase 3 demonstrate a more severe course of kidney disease in pediatric patients with Alport syndrome, who were predominantly male patients with X-linked genetic defects; their mean baseline UACR was higher, and despite their younger age, their mean baseline eGFR was similar to that of the adult population. Lastly, pediatric patients had an average annual eGFR decrease of −10.7 mL/min/1.73 m2 prior to enrollment in the trial.
Overall, the demographic and baseline characteristics of patients enrolled in the CARDINAL trial are representative of the Alport syndrome patient population and are generally consistent with the characteristics of patients enrolled in a prior natural history study in Alport syndrome (ATHENA; [31]). Notable differences included the distribution of male versus female patients; while more than one-half of patients randomized in CARDINAL were female, they comprised over two-thirds of the total enrolled population in ATHENA.
In CARDINAL, the diagnosis of Alport syndrome was confirmed either histologically or through genetic testing. More specifically, patients with a documented mutation in genes associated with Alport syndrome, including COL4A3, COL4A4, or COL4A5, were enrolled in the trial. Approximately 62% of randomized patients had X-linked Alport syndrome, with COL4A5 mutations. Because family history data were not collected as part of the study, we were unable to distinguish between autosomal dominant or autosomal recessive forms of the disease based on genetic testing alone. Nevertheless, the criteria used to define genetic confirmation of Alport syndrome are consistent with recent recommendations from the Alport Syndrome Classification Working Group. Notably, the Working Group proposed unifying the classification of all genetic disorders arising from COL4A mutations as an Alport Syndrome spectrum [4]. Accordingly, other known collagen-related kidney diseases, such as thin basement membrane nephropathy, benign familial hematuria, familial proteinuria, and some forms of FSGS, may also be classified as Alport syndrome under the proposed classification scheme. Furthermore, adopting this new classification system may also suggest that there are more patients with Alport syndrome who are undiagnosed or misdiagnosed [36].
The newly proposed classification scheme is based on genetic criteria instead of relying solely on histologic and clinical traits that are sex- and age-dependent and underscores the need for genetic testing in chronic kidney diseases. Indeed, recent expert consensus guidelines recommend genetic testing, which is more sensitive and specific than kidney biopsy, as the gold standard for the confirmation of an Alport syndrome diagnosis [37]. This idea has been supported by several recent studies that demonstrated significant diagnostic capabilities of genetic testing in patients with a variety of chronic kidney diseases. For example, genetic testing was shown to provide an accurate diagnosis in up to 40% of patients with kidney disease of unknown etiology [38]. Separately, recent gene sequencing studies have consistently demonstrated that variants in COL4A3, COL4A4, and COL4A5 commonly result in sporadic and familial adult FSGS, which should be identified as Alport syndrome based on the reclassification proposal [39, 40]. Likewise, Groopman et al. [41] demonstrated that almost 10% of patients with kidney disease tested by whole exome sequencing had a genetic defect, with COL4A gene variants being most common. In patients with suspected Alport syndrome, high throughput-targeted next-generation sequencing technologies with a customized panel for testing all 3 Alport genes − COL4A3, COL4A4, and COL4A5 − together can identify up to 95% of pathogenic COL4A variants [7, 37].
In conclusion, CARDINAL is the largest interventional, randomized controlled study in Alport syndrome that uniquely offers insights into the characteristics of an Alport syndrome trial population. Despite the frequent use of ACEi or ARB therapy, the CARDINAL trial population still exhibited varying degrees of compromised kidney function at baseline, with meaningful historical rates of kidney function loss. These findings emphasize the need for novel therapeutic agents in this population and renders CARDINAL results relevant to the broader population of patients with Alport syndrome.
Statement of Ethics
The study protocol was designed and implemented in accordance with the ICH Harmonized Tripartite Guidelines for Good Clinical Practice (as adapted by local health authorities), with applicable local regulations (including European Directive 2001/20/EC and UC Code of Federal Regulations Title 21) and with the ethical principles laid down in the Declaration of Helsinki. The study was registered at http://www.clinicaltrials.gov (NCT03019185).
Conflict of Interest Statement
G.B.A. reports personal fees from Alexion, Achillion, Ionis, Genentech, Mallinckrodt, Pfizer, Merck, Roche, Bristol-Myers Squibb, Up-to-Date, Genzyme-Sanofi, END, Serono, Omeros, Regulus, and Zyversa and grants from Sanofi-Genzyme, Achillion, Bristol-Myers Squibb, EMD Serono, Retrophin, Aurinia, Calliditas, ChemoCentryx, Zyversa, Mallinckrodt, Genentech, and NIH. S.A. has no disclosures to report. S.B. reports grants and personal fees from Reata Pharmaceuticals outside the submitted work. G.A.B. reports personal fees from Ardelyx and USRC during the conduct of the study and previous employment at Reata and KKC, as well as previous grants from Akebia and Keryx outside of the submitted work. G.M.C. reports personal fees from Reata, during the conduct of the study, personal fees from Akebia, Angion, Ardelyx, AstraZeneca, Bayer, Baxter, CloudCath, Cricket, DiaMedica, Durect, Gilead, Miromatrix, ReCor, Sanifit, Satellite Healthcare, and Vertex, and grants from Amgen, outside the submitted work. K.I. reports personal fees from Kyowa Kirin Co., Ltd. during the conduct of the study, grants and personal fees from Teijin Pharma Co., Ltd., Zenyaku Kogyo Co., Ltd., and Astellas Pharma Inc., grants from CSL Behring, Daiichi Sankyo, Co., Ltd., Biofermin Pharmaceutical Co., Ltd., Air Water Medical Inc., Otsuka Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., and Shionogi & Co., and personal fees from JCR Pharmaceuticals Co., Ltd., Chugai Pharmaceutical Co., Ltd., Asahi Kasei Pharma Corp., Takeda Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Sanofi K.K., Novartis Pharma K.K., Nippon Boehringer Ingelheim Co., Ltd., and Integrated Development Associates, outside the submitted work. L.A.I. reports grants from Reata Pharmaceuticals, Omeros Corporation, Retrophin, and Otsuka and other support from Omeros and Tricida, outside the submitted work. B.K. has no disclosures to report. L.H.M. reports personal fees from Reata during the conduct of the study, personal fees from Calliditas and Retrophin, and grants from Boehringer Ingelheim, outside the submitted work. K.N. reports personal fees from Kyowa Kirin Co. Ltd, outside the submitted work. P.E.P. reports personal fees from Abbvie, Akebia, AstraZeneca, Bayer, Corvidia, Gilead, and Reata during the study and is also an employee of Renal Associates, PA. A.L.S. reports research funding from Reata Pharmaceuticals during the conduct of the study. P.S. reports personal fees from Reata Pharmaceuticals, Baxter, AstraZeneca, VIFOR, FMC, and Astellas and grants from AstraZeneca and Bayer, outside the submitted work. R.T. has no disclosures to report. B.A.W. reports personal fees from Reata during the conduct of the study. M.P.C., A.S., and M.O. are employees of Reata Pharmaceuticals. C.J.M. is also an employee for Reata Pharmaceuticals and, in addition, as a patent on Methods of Treating Obesity Using Antioxidant Inflammation Modulators (Pending in US and a number of ex-US territories, granted in certain ex-US countries including Australia and Japan) licensed to Abbvie and Kyowa Hakko Kirin. G.A.B. reports employment at US Renal Care, past employment at Reata Pharmaceuticals, and previous consultant role at KKC.
Funding Sources
The study was sponsored by Reata Pharmaceuticals, Inc.
Author Contributions
G.M.C., G.B.A., S.A., S.B., G.A.B., A.B.C., M.P.C., K.L.G., A.G., K.I., L.A.I., K.N., B.K., L.H.M., C.J.M., M.O., A.L.S., P.S., R.T., B.A.W., and P.E.P. wrote the manuscript. M.P.C., A.G., C.J.M., and M.O. designed the research. G.M.C., G.B.A., S.A., S.B., G.A.B., A.B.C., K.L.G., K.I., L.A.I., K.N., B.K., L.H.M., A.L.S., P.S., R.T., B.A.W., and P.E.P. performed the research. M.P.C., A.G., C.J.M., and M.O. analyzed the data.
Acknowledgements
We acknowledge the supportive role of all CARDINAL investigators, support staff, and patients. We thank Svetlana Pitts, PhD, and Samantha D. Francis Stuart, PhD, of Reata Pharmaceuticals, for assistance in preparation of the manuscript.
References
- 1.European Medicines Agency Public summary of opinion on orphan designation: bardoxolone methyl for the treatment of Alport syndrome. 2018. Available from: https://www.ema.europa.eu/en/documents/orphan-designation/eu/3/18/2019-public-summary-opinion-orphan-designation-bardoxolone-methyl-treatment-alport-syndrome_en.pdf.
- 2.Alport Syndrome Foundation What is Alport syndrome. 2017. Available from: https://www.alportsyndrome.org/what-is-alport-syndrome/
- 3.USRDS. U.S Renal data system, USRDS 2014 Annual Data Report: atlas of chronic kidney disease and end stage renal disease in the United States. National Institutes of Health; National Institute of Diabetes and Digestive and Kidney Diseases. 2014 [Google Scholar]
- 4.Kashtan CE, Ding J, Garosi G, Heidet L, Massella L, Nakanishi K, et al. Alport syndrome: a unified classification of genetic disorders of collagen IV alpha345: a position paper of the Alport Syndrome Classification Working Group. Kidney Int. 2018 May;93((5)):1045–51. doi: 10.1016/j.kint.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 5.Kruegel J, Rubel D, Gross O. Alport syndrome: insights from basic and clinical research. Nat Rev Nephrol. 2013 Mar;9((3)):170–8. doi: 10.1038/nrneph.2012.259. [DOI] [PubMed] [Google Scholar]
- 6.Kashtan CE, Ding J, Gregory M, Gross O, Heidet L, Knebelmann B, et al. Clinical practice recommendations for the treatment of Alport syndrome: a statement of the Alport Syndrome Research Collaborative. Pediatr Nephrol. 2013 Jan;28((1)):5–11. doi: 10.1007/s00467-012-2138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savige J, Gregory M, Gross O, Kashtan C, Ding J, Flinter F. Expert guidelines for the management of Alport syndrome and thin basement membrane nephropathy. J Am Soc Nephrol. 2013 Feb;24((3)):364–75. doi: 10.1681/ASN.2012020148. [DOI] [PubMed] [Google Scholar]
- 8.Wardyn JD, Ponsford AH, Sanderson CM. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem Soc Trans. 2015 Aug;43((4)):621–6. doi: 10.1042/BST20150014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Hayashi M, Sekine H, et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun. 2016 May 23;7:11624. doi: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmad R, Raina D, Meyer C, Kharbanda S, Kufe D. Triterpenoid CDDO-Me blocks the NF-kappaB pathway by direct inhibition of IKKbeta on Cys-179. J Biol Chem. 2006 Nov 24;281((47)):35764–9. doi: 10.1074/jbc.M607160200. [DOI] [PubMed] [Google Scholar]
- 11.Yore MM, Liby KT, Honda T, Gribble GW, Sporn MB. The synthetic triterpenoid 1-[2-cyano-3, 12-dioxooleana-1, 9(11)-dien-28-oyl]imidazole blocks nuclear factor-kappaB activation through direct inhibition of IkappaB kinase beta. Mol Cancer Ther. 2006 Dec;5((12)):3232–9. doi: 10.1158/1535-7163.MCT-06-0444. [DOI] [PubMed] [Google Scholar]
- 12.Lee DF, Kuo HP, Liu M, Chou CK, Xia W, Du Y, et al. KEAP1 E3 ligase-mediated downregulation of NF-kappaB signaling by targeting IKKbeta. Mol Cell. 2009 Oct 9;36((1)):131–40. doi: 10.1016/j.molcel.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong ET, Tergaonkar V. Roles of NF-kappaB in health and disease: mechanisms and therapeutic potential. Clin Sci. 2009 Mar;116((6)):451–65. doi: 10.1042/CS20080502. [DOI] [PubMed] [Google Scholar]
- 14.Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW. When NRF2 talks, who's listening? Antioxid Redox Signal. 2010 Dec 1;13((11)):1649–63. doi: 10.1089/ars.2010.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011 Jan 5;13((1)):11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pergola PE, Krauth M, Huff JW, Ferguson DA, Ruiz S, Meyer CJ, et al. Effect of bardoxolone methyl on kidney function in patients with T2D and stage 3b-4 CKD. Am J Nephrol. 2011;33((5)):469–76. doi: 10.1159/000327599. [DOI] [PubMed] [Google Scholar]
- 17.de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013 Dec 26;369((26)):2492–503. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nangaku M, Kanda H, Takama H, Ichikawa T, Hase H, Akizawa T. Randomized clinical trial on the effect of bardoxolone methyl on GFR in diabetic kidney disease patients (TSUBAKI Study) Kidney Int Rep. 2020 Jun;5((6)):879–90. doi: 10.1016/j.ekir.2020.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chin MP, Bakris GL, Block GA, Chertow GM, Goldsberry A, Inker LA, et al. Bardoxolone methyl improves kidney function in patients with chronic kidney disease stage 4 and type 2 diabetes: post-hoc analyses from bardoxolone methyl evaluation in patients with chronic kidney disease and type 2 diabetes Study. Am J Nephrol. 2018;47((1)):40–7. doi: 10.1159/000486398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka Y, Aleksunes LM, Goedken MJ, Chen C, Reisman SA, Manautou JE, et al. Coordinated induction of Nrf2 target genes protects against iron nitrilotriacetate (FeNTA)-induced nephrotoxicity. Toxicol Appl Pharmacol. 2008 Sep 15;231((3)):364–73. doi: 10.1016/j.taap.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zoja C, Corna D, Gagliardini E, Conti S, Arnaboldi L, Benigni A, et al. Adding a statin to a combination of ACE inhibitor and ARB normalizes proteinuria in experimental diabetes, which translates into full renoprotection. Am J Physiol Renal Physiol. 2010 Nov;299((5)):F1203–11. doi: 10.1152/ajprenal.00045.2010. [DOI] [PubMed] [Google Scholar]
- 22.Wu QQ, Wang Y, Senitko M, Meyer C, Wigley WC, Ferguson DA, et al. Bardoxolone methyl (BARD) ameliorates ischemic AKI and increases expression of protective genes Nrf2, PPARgamma, and HO-1. Am J Physiol Renal Physiol. 2011 May;300((5)):F1180–92. doi: 10.1152/ajprenal.00353.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aminzadeh MA, Reisman SA, Vaziri ND, Khazaeli M, Yuan J, Meyer CJ, et al. The synthetic triterpenoid RTA dh404 (CDDO-dhTFEA) restores Nrf2 activity and attenuates oxidative stress, inflammation, and fibrosis in rats with chronic kidney disease. Xenobiotica. 2014 Jun;44((6)):570–8. doi: 10.3109/00498254.2013.852705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagasu H, Sogawa Y, Kidokoro K, Itano S, Yamamoto T, Satoh M, et al. Bardoxolone methyl analog attenuates proteinuria-induced tubular damage by modulating mitochondrial function. FASEB J. 2019 Nov;33((11)):12253–63. doi: 10.1096/fj.201900217R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey AS, Gansevoort RT, Coresh J, Inker LA, Heerspink HL, Grams ME, et al. Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the National Kidney Foundation in Collaboration with the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis. 2020 Jan;75((1)):84–104. doi: 10.1053/j.ajkd.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Jais JP, Knebelmann B, Giatras I, De Marchi M, Rizzoni G, Renieri A, et al. X-linked Alport syndrome: natural history in 195 families and genotype-phenotype correlations in males. J Am Soc Nephrol. 2000 Apr;11((4)):649–57. doi: 10.1681/ASN.V114649. [DOI] [PubMed] [Google Scholar]
- 27.Jedlicka J, Soleiman A, Draganovici D, Mandelbaum J, Ziegler U, Regele H, et al. Interstitial inflammation in Alport syndrome. Hum Pathol. 2010 Apr;41((4)):582–93. doi: 10.1016/j.humpath.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 28.Ding Y, Stidham RD, Bumeister R, Trevino I, Winters A, Sprouse M, et al. The synthetic triterpenoid, RTA 405, increases the glomerular filtration rate and reduces angiotensin II-induced contraction of glomerular mesangial cells. Kidney Int. 2013 May;83((5)):845–54. doi: 10.1038/ki.2012.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kidokoro K, Cherney DZI, Bozovic A, Nagasu H, Satoh M, Kanda E, et al. Evaluation of glomerular hemodynamic function by empagliflozin in diabetic mice using in vivo imaging. Circulation. 2019 Jul 23;140((4)):303–15. doi: 10.1161/CIRCULATIONAHA.118.037418. [DOI] [PubMed] [Google Scholar]
- 30.Chin MP, Wrolstad D, Bakris GL, Chertow GM, de Zeeuw D, Goldsberry A, et al. Risk factors for heart failure in patients with type 2 diabetes mellitus and stage 4 chronic kidney disease treated with bardoxolone methyl. J Card Fail. 2014 Dec;20((12)):953–8. doi: 10.1016/j.cardfail.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Chin MP, Wrolstad D, Bakris GL, Chertow GM, de Zeeuw D, Goldsberry A, et al. Risk factors for heart failure in patients with type 2 diabetes mellitus and stage 4 chronic kidney disease treated with bardoxolone methyl. J Card Fail. 2014 Dec;20((12)):953–8. doi: 10.1016/j.cardfail.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Wright JT, Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002 Nov 20;288((19)):2421–31. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 33.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Koch G, et al. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med. 2017 Nov 16;377((20)):1930–42. doi: 10.1056/NEJMoa1710030. [DOI] [PubMed] [Google Scholar]
- 34.Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018 Sep;6((9)):691–704. doi: 10.1016/S2213-8587(18)30141-4. [DOI] [PubMed] [Google Scholar]
- 35.Jais JP, Knebelmann B, Giatras I, De Marchi M, Rizzoni G, Renieri A, et al. X-linked Alport syndrome: natural history and genotype-phenotype correlations in girls and women belonging to 195 families: a “European Community Alport Syndrome Concerted Action” study. J Am Soc Nephrol. 2003 Oct;14((10)):2603–10. doi: 10.1097/01.asn.0000090034.71205.74. [DOI] [PubMed] [Google Scholar]
- 36.Warady BA, Agarwal F, Bangalore S, Champman A, Levin A, Stenvinkel P, et al. Alport syndrome classification and management. Kidney Med. 2020 Aug 7;2((5)):639–49. doi: 10.1016/j.xkme.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savige J, Ariani F, Mari F, Bruttini M, Renieri A, Gross O, et al. Expert consensus guidelines for the genetic diagnosis of Alport syndrome. Pediatr Nephrol. 2019 Jul;34((7)):1175–89. doi: 10.1007/s00467-018-3985-4. [DOI] [PubMed] [Google Scholar]
- 38.Hays T, Groopman EE, Gharavi AG. Genetic testing for kidney disease of unknown etiology. Kidney Int. 2020 Sep;98((3)):590–600. doi: 10.1016/j.kint.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gribouval O, Boyer O, Hummel A, Dantal J, Martinez F, Sberro-Soussan R, et al. Identification of genetic causes for sporadic steroid-resistant nephrotic syndrome in adults. Kidney Int. 2018 Nov;94((5)):1013–22. doi: 10.1016/j.kint.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 40.Yao T, Udwan K, John R, Rana A, Haghighi A, Xu L, et al. Integration of genetic testing and pathology for the diagnosis of adults with FSGS. Clin J Am Soc Nephrol. 2019 Feb 7;14((2)):213–23. doi: 10.2215/CJN.08750718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Groopman EE, Marasa M, Cameron-Christie S, Petrovski S, Aggarwal VS, Milo-Rasouly H, et al. Diagnostic utility of exome sequencing for kidney disease. N Engl J Med. 2019 Jan 10;380((2)):142–51. doi: 10.1056/NEJMoa1806891. [DOI] [PMC free article] [PubMed] [Google Scholar]